Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Hepatocellular Carcinoma
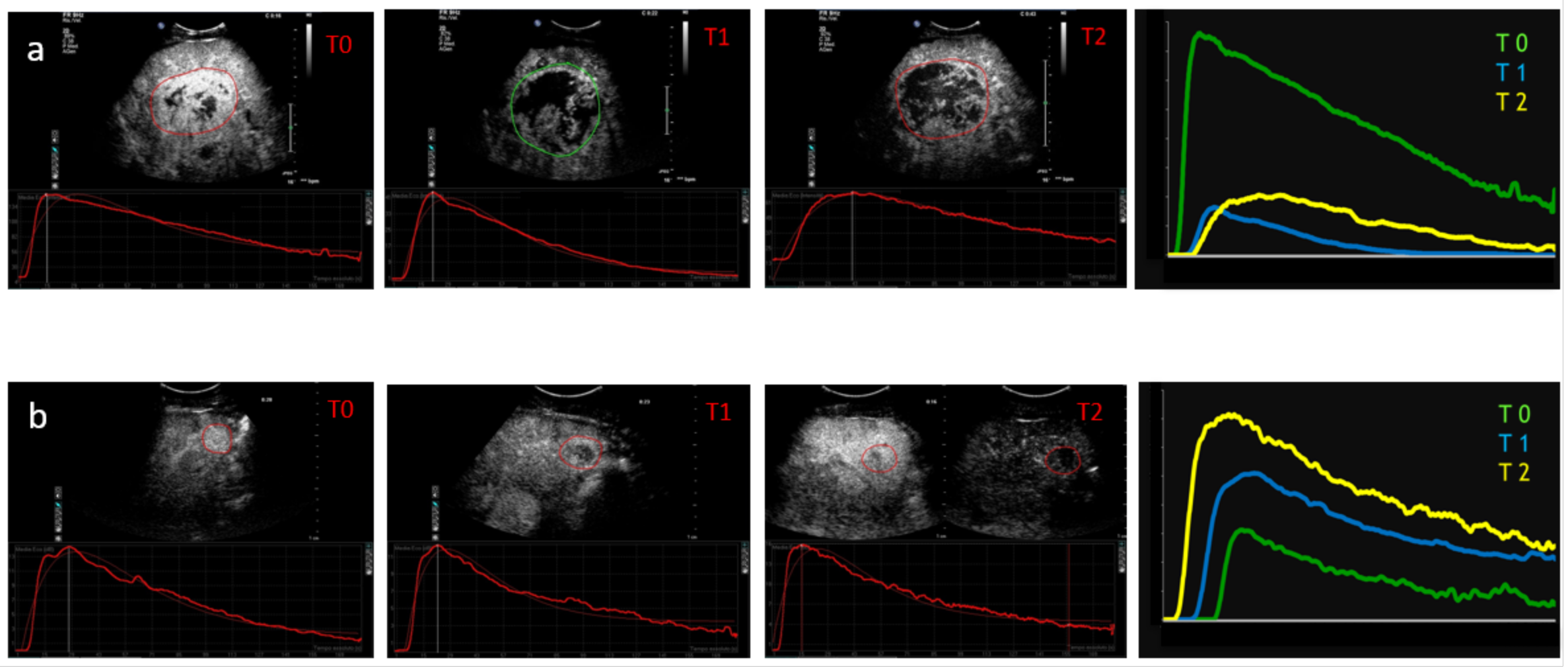
2. Technical Considerations Regarding Dynamic CEUS Application
- ‒
- Time to peak (TP) is the time from zero intensity (immediately before the contrast arrives in the ROI) to maximum intensity.
- ‒
- Wash-in time (WIT) is time from 5% intensity to 95% intensity.
- ‒
- Wash-out time (WOT) is the time from the peak of the curve to the zero value again.
- ‒
- Peak intensity (PI) is the maximum intensity on the time–intensity curve.
- ‒
- Mean transit time (MTT) describes the mean time taken by the CEUS bubbles (from the time of first arrival in the ROI) to pass through the ROI. Mathematically it is the first moment of the TIC curve. This parameter is easily calculated from the fitted mathematical model and is often given in a closed-form analytical expression.
- ‒
- Area under the curve (AUC) is the area under the time–intensity curve above baseline and is calculated numerically between the starting time and a predefined end time. This parameter may be related to blood volume.
