Radiotherapy in the Management of Non-Metastatic Inflammatory Breast Cancers: A Retrospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Method
3. Results
3.1. Population
3.2. Treatments
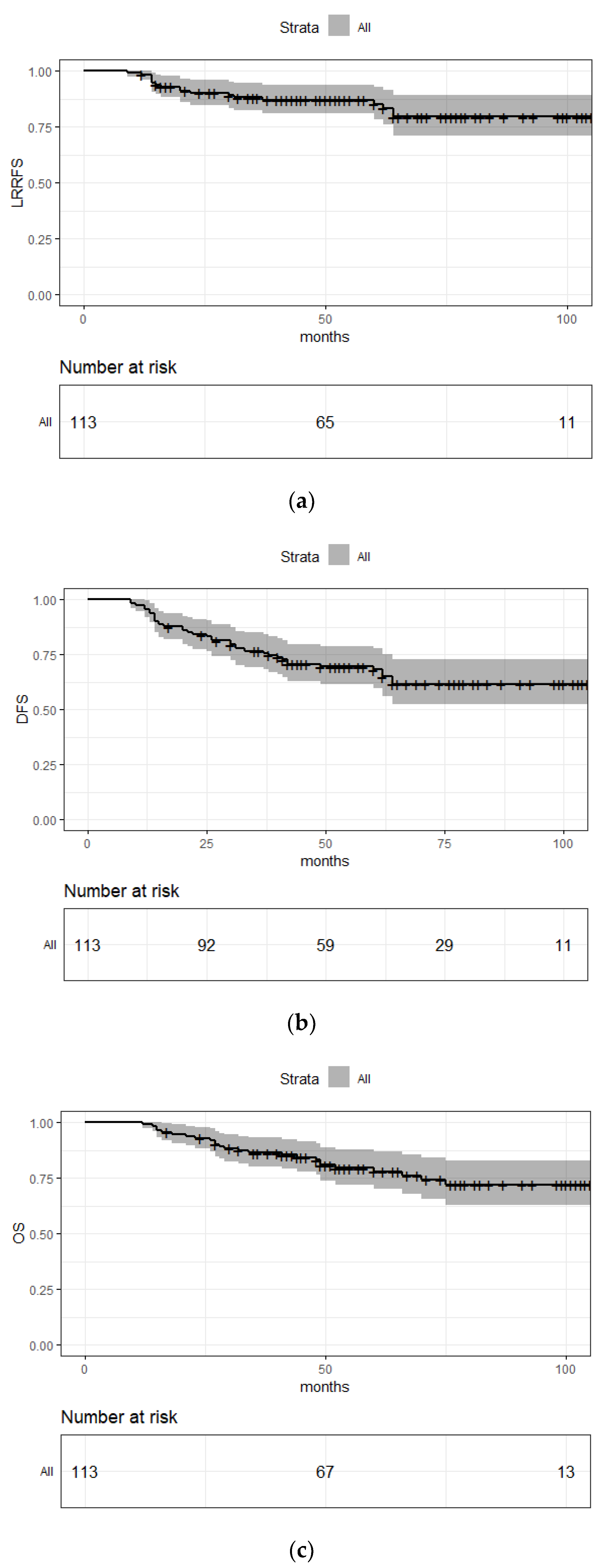
3.3. Outcomes
3.4. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Hance, K.W.; Anderson, W.F.; Devesa, S.S.; Young, H.A.; Levine, P.H. Trends in Inflammatory Breast Carcinoma Incidence and Survival: The Surveillance, Epidemiology, and End Results Program at the National Cancer Institute. J. Natl. Cancer Inst. 2005, 97, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.G.M.; Wittekind, C.H. TNM Classification of Malignant Tumours, 8th ed.; Wiley: Hoboken, NJ, USA, 2007; Available online: https://www.wiley.com/en-au/TNM+Classification+of+Malignant+Tumours%2C+8th+Edition-p-9781119263579 (accessed on 3 October 2021).
