Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Sweat Gland Neoplasms with Recent Advances in Their Diagnosis
2.1. Adenoid Cystic Carcinoma
2.2. Cutaneous Mixed Tumor (Chondroid Syringoma)
2.3. Cylindroma and Spiradenoma
2.4. Hidradenoma
2.5. Microcystic Adnexal Carcinoma
2.6. Myoepithelioma
2.7. Poroma
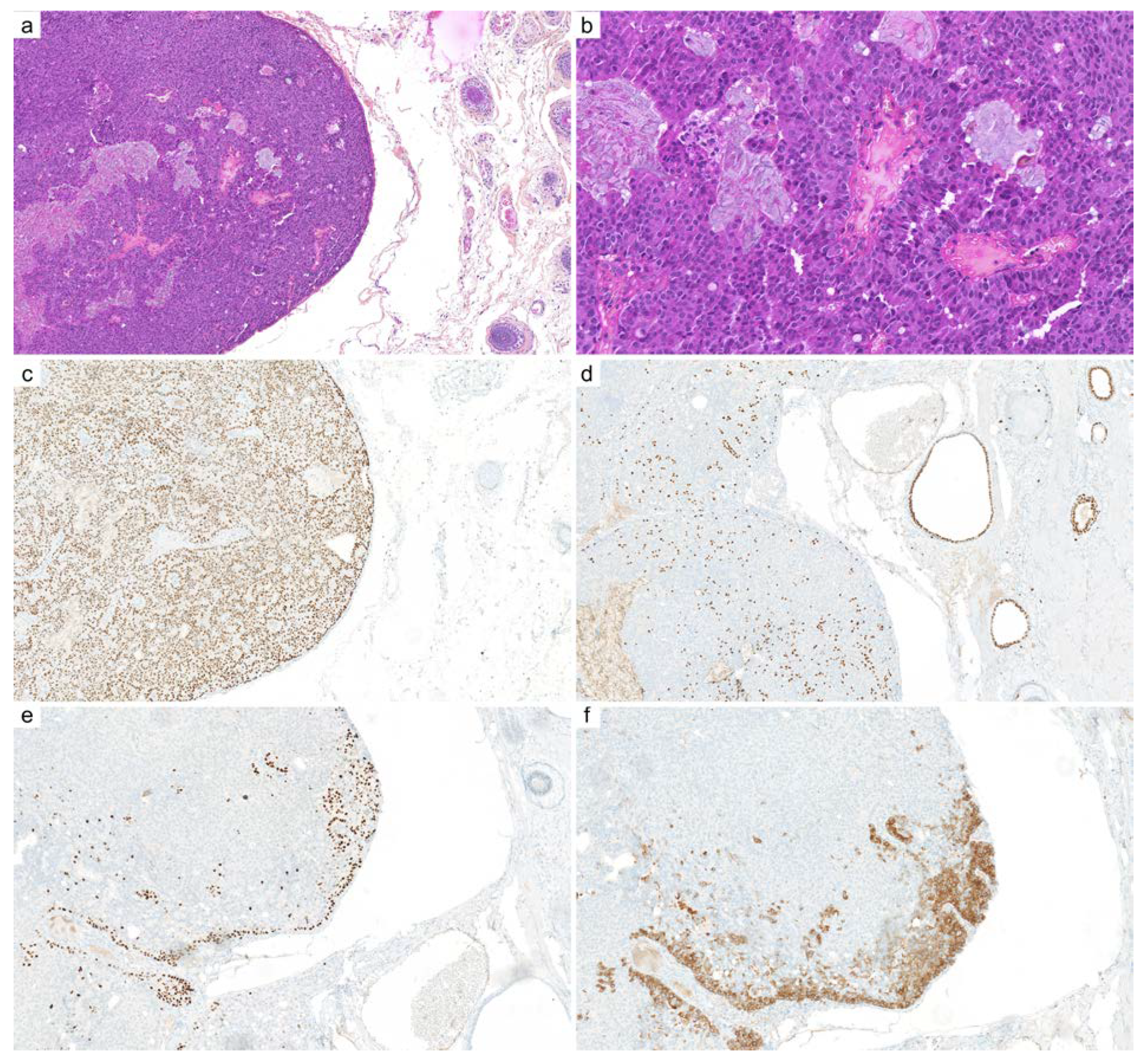
2.8. Secretory Carcinoma
2.9. Tubular Adenoma and Syringocystadenoma Papilliferum
2.10. Endocrine Mucin-Producing Sweat Gland Carcinoma
3. Main Clinical Settings to Use These Biomarkers and Their Limits
4. Other Sweat Gland Tumors Lacking Recent Data
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seris, A.; Battistella, M.; Beylot-Barry, M.; Dalle, S.; Mortier, L.; Lebbé, C.; Blom, A.; Neidhart-Berard, E.-M.; Kramkimel, N.; Dupuy, A.; et al. Creation, implementation and objectives of CARADERM, a national network for rare skin carcinomas—Adnexal neoplasm part. Ann. Dermatol. Venereol. 2019, 146, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumours; International Agency for Research on Cancer: Lyon, France, 2018; ISBN 978-92-832-2440-2. [Google Scholar]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer. Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.B.; Kanner, W.A.; Fehr, A.; Andrén, Y.; Moskaluk, C.A.; Löning, T.; Stenman, G.; Frierson, H.F. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod. Pathol. 2011, 24, 1169–1176. [Google Scholar] [CrossRef]
- Mitani, Y.; Rao, P.H.; Futreal, P.A.; Roberts, D.B.; Stephens, P.J.; Zhao, Y.-J.; Zhang, L.; Mitani, M.; Weber, R.S.; Lippman, S.M.; et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: Association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin. Cancer Res. 2011, 17, 7003–7014. [Google Scholar] [CrossRef]
- Stenman, G.; Persson, F.; Andersson, M.K. Diagnostic and therapeutic implications of new molecular biomarkers in salivary gland cancers. Oral. Oncol. 2014, 50, 683–690. [Google Scholar] [CrossRef]
- Hsieh, M.-S.; Lee, Y.-H.; Chang, Y.-L. SOX10-Positive salivary gland tumors: A growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum. Pathol. 2016, 56, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, H.J.; Yoo, C.W.; Park, W.S.; Ryu, J.S.; Jung, Y.-S.; Choi, S.W.; Park, J.Y.; Han, N. PLAG1, SOX10, and myb expression in benign and malignant salivary gland neoplasms. J. Pathol. Transl. Med. 2019, 53, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, L.; Sanati, S. SOX10 is a sensitive marker for breast and salivary gland adenoid cystic carcinoma: Immunohistochemical characterization of adenoid cystic carcinomas. Breast Cancer 2019, 13, 1178223419842185. [Google Scholar] [CrossRef]
- Adkins, B.D.; Geromes, A.; Zhang, L.Y.; Chernock, R.; Kimmelshue, K.; Lewis, J.; Ely, K. SOX10 and GATA3 in adenoid cystic carcinoma and polymorphous adenocarcinoma. Head Neck Pathol. 2020, 14, 406–411. [Google Scholar] [CrossRef]
- Prieto-Granada, C.N.; Zhang, L.; Antonescu, C.R.; Henneberry, J.M.; Messina, J.L. Primary cutaneous adenoid cystic carcinoma with MYB aberrations: Report of three cases and comprehensive review of the literature. J. Cutan. Pathol. 2017, 44, 201–209. [Google Scholar] [CrossRef]
- Andreadis, D.; Epivatianos, A.; Poulopoulos, A.; Nomikos, A.; Papazoglou, G.; Antoniades, D.; Barbatis, C. Detection of C-KIT (CD117) molecule in benign and malignant salivary gland tumours. Oral Oncol. 2006, 42, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Pardal, J.; Sundram, U.; Selim, M.A.; Hoang, M.P. GATA3 and MYB Expression in cutaneous adnexal neoplasms. Am. J. Derm. 2017, 39, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Houghton, S.L.; Biswas, A. A Comparative study of immunohistochemical myoepithelial cell markers in cutaneous benign cystic apocrine lesions. Am. J. Derm. 2016, 38, 475–483. [Google Scholar] [CrossRef]
- North, J.P.; McCalmont, T.H.; Fehr, A.; van Zante, A.; Stenman, G.; LeBoit, P.E. Detection of MYB Alterations and other immunohistochemical markers in primary cutaneous adenoid cystic carcinoma. Am. J. Surg. Pathol. 2015, 39, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Chaudhry, I.H.; Ramdial, P.; Lazar, A.J.; McMenamin, M.E.; Kazakov, D.; Brenn, T.; Calonje, E. Primary cutaneous adenoid cystic carcinoma: A clinicopathologic and immunohistochemical study of 27 cases. Am. J. Surg. Pathol. 2013, 37, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.T.P.; North, J.P. MYB, CD117 and SOX-10 expression in cutaneous adnexal tumors. J. Cutan. Pathol. 2017, 44, 444–450. [Google Scholar] [CrossRef]
- West, R.B.; Kong, C.; Clarke, N.; Gilks, T.; Lipsick, J.S.; Cao, H.; Kwok, S.; Montgomery, K.D.; Varma, S.; Le, Q.-T. MYB Expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am. J. Surg. Pathol. 2011, 35, 92–99. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.B.; Ko, J.J.; Siever, J.; Chan, A.M.Y.; Simpson, R.H.W.; Hao, D.; Lau, H.Y. MYB-NFIB Gene fusions identified in archival adenoid cystic carcinoma tissue employing nanostring analysis: An exploratory study. Diagn. Pathol. 2019, 14, 78. [Google Scholar] [CrossRef]
- Lv, J.-J.; Ren, M.; Cai, X.; Hu, J.; Kong, J.-C.; Kong, Y.-Y. Primary cutaneous adenoid cystic carcinoma: A clinicopathologic, immunohistochemical, and fluorescence in-situ hybridisation study of 13 cases. Histopathology 2021, 80, 407–419. [Google Scholar] [CrossRef]