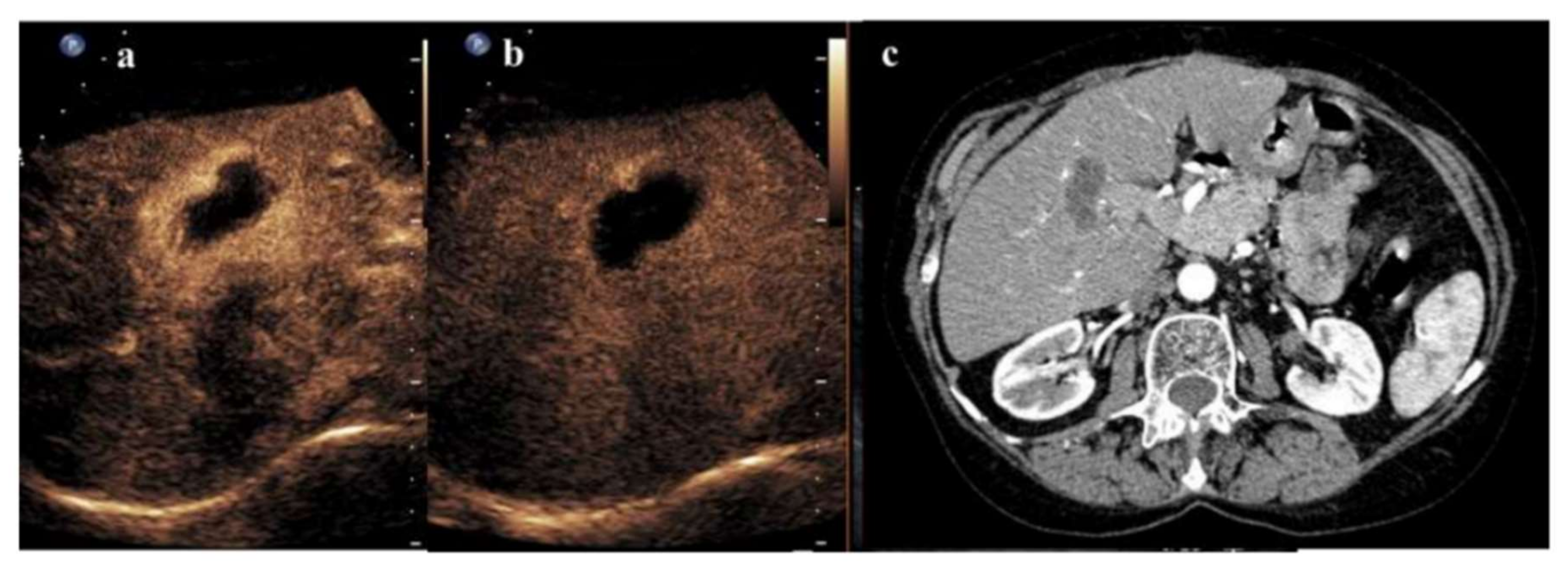
3. Radiofrequency Ablation and CEUS
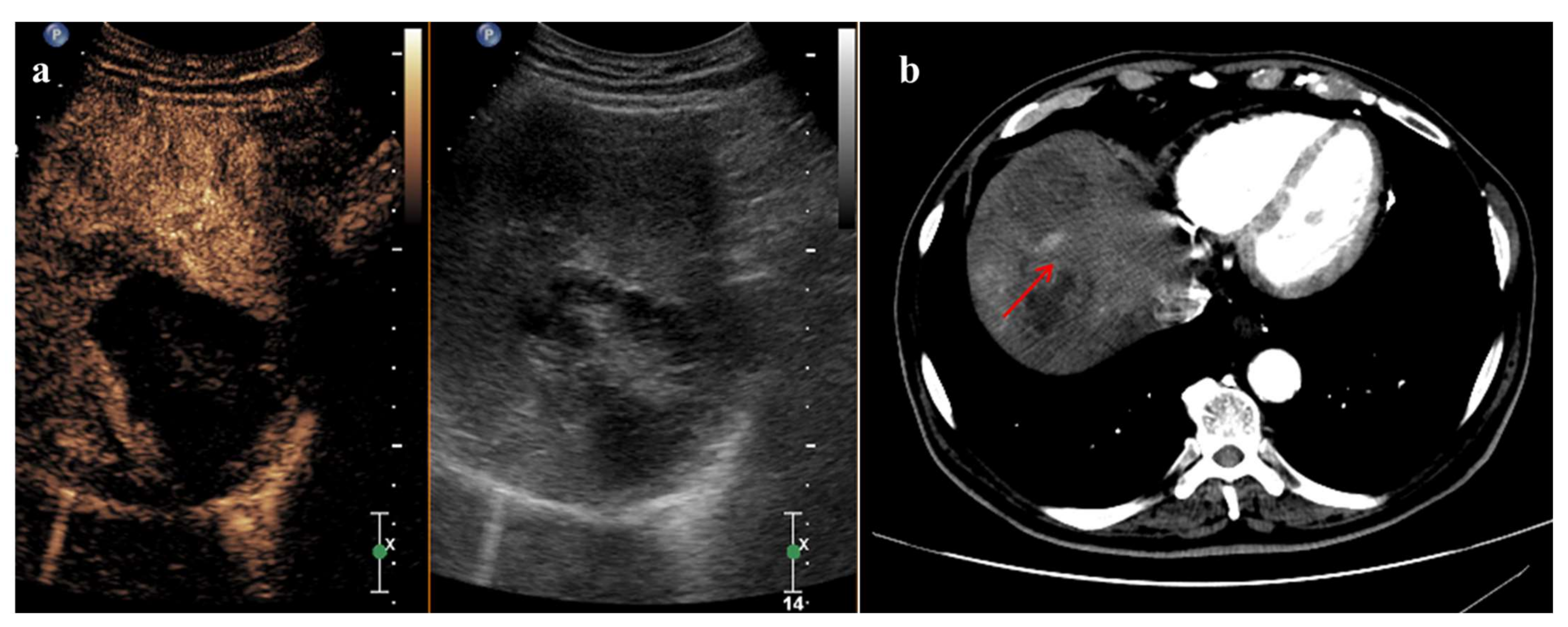
4. TACE and CEUS
5. Systemic Therapies and CEUS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceausu, M.; Socea, B.; Serban, D.; Smarandache, C.G.; Predescu, D.; Bacalbaşa, N.; Slavu, I.; Tulin, A.; Alecu, L.; Ceauşu, Z. Heterogeneity of antigenic constellation in human hepatocellular carcinoma. Exp. Ther. Med. 2021, 21, 270. [Google Scholar] [CrossRef]
- Paschoal, J.P.; Bernardo, V.; Canedo, N.H.; Ribeiro, O.D.; Caroli-Bottino, A.; Pannain, V.L. Microvascular density of regenerative nodule to small hepatocellular carcinoma by automated analysis using CD105 and CD34 immunoexpression. BMC Cancer 2014, 14, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver—Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012, 33, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Lorentzen, T.; Appelbaum, L.; Buscarini, E.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Gilja, O.H.; Hocke, M.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III—Abdominal Treatment Procedures (Short Version). Ultraschall Med. 2016, 37, 27–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bale, R.; Schullian, P.; Eberle, G.; Putzer, D.; Zoller, H.; Schneeberger, S.; Manzl, C.; Moser, P.; Oberhuber, G. Stereotactic Radiofrequency Ablation of Hepatocellular Carcinoma: A Histopathological Study in Explanted Livers. Hepatology 2019, 70, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.; Cantisani, V.; Drudi, F.; Pagliara, E.; Bezzi, M.; Meloni, F.; Calliada, F.; Erturk, S.M.; D’Andrea, V.; D’Ambrosio, U.; et al. Is contrast-enhanced US alternative to spiral CT in the assessment of treatment outcome of radiofrequency ablation in hepatocellular carcinoma? Ultraschall Med. Eur. J. Ultrasound 2009, 30, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.D.; Yu, X.L.; Li, A.H.; Jiang, T.A.; Chen, M.H.; Zhao, B.Z.; Zhou, X.D.; Wang, J.R. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: A multi-center study in China. Ultrasound Med. Biol. 2007, 33, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.G.; Xu, H.X.; Lu, M.D.; Xie, X.Y.; Xu, Z.F.; Liu, G.J.; Liu, L.N. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 855–865. [Google Scholar] [CrossRef]
- Dill-Macky, M.J.; Asch, M.; Burns, P.; Wilson, S. Radiofrequency ablation of hepatocellular carcinoma: Predicting success using contrast-enhanced sonography. Am. J. Roentgenol. 2006, 186 (Suppl. S5), S287–S295. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.F.; Andreano, A.; Franza, E.; Passamonti, M.; Lazzaroni, S. Contrast enhanced ultrasound: Should it play a role in immediate evaluation of liver tumors following thermal ablation? Eur. J. Radiol. 2012, 81, e897–e902. [Google Scholar] [CrossRef]