- Bristol, I.J.; Woodward, W.A.; Strom, E.A.; Cristofanilli, M.; Domain, D.; Singletary, S.E.; Perkins, G.H.; Oh, J.L.; Yu, T.-K.; Terrefe, W.; et al. Locoregional Treatment Outcomes After Multimodality Management of Inflammatory Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 474–484. [Google Scholar] [CrossRef]
- Damast, S.; Ho, A.Y.; Montgomery, L.; Fornier, M.N.; Ishill, N.; Elkin, E.; Beal, K.; McCormick, B. Locoregional Outcomes of Inflammatory Breast Cancer Patients Treated with Standard Fractionation Radiation and Daily Skin Bolus in the Taxane Era. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Abrous-Anane, S.; Savignoni, A.; Daveau, C.; Pierga, J.-Y.; Gautier, C.; Reyal, F.; Dendale, R.; Campana, F.; Kirova, Y.M.; Fourquet, A.; et al. Management of Inflammatory Breast Cancer after Neoadjuvant Chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Harmsen, W.; Blanchard, M.; Goetz, M.; Jakub, J.; Mutter, R.; Petersen, I.; Rooney, J.; Stauder, M.; Yan, E.; et al. Once-Daily Radiation Therapy for Inflammatory Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 997–1003. [Google Scholar] [CrossRef]
- Genet, D.; Lejeune, C.; Bonnier, P.; Aubard, Y.; Venat-Bouvet, L.; Adjadj, D.J.; Martin, J.; Labourey, J.L.; Benyoub, A.; Clavère, P.; et al. Concomitant Intensive Chemoradiotherapy Induction in Non-Metastatic Inflammatory Breast Cancer: Long-Term Follow-Up. Br. J. Cancer 2007, 97, 883–887. [Google Scholar] [CrossRef][Green Version]
- Biswas, T.; Jindal, C.; Fitzgerald, T.L.; Efird, J.T. Pathologic Complete Response (PCR) and Survival of Women with Inflammatory Breast Cancer (IBC): An Analysis Based on Biologic Subtypes and Demographic Characteristics. Int. J. Environ. Res. Public Health 2019, 16, 124. [Google Scholar] [CrossRef]
- Van Uden, D.J.P.; van Maaren, M.C.; Bult, P.; Strobbe, L.J.A.; van der Hoeven, J.J.M.; Blanken-Peeters, C.F.J.M.; Siesling, S.; de Wilt, J.H.W. Pathologic Complete Response and Overall Survival in Breast Cancer Subtypes in Stage III Inflammatory Breast Cancer. Breast Cancer Res. Treat. 2019, 176, 217–226. [Google Scholar] [CrossRef]