- Goto, K.; Kajimoto, K.; Sugino, T.; Nakatsuka, S.-I.; Yoshida, M.; Noto, M.; Kono, M.; Takai, T. MYB Translocations in both myoepithelial and ductoglandular epithelial cells in adenoid cystic carcinoma: A histopathologic and genetic reappraisal in six primary cutaneous cases. Am. J. Derm. 2021, 43, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Dobashi, A.; Sakata, S.; Sato, Y.; Baba, S.; Seto, A.; Mitani, H.; Kawabata, K.; Takeuchi, K. MYB and MYBL1 in adenoid cystic carcinoma: Diversity in the mode of genomic rearrangement and transcripts. Mod. Pathol. 2018, 31, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Kyrpychova, L.; Vanecek, T.; Grossmann, P.; Martinek, P.; Steiner, P.; Hadravsky, L.; Belousova, I.E.; Shelekhova, K.V.; Svajdler, M.; Dubinsky, P.; et al. Small subset of adenoid cystic carcinoma of the skin is associated with alterations of the MYBL1 gene similar to their extracutaneous counterparts. Am. J. Derm. 2018, 40, 721–726. [Google Scholar] [CrossRef]
- Kim, J.; Geyer, F.C.; Martelotto, L.G.; Ng, C.K.; Lim, R.S.; Selenica, P.; Li, A.; Pareja, F.; Fusco, N.; Edelweiss, M.; et al. MYBL1 rearrangements and MYB amplification in breast adenoid cystic carcinomas lacking the MYB-NFIB fusion gene. J. Pathol. 2018, 244, 143–150. [Google Scholar] [CrossRef]
- George, O.L.; Ness, S.A. Situational awareness: Regulation of the myb transcription factor in differentiation, the cell cycle and oncogenesis. Cancers 2014, 6, 2049–2071. [Google Scholar] [CrossRef] [PubMed]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary gland carcinoma: Novel targets to overcome treatment resistance in advanced disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.; Kovács, A.; Löning, T.; Frierson, H.; Van den Oord, J.; Stenman, G. The MYB-NFIB gene fusion—A novel genetic link between adenoid cystic carcinoma and dermal cylindroma. J. Pathol. 2011, 224, 322–327. [Google Scholar] [CrossRef]
- Rashid, M.; van der Horst, M.; Mentzel, T.; Butera, F.; Ferreira, I.; Pance, A.; Rütten, A.; Luzar, B.; Marusic, Z.; de Saint Aubain, N.; et al. ALPK1 hotspot mutation as a driver of human spiradenoma and spiradenocarcinoma. Nat. Commun. 2019, 10, 2213. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Dubuc, A.; Hanna, J. Differential expression of PLAG1 in apocrine and eccrine cutaneous mixed tumors: Evidence for distinct molecular pathogenesis. Am. J. Derm. 2020, 42, 251–257. [Google Scholar] [CrossRef]
- Panagopoulos, I.; Gorunova, L.; Andersen, K.; Lund-Iversen, M.; Lobmaier, I.; Micci, F.; Heim, S. NDRG1-PLAG1 and TRPS1-PLAG1 fusion genes in chondroid syringoma. Cancer Genom. Proteom. 2020, 17, 237–248. [Google Scholar] [CrossRef]
- Matsuyama, A.; Hisaoka, M.; Hashimoto, H. PLAG1 Expression in Mesenchymal Tumors: An Immunohistochemical Study with Special Emphasis on the Pathogenetical Distinction between Soft Tissue Myoepithelioma and Pleomorphic Adenoma of the Salivary Gland. Pathol. Int. 2012, 62, 1–7. [Google Scholar] [CrossRef]
- Katabi, N.; Ghossein, R.; Ho, A.; Dogan, S.; Zhang, L.; Sung, Y.-S.; Antonescu, C.R. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum. Pathol. 2015, 46, 26–33. [Google Scholar] [CrossRef]
- Stenman, G. Fusion oncogenes and tumor type specificity—Insights from salivary gland tumors. Semin. Cancer Biol. 2005, 15, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Stenman, G. Fusion Oncogenes in salivary gland tumors: Molecular and clinical consequences. Head Neck Pathol. 2013, 7 (Suppl. 1), S12–S19. [Google Scholar] [CrossRef]
- Matsuyama, A.; Hisaoka, M.; Hashimoto, H. PLAG1 Expression in cutaneous mixed tumors: An immunohistochemical and molecular genetic study. Virchows Arch. 2011, 459, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, I.; Gorunova, L.; Lund-Iversen, M.; Bassarova, A.; Heim, S. Fusion of the genes PHF1 and TFE3 in malignant chondroid syringoma. Cancer Genom. Proteom. 2019, 16, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Iwai, S.; Hosaka, H.; Kitami, Y.; Akiyama, M.; Suzuki, T.; Sueki, H. Immunohistochemical characterization of non-epithelial cells in spiradenoma. J. Derm. 2013, 40, 896–900. [Google Scholar] [CrossRef]
- Yiğit, N.; Çelik, E.; Yavan, İ.; Günal, A.; Kurt, B.; Karslıoğlu, Y.; Öngürü, Ö.; Özcan, A. Distinctive immunostaining of claudin-4 in spiradenomas. Ann. Diagn. Pathol. 2016, 20, 44–47. [Google Scholar] [CrossRef]
- Petersson, F.; Nga, M.E. Spiradenocarcinoma with low-grade basal cell adenocarcinoma pattern: Report of a Case with varied morphology and wild type TP53. J. Cutan. Pathol. 2012, 39, 372–376. [Google Scholar] [CrossRef]
- Van der Horst, M.P.J.; Marusic, Z.; Hornick, J.L.; Luzar, B.; Brenn, T. Morphologically low-grade spiradenocarcinoma: A Clinicopathologic study of 19 cases with emphasis on outcome and myb expression. Mod. Pathol. 2015, 28, 944–953. [Google Scholar] [CrossRef]
- Bignell, G.R.; Warren, W.; Seal, S.; Takahashi, M.; Rapley, E.; Barfoot, R.; Green, H.; Brown, C.; Biggs, P.J.; Lakhani, S.R.; et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat. Genet. 2000, 25, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Biernat, W.; Peraud, A.; Wozniak, L.; Ohgaki, H. P53 Mutations in sweat gland carcinomas. Int. J. Cancer 1998, 76, 317–320. [Google Scholar] [CrossRef]
- Rajan, N.; Andersson, M.K.; Sinclair, N.; Fehr, A.; Hodgson, K.; Lord, C.J.; Kazakov, D.V.; Vanecek, T.; Ashworth, A.; Stenman, G. Overexpression of MYB drives proliferation of CYLD-defective cylindroma cells. J. Pathol. 2016, 239, 197–205. [Google Scholar] [CrossRef]
- Williams, E.A.; Montesion, M.; Sharaf, R.; Corines, J.; Patel, P.J.; Gillespie, B.J.; Pavlick, D.C.; Sokol, E.S.; Alexander, B.M.; Williams, K.J.; et al. CYLD-mutant cylindroma-like basaloid carcinoma of the anus: A Genetically and morphologically distinct class of HPV-related anal carcinoma. Mod. Pathol. 2020, 33, 2614–2625. [Google Scholar] [CrossRef]