- Ainora, M.E.; Iezzi, R.; Ponziani, F.R.; Garcovich, M.; Di Stasio, E.; Riccardi, L.; Annicchiarico, B.E.; Abbate, V.; De Gaetano, A.M.; Siciliano, M.; et al. Contrast-Enhanced Ultrasound in the Short-Term Evaluation of Hepatocellular Carcinoma after Locoregional Treatment. Dig. Dis. 2020, 38, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Leoni, S.; Piscaglia, F.; Granito, A.; Borghi, A.; Galassi, M.; Marinelli, S.; Terzi, E.; Bolondi, L. Characterization of primary and recurrent nodules in liver cirrhosis using contrast-enhanced ultrasound: Which vascular criteria should be adopted? Ultraschall Med. Eur. J. Ultrasound 2013, 34, 280–287. [Google Scholar] [CrossRef]
- Wu, J.Y.; Bai, X.M.; Wang, H.; Xu, Q.; Wang, S.; Wu, W.; Yan, K.; Yang, W. The Perfusion Features of Recurrent Hepatocellular Carcinoma After Radiofrequency Ablation Using Contrast-Enhanced Ultrasound and Pathological Stemness Evaluation: Compared to Initial Tumors. Front. Oncol. 2020, 10, 1464. [Google Scholar] [CrossRef]
- Han, X.; Dong, J.; Liu, Z.; Wu, B.; Tian, Y.; Tan, H.; Cheng, W. Quantitative dynamic contrast-enhanced ultrasound to predict intrahepatic recurrence of hepatocellular carcinoma after radiofrequency ablation: A cohort study. Int. J. Hyperth. 2020, 37, 1066–1073. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, D.Y.; Cui, Z.; Ma, Y.; Liu, Y.Z.; Zhang, W. Predictive value of quantitative contrast-enhanced ultrasound in hepatocellular carcinoma recurrence after ablation. World J. Gastroenterol. 2015, 21, 10418–10426. [Google Scholar] [CrossRef]
- Maruyama, H.; Takahashi, M.; Shimada, T.; Sekimoto, T.; Kamesaki, H.; Kanai, F.; Yokosuka, O. Pretreatment microbubble-induced enhancement in hepatocellular carcinoma predicts intrahepatic distant recurrence after radiofrequency ablation. Am. J. Roentgenol. 2013, 200, 570–577. [Google Scholar] [CrossRef]
- Solbiati, L.; Ierace, T.; Tonolini, M.; Cova, L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur. J. Radiol. 2004, 51 (Supplement), S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Burrel, M.; Reig, M.; Forner, A.; Barrufet, M.; Lope, C.R.D.; Tremosini, S.; Ayuso, C.; Llovet, J.M.; Real, M.I.; Bruix, J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J. Hepatol. 2012, 56, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.B.; Nikolic, B.; Covey, A.M.; Nutting, C.W.; Saad, W.E.A.; Salem, R.; Sofocleous, C.T.; Sze, D.Y. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J. Vasc. Interv. Radiol. 2012, 23, 287–294. [Google Scholar] [CrossRef]
