The Clinical, Pathological, and Prognostic Value of High PD-1 Expression and the Presence of Epstein–Barr Virus Reactivation in Patients with Laryngeal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Group
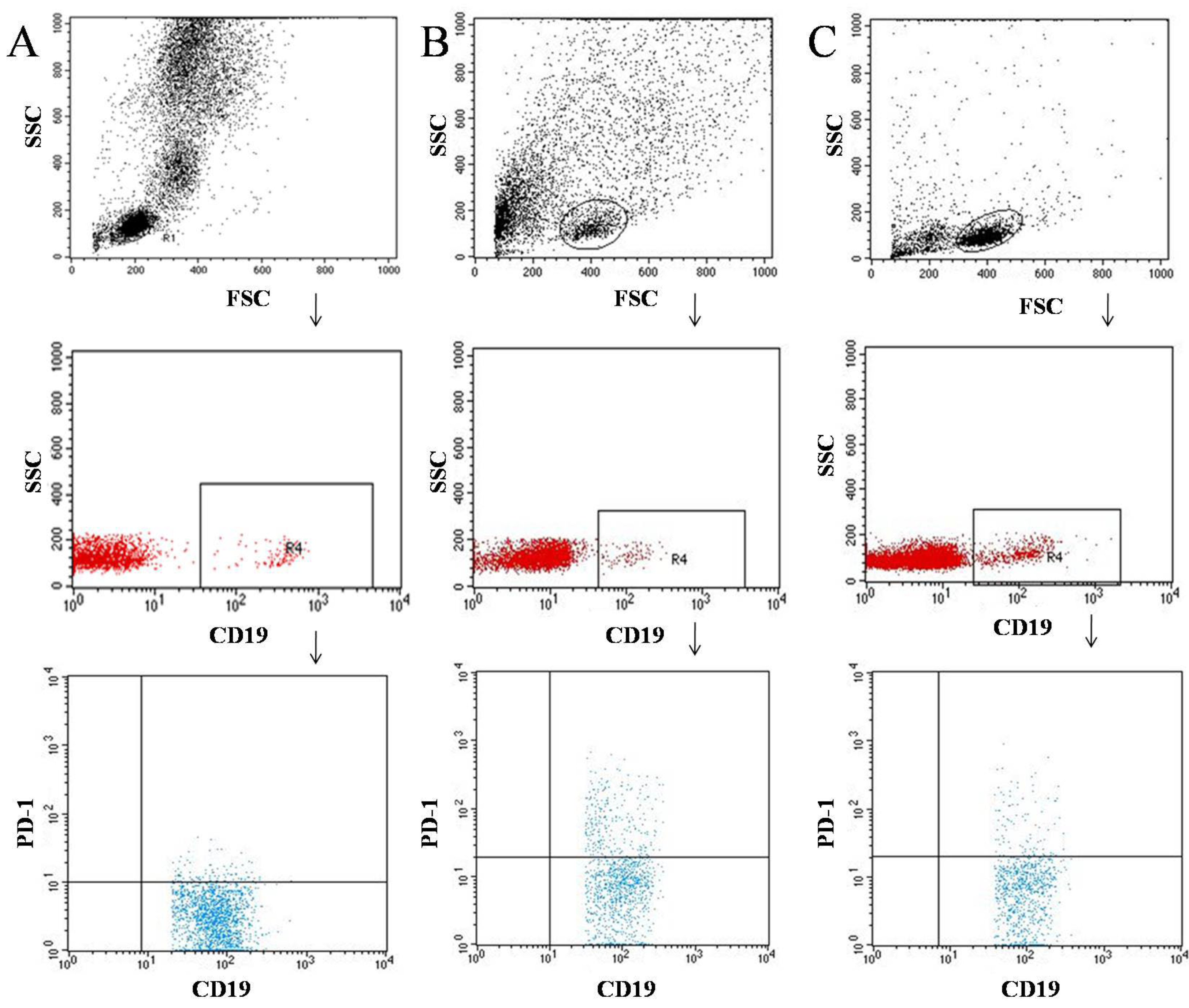
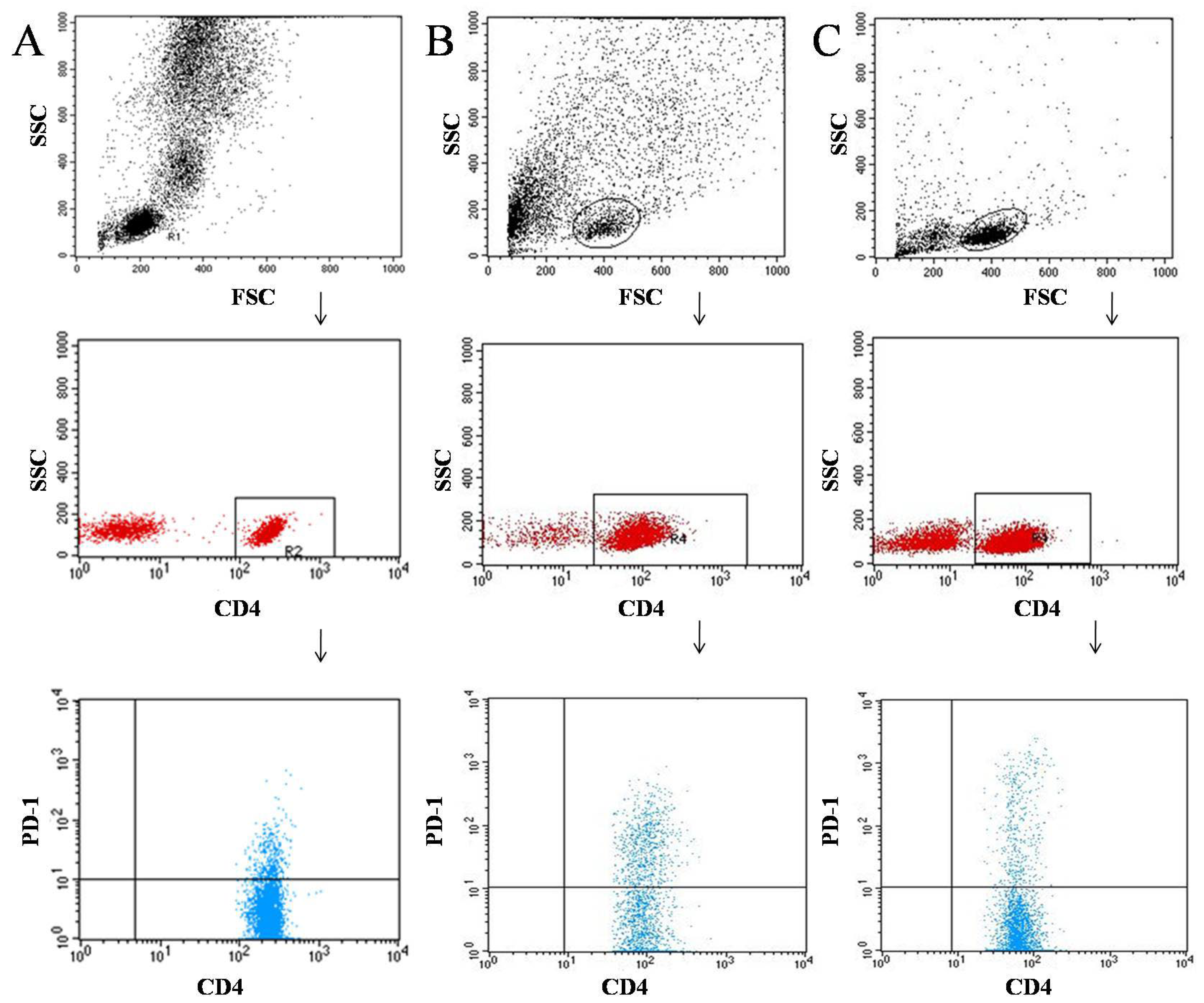
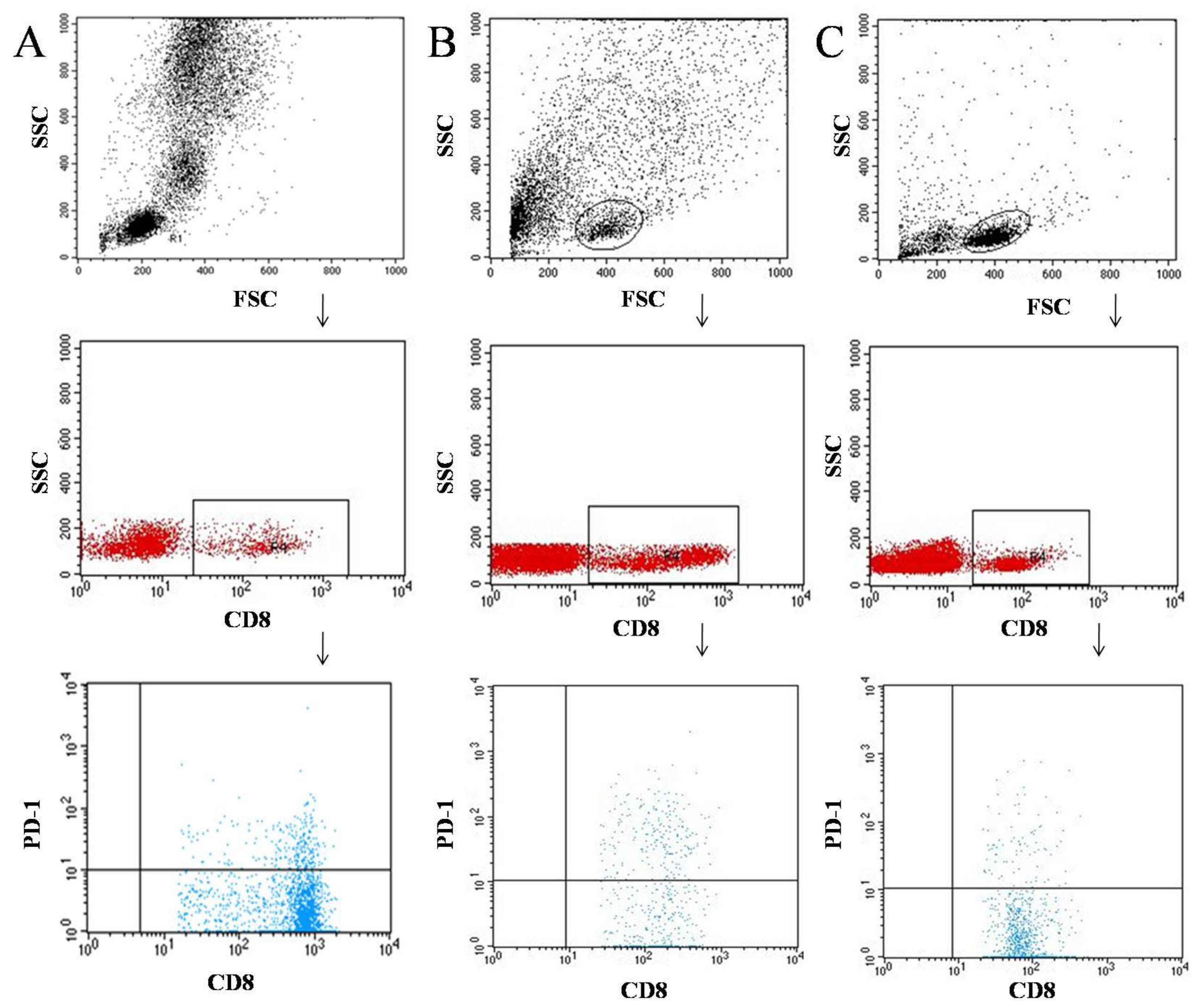
2.2. Flow Cytometric Analysis of CD69+, CD25+, and PD-1 Cells in Peripheral Blood
2.3. Flow Cytometric Analysis of CD69+, CD25+, and PD-1 Cells in Tumor Tissue and Lymph Nodes
2.4. DNA Isolation, RT-PCR, and Calculation of EBV Load
2.5. Evaluation of IgM VCA and IgG VCA Concentrations
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Immunophenotype in Patients Diagnosed with Cancer of the Larynx and in the Control Group
3.2. Characterization of the Immunophenotype of Tumor and Lymph Node Samples in Patients Diagnosed with Laryngeal Cancer
3.3. Analysis of the Relationship among the Expression of the PD-1 Receptor in the Tested Blood, Tumor, and Lymph Node Samples of Patients Diagnosed with Laryngeal Cancer and the Stage of the Disease and the TNM Scale
3.4. Analysis of PD-1 Expression in the Studied Biological Material Regarding Life Expectancy of Patients Diagnosed with Laryngeal Cancer Based on the Cox Proportional Hazard Regression Model
3.5. Analysis of the Presence of EBV in the Tested Biological Materials and Related to the Grade (G) and TNM Scale of Laryngeal Cancer Classification
3.6. Analysis of the Correlation between the Presence of EBV Genetic Material and the Expression of PD-1 in the Tested Biological Material from Patients Diagnosed with Laryngeal Cancer