- Wingo, P.A.; Jamison, P.M.; Young, J.L.; Gargiullo, P. Population-Based Statistics for Women Diagnosed with Inflammatory Breast Cancer (United States). Cancer Causes Control 2004, 15, 321–328. [Google Scholar] [CrossRef]
- Rueth, N.M.; Lin, H.Y.; Bedrosian, I.; Shaitelman, S.F.; Ueno, N.T.; Shen, Y.; Babiera, G. Underuse of Trimodality Treatment Affects Survival for Patients with Inflammatory Breast Cancer: An Analysis of Treatment and Survival Trends from the National Cancer Database. J. Clin. Oncol. 2014, 32, 2018–2024. [Google Scholar] [CrossRef]
- Grellier Adedjouma, N.; Chevrier, M.; Fourquet, A.; Costa, E.; Xu, H.; Berger, F.; Campana, F.; Laki, F.; Beuzeboc, P.; Lefeuvre, D.; et al. Long-Term Results of a Highly Performing Conformal Electron Therapy Technique for Chest Wall Irradiation after Mastectomy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Boulle, G.; Saint-Martin, C.; De La Lande, B.; Laki, F.; Bidoz, N.F.; Berger, F.; Veret, A.; Bragard, C.; Fourquet, A.; Kirova, Y.M. Photons without Bolus Versus Electrons with Bolus after Upfront Mastectomy without Immediate Reconstruction in Breast Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 877–884. [Google Scholar] [CrossRef]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.-A.W.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials: The STEEP System. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Barrera, A.M.; Fouad, T.M.; Song, J.; Webster, R.; Elsayegh, N.; Wood, A.L.; Demir, A.; Litton, J.K.; Ueno, N.T.; Arun, B.K. BRCA Mutations in Women with Inflammatory Breast Cancer. Cancer 2018, 124, 466–474. [Google Scholar] [CrossRef]
- Li, C.I.; Anderson, B.O.; Daling, J.R.; Moe, R.E. Trends in Incidence Rates of Invasive Lobular and Ductal Breast Carcinoma. JAMA 2003, 289, 1421–1424. [Google Scholar] [CrossRef]
- Raghav, K.; French, J.T.; Ueno, N.T.; Lei, X.; Krishnamurthy, S.; Reuben, J.M.; Valero, V.; Ibrahim, N.K. Inflammatory Breast Cancer: A Distinct Clinicopathological Entity Transcending Histological Distinction. PLoS ONE 2016, 11, e0145534. [Google Scholar] [CrossRef]