- Bargellini, I.; Bozzi, E.; Campani, D.; Carrai, P.; De Simone, P.; Pollina, L.; Cioni, R.; Filipponi, F.; Bartolozzi, C. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur. J. Radiol. 2013, 82, e212–e218. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Numata, K.; Morimoto, M.; Kondo, M.; Nozaki, A.; Moriya, S.; Ishii, T.; Oshima, T.; Fukuda, H.; Okada, M.; et al. Use of contrast-enhanced ultrasonography with a perflubutane-based contrast agent performed one day after transarterial chemoembolization for the early assessment of residual viable hepatocellular carcinoma. Eur. J. Radiol. 2013, 82, 1471–1480. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ogawa, M.; Kumagawa, M.; Hirayama, M.; Miura, T.; Matsumoto, N.; Nakaga-wara, H.; Yamamoto, T.; Moriyama, M. Utility of Contrast-Enhanced Ultrasound for Early Therapeutic Evaluation of Hepatocellular Carcinoma After Transcatheter Arterial Che-moembolization. J. Ultrasound Med. 2020, 39, 431–440. [Google Scholar] [CrossRef]
- Xia, Y.; Kudo, M.; Minami, Y.; Ueshima, K.; Chung, H.; Hagiwara, S.; Inoue, T.; Ishikawa, E.; Kitai, S.; Takahashi, S.; et al. Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: The usefulness of Sonazoid-enhanced harmonic sonography. Oncology 2008, 75 (Suppl. S1), 99–105. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.X.; Xu, Z.F.; Zheng, K.; Wang, W.; Kuang, M.; Zhuang, W.; Xie, X. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluat-ing the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur. Radiol. 2015, 25, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.M.; Eisenbrey, J.R.; Lyshchik, A.; O’Kane, L.O.; Merton, D.A.; Machado, P.; Pino, L.; Brown, D.B.; Forsberg, F. Contrast-enhanced ultrasound evaluation of residual blood flow to hepatocellular carcinoma after treatment with transarterial chemoembolization using drug-eluting beads: A prospective study. J. Ultrasound Med. 2015, 34, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, K.; Matsui, T.; Murakami, T.; Watanabe, M.; Maetani, I. Predicting Therapeutic Efficacy of Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound. Diagnostics 2021, 11, 291. [Google Scholar] [CrossRef]
- Moschouris, H.; Malagari, K.; Dimakis, A.; Kiakidis, T.; Anagnostopoulou, A. Transarterial Chemoembolization of HCC with Radiopaque Microspheres: Evaluation with Computed Tomography and the Complementary Role of Contrast-Enhanced Ultrasonography. Cardiovasc. Interv. Radiol. 2020, 43, 1075–1083. [Google Scholar] [CrossRef]