4. Discussion
4.1. Immunophenotype of Patients Diagnosed with Cancer of the Larynx
4.2. PD-1 Expression on T and B Lymphocytes
4.3. The Role of EBV in the Development of Laryngeal Cancer
4.4. Limitations of Conducted Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 24 October 2021).
- Olofsson, J.; Sökjer, H. Radiology and Laryngoscopy for the Diagnosis of Laryngeal Carcinoma. Acta Radiol. Diagn. 1977, 18, 449–468. [Google Scholar] [CrossRef]
- Thekdi, A.A.; Ferris, R.L. Diagnostic Assessment of Laryngeal Cancer. Otolaryngol. Clin. N. Am. 2002, 35, 953–969. [Google Scholar] [CrossRef]
- Maurizi, M.; Almadori, G.; Ferrandina, G.; Distefano, M.; Romanini, M.E.; Cadoni, G.; Benedetti-Panici, P.; Paludetti, G.; Scambia, G.; Mancuso, S. Prognostic Significance of Epidermal Growth Factor Receptor in Laryngeal Squamous Cell Carcinoma. Br. J. Cancer 1996, 74, 1253–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almadori, G.; Cadoni, G.; Galli, J.; Ferrandina, G.; Scambia, G.; Exarchakos, G.; Paludetti, G.; Ottaviani, F. Epidermal Growth Factor Receptor Expression in Primary Laryngeal Cancer: An Independent Prognostic Factor of Neck Node Relapse. Int. J. Cancer 1999, 84, 188–191. [Google Scholar] [CrossRef]
- Nijkamp, M.M.; Span, P.N.; Terhaard, C.H.J.; Doornaert, P.A.H.; Langendijk, J.A.; van den Ende, P.L.A.; de Jong, M.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H.A.M. Epidermal Growth Factor Receptor Expression in Laryngeal Cancer Predicts the Effect of Hypoxia Modification as an Additive to Accelerated Radiotherapy in a Randomised Controlled Trial. Eur. J. Cancer 2013, 49, 3202–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yang, B.; Zhang, X. Effects of Telomerase Activity on Carcinogenesis and Survival in Laryngeal Carcinoma. J. Int. Med. Res. 2008, 36, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zand, V.; Binesh, F.; Meybodian, M.; Safi Dahaj, F.; Alamdar Yazdi, A. Cyclin D1 Expression in Patients with Laryngeal Squamous Cell Carcinoma. Iran. J. Pathol. 2020, 15, 245–250. [Google Scholar] [CrossRef]
- Lentari, I.; Segas, I.; Kandiloros, D. The Importance of Cathepsin’s-D Tissular Detection in Laryngeal Squamous Cell Carcinoma. Acta Otorhinolaryngol. Belg. 2002, 56, 383–389. [Google Scholar] [PubMed]
- Verma, A.; Schwartz, N.; Cohen, D.J.; Patel, V.; Nageris, B.; Bachar, G.; Boyan, B.D.; Schwartz, Z. Loss of Estrogen Receptors Is Associated with Increased Tumor Aggression in Laryngeal Squamous Cell Carcinoma. Sci Rep. 2020, 10, 4227. [Google Scholar] [CrossRef]
- Zha, C.; Jiang, X.H.; Peng, S.F. ITRAQ-Based Quantitative Proteomic Analysis on S100 Calcium Binding Protein A2 in Metastasis of Laryngeal Cancer. PLoS ONE 2015, 10, e0122322. [Google Scholar] [CrossRef] [PubMed]
- Celenk, F.; Bayramoglu, I.; Yilmaz, A.; Menevse, A.; Bayazit, Y. Expression of Cyclooxygenase-2, 12-Lipoxygenase, and Inducible Nitric Oxide Synthase in Head and Neck Squamous Cell Carcinoma. J. Craniofac. Surg. 2013, 24, 1114–1117. [Google Scholar] [CrossRef]
- Frejborg, E.; Salo, T.; Salem, A. Role of Cyclooxygenase-2 in Head and Neck Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 9246. [Google Scholar] [CrossRef]
- Miranda, F.A.; Hassumi, M.K.; Guimarães, M.C.M.; Simões, R.T.; Silva, T.G.A.; Lira, R.C.P.; Rocha, A.M.; Mendes, C.T.; Donadi, E.A.; Soares, C.P.; et al. Galectin-3 Overexpression in Invasive Laryngeal Carcinoma, Assessed by Computer-Assisted Analysis. J. Histochem. Cytochem. 2009, 57, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, S.; Tian, Y.; Wang, Y.; Zhang, Q.; Zhou, X.; Meng, X.; Song, N. Prognostic Role of Galectin-3 Expression in Patients with Solid Tumors: A Meta-Analysis of 36 Eligible Studies. Cancer Cell Int. 2018, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Hymos, A.; Grywalska, E.; Klatka, J.; Klatka, M.; Korona-Głowniak, I.; Roliński, J. ThymicPeptides Reverse Immune Exhaustion in Patients with Reactivated Human Alphaherpesvirus1 Infections. Int. J. Mol. Sci. 2020, 21, 2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatka, J.; Hymos, A.; Szkatuła-Łupina, A.; Grywalska, E.; Klatka, B.; Terpiłowski, M.; Stepulak, A. T-Lymphocyte Activation Is Correlated With the Presence of Anti-EBV in Patients With Laryngeal Squamous Cell Carcinoma. Vivo 2019, 33, 2007–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Shi, Y.; Tang, Y.; Yin, H.; Guo, Y.; Wen, S.; Wang, B.; An, C.; Wu, Y.; Gao, W. Effect of HPV Infection on the Occurrence and Development of Laryngeal Cancer: A Review. J. Cancer 2019, 10, 4455–4462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onerci Celebi, O.; Sener, E.; Hosal, S.; Cengiz, M.; Gullu, I.; Guler Tezel, G. Human Papillomavirus Infection in Patients with Laryngeal Carcinoma. BMC Cancer 2018, 18, 1005. [Google Scholar] [CrossRef] [PubMed]
- Mes, S.W.; Leemans, C.R.; Brakenhoff, R.H. Applications of Molecular Diagnostics for Personalized Treatment of Head and Neck Cancer: State of the Art. Expert Rev. Mol. Diagn. 2016, 16, 205–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliabue, M.; Maffini, F.; Fumagalli, C.; Gandini, S.; Lepanto, D.; Corso, F.; Cacciola, S.; Ranghiero, A.; Rappa, A.; Vacirca, D.; et al. A Role for the Immune System in Advanced Laryngeal Cancer. Sci. Rep. 2020, 10, 18327. [Google Scholar] [CrossRef]
- Klatka, J.; Grywalska, E.; Hymos, A.; Krasowska, E.; Mielnik, M.; Siwicka-Gieroba, D.; Markowicz, J.; Trojanowski, P.; Olszański, W.; Roliński, J. Subpopulations of Natural Killer-T-like Cells before and after Surgical Treatment of Laryngeal Cancer. Cent. Eur. J. Immunol. 2017, 42, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batur, S.; Kain, Z.; Gozen, E.; Kepil, N.; Aydin, O.; Comunoglu, N. Programmed Death Ligand 1 Expression in Laryngeal Squamous Cell Carcinomas and Prognosis. Clin. Pathol. 2020, 13, 2632010X2096484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Sharpe, A.H.; Pauken, K.E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of Immune Suppression by Interleukin-10 and Transforming Growth Factor-Beta: The Role of T Regulatory Cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Hoffman, B.E.; Martino, A.T.; Sack, B.K.; Cao, O.; Liao, G.; Terhorst, C.; Herzog, R.W. Nonredundant Roles of IL-10 and TGF-β in Suppression of Immune Responses to Hepatic AAV-Factor IX Gene Transfer. Mol. Ther. 2011, 19, 1263–1272. [Google Scholar] [CrossRef]
- Thepmalee, C.; Panya, A.; Junking, M.; Chieochansin, T.; Yenchitsomanus, P. Inhibition of IL-10 and TGF-β Receptors on Dendritic Cells Enhances Activation of Effector T-Cells to Kill Cholangiocarcinoma Cells. Hum. Vaccines Immunother. 2018, 14, 1423–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalecenia Postępowania Diagnostyczno-Terapeutycznego w Nowotworach Złośliwych. 2019. Available online: http://onkologia.zalecenia.med.pl/ (accessed on 24 October 2021).