- Matro, J.M.; Li, T.; Cristofanilli, M.; Hughes, M.E.; Ottesen, R.A.; Weeks, J.C.; Wong, Y.-N. Inflammatory Breast Cancer Management in the National Comprehensive Cancer Network: The Disease, Recurrence Pattern, and Outcome. Clin. Breast Cancer 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Masuda, H.; Brewer, T.M.; Liu, D.D.; Iwamoto, T.; Shen, Y.; Hsu, L.; Willey, J.S.; Gonzalez-Angulo, A.M.; Chavez-MacGregor, M.; Fouad, T.M.; et al. Long-Term Treatment Efficacy in Primary Inflammatory Breast Cancer by Hormonal Receptor- and HER2-Defined Subtypes. Ann. Oncol. 2014, 25, 384–391. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Bertucci, F.; Fekih, M.; Autret, A.; Petit, T.; Dalenc, F.; Levy, C.; Romieu, G.; Bonneterre, J.; Ferrero, J.-M.; Kerbrat, P.; et al. Bevacizumab plus Neoadjuvant Chemotherapy in Patients with HER2-Negative Inflammatory Breast Cancer (BEVERLY-1): A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2016, 17, 600–611. [Google Scholar] [CrossRef]
- Pierga, J.-Y.; Petit, T.; Delozier, T.; Ferrero, J.-M.; Campone, M.; Gligorov, J.; Lerebours, F.; Roché, H.; Bachelot, T.; Charafe-Jauffret, E.; et al. Neoadjuvant Bevacizumab, Trastuzumab, and Chemotherapy for Primary Inflammatory HER2-Positive Breast Cancer (BEVERLY-2): An Open-Label, Single-Arm Phase 2 Study. Lancet Oncol. 2012, 13, 375–384. [Google Scholar] [CrossRef]
- Gonçalves, A.; Pierga, J.-Y.; Ferrero, J.-M.; Mouret-Reynier, M.-A.; Bachelot, T.; Delva, R.; Fabbro, M.; Lerebours, F.; Lotz, J.-P.; Linassier, C.; et al. UNICANCER-PEGASE 07 Study: A Randomized Phase III Trial Evaluating Postoperative Docetaxel-5FU Regimen after Neoadjuvant Dose-Intense Chemotherapy for Treatment of Inflammatory Breast Cancer. Ann. Oncol. 2015, 26, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Viens, P.; Palangié, T.; Janvier, M.; Fabbro, M.; Roché, H.; Delozier, T.; Labat, J.P.; Linassier, C.; Audhuy, B.; Feuilhade, F.; et al. First-Line High-Dose Sequential Chemotherapy with RG-CSF and Repeated Blood Stem Cell Transplantation in Untreated Inflammatory Breast Cancer: Toxicity and Response (PEGASE 02 Trial). Br. J. Cancer 1999, 81, 449–456. [Google Scholar] [CrossRef]