- Williams, E.A.; Montesion, M.; Alexander, B.M.; Ramkissoon, S.H.; Elvin, J.A.; Ross, J.S.; Williams, K.J.; Glomski, K.; Bledsoe, J.R.; Tse, J.Y.; et al. CYLD Mutation characterizes a subset of HPV-positive head and neck squamous cell carcinomas with distinctive genomics and frequent cylindroma-like histologic features. Mod. Pathol. 2021, 34, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Wiedemeyer, K.; Gill, P.; Schneider, M.; Kind, P.; Brenn, T. Clinicopathologic characterization of hidradenoma on acral sites: A Diagnostic pitfall with digital papillary adenocarcinoma. Am. J. Surg. Pathol. 2019, 44, 711–717. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Su, A.; Robbins, B.A.; Altree-Tacha, D.; Ra, S. SOX10 Immunohistochemistry in sweat ductal/glandular neoplasms. J. Cutan. Pathol. 2017, 44, 544–547. [Google Scholar] [CrossRef]
- Nazarian, R.M.; Kapur, P.; Rakheja, D.; Piris, A.; Duncan, L.M.; Mihm, M.C.; Hoang, M.P. Atypical and malignant hidradenomas: A histological and immunohistochemical study. Mod. Pathol. 2009, 22, 600–610. [Google Scholar] [CrossRef]
- Yoshimi, K.; Goto, H.; Otsuka, M.; Yoshikawa, S.; Omodaka, T.; Kiyohara, Y. Translocation of the MAML2 gene in hidradenocarcinoma. J. Derm. 2017, 44, e190–e191. [Google Scholar] [CrossRef]
- Seethala, R.R.; Dacic, S.; Cieply, K.; Kelly, L.M.; Nikiforova, M.N. A Reappraisal of the MECT1/MAML2 Translocation in salivary mucoepidermoid carcinomas. Am. J. Surg. Pathol. 2010, 34, 1106–1121. [Google Scholar] [CrossRef]
- Jee, K.J.; Persson, M.; Heikinheimo, K.; Passador-Santos, F.; Aro, K.; Knuutila, S.; Odell, E.W.; Mäkitie, A.; Sundelin, K.; Stenman, G.; et al. Genomic Profiles and CRTC1-MAML2 Fusion distinguish different subtypes of mucoepidermoid carcinoma. Mod. Pathol. 2013, 26, 213–222. [Google Scholar] [CrossRef]
- Nakayama, T.; Miyabe, S.; Okabe, M.; Sakuma, H.; Ijichi, K.; Hasegawa, Y.; Nagatsuka, H.; Shimozato, K.; Inagaki, H. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod. Pathol. 2009, 22, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, D.V.; Ivan, D.; Kutzner, H.; Spagnolo, D.V.; Grossmann, P.; Vanecek, T.; Sima, R.; Kacerovska, D.; Shelekhova, K.V.; Denisjuk, N.; et al. Cutaneous hidradenocarcinoma: A clinicopathological, immunohistochemical, and molecular biologic study of 14 Cases, including Her2/Neu gene expression/amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am. J. Derm. 2009, 31, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Kyrpychova, L.; Kacerovska, D.; Vanecek, T.; Grossmann, P.; Michal, M.; Kerl, K.; Kazakov, D.V. Cutaneous Hidradenoma: A study of 21 neoplasms revealing neither correlation between the cellular composition and CRTC1-MAML2 fusions nor presence of CRTC3-MAML2 fusions. Ann. Diagn. Pathol. 2016, 23, 8–13. [Google Scholar] [CrossRef]
- Yan, K.; Yesensky, J.; Hasina, R.; Agrawal, N. Genomics of mucoepidermoid and adenoid cystic carcinomas. Laryngoscope Investig. Otolaryngol. 2018, 3, 56–61. [Google Scholar] [CrossRef]
- Wu, Y.; He, Z.; Li, S.; Tang, H.; Wang, L.; Yang, S.; Dong, B.; Qin, J.; Sun, Y.; Yu, H.; et al. Gefitinib represses JAK-STAT signaling activated by CRTC1-MAML2 fusion in mucoepidermoid carcinoma cells. Curr. Cancer Drug Targets 2019, 19, 796–806. [Google Scholar] [CrossRef]
- Möller, E.; Stenman, G.; Mandahl, N.; Hamberg, H.; Mölne, L.; van den Oord, J.J.; Brosjö, O.; Mertens, F.; Panagopoulos, I. POU5F1, encoding a key regulator of stem cell pluripotency, is fused to EWSR1 in Hidradenoma of the skin and mucoepidermoid carcinoma of the salivary glands. J. Pathol. 2008, 215, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Bozdogan, O.; Kadan, E.; Bozdogan, N. Are there EWSR1 rearranged cutaneous hidradenomas and mucoepidermoid carcinomas of salivary glands? A fish study and review of the literature. Pol. J. Pathol. 2020, 71, 99–106. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Zhang, L.; Chang, N.-E.; Pawel, B.R.; Travis, W.; Katabi, N.; Edelman, M.; Rosenberg, A.E.; Nielsen, G.P.; Dal Cin, P.; et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 2010, 49, 1114–1124. [Google Scholar] [CrossRef]
- Llamas-Velasco, M.; Pérez-Gónzalez, Y.C.; Bosch-Príncep, R.; Fernández-Figueras, M.-T.; Rütten, A. Solid Carcinoma Is a Variant of microcystic adnexal carcinoma: A 14-case series. J. Cutan. Pathol. 2018, 45, 897–904. [Google Scholar] [CrossRef]
- Torre-Castro, J.; Moya-Martinez, C.; Sangueza, O.; Raquena, L. Microcystic Adnexal Adenoma: The Benign Counterpart of Microcystic Adnexal Carcinoma. Am. J. Dermatopathol. 2021, 43. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, J.; Wetzig, T.; Rutten, A.; Hortnagel, K.; Tischkowitz, M.; Ziemer, M. Sweat Duct Proliferation Associated with Aggregation of Elastic Tissue and Atrophodermia Vermiculata: A Simulator of Microcystic Adnexal Carcinoma—A Family with MALTA-Syndrome. J. Der Dtsch. Dermatol. Gesellschaft. J. Ger. Soc. Dermatol. 2021, 19, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.P.; Dresser, K.A.; Kapur, P.; High, W.A.; Mahalingam, M. Microcystic adnexal carcinoma: An immunohistochemical reappraisal. Mod. Pathol. 2008, 21, 178–185. [Google Scholar] [CrossRef]
- Sellheyer, K.; Nelson, P.; Kutzner, H.; Patel, R.M. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers: BerEP4 and stem cell markers in adnexal neoplasms. J. Cutan. Pathol. 2013, 40, 363–370. [Google Scholar] [CrossRef]
- Gill, P.; Naugler, C.; Abi Daoud, M.S. Utility of Ber-EP4 and MOC-31 in basaloid skin tumor detection. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Krahl, D.; Sellheyer, K. Monoclonal Antibody Ber-EP4 Reliably discriminates between microcystic adnexal carcinoma and basal cell carcinoma. J Cutan. Pathol. 2007, 34, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Frouin, E.; Vignon-Pennamen, M.D.; Balme, B.; Cavelier-Balloy, B.; Zimmermann, U.; Ortonne, N.; Carlotti, A.; Pinquier, L.; André, J.; Cribier, B. Anatomoclinical Study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J. Eur. Acad. Derm. Venereol. 2015, 29, 1978–1994. [Google Scholar] [CrossRef]