- Kuss, I.; Hathaway, B.; Ferris, R.L.; Gooding, W.; Whiteside, T.L. Decreased Absolute Counts of T Lymphocyte Subsets and Their Relation to Disease in Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2004, 10, 3755–3762. [Google Scholar] [CrossRef] [Green Version]
- Starska, K.; Głowacka, E.; Kulig, A.; Lewy-Trenda, I.; Bryś, M.; Lewkowicz, P. The role of tumorcells in the modification of Tlymphocytesactivity—The expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activationmarkers on T CD4+ and CD8+ cells in squamouscelllaryngeal carcinoma. Part I. Folia Histochem. Cytobiol. 2011, 49, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Sheu, B.; Lin, R.; Ho, H.; Huang, S. Down-regulation of CD25 expression on the surface of activated tumor infiltrating lymphocytes in human cervical carcinoma. Hum. Immunol. 1997, 56, 39–48. [Google Scholar] [CrossRef]
- Bose, A.; Chakraborty, T.; Chakraborty, K.; Pal, S.; Baral, R. Dysregulation in Immune Functions Is Reflected in Tumor Cell Cytotoxicity by Peripheral Blood Mononuclear Cells from Head and Neck Squamous Cell Carcinoma Patients. Cancer Immun. 2008, 8, 10. [Google Scholar] [PubMed]
- Aarstad, H.J.; Heimdal, J.-H.; Klementsen, B.; Olofsson, J.; Ulvestad, E. Presence of Activated T Lymphocytes in Peripheral Blood of Head and Neck Squamous Cell Carcinoma Patients Predicts Impaired Prognosis. Acta Otolaryngol. 2006, 126, 1326–1333. [Google Scholar] [CrossRef]
- Schaefer, C.; Kim, G.G.; Albers, A.; Hoermann, K.; Myers, E.N.; Whiteside, T.L. Characteristics of CD4+CD25+ Regulatory T Cells in the Peripheral Circulation of Patients with Head and Neck Cancer. Br. J. Cancer 2005, 92, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.Y.; Chu, C.S.; Goletz, T.J.; Schlienger, K.; Yeh, H.; Coukos, G.; Rubin, S.C.; Kaiser, L.R.; June, C.H. Regulatory CD4(+)CD25(+) T Cells in Tumors from Patients with Early-Stage Non-Small Cell Lung Cancer and Late-Stage Ovarian Cancer. Cancer Res. 2001, 61, 4766–4772. [Google Scholar]
- Liyanage, U.K.; Moore, T.T.; Joo, H.-G.; Tanaka, Y.; Herrmann, V.; Doherty, G.; Drebin, J.A.; Strasberg, S.M.; Eberlein, T.J.; Goedegebuure, P.S.; et al. Prevalence of Regulatory T Cells Is Increased in Peripheral Blood and Tumor Microenvironment of Patients with Pancreas or Breast Adenocarcinoma. J. Immunol. 2002, 169, 2756–2761. [Google Scholar] [CrossRef]
- Weinstock, M.; McDermott, D. Targeting PD-1/PD-L1 in the Treatment of Metastatic Renal Cell Carcinoma. Ther. Adv. Urol. 2015, 7, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xerri, L.; Chetaille, B.; Serriari, N.; Seriari, N.; Attias, C.; Guillaume, Y.; Arnoulet, C.; Olive, D. Programmed Death 1 Is a Marker of Angioimmunoblastic T-Cell Lymphoma and B-Cell Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia. Hum. Pathol. 2008, 39, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Pasiarski, M.; Sosnowska-Pasiarska, B.; Macek, P.; Rolińska, A.; Samardakiewicz, M.; Ludian, J.; Góźdź, S.; Roliński, J. Programmed Cell Death 1 Expression and Epstein-Barr Virus Infection in Chronic Lymphocytic Leukaemia: A Prospective Cohort Study. Cancer Manag. Res. 2019, 11, 7605–7618. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hamrouni, A.; Wolowiec, D.; Coiteux, V.; Kuliczkowski, K.; Hetuin, D.; Saudemont, A.; Quesnel, B. Plasma Cells from Multiple Myeloma Patients Express B7-H1 (PD-L1) and Increase Expression after Stimulation with IFN-γ and TLR Ligands via a MyD88-, TRAF6-, and MEK-Dependent Pathway. Blood 2007, 110, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Ghebeh, H.; Mohammed, S.; Al-Omair, A.; Qattan, A.; Lehe, C.; Al-Qudaihi, G.; Elkum, N.; Alshabanah, M.; Bin Amer, S.; Tulbah, A.; et al. The B7-H1 (PD-L1) T Lymphocyte-Inhibitory Molecule Is Expressed in Breast Cancer Patients with Infiltrating Ductal Carcinoma: Correlation with Important High-Risk Prognostic Factors. Neoplasia 2006, 8, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 Expression on Non-Small Cell Lung Cancer Cells and Its Relationship with Tumor-Infiltrating Lymphocytes and Their PD-1 Expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Jiang, J.; Pang, X.; Huang, M.; Tang, Y.; Liang, X.; Tang, Y. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Hsiao, J.-R.; Chang, K.-C.; Wu, Y.-H.; Su, I.-J.; Jin, Y.-T.; Chang, Y. Increase of Programmed Death-1-Expressing Intratumoral CD8 T Cells Predicts a Poor Prognosis for Nasopharyngeal Carcinoma. Mod. Pathol. 2010, 23, 1393–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Shi, D.; Miao, J.; Wu, H.; Chen, J.; Zhou, X.; Hu, D.; Zhao, C.; Deng, W.; Xie, C. PD-L1 Predicts Poor Prognosis for Nasopharyngeal Carcinoma Irrespective of PD-1 and EBV-DNA Load. Sci. Rep. 2017, 7, 43627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kang, S.; Shen, J.; He, J.; Jiang, L.; Wang, W.; Guo, Z.; Peng, G.; Chen, G.; He, J.; et al. Prognostic Significance of Programmed Cell Death 1 (PD-1) or PD-1 Ligand 1 (PD-L1) Expression in Epithelial-Originated Cancer: A Meta-Analysis. Medicine 2015, 94, e515. [Google Scholar] [CrossRef]