- Monneur, A.; Goncalves, A.; Gilabert, M.; Finetti, P.; Tarpin, C.; Zemmour, C.; Extra, J.-M.; Tallet, A.; Lambaudie, E.; Jacquemier, J.; et al. Similar Response Profile to Neoadjuvant Chemotherapy, but Different Survival, in Inflammatory versus Locally Advanced Breast Cancers. Oncotarget 2017, 8, 66019–66032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk after Neoadjuvant Chemotherapy Associated with Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Loap, P.; Loirat, D.; Berger, F.; Ricci, F.; Vincent-Salomon, A.; Ezzili, C.; Mosseri, V.; Fourquet, A.; Ezzalfani, M.; Kirova, Y. Combination of Olaparib and Radiation Therapy for Triple Negative Breast Cancer: Preliminary Results of the RADIOPARP Phase 1 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 436–440. [Google Scholar] [CrossRef]
- Bertucci, A.; Bertucci, F.; Zemmour, C.; Lerebours, F.; Pierga, J.-Y.; Levy, C.; Dalenc, F.; Grenier, J.; Petit, T.; Berline, M.; et al. PELICAN-IPC 2015-016/Oncodistinct-003: A Prospective, Multicenter, Open-Label, Randomized, Non-Comparative, Phase II Study of Pembrolizumab in Combination with Neo Adjuvant EC-Paclitaxel Regimen in HER2-Negative Inflammatory Breast Cancer. Front. Oncol. 2020, 10, 575978. [Google Scholar] [CrossRef]
| Median (Range) | Number of Patients (n = 113), Proportion | ||
|---|---|---|---|
| Age (median (range)) | 51 (26–89) | ||
| BMI (median (range)) | 26.7 (18.4–45.0) | ||
| Genetic predisposition | |||
| BRCA1 mutation | 5 | 4.4% | |
| BRCA2 mutation | 1 | 0.1% | |
| Unknown | 107 | 96.5% | |
| Clinical stage | |||
| T4d N− | 23 | 20.3% | |
| T4d N+ | 90 | 79.7% | |
| Histological type | |||
| Ductal | 106 | 93.8% | |
| Lobular | 7 | 6.2% | |
| Ki67 (median (range)) | 40 (0–95) | ||
| Histological grade (SBR) | |||
| I | 3 | 2.7% | |
| II | 40 | 35.4% | |
| III | 68 | 60.2% | |
| NA 1 | 2 | 1.7% | |
| Receptor status | |||
| HR+/HER2− | 50 | 44.2% | |
| HER2+ | 24 | 21.2% | |
| HR+/HER2+ | 12 | 10.6% | |
| HR−/HER2+ | 12 | 10.6% | |
| Triple-negative | 39 | 34.6% | |
| Pathological response | |||
| Pathological complete response (pCR) | 37 | 32.7% | |
| Non-pCR | 76 | 67.3% | |