- Moschouris, H.; Malagari, K.; Papadaki, M.G.; Kornezos, I.; Gkoutzios, P.; Tepelenis, N.; Matsaidonis, D. Short-term evaluation of liver tumors after transarterial chemoembolization: Limitations and feasibility of contrast-enhanced ultrasonography. Abdom. Imaging 2011, 36, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Moschouris, H.; Malagari, K.; Kornezos, I.; Papadaki, M.G.; Gkoutzios, P.; Matsaidonis, D. Unenhanced and contrast-enhanced ultrasonography during hepatic transarterial embolization and chemoembolization with drug-eluting beads. Cardiovasc. Interv. Radiol. 2010, 33, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Stanczak, M.; Lyshchik, A.; Machado, P.; Kono, Y.; Forsberg, F.; Shaw, C.M.; Eisenbrey, J.R. Evaluation of Hepatocellular Carcinoma Transarterial Chemoembolization using Quantitative Analysis of 2D and 3D Real-time Contrast Enhanced Ultrasound. Biomed. Phys. Eng. Express 2018, 4, 035039. [Google Scholar] [CrossRef]
- Rennert, J.; Wiesinger, I.; Schicho, A.; Beyer, L.P.; Wiggermann, P.; Stroszczynski, C.; Jung, E.M. Color coded perfusion imaging with contrast enhanced ultrasound (CEUS) for post-interventional success control following trans-arterial chemoembolization (TACE) of hepatocellular carcinoma. PLoS ONE 2019, 14, e0217599. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhao, Q.; Huang, M.; Sun, J.; Tian, G. Contrast-Enhanced Ultrasound in Residual Tumor of Hepatocellular Carcinoma following Transarterial Chemoembolization: Is It Helpful for Tumor Response? BioMed Res. Int. 2018, 2018, 8632069. [Google Scholar] [CrossRef] [Green Version]
- Wessner, C.E.; Shaw, C.M.; Stanczak, M.; Forsberg, F.; Lyshchik, A.; Tan, A.; Eisenbrey, J.R. Contrast-enhanced Ultrasound Identifies Patent Feeding Vessels in Transarterial Chemoembolization Patients With Residual Tumor Vascularity. Ultrasound Q. 2020, 36, 218–223. [Google Scholar] [CrossRef]
- Moschouris, H.; Malagari, K.; Papadaki, M.G.; Kornezos, I.; Stamatiou, K.; Anagnostopoulos, A.; Chatzimichael, K.; Kelekis, N. mRECIST criteria and contrast- enhanced US for the assessment of the response of hepatocellular carcinoma to transarterial chemoembolization. Diagn. Interv. Radiol. 2014, 20, 136–142. [Google Scholar] [CrossRef]
- Lamuraglia, M.; Escudier, B.; Chami, L.; Schwartz, B.; Leclère, J.; Roche, A.; Lassau, N. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: Pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur. J. Cancer 2006, 42, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Lamuraglia, M.; Chami, L.; Leclère, J.; Bonvalot, S.; Terrier, P.; Roche, A.; Le Cesne, A. Gastrointestinal stromal tumors treated with imatinib: Monitoring response with contrast-enhanced sonography. Am. J. Roentgenol. 2006, 187, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Moschouris, H.; Malagari, K.; Gkoutzios, P.; Kalokairinou, M.; Stamatiou, K.; Chatzimichail, K.; Kornezos, I.; Karagiannis, E.; Kiltenis, M.; Papadaki, M.G. Intermediate and advanced hepatocellular carcinoma treated with the antiangiogenic agent sorafenib. Evaluation with unenhanced and contrast-enhanced ultrasonography. Med. Ultrason. 2012, 14, 87–94. [Google Scholar]