- Smith, C.; McGrath, M.; Neller, M.A.; Matthews, K.K.; Crooks, P.; Le Texier, L.; Panizza, B.; Porceddu, S.; Khanna, R. Complete Response to PD-1 Blockade Following EBV-Specific T-Cell Therapy in Metastatic Nasopharyngeal Carcinoma. NPJ Precis. Oncol. 2021, 5, 24. [Google Scholar] [CrossRef]
- Lu, J.; Lee-Gabel, L.; Nadeau, M.C.; Ferencz, T.M.; Soefje, S.A. Clinical Evaluation of Compounds Targeting PD-1/PD-L1 Pathway for Cancer Immunotherapy. J. Oncol. Pharm. Pr. 2015, 21, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fang, W.; Qin, T.; Yang, Y.; Hong, S.; Liang, W.; Ma, Y.; Zhao, H.; Huang, Y.; Xue, C.; et al. Co-Expression of PD-1 and PD-L1 Predicts Poor Outcome in Nasopharyngeal Carcinoma. Med. Oncol. 2015, 32, 86. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Larkin, J. Combination Immune Checkpoint Blockade with Ipilimumab and Nivolumab in the Management of Advanced Melanoma. Expert Opin. Biol Ther. 2016, 16, 389–396. [Google Scholar] [CrossRef] [PubMed]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 Expression in Molecularly Selected Non-Small-Cell Lung Cancer Patients. Br. J. Cancer 2015, 112, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Price, D.A.; Casazza, J.P.; Ferrari, G.; Nason, M.; Chattopadhyay, P.K.; Roederer, M.; Gostick, E.; Katsikis, P.D.; Douek, D.C.; et al. Surface Expression Patterns of Negative Regulatory Molecules Identify Determinants of Virus-Specific CD8+ T-Cell Exhaustion in HIV Infection. Blood 2011, 117, 4805–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, T.T.C.; Ngan, R.K.C.; Fong, A.H.W.; Law, S.C.K. Application of Circulating Plasma/Serum EBV DNA in the Clinical Management of Nasopharyngeal Carcinoma. Oral Oncol. 2014, 50, 527–538. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Liu, S.; Wang, G.-N.; Zheng, S.-H.; Ding, S.-R.; Tao, Y.; Chen, C.; Liu, S.-R.; Yang, X.; Chang, H.; et al. The Prognostic Significance of PD-L1 and PD-1 Expression in Patients with Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. Cancer Cell Int. 2019, 19, 141. [Google Scholar] [CrossRef]
- Scognamiglio, G.; De Chiara, A.; Di Bonito, M.; Tatangelo, F.; Losito, N.S.; Anniciello, A.; De Cecio, R.; D’Alterio, C.; Scala, S.; Cantile, M.; et al. Variability in Immunohistochemical Detection of Programmed Death Ligand 1 (PD-L1) in Cancer Tissue Types. Int. J. Mol. Sci. 2016, 17, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massini, G.; Siemer, D.; Hohaus, S. EBV in Hodgkin Lymphoma. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009013. [Google Scholar] [CrossRef]
- Pannone, G.; Zamparese, R.; Pace, M.; Pedicillo, M.C.; Cagiano, S.; Somma, P.; Errico, M.E.; Donofrio, V.; Franco, R.; De Chiara, A.; et al. The Role of EBV in the Pathogenesis of Burkitt’s Lymphoma: An Italian Hospital Based Survey. Infect. Agents Cancer 2014, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Jia, K.; Lv, H.; Wang, S.-Q.; Wu, Y.; Lei, H.; Chen, X. EBV-Positive Gastric Cancer: Current Knowledge and Future Perspectives. Front. Oncol. 2020, 10, 2096. [Google Scholar] [CrossRef]
- Hau, P.M.; Lung, H.L.; Wu, M.; Tsang, C.M.; Wong, K.-L.; Mak, N.K.; Lo, K.W. Targeting Epstein-Barr Virus in Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10, 600. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Grywalska, E.; Smarz-Widelska, I.; Korona-Głowniak, I.; Mertowski, S.; Gosik, K.; Hymos, A.; Ludian, J.; Niedźwiedzka-Rystwej, P.; Roliński, J.; Załuska, W. PD-1 and PD-L1 Expression on Circulating Lymphocytes as a Marker of Epstein-Barr Virus Reactivation-Associated Proliferative Glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 8001. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; Marijt, K.A.; Schoonderwoerd, M.J.A.; van Hall, T.; Ossendorp, F.; Fransen, M.F. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology 2017, 6, e1294299. [Google Scholar] [CrossRef]
| Characteristics | Scale | Laryngeal Cancer Patients (n = 45) |
|---|---|---|
| Age, Mean ± SD Years | 62.27 ± 6.40 | |
| Gender (Male/Female) | 45/0 | |
| Tumor stage | TI | 0 |
| TII | 6.7% (3) | |
| TIII | 28.9% (13) | |
| TIV | 64.4% (29) | |
| Nodal stage | N0 | 8.9% (4) |
| NI | 22.2% (10) | |
| NII | 57.8% (26) | |
| NIII | 11.1% (5) | |
| M stage | M0 | 80% (36) |
| MI | 20% (9) | |
| Grading | GI | 26.7% (12) |
| GII | 37.8% (17) | |
| GIII | 35.5 (16) | |
| Anti-VCA IgM | Positive | 49% (22) |
| Negative | 51% (23) | |
| Anti-VCA IgM (U/mL) | Mean ± SD | 25.59 ± 10.36 |
| Median (min–max) | 23.07 (15.31–49.91) | |
| Anti-VCA IgG | Positive | 100% (45) |
| Anti-VCA IgG (U/mL) | Mean ± SD | 90.51 ± 51.79 |
| Median (min–max) | 74.36 (26.22–242.62) | |
| EBV DNA | Positive | 48.9% (22) |
| Negative | 51.1% (23) | |
| EBV DNA copy number/µg DNA in the blood | Mean ± SD | 259.42 ± 207.96 |
| Median (min–max) | 162.66 (32.47–718.92) | |
| EBV DNA copy number/µg DNA in the tumor tissue | Mean ± SD | 582.71 ± 398.91 |
| Median (min–max) | 556.57 (75.76–1542.67) | |
| EBV DNA copy number/µg DNA in the lymph node | Mean ± SD | 430.45 ± 295.70 |