| Median (Range) | Number of Patients (n = 113), Proportion | ||
|---|---|---|---|
| Systemic treatment | |||
| Neoadjuvant | 113 | 100% | |
| Anthracycline-containing | 104 | 92.0% | |
| Taxane-containing | 111 | 98.2% | |
| Cyclophosphamide-containing | 108 | 95.6% | |
| 5FU-containing | 68 | 60.2% | |
| Carboplatin-containing | 1 | 0.9% | |
| Bevacizumab-containing | 2 | 1.8% | |
| Concomitant with radiotherapy | 17 | 15.0% | |
| 5FU-Vinorelbine | 12 | ||
| Capecitabine | 5 | ||
| Bevacizumab | 1 | ||
| Adjuvant | 6 | 5.3% | |
| Capecitabine | 3 | 2,7% | |
| Other 1 | 3 | 2.7% | |
| HER2 inhibitors | 24 | 21.2% | |
| Trastuzumab-Pertuzumab | 6 | 5.3% | |
| TDM1 | 1 | 0.9% | |
| Hormone therapy | 62 | 5.3% | |
| Surgery | |||
| Mastectomy | 110 | 97.3% | |
| Breast-conserving surgery | 2 | 2.7% | |
| Axillary dissection | 109 | 96.5% | |
| Sentinel lymph node biopsy | 3 | 3.5% | |
| No surgery | 1 | 0.1% | |
| Radiotherapy | |||
| Setting | |||
| Preoperative | 10 | 8.8% | |
| Adjuvant | 103 | 91.2% | |
| Regimen | |||
| Dose (Gy (range)) | 50 (36–52) | ||
| Fractions (range) | 25 (18–29) | ||
| Target volumes | |||
| Chest wall/breast | 113 | 100% | |
| Boost (scar/nodules) | 6 (4/2) | 5.3% | |
| Berg’s I lymph node | 24 | 21.2% | |
| Berg’s II-III lymph node | 100 | 88.5% | |
| Berg’s IV lymph node | 101 | 89.4% | |
| Internal mammary chain | 96 | 85.0% | |
| Technique | |||
| 3D | 102 | 90.3% | |
| Electrons (chest wall) | 34 | 30.1% | |
| Photons (chest wall) | 52 | 46% | |
| With photons and electrons to IMC | 30 | 26.5% | |
| With electrons to IMC | 4 | 3.5% | |
| With photons to IMC | 13 | 11.5% | |
| NA 2 | 14 | 12.4% | |
| VMAT | 3 | 2.7% | |
| Tomotherapy | 8 | 7.0% | |
| 5-Year LRRFS | 5-Year DFS | 5-Year OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | p-Value | HR | p-Value | HR | p Value | ||||
| Age | 1.09 (0.43–2.74) | 0.859 | 1.14 (0.6–2.15) | 0.687 | 0.85 (0.39–1.88) | 0.69 | |||
| <50 years | 85.77% (75.91–96.91%) | 69.36% (57.87–83.13%) | 73.74% (62.44–87.09%) | ||||||
| >50 years | 84.25% (74.77–94.93%) | 65.95% (53.89-80.72%) | 82.74% (72.31–94.66%) | ||||||