- Frampas, E.; Lassau, N.; Zappa, M.; Vulliermec, M.P.; Koscielnyd, S.; Vilgraince, V. Advanced Hepatocellular Carcinoma: Early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary results. Eur. J. Radiol. 2013, 82, e205–e211. [Google Scholar] [CrossRef]
- Lassau, N.; Bonastre, J.; Kind, M.; Vilgrain, V.; Lacroix, J.; Cuinet, M.; Taieb, S.; Aziza, R.; Sarran, A.; Labbe-Devilliers, C.; et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: The French multicenter support for innovative and expensive techniques study. Investig. Radiol. 2014, 49, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Moriyasu, F.; Saito, K.; Rognin, N.; Kamiyama, N.; Furuichi, Y.; Imai, Y. Hepatocellular carcinoma treated with sorafenib: Early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013, 33, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Zocco, M.A.; Garcovich, M.; Lupascu, A.; Di Stasio, E.; Roccarina, D.; Annicchiarico, B.E.; Riccardi, L.; Ainora, M.E.; Ponziani, F.; Caracciolo, G.; et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: The role of dynamic contrast enhanced ultrasound. J. Hepatol. 2013, 59, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, G.; Lu, M.D.; Xie, X.; Kuang, M.; Wang, W.; Xu, Z.; Lin, M.; Chen, L. Role of portal vein tumor thrombosis in quantitative perfusion analysis of contrast-enhanced ultrasound of hepatocellular carcinoma. Ultrasound Med. Biol. 2015, 41, 1277–1286. [Google Scholar] [CrossRef]
- Kuorda, H.; Abe, T.; Fujiwara, Y.; Okamoto, T.; Yonezawa, M.; Sato, H.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2365–2372. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccia, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers 2022, 14, 481. https://doi.org/10.3390/cancers14030481
Faccia M, Garcovich M, Ainora ME, Riccardi L, Pompili M, Gasbarrini A, Zocco MA. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers. 2022; 14(3):481. https://doi.org/10.3390/cancers14030481
Chicago/Turabian StyleFaccia, Mariella, Matteo Garcovich, Maria Elena Ainora, Laura Riccardi, Maurizio Pompili, Antonio Gasbarrini, and Maria Assunta Zocco. 2022. "Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma" Cancers 14, no. 3: 481. https://doi.org/10.3390/cancers14030481
APA StyleFaccia, M., Garcovich, M., Ainora, M. E., Riccardi, L., Pompili, M., Gasbarrini, A., & Zocco, M. A. (2022). Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers, 14(3), 481. https://doi.org/10.3390/cancers14030481