| Median (min–max) | 379.75 (59.89–867.91) |
| Characteristic/Frequency of Occurrence [%] | Patients Diagnosed with Laryngeal Cancer (n = 45) | Control Group (n = 20) | p Value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Age | 63.000 | 50.000–79.000 | 59.000 | 44.000–69.000 | 0.03524 * |
| NK cells | 14.780 | 4.160–24.930 | 13.225 | 4.900–23.140 | 0.17752 |
| Lymphocytes T CD3+ | 70.540 | 57.270–84.200 | 76.110 | 63.340–87.660 | 0.00157 * |
| Lymphocytes B CD19+ | 14.170 | 7.110–22.660 | 9.940 | 4.350–22.120 | 0.00185 * |
| Lymphocytes T CD4+ CD3+ | 43.370 | 29.910–63.960 | 48.285 | 38.970–61.220 | 0.00196 * |
| Lymphocytes T CD8+ CD3+ | 25.150 | 16.660–41.760 | 25.615 | 20.580–36.710 | 0.97410 |
| Ratio of lymphocytes T CD4+ CD3+ to CD8+ CD3+ | 1.692 | 0.716–3.839 | 1.858 | 1.125–2.688 | 0.42480 |
| Lymphocytes T CD3+ CD69+ | 4.970 | 1.400–12.320 | 2.610 | 1.770–5.740 | 0.00070 * |
| Lymphocytes B CD19+ CD69+ | 5.890 | 1320–15,420 | 6755 | 2170–15,400 | 0.50109 |
| Lymphocytes T CD3+ CD25+ | 45.590 | 12.970–89.930 | 30.015 | 23.460–51.120 | 0.00010 * |
| Lymphocytes B CD19+ CD25+ | 31.520 | 10.000–74.640 | 25.275 | 11.860–45.480 | 0.32289 |
| Lymphocytes T CD4+ CD69+ | 4.700 | 1.230–13.840 | 2.135 | 1.170–7.580 | 0.00153 * |
| Lymphocytes T CD8+ CD69+ | 3.700 | 1.060–10.990 | 1.095 | 0.290–4.190 | <0.00001 * |
| Lymphocytes T CD4+ CD25+ | 58.000 | 29.610–96.690 | 45.095 | 29.980–67.740 | 0.00698 * |
| Lymphocytes T CD8+ CD25+ | 5.960 | 1.450–50.410 | 1.760 | 0.550–4.590 | 0.00241 * |
| Lymphocytes B CD19+ PD-1 | 4.150 | 1.140–10.870 | 7.105 | 1.730–11.340 | 0.00030 * |
| Lymphocytes T CD4+ PD-1 | 20.740 | 12.210–32.160 | 7.800 | 5.090–10.240 | <0.00001 * |
| Lymphocytes T CD8+ PD-1 | 17.450 | 10.120–32.450 | 4.015 | 1.320–8.360 | <0.00001 * |
| Frequency of Occurrence [%] | Tumor Sample (n = 45) | Lymph Node Sample (n = 45) | Blood Sample (n = 45) | ANOVA p | Statistically Significant Difference | |||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | |||
| Lymphocytes T CD3+ CD69+ | 38.920 | 5.630–74.510 | 25.740 | 2.740–76.580 | 4.970 | 1.400–12.320 | <0.00001 | a–c |
| Lymphocytes B CD19+ CD69+ | 22.350 | 10.870–56.670 | 25.410 | 10.230–65.150 | 5.890 | 1.320–15.420 | <0.00001 | b,c |
| Lymphocytes T CD3+ CD25+ | 28.950 | 9.250–67.180 | 27.970 | 7.360–51.720 | 45.590 | 12.970–89.930 | <0.00001 | b,c |
| Lymphocytes B CD19+ CD25+ | 20.450 | 4.670–81.110 | 22.210 | 12.210–39.920 | 31.520 | 10.000–74.640 | 0.03831 | c |
| Lymphocytes T CD4+ CD69+ | 45.450 | 15.670–81.510 | 13.270 | 2.850–38.590 | 4.700 | 1.230–13.840 | <0.00001 | a–c |
| Lymphocytes T CD8+ CD69+ | 38.890 | 11.250–89.920 | 15.580 | 2.390–47.970 | 3.700 | 1.060–10.990 | 0.00001 | a–c |
| Lymphocytes T CD4+ CD25+ | 27.460 | 6.570–64.140 | 32.140 | 10.230–60.470 | 58.000 | 29.610–96.690 | <0.00001 | b,c |
| Lymphocytes T CD8+ CD25+ | 8.780 | 2.120–21.120 | 6.410 | 1.960–34.160 | 5.960 | 1.450–50.410 | 0.04731 | a |
| Lymphocytes B CD19+ PD-1 | 22.470 | 12.250–50.250 | 9.210 | 3.470–22.140 | 4.150 | 1.140–10.870 | <0.00001 | a–c |
| Lymphocytes T CD4+ PD-1 | 37.140 | 15.890–75.450 | 21.120 | 9.180–42.870 | 20.740 | 12.210–32.160 | <0.00001 | a,b |
| Lymphocytes T CD8+ PD-1 | 40.410 | 10.450–79.450 | 20.870 | 7.120–32.460 | 17.450 | 10.120–32.450 | <0.00001 | a,b |
| Characteristic Parameter | G = 1 | G = 2 | G = 3 | ANOVA p | Statistically Significant Difference | |||
|---|---|---|---|---|---|---|---|---|
| Blood | Median | Range | Median | Range | Median | Range | ||
| Lymphocytes B CD19+ PD-1 | 3.435 | 1.140–6.610 | 4.690 | 1.890–10.870 | 4.250 | 1.490–6.470 | 0.2060 | NS |
| Lymphocytes T CD4+ PD-1 | 20.925 | 15.470–30.270 | 20.120 | 12.350–30.060 | 21.670 | 12.210–32.160 | 0.6110 | NS |
| Lymphocytes T CD8+ PD-1 | 16.500 | 11.670–29.470 | 14.670 | 10.120–19.450 | 24.120 | 15.010–32.450 | <0.0001 * | b,c |
| Tumor | ||||||||
| Lymphocytes B CD19+ PD-1 | 22.425 | 12.250–47.150 | 27.120 | 15.460–50.250 | 21.070 | 12.450–38.740 | 0.3480 | NS |
| Lymphocytes T CD4+ PD-1 | 23.340 | 15.890–29.140 | 36.170 | 31.020–48.970 | 58.670 | 49.450–75.450 | <0.0001 * | a–c |
| Lymphocytes T CD8+ PD-1 | 34.220 | 10.450–49.680 | 32.140 | 21.120–70.170 | 66.7950 | 29.460–79.450 | <0.0001* | b,c |
| Lymph node | ||||||||
| Lymphocytes B CD19+ PD-1 | 9.530 | 3.470–22.140 | 6.570 | 3.470–14.460 | 10.520 | 6.150–21.120 | 0.0680 | NS |
| Lymphocytes T CD4+ PD-1 | 15.735 | 11.360–24.740 | 21.120 | 9.180–37.460 | 31.9050 | 11.970–42.870 | <0.0001 * | b,c |
| Lymphocytes T CD8+ PD-1 | 15.005 | 7.120–28.190 | 20.010 | 7.150–29.640 | 25.2450 | 22.120–32.460 | <0.0001 * | b,c |
| Characteristic Parameter | T = 2 | T = 3 | T = 4 | ANOVA p | Statistically Significant Difference | |||
|---|---|---|---|---|---|---|---|---|
| Blood | Median | Range | Median | Range | Median | Range | ||
| Lymphocytes B CD19+ PD-1 | 5.250 | 3.110–7.180 | 4.250 | 2.210–10.870 | 3.680 | 1.140–8.170 | 0.3568 | NS |
| Lymphocytes T CD4+ PD-1 | 20.120 | 19.470–22.120 | 17.490 | 12.350–30.270 | 22.120 | 12.210–32.160 | 0.5243 | NS |