- Vidal, C.I.; Goldberg, M.; Burstein, D.E.; Emanuel, H.J.; Emanuel, P.O. P63 immunohistochemistry is a useful adjunct in distinguishing sclerosing cutaneous tumors. Am. J. Dermatopathol. 2010, 32, 257–261. [Google Scholar] [CrossRef]
- Plaza, J.A.; Ortega, P.F.; Stockman, D.L.; Suster, S. Value of P63 and Podoplanin (D2-40) Immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: A clinicopathologic and immunohistochemical study of 79 cases. J. Cutan. Pathol. 2010, 37, 403–410. [Google Scholar] [CrossRef]
- Smith, K.J.; Williams, J.; Corbett, D.; Skelton, H. Microcystic adnexal carcinoma: An immunohistochemical study including markers of proliferation and apoptosis. Am. J. Surg. Pathol. 2001, 25, 464–471. [Google Scholar] [CrossRef]
- Evangelista, M.T.P.; North, J.P. Comparative analysis of cytokeratin 15, TDAG51, cytokeratin 20 and androgen receptor in sclerosing adnexal neoplasms and variants of basal cell carcinoma: Comparative analysis of cytokeratin 15, TDAG51, cytokeratin 20 and androgen receptor. J. Cutan. Pathol. 2015, 42, 824–831. [Google Scholar] [CrossRef]
- Bush, J.W.; Gru, A.A.; Wick, M.R. Immunoreactivity for Sox10 in basaloid neoplasms of the skin. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 114–118. [Google Scholar] [CrossRef]
- Chen, M.-B.; Laber, D.A. Metastatic microcystic adnexal carcinoma with DNA sequencing results and response to systemic antineoplastic chemotherapy. Anticancer. Res. 2017, 37, 5109–5111. [Google Scholar] [CrossRef][Green Version]
- Gambichler, T.; Hartenstein, I.; Dreißigacker, M.; Stockfleth, E.; Stücker, M.; Schaller, J.; Schulze, H.-J.; Becker, J.C.; Käfferlein, H.U.; Brüning, T.; et al. Expression of hedgehog signalling molecules in microcystic adnexal carcinoma. Clin. Exp. Derm. 2021, 46, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Suurmeijer, A.J.H.; Dickson, B.C.; Swanson, D.; Zhang, L.; Sung, Y.-S.; Fletcher, C.D.; Antonescu, C.R. A Morphologic and molecular reappraisal of myoepithelial tumors of soft tissue, bone, and viscera with EWSR1 and FUS gene rearrangements. Genes Chromosomes Cancer 2020, 59, 348–356. [Google Scholar] [CrossRef]
- Michal, M.; Miettinen, M. Myoepitheliomas of the skin and soft tissues. Report of 12 Cases. Virchows Arch. 1999, 434, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, T.; Requena, L.; Kaddu, S.; Soares de Aleida, L.M.; Sangueza, O.P.; Kutzner, H. Cutaneous myoepithelial neoplasms: Clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J. Cutan. Pathol. 2003, 30, 294–302. [Google Scholar] [CrossRef]
- Hornick, J.L.; Fletcher, C.D.M. Cutaneous Myoepithelioma: A clinicopathologic and immunohistochemical study of 14 cases. Hum. Pathol. 2004, 35, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Antonescu, C.R.; Zhang, L.; Dal Cin, P.; Hornick, J.L.; Fletcher, C.D.M. Cutaneous syncytial myoepithelioma: Clinicopathologic characterization in a series of 38 cases. Am. J. Surg. Pathol. 2013, 37, 710–718. [Google Scholar] [CrossRef]
- MacKinnon, W.F.; Carter, M.D.; Bridge, J.A.; Tremaine, R.D.; Walsh, N.M.G. EWSR1-PBX3 Gene fusion in cutaneous syncytial myoepithelioma. J. Cutan. Pathol. 2019, 46, 421–424. [Google Scholar] [CrossRef]
- Jo, V.Y.; Antonescu, C.R.; Dickson, B.C.; Swanson, D.; Zhang, L.; Fletcher, C.D.M.; Demicco, E.G. Cutaneous syncytial myoepithelioma is characterized by recurrent EWSR1-PBX3 fusions. Am. J. Surg. Pathol. 2019, 43, 1349–1354. [Google Scholar] [CrossRef]
- Komatsu, M.; Kawamoto, T.; Kanzawa, M.; Kawakami, Y.; Hara, H.; Akisue, T.; Kuroda, R.; Nakamura, H.; Hokka, D.; Jimbo, N.; et al. A novel EWSR1-VGLL1 gene fusion in a soft tissue malignant myoepithelial tumor. Genes Chromosomes Cancer 2020, 59, 249–254. [Google Scholar] [CrossRef]
- Cajaiba, M.M.; Jennings, L.J.; Rohan, S.M.; Leuer, K.M.; Anagnost, M.R.; Fahner, J.B.; Fulton, B.K.; Geller, J.I.; Perlman, E.J. Expanding the spectrum of renal tumors in children: Primary renal myoepithelial carcinomas with a novel EWSR1-KLF15 fusion. Am. J. Surg. Pathol. 2016, 40, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Wales, C.; Diamond, S.; Hinds, B. Cutaneous syncytial myoepithelioma: A nondescript skin tumor with serious diagnostic pitfalls. Int. J. Surg. Pathol. 2020, 28, 63–67. [Google Scholar] [CrossRef]
- Huang, S.-C.; Chen, H.-W.; Zhang, L.; Sung, Y.-S.; Agaram, N.P.; Davis, M.; Edelman, M.; Fletcher, C.D.M.; Antonescu, C.R. Novel FUS-KLF17 and EWSR1-KLF17 fusions in myoepithelial tumors. Genes Chromosomes Cancer 2015, 54, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Agaram, N.P.; Chen, H.-W.; Zhang, L.; Sung, Y.-S.; Panicek, D.; Healey, J.H.; Nielsen, G.P.; Fletcher, C.D.M.; Antonescu, C.R. EWSR1-PBX3: A novel gene fusion in myoepithelial tumors: A novel gene fusion in myoepithelial tumors. Genes Chromosomes Cancer 2015, 54, 63–71. [Google Scholar] [CrossRef]
- Flucke, U.; Mentzel, T.; Verdijk, M.A.; Slootweg, P.J.; Creytens, D.H.; Suurmeijer, A.J.H.; Tops, B.B.J. EWSR1-ATF1 Chimeric transcript in a myoepithelial tumor of soft tissue: A case report. Hum. Pathol. 2012, 43, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Gleason, B.C.; Fletcher, C.D.M. Myoepithelial carcinoma of soft tissue in children: An aggressive neoplasm analyzed in a series of 29 cases. Am. J. Surg. Pathol. 2007, 31, 1813–1824. [Google Scholar] [CrossRef]
- Hornick, J.L.; Dal Cin, P.; Fletcher, C.D.M. Loss of INI1 Expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am. J. Surg. Pathol. 2009, 33, 542–550. [Google Scholar] [CrossRef]
- Le Loarer, F.; Zhang, L.; Fletcher, C.D.; Ribeiro, A.; Singer, S.; Italiano, A.; Neuville, A.; Houlier, A.; Chibon, F.; Coindre, J.-M.; et al. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer 2014, 53, 475–486. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Zhang, L.; Shao, S.Y.; Mosquera, J.-M.; Weinreb, I.; Katabi, N.; Fletcher, C.D.M. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer 2013, 52, 675–682. [Google Scholar] [CrossRef]