| cN | 2.17 (0.5–9.45) | 0.301 | 0.79 (0.37–1.66) | 0.526 | 0.45 (0.2–1.01) | 0.052 | |||
| N- | 91.3% (80.49–100%) | 57.54% (39.55–83.72%) | 60.47% (42.14–86.78%) | ||||||
| N+ | 83.71% (75.7–92.57%) | 70.63% (61.41–81.24%) | 82.87% (74.97–91.59%) | ||||||
| Tumoral diameter on imaging (mm) | 1 (0.98–1.02) | 0.910 | 0.99 (0.98–1.01) | 0.367 | 1 (0.98–1.02) | 0.763 | |||
| Grade | 1.15 (0.43–3.08) | 0.778 | 0.75 (0.39–1.43) | 0.380 | 0.82 (0.36–1.84) | 0.623 | |||
| I-II | 83.88% (70.32–100%) | 59.4% (44.5–79.29%) | 78.65% (66.22–93.4%) | ||||||
| III | 84.5% (76.07–93.86%) | 71.45% (61.35–83.22%) | 77.14% (67.11–88.67%) | ||||||
| Histology | 1.72 (0.68–4.35) | 0.255 | 1.46 (0.76–2.81) | 0.253 | 2.12 (0.97–4.66) | 0.061 | |||
| HR+ or HER2+ | 88.99% (81.3–97.41%) | 71.53% (61.27–83.5%) | 83.64% (74.65–93.72%) | ||||||
| TNBC | 77.93% (65.46–92.77%) | 61.54% (48.02–78.87%) | 67.76% (54.06–84.92%) | ||||||
| RECIST | 2.04 (0.52–7.97) | 0.304 | 1.79 (0.74–4.31) | 0.193 | 2.26 (0.77–6.64) | 0.138 | |||
| CR/PR | 88.82% (80.44–98.06%) | 71.86% (61.14–84.45%) | 85.19% (76.65–94.68%) | ||||||
| SD/PD | 88.24% (74.18–100%) | 63.09% (43.32–91.88%) | 72.4% (52.11–100%) | ||||||
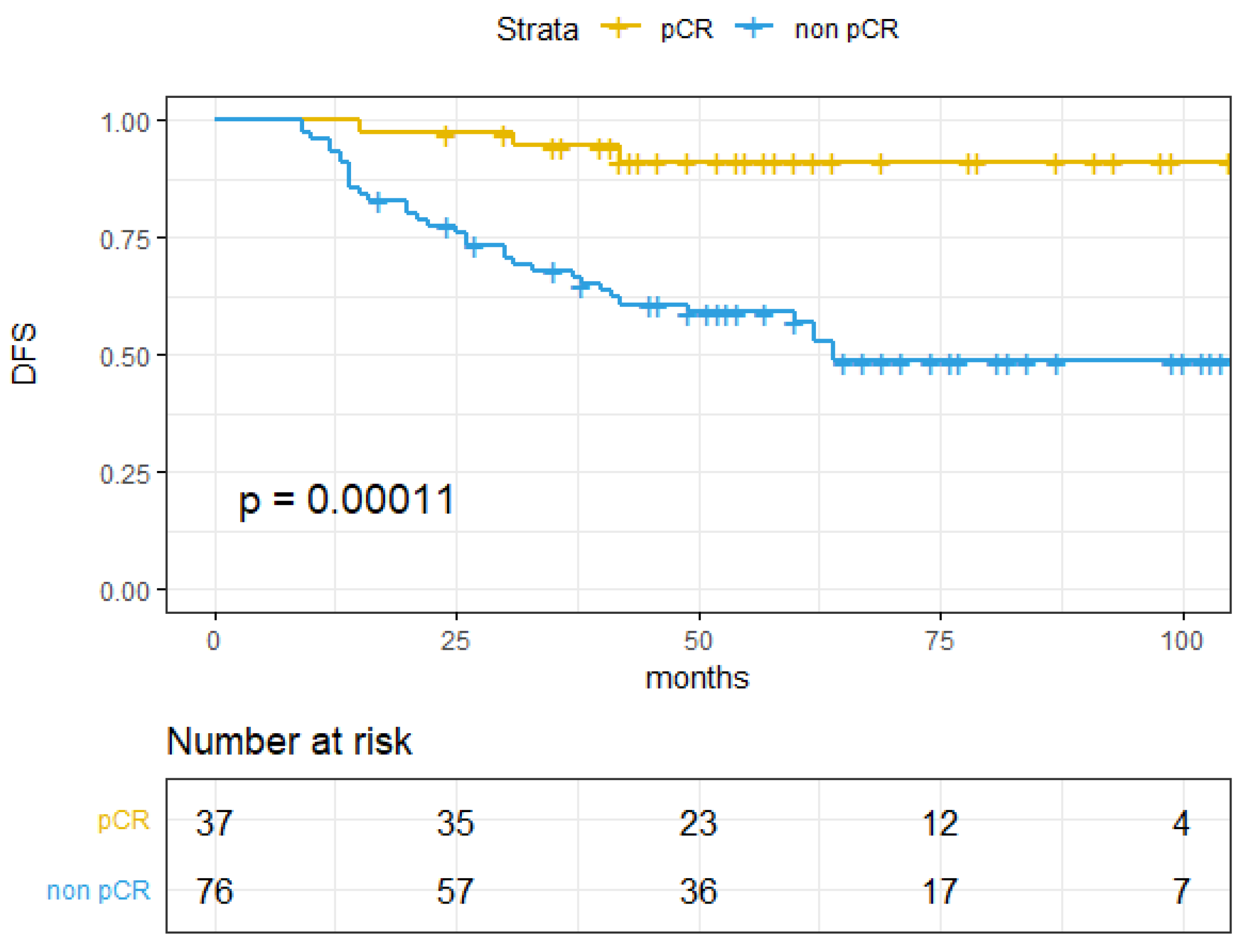
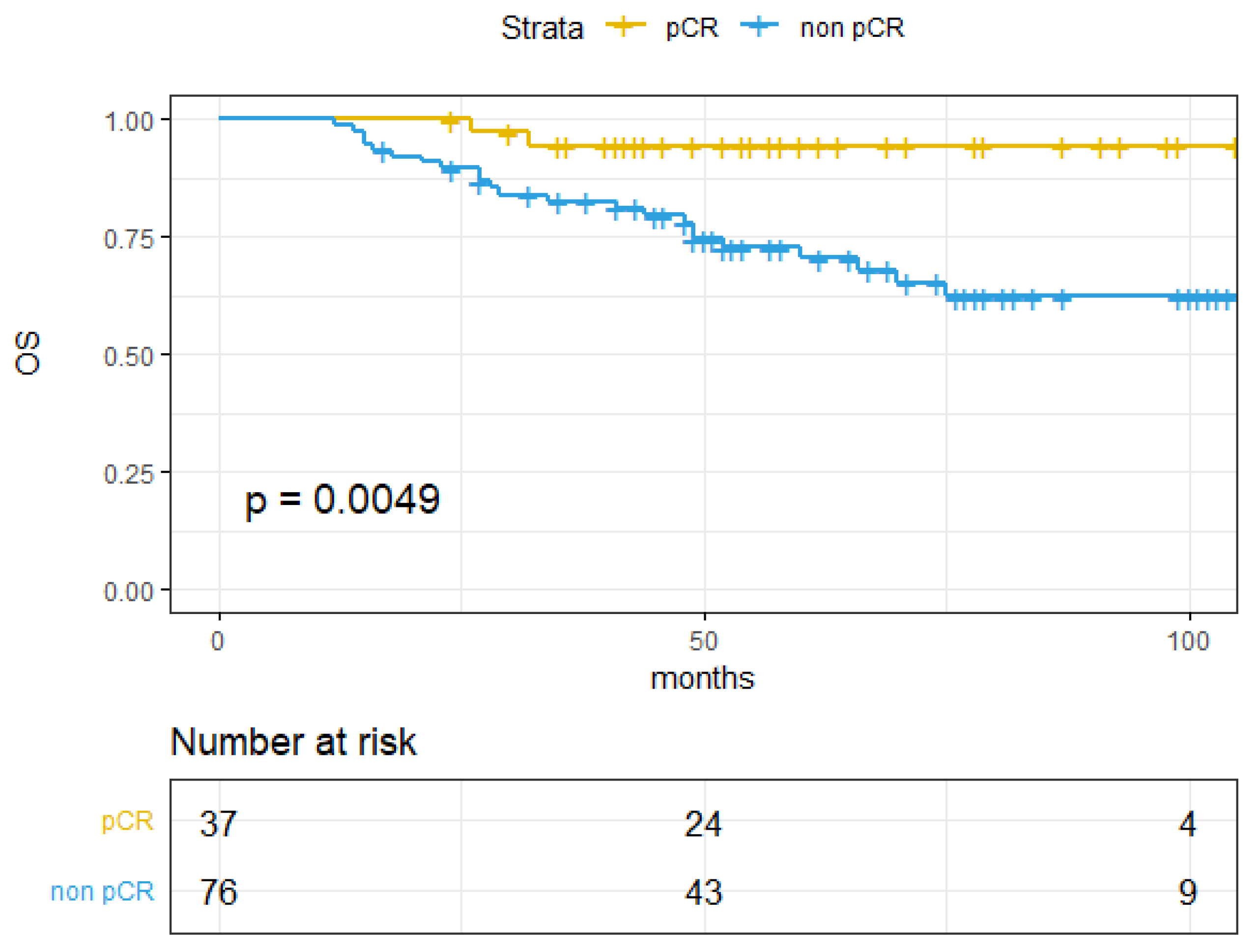
| Tumor response | 3.03 (1.47–20.63) | <0.01 | 7.28 (2.24–23.66) | 0.001 | 6.14 (1.45–26.03) | 0.014 | |||
| pCR | 100% (100–100%) | 91.18% (82.09–100%) | 94.36% (87.07–100%) | ||||||
| Non-pCR | 77.86% (68.26–88.8%) | 57.11% (46.6–70%) | 70.32% (59.94–82.49%) | ||||||
| Capsular rupture | 3.46 (1.3–9.22) | 0.013 | 1.95 (0.89–4.25) | 0.095 | 1.29 (0.44–3.75) | 0.645 | |||
| No | 89.59% (83.67–95.92%) | 71.56% (62.97–81.32%) | 79.77% (71.62–88.86%) | ||||||
| Yes | 60.11% (37.82–95.55%) | 46.75% (26.17–83.53%) | 67.53% (45.73–99.74%) | ||||||
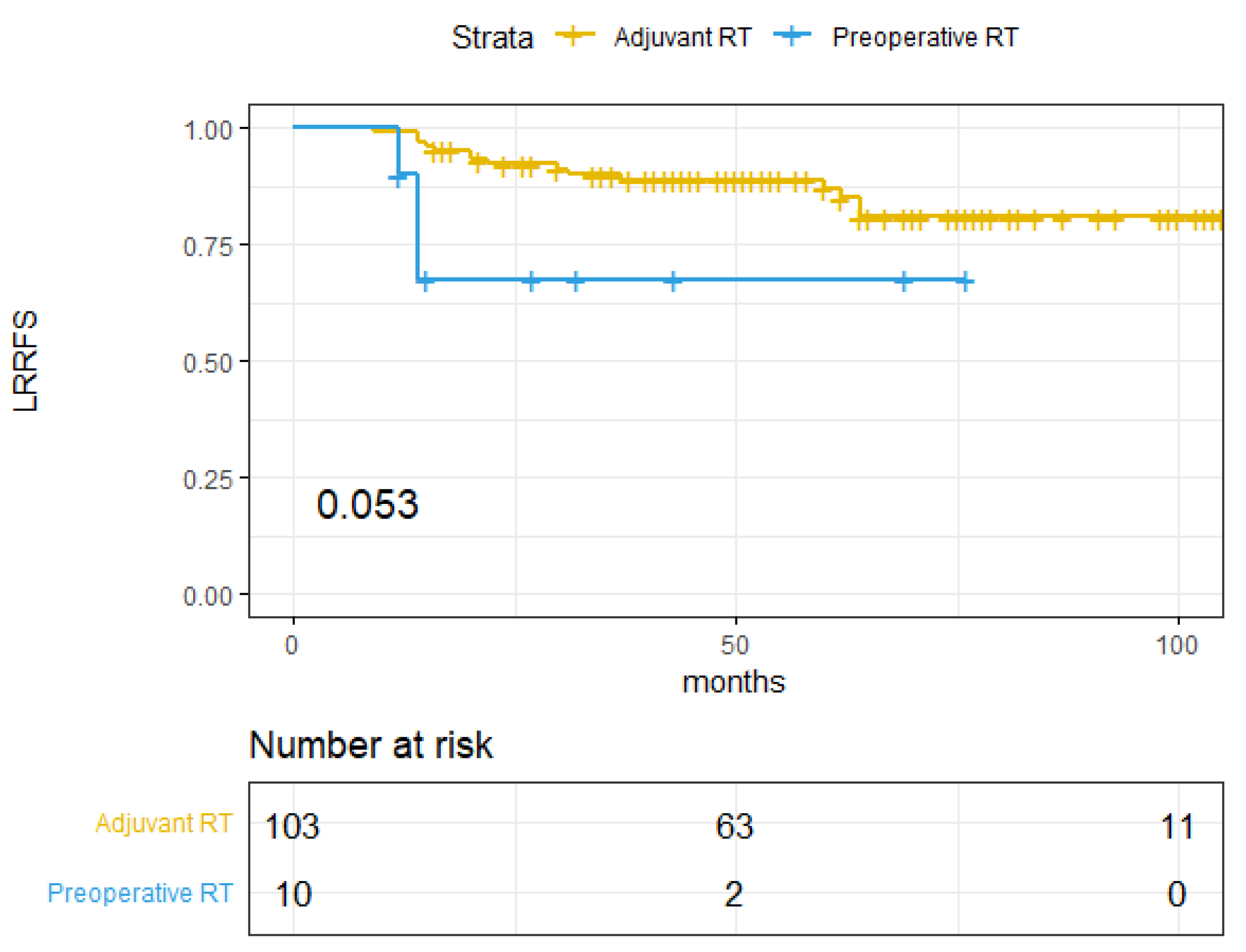
| RT setting | 3.42 (0.98–11.89) | 0.053 | 4.03 (1.77–9.2) | 0.001 | 6.03 (2.37–15.32) | <0.01 | |||
| Adjuvant RT | 86.98% (80.16–94.38%) | 71.78% (63.14–81.59%) | 81.91% (74.08–90.57%) | ||||||
| Pre-operative RT | 67.5% (43.03–100%) | 30% (11.64–77.32%) | 36% (14.98–86.49%) | ||||||