| Lymphocytes T CD8+ PD-1 | 10.120 | 10.120–11.450 | 14.240 | 11.670–15.320 | 19.450 | 15.670–32.450 | 0.0000 * | a,b |
| Tumor | ||||||||
| Lymphocytes B CD19+ PD-1 | 32.750 | 22.140–50.250 | 23.240 | 12.750–44.160 | 22.140 | 12.250–47.150 | 0.3092 | NS |
| Lymphocytes T CD4+ PD-1 | 36.790 | 32.150–38.740 | 31.750 | 15.890–67.480 | 40.230 | 17.240–75.450 | 0.4364 | NS |
| Lymphocytes T CD8+ PD-1 | 36.890 | 36.470–45.170 | 27.940 | 10.450–70.250 | 42.120 | 17.690–79.450 | 0.4193 | NS |
| Lymph node | ||||||||
| Lymphocytes B CD19+ PD-1 | 6.140 | 5.460–13.780 | 8.180 | 4.310–17.640 | 9.470 | 3.470–22.140 | 0.6771 | NS |
| Lymphocytes T CD4+ PD-1 | 16.120 | 10.140–16.660 | 24.740 | 11.140–37.890 | 21.120 | 9.180–42.870 | 0.3093 | NS |
| Lymphocytes T CD8+ PD-1 | 12.690 | 9.340–29.640 | 20.120 | 7.120–31.460 | 22.110 | 9.780–32.460 | 0.5419 | NS |
| Characteristic Parameter | N = 0 | N = 1 | N = 2 | N = 3 | ANOVA p | Statistically Significant Difference |
|---|---|---|---|---|---|---|
| Blood | Median (Range) | Median (Range) | Median (Range) | Median (Range) | ||
| Lymphocytes B CD19+ PD-1 | 5.480 (3.680–10.870) | 3.1250 (1.140–6.140) | 3.950 (1.490–8.170) | 5.250 (4.460–6.470) | 0.0202 * | a,b,e |
| Lymphocytes T CD4+ PD-1 | 20.620 (13.310–27.450) | 21.1950 (16.670–30.270) | 20.5550 (12.210–32.160) | 19.470 (18.720–27.150) | 0.9539 | NS |
| Lymphocytes T CD8+ PD-1 | 11.890 (10.120–15.670) | 16.50 (10.120–17.450) | 19.0750 (12.650–32.450) | 29.470 (11.450–31.170) | 0.0035 * | b–e |
| Tumor | ||||||
| Lymphocytes B CD19+ PD-1 | 37.310 (32.750–46.660) | 21.6150 (12.250–34.120) | 22.810 (12.250–44.160) | 38.740 (21.020–50.250) | 0.0025 * | a,b |
| Lymphocytes T CD4+ PD-1 | 31.6750 (15.890–40.020) | 30.080 (17.240–48.970) | 44.840 (20.670–75.450) | 67.270 (22.120–72.680) | 0.0104 * | c–e |
| Lymphocytes T CD8+ PD-1 | 30.140 (10.450–36.470) | 31.8750 (17.690–57.890) | 45.440 (21.120–79.450) | 55.460 (36.890–70.290) | 0.0206 * | b,c |
| Lymph node | ||||||
| Lymphocytes B CD19+ PD-1 | 7.360 (6.140–12.210) | 6.870 (5.460–22.140) | 10.520 (3.470–21.140) | 9.470 (8.180–16.780) | 0.8375 | NS |
| Lymphocytes T CD4+ PD-1 | 14.9450 (11.140–16.660) | 16.8950 (9.180–25.340) | 23.430 (11.250–42.870) | 34.620 (10.140–39.460) | 0.0267 * | b–e |
| Lymphocytes T CD8+ PD-1 | 8.2450 (7.120–9.780) | 12.340 (10.120–17.560) | 22.1150 (17.690–27.650) | 31.460 (28.190–32.460) | <0.0001 * | b–f |
| Characteristic Parameter | M = 0 | M = 1 | p | ||
|---|---|---|---|---|---|
| Blood | Median | Range | Median | Range | |
| Lymphocytes B CD19+ PD-1 | 3.965 | 1.140–10.870 | 4.250 | 2.110–6.470 | 0.7517 |
| Lymphocytes T CD4+ PD-1 | 20.005 | 12.210–30.270 | 25.150 | 15.670–32.160 | 0.1238 |
| Lymphocytes T CD8+ PD-1 | 17.185 | 10.120–29.470 | 27.450 | 14.470–32.450 | 0.0008 * |
| Tumor | |||||
| Lymphocytes B CD19+ PD-1 | 21.960 | 12.250–50.250 | 31.170 | 21.020–38.740 | 0.2723 |
| Lymphocytes T CD4+ PD-1 | 32.655 | 15.890–60.870 | 68.900 | 33.250–75.450 | <0.0001 * |
| Lymphocytes T CD8+ PD-1 | 35.310 | 10.450–67.450 | 70.170 | 55.460–79.450 | <0.0001 * |
| Lymph node | |||||
| Lymphocytes B CD19+ PD-1 | 10.070 | 3.470–22.140 | 8.210 | 5.640–16.780 | 0.3755 |
| Lymphocytes T CD4+ PD-1 | 16.990 | 9.180–32.150 | 37.150 | 27.460–42.870 | <0.0001 * |
| Lymphocytes T CD8+ PD-1 | 19.675 | 7.120–29.640 | 27.120 | 20.870–32.460 | 0.0002 * |
| Type of Sample | Frequency of Occurrence [%] | The Cox Proportional Hazard Regression Model Chi-Squared: 64.60644; df = 9; p = 0.00000 | |||
|---|---|---|---|---|---|
| p | Relative Hazard | Relative Hazard 95% Lower | Hazard Relative 95% Upper | ||
| Blood | Lymphocytes B CD19+ PD-1 | 0.060 | 0.516 | 0.259 | 1.028 |
| Lymphocytes T CD4+ PD-1 | 0.374 | 1.063 | 0.929 | 1.218 | |
| Lymphocytes T CD8+ PD-1 | 0.438 | 0.944 | 0.815 | 1.093 | |
| Tumor | Lymphocytes B CD19+ PD-1 | 0.297 | 0.942 | 0.843 | 1.054 |
| Lymphocytes T CD4+ PD-1 | 0.994 | 1.000 | 0.921 | 1.086 | |
| Lymphocytes T CD8+ PD-1 | 0.853 | 0.995 | 0.939 | 1.053 | |
| Lymph node | Lymphocytes B CD19+ PD-1 | 0.180 | 0.859 | 0.688 | 1.073 |
| Lymphocytes T CD4+ PD-1 | 0.000 * | 1.480 | 1.189 | 1.842 | |
| Lymphocytes T CD8+ PD-1 | 0.061 | 1.352 | 0.986 | 1.854 | |
| Characteristic Parameter | G = 1 | G = 2 | G = 3 | ANOV p | Statistically Significant Difference | |||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | |||
| EBV DNA copy number/µg DNA in the tumor tissue | 514.117 | 213.203–604.152 | 367.46 | 112.884–715.071 | 942.979 | 75.762–1542.67 | 0.1100 | NS |
| EBV DNA copy number/µg DNA in the lymph node | 376.585 | 127.357–454.85 | 232.516 | 78.20–473.254 | 810.267 | 59.894–867.907 | 0.0470 * | a |
| EBV DNA copy number/µg DNA in the blood | 159.315 | 83.984–269.63 | 105.616 | 43.873–305.320 | 445.997 | 32.471–718.919 | 0.0220 * | a |
| IgM VCA EBV | 20.120 | 16.070–49.910 | 16.540 | 15.310–30.000 | 29.055 | 19.510–49.740 | 0.0611 | NS |
| IgG VCA EBV | 95.40 | 27.860–103.410 | 64.420 | 37.150–135.610 | 77.035 | 31.560–148.30 | 0.9771 | NS |