- Requena, L.; Sánchez, M. Poroid hidradenoma: A light microscopic and immunohistochemical study. Cutis 1992, 50, 43–46. [Google Scholar] [PubMed]
- Parra, O.; Kerr, D.A.; Bridge, J.A.; Loehrer, A.P.; Linos, K. A Case of YAP1 and NUTM1 Rearranged Porocarcinoma with corresponding immunohistochemical expression: Review of recent advances in poroma and porocarcinoma pathogenesis with potential diagnostic utility. J. Cutan. Pathol. 2021, 48, 95–101. [Google Scholar] [CrossRef]
- Macagno, N.; Kervarrec, T.; Sohier, P.; Poirot, B.; Haffner, A.; Carlotti, A.; Balme, B.; Castillo, C.; Jullie, M.-L.; Osio, A.; et al. NUT is a specific immunohistochemical marker for the diagnosis of YAP1-NUTM1-rearranged cutaneous poroid neoplasms. Am. J. Surg. Pathol. 2021, 45, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Hornick, J.L.; Hanna, J. Utility of YAP1 and NUT Immunohistochemistry in the diagnosis of porocarcinoma. J. Cutan. Pathol. 2020, 48, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Haack, H.; Johnson, L.A.; Fry, C.J.; Crosby, K.; Polakiewicz, R.D.; Stelow, E.B.; Hong, S.-M.; Schwartz, B.E.; Cameron, M.J.; Rubin, M.A.; et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am. J. Surg. Pathol. 2009, 33, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Amatore, F.; Pissaloux, D.; Paindavoine, S.; Legrand, E.; Lehmann-Che, J.; Battistella, M.; Macagno, N. Expanding the spectrum of primary cutaneous carcinoma with BRD3-NUTM1 fusion. Am. J. Surg. Pathol. 2021, 45, 1548–1586. [Google Scholar] [CrossRef]
- Nishimura, Y.; Ryo, E.; Yamazaki, N.; Yatabe, Y.; Mori, T. Cutaneous primary NUT carcinoma with BRD3-NUTM1 fusion. Am. J. Surg. Pathol. 2021, 45, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Pan, X.; Lu, Y.; Pan, D.; Ma, Y.; Zhan, R. A case of eccrine porocarcinoma characterized by a progressive increase in the level of Ki-67 index: Case report and review of literature. BMC Surg. 2019, 19, 142. [Google Scholar] [CrossRef]
- Ansai, S.; Koseki, S.; Hozumi, Y.; Kondo, S. Assessment of cellular proliferation of eccrine acrospiromas and eccrine sweat gland carcinomas by AgNOR counting and immunohistochemical demonstration of proliferating cell nuclear antigen (PCNA) and Ki-67. Clin. Exp. Derm. 1995, 20, 27–34. [Google Scholar] [CrossRef]
- Zahn, J.; Chan, M.P.; Wang, G.; Patel, R.M.; Andea, A.A.; Bresler, S.C.; Harms, P.W. Altered Rb, P16, and P53 Expression is specific for porocarcinoma relative to poroma. J. Cutan. Pathol. 2019, 46, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Takai, T.; Fukumoto, T.; Anan, T.; Kimura, T.; Ansai, S.; Oshitani, Y.; Murata, Y.; Sakuma, T.; Hirose, T. CD117 (KIT) is a useful immunohistochemical marker for differentiating porocarcinoma from squamous cell carcinoma. J. Cutan. Pathol. 2016, 43, 219–226. [Google Scholar] [CrossRef]
- Goto, K.; Ishikawa, M.; Hamada, K.; Muramatsu, K.; Naka, M.; Honma, K.; Sugino, T. Comparison of immunohistochemical expression of cytokeratin 19, c-KIT, BerEP4, GATA3, and NUTM1 between porocarcinoma and squamous cell carcinoma. Am. J. Derm. 2021, 43, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Kunisada, M.; Fujiwara, N.; Oka, M.; Funasaka, Y.; Nishigori, C. Multiple apocrine poromas: A new case report. J. Cutan. Pathol. 2015, 42, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O.; Hisaoka, M.; Yasuda, H.; Kasai, T.; Hashimoto, H. Cytokeratin expression of apocrine and eccrine poromas with special reference to its expression in cuticular cells. J. Cutan. Pathol. 2000, 27, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Kuo, T.-T.; Chang, Y.-C. Comparative Immunohistochemical study of hidroacanthoma simplex and clonal seborrheic keratosis with GATA3 and P63. Am. J. Derm. 2021, 44, 17–20. [Google Scholar] [CrossRef]
- Mazoujian, G.; Margolis, R. Immunohistochemistry of gross cystic disease fluid protein (GCDFP-15) in 65 benign sweat gland tumors of the skin. Am. J. Derm. 1988, 10, 28–35. [Google Scholar] [CrossRef]
- Sekine, S.; Kiyono, T.; Ryo, E.; Ogawa, R.; Wakai, S.; Ichikawa, H.; Suzuki, K.; Arai, S.; Tsuta, K.; Ishida, M.; et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J. Clin. Invest. 2019, 129, 3827–3832. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Tögel, L.; Haller, F.; Zenk, J.; Hornung, J.; Märkl, B. YAP1-NUTM1 Gene fusion in porocarcinoma of the external auditory canal. Head Neck Pathol. 2020, 14, 982–990. [Google Scholar] [CrossRef]
- Agaimy, A.; Stoehr, R.; Tögel, L.; Hartmann, A.; Cramer, T. YAP1-MAML2-Rearranged poroid squamous cell carcinoma (squamoid porocarcinoma) presenting as a primary parotid gland tumor. Head Neck Pathol. 2021, 15, 361–367. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev. Biol. 2021, 475, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, S.; Busico, A.; Capone, I.; Conca, E.; Dallera, E.; Quattrone, P.; Licitra, L.; Pruneri, G.; Bossi, P.; Perrone, F. Identification of potentially druggable molecular alterations in skin adnexal malignancies. J. Derm. 2019, 46, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Hovelson, D.H.; Cani, A.K.; Omata, K.; Haller, M.J.; Wang, M.L.; Arps, D.; Patel, R.M.; Fullen, D.R.; Wang, M.; et al. Porocarcinomas harbor recurrent hras-activating mutations and tumor suppressor inactivating mutations. Hum. Pathol. 2016, 51, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bosic, M.; Kirchner, M.; Brasanac, D.; Leichsenring, J.; Lier, A.; Volckmar, A.