| RT interruption | 1.39 (0.32–6.05) | 0.66 | 1.72 (0.67–4.41) | 0.26 | 2.81 (1.05–7.48) | 0.039 | |||
| No | 86.03% (79.02–93.66%) | 69.84% (61.07–79.87%) | 81.31% (73.47–89.99%) | ||||||
| Yes | 76.19% (52.08–100%) | 50% (26.9–92.93%) | 50% (26.9–92.93%) | ||||||
| RT beam type | 0.66 (0.21–2.11) | 0.482 | 0.56 (0.25–1.26) | 0.164 | 0.4 (0.14–1.2) | 0.104 | |||
| Photons | 85.58% (76.38–95.89%) | 66.87% (55.72–80.25%) | 73.66% (62.76–86.46%) | ||||||
| Electrons | 90.8% (81.39–100%) | 78.98% (66.25–94.14%) | 90.63% (81.03–100%) | ||||||
| Toxicity Grade (CTCAE v5) | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4–5 | |
| Acute | ||||
| Dermatitis | n = 40 (35%) | n = 25 (22%) | n = 2 (2%) | - |
| Dysphagia | n = 1 (1%) | - | - | - |
| Pain | - | n = 1 (1%) | - | - |
| Cardiovascular | - | - | n = 1 (1%) * | - |
| Late | ||||
| Edema | n = 1 (1%) | - | - | - |
| Pulmonary | n = 1 (1%) ** | - | - | - |
| Asthenia | - | n = 1 (1%) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicaise, B.; Loap, P.; Loirat, D.; Laki, F.; Pierga, J.-Y.; Fourquet, A.; Kirova, Y. Radiotherapy in the Management of Non-Metastatic Inflammatory Breast Cancers: A Retrospective Observational Study. Cancers 2022, 14, 107. https://doi.org/10.3390/cancers14010107
Nicaise B, Loap P, Loirat D, Laki F, Pierga J-Y, Fourquet A, Kirova Y. Radiotherapy in the Management of Non-Metastatic Inflammatory Breast Cancers: A Retrospective Observational Study. Cancers. 2022; 14(1):107. https://doi.org/10.3390/cancers14010107
Chicago/Turabian StyleNicaise, Benjamin, Pierre Loap, Delphine Loirat, Fatima Laki, Jean-Yves Pierga, Alain Fourquet, and Youlia Kirova. 2022. "Radiotherapy in the Management of Non-Metastatic Inflammatory Breast Cancers: A Retrospective Observational Study" Cancers 14, no. 1: 107. https://doi.org/10.3390/cancers14010107
APA StyleNicaise, B., Loap, P., Loirat, D., Laki, F., Pierga, J.-Y., Fourquet, A., & Kirova, Y. (2022). Radiotherapy in the Management of Non-Metastatic Inflammatory Breast Cancers: A Retrospective Observational Study. Cancers, 14(1), 107. https://doi.org/10.3390/cancers14010107