| Characteristic Parameter | T = 2 | T = 3 | T = 4 | ANOV p | Statistically Significant Difference | |||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | |||
| EBV DNA copy number/µg DNA in the tumor tissue | 160.807 | 112.884–208.729 | 202.499 | 75.762–499.238 | 715.91 | 102.872–1542.67 | 0.0024 * | NS |
| EBV DNA copy number/µg DNA in the lymph node | 101.686 | 78.2–125.171 | 154.125 | 59.894–361.409 | 519.145 | 70.562–867.907 | 0.0013 * | a |
| EBV DNA copy number/µg DNA in the blood | 53.878 | 43.873–63.884 | 99.838 | 32.471–146.741 | 357.678 | 52.55–718.919 | 0.0062 * | a |
| IgM VCA EBV | 16.135 | 15.4–16.87 | 24.825 | 16.54–40.3 | 24.47 | 15.31–49.91 | 0.4190 | NS |
| IgG VCA EBV | 50.785 | 37.15–64.42 | 80.38 | 37.18–100.12 | 77.035 | 27.86–148.3 | 0.4370 | NS |
| Characteristic Parameter | N = 0 | N = 1 | N = 2 | N = 3 | ANOV p | Statistically Significant Difference |
|---|---|---|---|---|---|---|
| Median Range | Median Range | Median Range | Median Range | |||
| EBV DNA copy number/µg DNA in the tumor tissue | 208.729 208.729–208.729 | 451.782 112.884–715.071 | 556.572 102.872–1542.67 | 800.029 75.762–1000.468 | 0.6480 | NS |
| EBV DNA copy number/µg DNA in the lymph node | 125.171 125.171–125.171 | 347.553 78.2–473.254 | 379.745 70.562–867.907 | 651.149 59.894–858.16 | 0.4790 | NS |
| EBV DNA copy number/µg DNA in the blood | 63.884 63.884–63.884 | 122.811 43.873–305.32 | 162.658 52.55–718.919 | 364.969 32.471–504.924 | 0.5032 | NS |
| IgM VCA EBV | 16.87 16.87–16.87 | 15.57 15.31–30 | 24.47 16.07–32.2 | 45.02 22.85–49.91 | 0.0011 * | a,b |
| IgG VCA EBV | 37.15 37.15–37.15 | 72.65 59.22–135.61 | 76.59 31.56–148.3 | 77.035 27.86–88.11 | 0.6233 | NS |
| Characteristic Parameter | M = 0 | M = 1 | p | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| EBV DNA copy number/µg DNA in the tumor tissue | 514.117 | 112.884–1542.67 | 716.748 | 75.762–1212.282 | 0.8446 |
| EBV DNA copy number/µg DNA in the lymph node | 376.585 | 78.2–867.907 | 565.037 | 59.894–863.076 | 0.4596 |
| EBV DNA copy number/µg DNA in the blood | 159.315 | 43.873–718.919 | 410.037 | 32.471–675.295 | 0.3182 |
| IgM VCA EBV | 18.940 | 15.31–49.910 | 28.830 | 16.54–49.740 | 0.14942 |
| IgG VCA EBV | 72.650 | 27.86–135.610 | 73.790 | 31.56–148.30 | 0.94265 |
| Type of Sample | Frequency of Occurrence [%] | EBV DNA Copy Number/µg DNA in the Blood | EBV DNA Copy Number/µg DNA in the Tumor Tissue | EBV DNA Copy Number/µg DNA in the Lymph Node |
|---|---|---|---|---|
| Blood | Lymphocytes B CD19+ PD-1 | −0.0489 p = 0.762 | −0.0462 p = 0.774 | −0.0043 p = 0.979 |
| Lymphocytes T CD4+ PD-1 | 0.2952 p = 0.061 | 0.3385 p = 0.030 * | 0.3705 p = 0.0017 * | |
| Lymphocytes T CD8+ PD-1 | 0.6969 p = 0.000 * | 0.6282 p = 0.000 * | 0.6760 p = 0.000 * | |
| Tumor | Lymphocytes B CD19+ PD-1 | 0.1275 p = 0.427 | 0.0791 p = 0.623 | 0.1155 p = 0.472 |
| Lymphocytes T CD4+ PD-1 | 0.5289 p = 0.000 * | 0.4304 p = 0.005 * | 0.4850 p = 0.001 * | |
| Lymphocytes T CD8+ PD-1 | 0.4469 p = 0.003 * | 0.3727 p = 0.016 * | 0.3902 p = 0.012 * | |
| Lymph node | Lymphocytes B CD19+ PD-1 | 0.0496 p = 0.758 | 0.0870 p = 0.589 | 0.0852 p = 0.596 |
| Lymphocytes T CD4+ PD-1 | 0.5461 p = 0.000 * | 0.4523 p = 0.003 * | 0.4817 p = 0.001 * | |
| Lymphocytes T CD8+ PD-1 | 0.4173 p = 0.007 * | 0.3604 p = 0.021 | 0.4193 p = 0.006 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klatka, J.; Szkatuła-Łupina, A.; Hymos, A.; Klatka, M.; Mertowska, P.; Mertowski, S.; Grywalska, E.; Charytanowicz, M.; Błażewicz, A.; Poniewierska-Baran, A.; et al. The Clinical, Pathological, and Prognostic Value of High PD-1 Expression and the Presence of Epstein–Barr Virus Reactivation in Patients with Laryngeal Cancer. Cancers 2022, 14, 480. https://doi.org/10.3390/cancers14030480
Klatka J, Szkatuła-Łupina A, Hymos A, Klatka M, Mertowska P, Mertowski S, Grywalska E, Charytanowicz M, Błażewicz A, Poniewierska-Baran A, et al. The Clinical, Pathological, and Prognostic Value of High PD-1 Expression and the Presence of Epstein–Barr Virus Reactivation in Patients with Laryngeal Cancer. Cancers. 2022; 14(3):480. https://doi.org/10.3390/cancers14030480
Chicago/Turabian StyleKlatka, Janusz, Anna Szkatuła-Łupina, Anna Hymos, Maria Klatka, Paulina Mertowska, Sebastian Mertowski, Ewelina Grywalska, Małgorzata Charytanowicz, Anna Błażewicz, Agata Poniewierska-Baran, and et al. 2022. "The Clinical, Pathological, and Prognostic Value of High PD-1 Expression and the Presence of Epstein–Barr Virus Reactivation in Patients with Laryngeal Cancer" Cancers 14, no. 3: 480. https://doi.org/10.3390/cancers14030480
APA StyleKlatka, J., Szkatuła-Łupina, A., Hymos, A., Klatka, M., Mertowska, P., Mertowski, S., Grywalska, E., Charytanowicz, M., Błażewicz, A., Poniewierska-Baran, A., Bębnowska, D., & Niedźwiedzka-Rystwej, P. (2022). The Clinical, Pathological, and Prognostic Value of High PD-1 Expression and the Presence of Epstein–Barr Virus Reactivation in Patients with Laryngeal Cancer. Cancers, 14(3), 480. https://doi.org/10.3390/cancers14030480