-L.; Oliveira, C.; Buchhalter, I.; Stögbauer, F.; Zivkovic-Perisic, S.; et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology 2018, 50, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Denisova, E.; Westphal, D.; Surowy, H.M.; Meier, F.; Hutter, B.; Reifenberger, J.; Rütten, A.; Schulz, A.; Sergon, M.; Ziemer, M.; et al. Whole-exome sequencing in eccrine porocarcinoma indicates promising therapeutic strategies. Cancer Gene 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Harrison, B.T.; Fowler, E.; Krings, G.; Chen, Y.-Y.; Bean, G.R.; Vincent-Salomon, A.; Fuhrmann, L.; Barnick, S.E.; Chen, B.; Hosfield, E.M.; et al. Pan-TRK Immunohistochemistry: A useful diagnostic adjunct for secretory carcinoma of the breast. am. J. Surg. Pathol. 2019, 43, 1693–1700. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nozaki, Y.; Sugii, A.; Taguchi, K.; Hongo, T.; Jiromaru, R.; Sato, M.; Nakano, T.; Hashimoto, K.; Fujiwara, M.; et al. Pan-tropomyosin receptor kinase immunoreactivity, ETV6-NTRK3 fusion subtypes, and RET rearrangement in salivary secretory carcinoma. Hum. Pathol. 2021, 109, 37–44. [Google Scholar] [CrossRef]
- Csanyi-Bastien, M.; Lanic, M.-D.; Beaussire, L.; Ferric, S.; François, A.; Meseure, D.; Jardin, F.; Wassef, M.; Ruminy, P.; Laé, M. Pan-TRK immunohistochemistry is highly correlated with NTRK3 gene rearrangements in salivary gland tumors. Am. J. Surg. Pathol. 2021, 45, 1487–1498. [Google Scholar] [CrossRef]
- De la Fouchardière, A.; Tee, M.K.; Peternel, S.; Valdebran, M.; Pissaloux, D.; Tirode, F.; Busam, K.J.; LeBoit, P.E.; McCalmont, T.H.; Bastian, B.C.; et al. Fusion partners of NTRK3 affect subcellular localization of the fusion kinase and cytomorphology of melanocytes. Mod. Pathol. 2021, 34, 735–747. [Google Scholar] [CrossRef]
- Baghai, F.; Yazdani, F.; Etebarian, A.; Garajei, A.; Skalova, A. Clinicopathologic and molecular characterization of mammary analogue secretory carcinoma of salivary gland origin. Pathol. Res. Pr. 2017, 213, 1112–1118. [Google Scholar] [CrossRef]
- Taverna, C.; Baněčková, M.; Lorenzon, M.; Palomba, A.; Franchi, A.; Skalova, A.; Agaimy, A. MUC4 Is a valuable marker for distinguishing secretory carcinoma of the salivary glands from its mimics. Histopathol. 2021, 79, 315–324. [Google Scholar] [CrossRef]
- Bishop, J.A.; Taube, J.M.; Su, A.; Binder, S.W.; Kazakov, D.V.; Michal, M.; Westra, W.H. Secretory carcinoma of the skin harboring ETV6 gene fusions: A cutaneous analogue to secretory carcinomas of the breast and salivary glands. Am. J. Surg. Pathol. 2017, 41, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.M.; Beattie, A.; Ling, X.; Jennings, L.J.; Guitart, J. Primary Cutaneous mammary analog secretory carcinoma with ETV6-NTRK3 translocation. Am. J. Derm. 2016, 38, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Kastnerova, L.; Luzar, B.; Goto, K.; Grishakov, V.; Gatalica, Z.; Kamarachev, J.; Martinek, P.; Hájková, V.; Grossmann, P.; Imai, H.; et al. Secretory carcinoma of the skin: Report of 6 cases, including a case with a novel NFIX-PKN1 translocation. Am. J. Surg. Pathol. 2019, 43, 1092–1098. [Google Scholar] [CrossRef]
- Krings, G.; Joseph, N.M.; Bean, G.R.; Solomon, D.; Onodera, C.; Talevich, E.; Yeh, I.; Grenert, J.P.; Hosfield, E.; Crawford, E.D.; et al. Genomic profiling of breast secretory carcinomas reveals distinct genetics from other breast cancers and similarity to mammary analog secretory carcinomas. Mod. Pathol. 2017, 30, 1086–1099. [Google Scholar] [CrossRef]
- Suurmeijer, A.J.; Dickson, B.C.; Swanson, D.; Zhang, L.; Sung, Y.-S.; Huang, H.-Y.; Fletcher, C.D.; Antonescu, C.R. The histologic spectrum of soft tissue spindle cell tumors with NTRK3 gene rearrangements. Genes Chromosomes Cancer 2019, 58, 739–746. [Google Scholar] [CrossRef]
- Rubin, B.P.; Chen, C.J.; Morgan, T.W.; Xiao, S.; Grier, H.E.; Kozakewich, H.P.; Perez-Atayde, A.R.; Fletcher, J.A. Congenital mesoblastic nephroma t (12;15) is associated with ETV6-NTRK3 gene fusion: Cytogenetic and Molecular relationship to congenital (infantile) fibrosarcoma. Am. J. Pathol. 1998, 153, 1451–1458. [Google Scholar] [CrossRef]
- Knezevich, S.R.; Garnett, M.J.; Pysher, T.J.; Beckwith, J.B.; Grundy, P.E.; Sorensen, P.H. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998, 58, 5046–5048. [Google Scholar]
- Watanabe, N.; Kobayashi, H.; Hirama, T.; Kikuta, A.; Koizumi, S.; Tsuru, T.; Kaneko, Y. Cryptic t(12;15)(P13;Q26) Producing the ETV6-NTRK3 fusion gene and no loss of IGF2 imprinting in congenital mesoblastic nephroma with trisomy 11: Fluorescence in situ hybridization and IGF2 allelic expression analysis. Cancer Genet. Cytogenet. 2002, 136, 10–16. [Google Scholar] [CrossRef]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.W.; Passador-Santos, F.; et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef]
- Fehr, A.; Löning, T.; Stenman, G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am. J. Surg. Pathol. 2011, 35, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef]
- Makretsov, N.; He, M.; Hayes, M.; Chia, S.; Horsman, D.E.; Sorensen, P.H.B.; Huntsman, D.G. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer 2004, 40, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yoshida, A.; Taguchi, K.; Kohashi, K.; Hatanaka, Y.; Yamashita, A.; Mori, D.; Oda, Y. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology 2016, 69, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Alassiri, A.H.; Ali, R.H.; Shen, Y.; Lum, A.; Strahlendorf, C.; Deyell, R.; Rassekh, R.; Sorensen, P.H.; Laskin, J.; Marra, M.; et al. ETV6-NTRK3 is expressed in a subset of ALK-negative inflammatory myofibroblastic tumors. Am. J. Surg. Pathol. 2016, 40, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Brenca, M.; Rossi, S.; Polano, M.; Gasparotto, D.; Zanatta, L.; Racanelli, D.; Valori, L.; Lamon, S.; Dei Tos, A.P.; Maestro, R. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J. Pathol. 2016, 238, 543–549. [Google Scholar] [CrossRef]
- Punnett, H.H.; Tomczak, E.Z.; Pawel, B.R.; de Chadarevian, J.P.; Sorensen, P.H. ETV6-NTRK3 Gene fusion in metastasizing congenital fibrosarcoma. Med. Pediatr. Oncol. 2000, 35, 137–139. [Google Scholar] [CrossRef]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Tee, M.K.; Botton, T.; Shain, A.H.; Sparatta, A.J.; Gagnon, A.; Vemula, S.S.; Garrido, M.C.; Nakamaru, K.; Isoyama, T.; et al. NTRK3 kinase fusions in spitz tumours. J. Pathol. 2016, 240, 282–290. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, G.; Miller, C.P.; Tatevossian, R.G.; Dalton, J.D.; Tang, B.; Orisme, W.; Punchihewa, C.; Parker, M.; Qaddoumi, I.; et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 2013, 45, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Diaz, A.K.; Paugh, B.S.; Rankin, S.L.; Ju, B.; Li, Y.; Zhu, X.; Qu, C.; Chen, X.; Zhang, J.; et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014, 46, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Kelly, L.M.; Liu, P.; Brenner, A.V.; Little, M.P.; Bogdanova, T.I.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014, 120, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Skálová, A.; Agaimy, A.; Bishop, J.A.; Laco, J.; Leivo, I.; Franchi, A.; Larsen, S.R.; Erentaite, D.; Ulhøi, B.P.; et al. ETV6 Gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: A novel translocation-associated carcinoma restricted to the sinonasal tract. Am. J. Surg. Pathol. 2017, 41, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, D.V.; Bisceglia, M.; Calonje, E.; Hantschke, M.; Kutzner, H.; Mentzel, T.; Michal, M.; Mukensnabl, P.; Spagnolo, D.V.; Rütten, A.; et al. Tubular adenoma and syringocystadenoma papilliferum: A reappraisal of their relationship. an interobserver study of a series, by a panel of dermatopathologists. Am. J. Derm. 2007, 29, 256–263. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shido, K.; Niihori, T.; Niizuma, H.; Katata, Y.; Iizuka, C.; Oba, D.; Moriya, K.; Saito-Nanjo, Y.; Onuma, M.; et al. Somatic BRAF c.1799T>A p.V600E mosaicism syndrome characterized by a linear syringocystadenoma papilliferum, anaplastic astrocytoma, and ocular abnormalities. Am. J. Med. Genet. A 2016, 170, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Salam, A.; Griffiths, C.E.M.; McGrath, J.A. Naevus sebaceus: A mosaic rasopathy. Clin. Exp. Derm. 2014, 39, 1–6. [Google Scholar] [CrossRef]
- Friedman, B.J.; Sahu, J.; Solomides, C.C.; Connolly, D.M.; Lee, J.B. Contiguous verrucous proliferations in syringocystadenoma papilliferum: A retrospective analysis with additional evaluation via mutation-specific BRAFV600E immunohistochemistry. J. Cutan. Pathol. 2018, 45, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Groesser, L.; Herschberger, E.; Ruetten, A.; Ruivenkamp, C.; Lopriore, E.; Zutt, M.; Langmann, T.; Singer, S.; Klingseisen, L.; Schneider-Brachert, W.; et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and schimmelpenning syndrome. Nat. Genet. 2012, 44, 783–787. [Google Scholar] [CrossRef]
- Shen, A.-S.; Peterhof, E.; Kind, P.; Rütten, A.; Zelger, B.; Landthaler, M.; Berneburg, M.; Hafner, C.; Groesser, L. Activating mutations in the RAS/mitogen-activated protein kinase signaling pathway in sporadic trichoblastoma and syringocystadenoma papilliferum. Hum. Pathol. 2015, 46, 272–276. [Google Scholar] [CrossRef]
- Liau, J.-Y.; Tsai, J.-H.; Huang, W.-C.; Lan, J.; Hong, J.-B.; Yuan, C.-T. BRAF and KRAS Mutations in tubular apocrine adenoma and papillary eccrine adenoma of the skin. Hum. Pathol. 2018, 73, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, A.M.; Kyrpychova, L.; Nemcova, J.; Sedivcova, M.; Bisceglia, M.; Kutzner, H.; Zamecnik, M.; Sehnalkova, E.; Pavlovsky, M.; Zateckova, K.; et al. Syringocystadenoma papilliferum of the anogenital area and buttocks: A report of 16 cases, including human papillomavirus analysis and HRAS and BRAF V600 mutation studies. Am. J. Derm. 2019, 41, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Bonomo, B.; Shearer, S.; Welton, W.; Massullo, R.; Gibbons, G. Is syringocystadenoma papilliferum incidental in this verrucous carcinoma? Case. Rep. Pathol. 2019, 2019, 1783758. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Landa, V.; Jo-Velasco, M.; Santonja, C.; Eraña, I.; Vergara-Sanchez, A.; Kutzner, H.; Requena, L. Syringocystadenoma papilliferum associated with verrucous carcinoma of the skin in the same lesion: Report of four cases. J. Cutan. Pathol. 2020, 47, 12–16. [Google Scholar] [CrossRef]
- Hsieh, M.-S.; Bishop, J.A.; Wang, Y.-P.; Poh, C.F.; Cheng, Y.-S.L.; Lee, Y.-H.; Jin, Y.-T.; Chang, J.Y.F. Salivary Sialadenoma papilliferum consists of two morphologically, immunophenotypically, and genetically distinct subtypes. Head Neck Pathol. 2020, 14, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-S.; Bishop, J.A.; Yu Fong Chang, J. Sialadenoma papilliferum. Surg. Pathol. Clin. 2021, 14, 43–51. [Google Scholar] [CrossRef]
- Trager, M.H.; Jurkiewicz, M.; Khan, S.; Niedt, G.W.; Geskin, L.J.; Carvajal, R.D. A case report of papillary digital adenocarcinoma with BRAFV600E mutation and quantified mutational burden. Am. J. Derm. 2021, 43, 57–59. [Google Scholar] [CrossRef]
- Agni, M.; Raven, M.L.; Bowen, R.C.; Laver, N.V.; Chevez-Barrios, P.; Milman, T.; Eberhart, C.G.; Couch, S.; Bennett, D.D.; Albert, D.M.; et al. An update on endocrine mucin-producing sweat gland carcinoma: Clinicopathologic study of 63 cases and comparative analysis. Am. J. Surg. Pathol. 2020, 44, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Zembowicz, A.; Garcia, C.F.; Tannous, Z.S.; Mihm, M.C.; Koerner, F.; Pilch, B.Z. Endocrine mucin-producing sweat gland carcinoma: Twelve new cases suggest that it is a precursor of some invasive mucinous carcinomas. Am. J. Surg. Pathol. 2005, 29, 1330–1339. [Google Scholar] [CrossRef]
- Goto, K.; Anan, T.; Nakatsuka, T.; Kaku, Y.; Sakurai, T.; Fukumoto, T.; Kimura, T.; Shibata, A. Low-grade neuroendocrine carcinoma of the skin (primary cutaneous carcinoid tumor) as a distinctive entity of cutaneous neuroendocrine tumors: A clinicopathologic study of 3 cases with literature review. Am. J. Derm. 2017, 39, 250–258. [Google Scholar] [CrossRef]
- Chou, Y.-H.; Chang, Y.-C.; Huang, Y.-L.; Wu, C.-T. Endocrine mucin-producing sweat gland carcinoma with GATA3 expression: Report of two cases. Pathol. 2017, 49, 805–808. [Google Scholar] [CrossRef]
- Quattrochi, B.; Russell-Goldman, E. Utility of Insulinoma-Associated Protein 1 (INSM1) and Mucin 2 (MUC2) Immunohistochemistry in the distinction of endocrine mucin-producing sweat gland carcinoma from morphologic mimics. Am. J. Derm. 2021. [Google Scholar] [CrossRef] [PubMed]
- Held, L.; Ruetten, A.; Kutzner, H.; Palmedo, G.; John, R.; Mentzel, T. Endocrine mucin-producing sweat gland carcinoma: Clinicopathologic, immunohistochemical, and molecular analysis of 11 cases with emphasis on myb immunoexpression. J. Cutan. Pathol. 2018, 28, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, M.; Kuhar, M.; Hattab, E.; Linos, K. GATA3 positivity in endocrine mucin-producing sweat gland carcinoma and invasive mucinous carcinoma of the eyelid: Report of 2 cases. Am. J. Derm. 2016, 38, 789–791. [Google Scholar] [CrossRef]
- Shon, W.; Salomão, D.R. WT1 Expression in endocrine mucin-producing sweat gland carcinoma: A study of 13 cases. Int. J. Derm. 2014, 53, 1228–1234. [Google Scholar] [CrossRef]
- Mathew, J.G.; Bowman, A.S.; Saab, J.; Busam, K.J.; Nehal, K.; Pulitzer, M. Next generation sequencing analysis suggests varied multistep mutational pathogenesis for endocrine mucin producing sweat gland carcinoma with comments on INSM1 and MUC2 suggesting a conjunctival origin. J. Am. Acad. Derm. 2021. [Google Scholar] [CrossRef]
- Qin, H.; Moore, R.F.; Ho, C.-Y.; Eshleman, J.; Eberhart, C.G.; Cuda, J. Endocrine mucin-producing sweat gland carcinoma: A study of 11 cases with molecular analysis. J. Cutan. Pathol. 2018, 45, 681–687. [Google Scholar] [CrossRef]
- Cornejo, K.M.; Hutchinson, L.; Meng, X.; OʼDonnell, P.; Deng, A. Endocrine mucin-producing sweat gland carcinoma of the eyelid: A report of a case with molecular analysis. Am. J. Derm. 2016, 38, 636–638. [Google Scholar] [CrossRef] [PubMed]
- De Pinieux, G.; Karanian, M.; Le Loarer, F.; Le Guellec, S.; Chabaud, S.; Terrier, P.; Bouvier, C.; Batistella, M.; Neuville, A.; Robin, Y.-M.; et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef] [PubMed]
- Battistella, M.; Balme, B.; Jullie, M.-L.; Zimmermann, U.; Carlotti, A.; Crinquette, M.; Crinquette, M.; Frouin, E.; Macagno, N.; Lamant, L.; et al. Impact of expert pathology review in skin adnexal carcinoma diagnosis: Analysis of 2573 patients from the french caraderm network. Eur. J. Cancer 2021, in press. [Google Scholar]


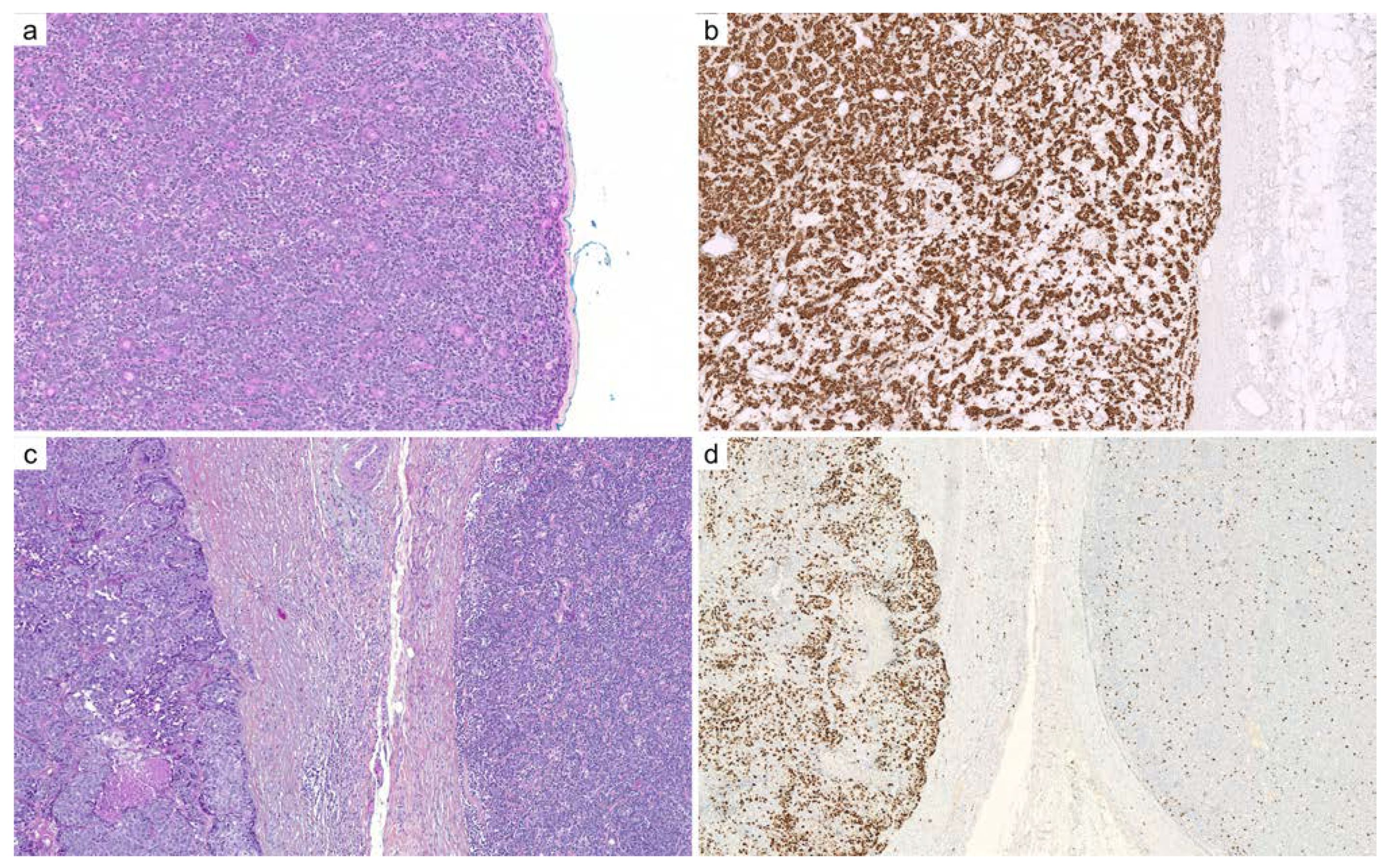
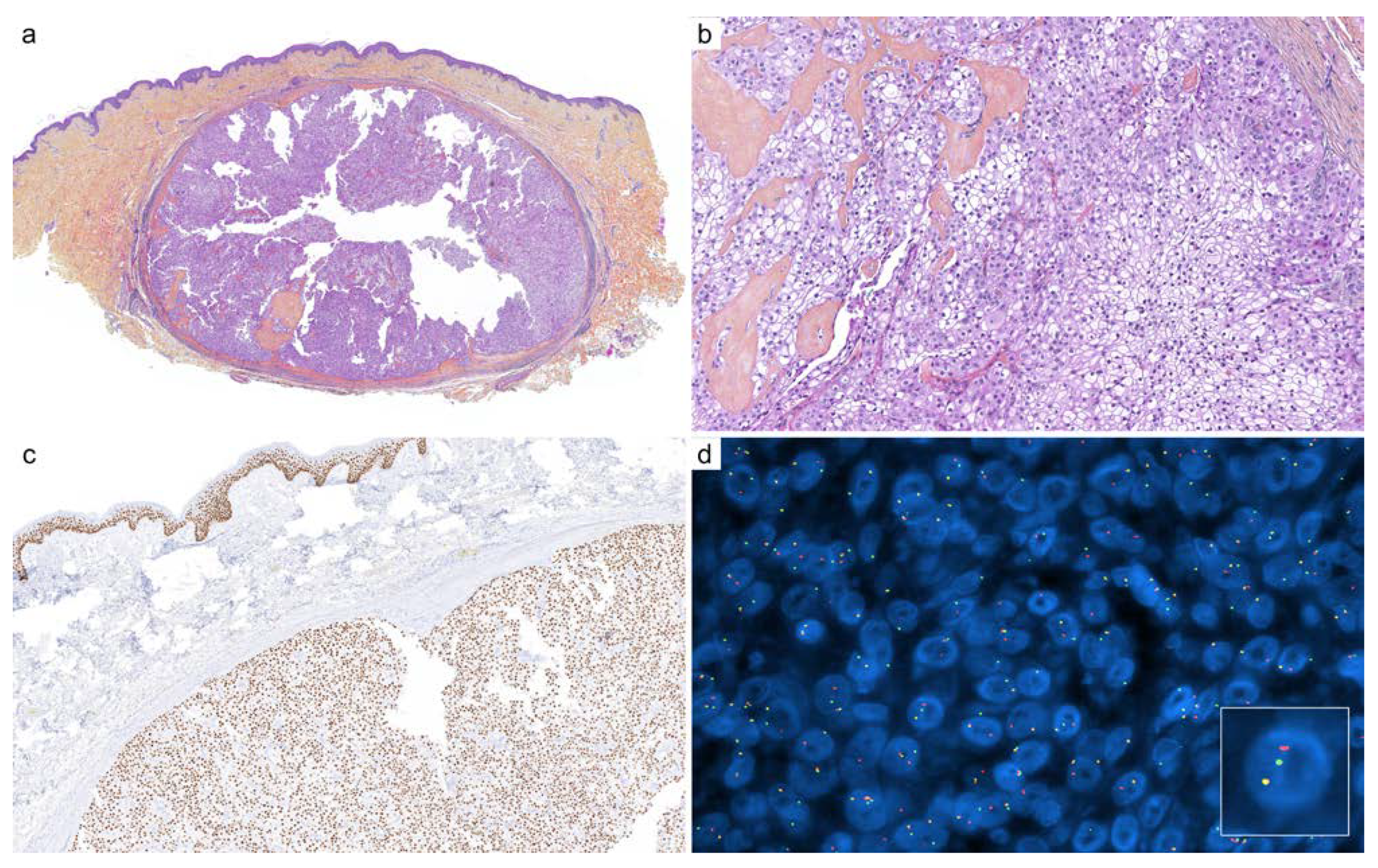
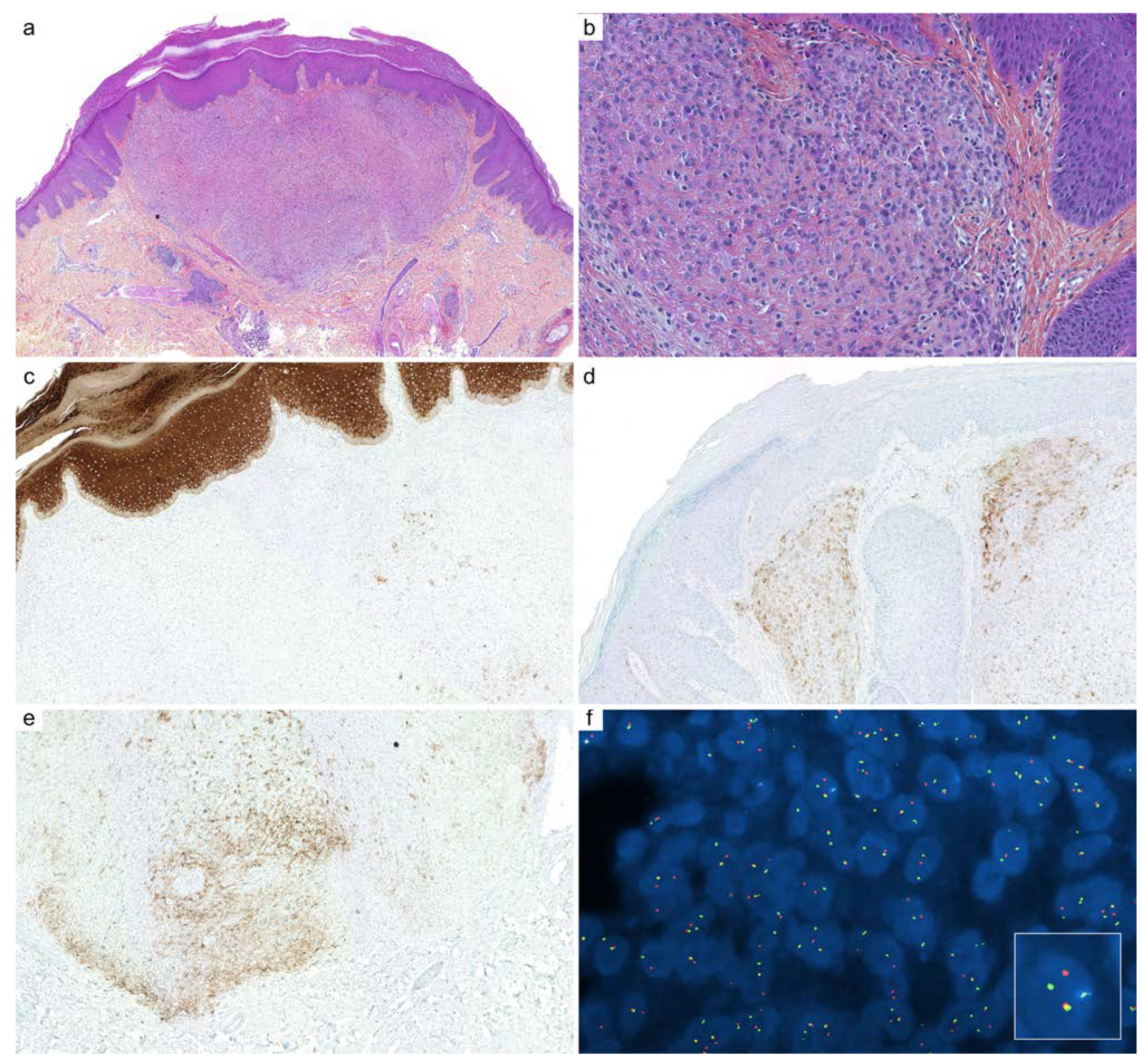

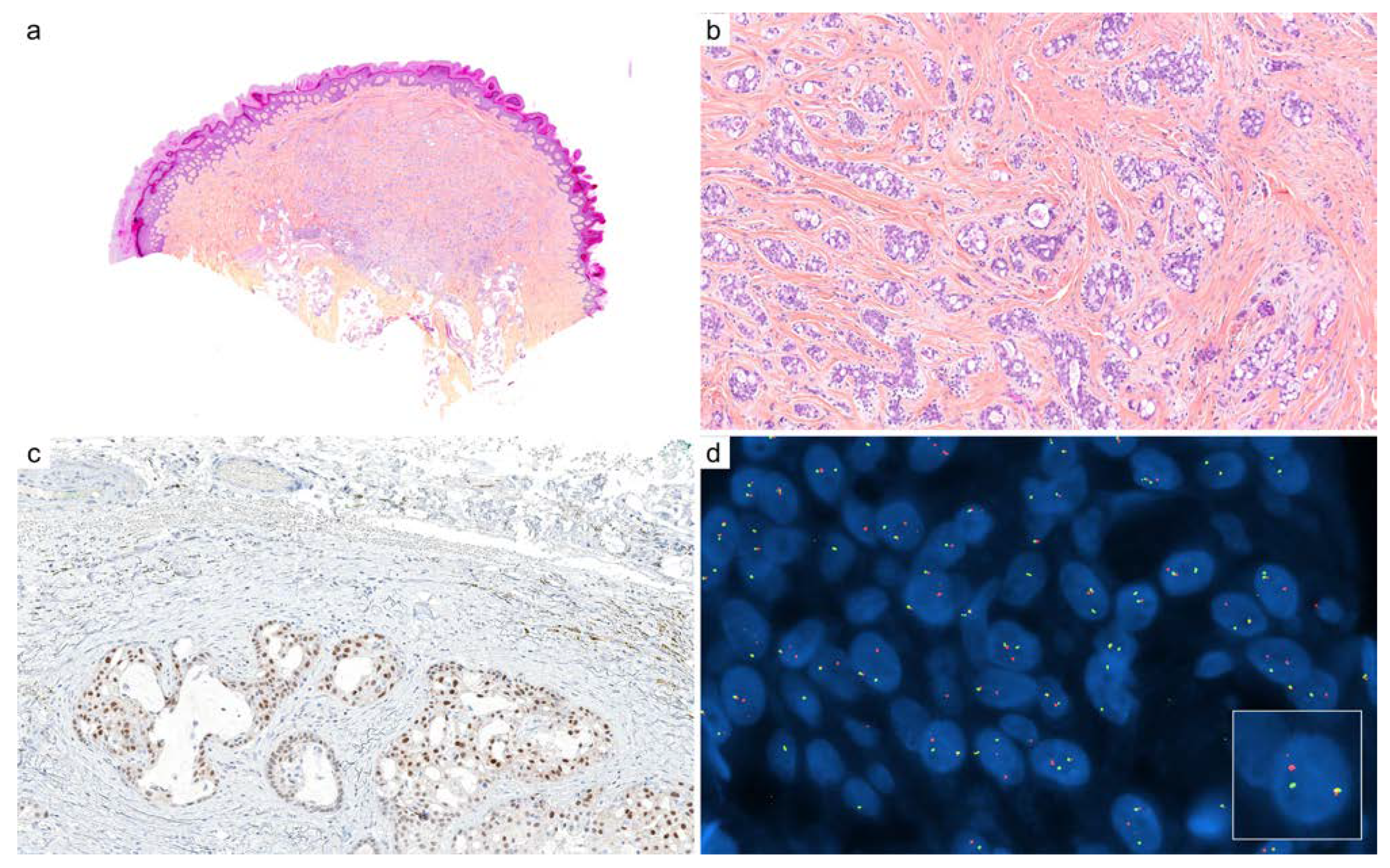
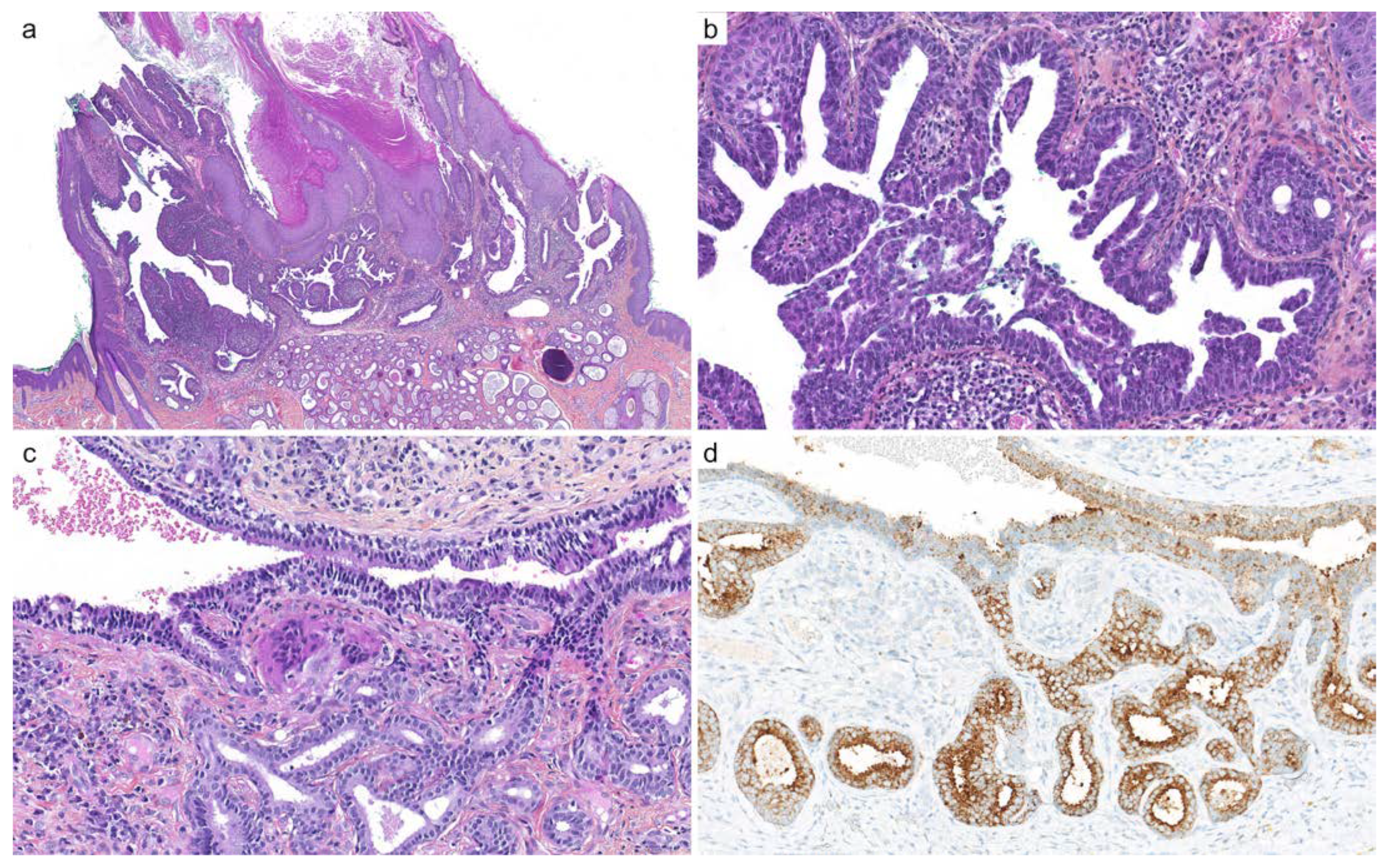
| Diagnoses Considered | Antibody | Staining Pattern | Reported Positivity |
|---|---|---|---|
| Adenoid cystic carcinoma, cylindroma, and spiradenoma | SOX10 MYB | nuclear nuclear | 73–100% 70–90% |
| Cutaneous mixed tumor | PLAG1 HMGA2 | nuclear nuclear | 87–100% unknown |
| Poroma, porocarcinoma, poroid hidradenoma | YAP1 NUT | cytoplasmic (loss) nuclear | 58–80% 29–32% |
| Secretory carcinoma | panTRK | nuclear | 100% |
| Syringocystadenoma papilliferum and tubular adenoma | BRAFV600E | cytoplasmic | 50–64% |
| Endocrine mucin-producing sweat gland carcinoma | INSM1 | nuclear | 100% |
| Diagnosis | Molecular Alteration | Frequency (%) |
|---|---|---|
| Adenoid cystic carcinoma | MYB::NFIB fusion MYBL1::NFIB fusion | 73–83% 20–23% |
| Cutaneous mixed tumor | PLAG1 fusion HMGA2 fusion | 33% unknown |
| Cylindroma | CYLD inactivation | near 100% |
| Spiradenoma | CYLD inactivation ALPK1 p.V1092A mutation | 29% 43% |
| Spiradenocarcinoma | CYLD inactivation ALPK1 p.V1092A mutation | 8% 33% |
| Hidradenoma | CRTC1::MAML2 fusion CRTC3::MAML2 fusion | 50–75% rare |
| Hidradenocarcinoma | CRTC1::MAML2 fusion | unknown |
| Myoepithelioma | EWSR1 fusion FUS fusion | 82% 18% |
| Poroma | YAP1 fusion NUTM1 fusion | 88% 17–55% |
| Porocarcinoma | YAP1 fusion NUTM1 fusion | 8–63% 11–54% |
| Secretory carcinoma | ETV6:NTRK3 fusion | near 100% |
| Syringocystadenoma papilliferum and tubular adenoma | BRAF p.V600E mutation HRAS p.G13R mutation KRAS p.G12D mutation | 50–64% 7–26% rare |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macagno, N.; Sohier, P.; Kervarrec, T.; Pissaloux, D.; Jullie, M.-L.; Cribier, B.; Battistella, M. Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors. Cancers 2022, 14, 476. https://doi.org/10.3390/cancers14030476
Macagno N, Sohier P, Kervarrec T, Pissaloux D, Jullie M-L, Cribier B, Battistella M. Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors. Cancers. 2022; 14(3):476. https://doi.org/10.3390/cancers14030476
Chicago/Turabian StyleMacagno, Nicolas, Pierre Sohier, Thibault Kervarrec, Daniel Pissaloux, Marie-Laure Jullie, Bernard Cribier, and Maxime Battistella. 2022. "Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors" Cancers 14, no. 3: 476. https://doi.org/10.3390/cancers14030476
APA StyleMacagno, N., Sohier, P., Kervarrec, T., Pissaloux, D., Jullie, M.-L., Cribier, B., & Battistella, M. (2022). Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors. Cancers, 14(3), 476. https://doi.org/10.3390/cancers14030476







