Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
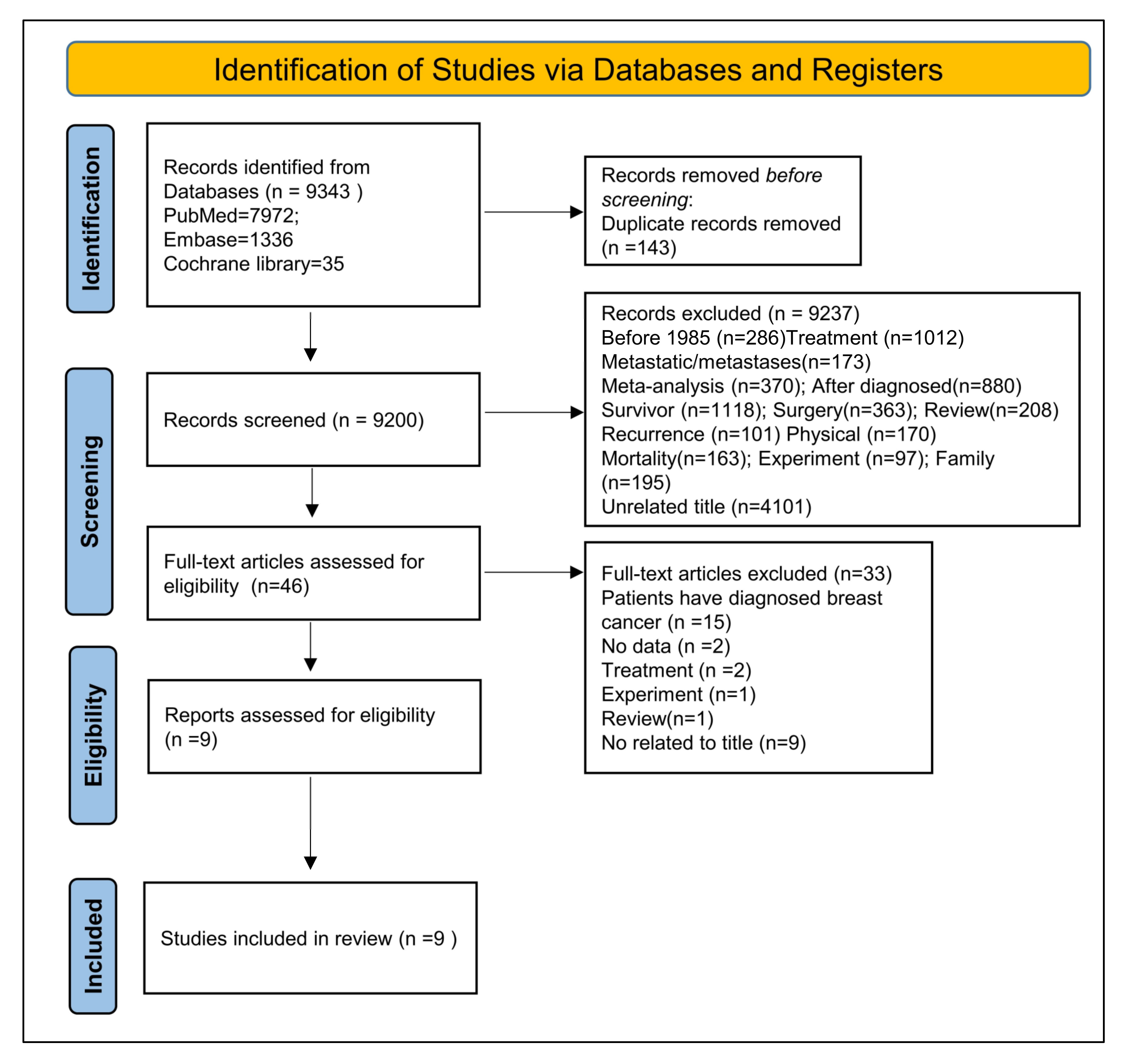
3.1. Study Selection and Characteristics
| Authors | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest Was Not Present at the Start of the Study | Comparability of Cohorts Based on the Design or Analysis Controlled for Confounders | Assessment of the Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of the Follow-Up of Cohorts | Total Score | |
| Aro et al., 2005 [43] | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| Mitchell et al. 2017 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Chang et al., 2015 [45] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Jacobs and Bovasso, 2000 [46] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Hjerl et al., 1999 [47] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Wakai et al., 2007 [48] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Yeh and Lee, 2016 (Anxiety) [49] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Yeh and Lee, 2016 (Depression) [49] | SAA | SAA | SAA | SAA | SAA | SAA | SAA | SAA | SAA |
| Hahn and Petitti, 1988 [50] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Peled et al., 2008 [51] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Authors | Year | Country | RR Ratio | Sample Size | BC Cases | Follow-Up Years | Age | Emotion Type | Diagnosis of NEs |
|---|---|---|---|---|---|---|---|---|---|
| Aro et al., 2005 [43] | 2005 | Finland | 2.83 (1.26–6.36) | 10,893 | 278 | 6–9 | 48–50 | Psychological risk factors | BDI |
| Mitchell et al., 2017 [44] | 2017 | USA | 1.36 (0.31–5.94) | 1067 | 43 | 24 | 19–79 | Depression | DSM-MD III |
| Chang et al., 2015 [45] | 2015 | USA | 3.8 (1.0–14.2) | 3109 | 25 | 13 | >18 | Depression | DSM-MD III |
| Jacobs and Bovasso, 2000 [46] | 2000 | USA | 17.2 (3.76–77.08) | 1533 | 40 | 15 | 40 | Stress | MDD |
| Hjerl et al., 1999 [47] | 1999 | Denmark | 1.02 (0.97–1.08) | 66,648 | 1270 | 24 | >15 | Affective orneurotic disorders | DSM-MD III |
| Wakai et al., 2007 [48] | 2007 | Japan | 1.01 (0.69–1.5) | 34,497 | 34 | 7.5 | 40–79 | Stress | Questionnaire |
| Yeh and Lee, 2016 (Anxiety) [49] | 2016 | Taiwan | 2.173 (1.009–4.648) | 1160 | 10 | 5.19 | 17–70 | Anxiety | PSS |
| Yeh and Lee, 2016 (Depression) [49] | 2016 | Taiwan | 4.979 (1.643–12.303) | 1160 | 5 | 5.19 | 17–70 | Depression | PSS |
| Hahn and Petitti, 1988 [50] | 1988 | USA | 1.5 (0.9–2.5) | 8932 | 120 | 18 | - | Depression | ICD-8 |
| Peled et al., 2008 [51] | 2008 | Israel | 1.62 (1.09–2.4) | 622 | 255 | 4 | <45 | Psychological distress | BSI |
| Total | 129,621 | 2080 |
| Authors | Country | Type of Emotion | Follow-Up Years | Outcome | Diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | Europe | Asia | Depression | Psychosis | Psychological Factor | <10 | 10–20 | >20 | Positive | Negative | ICD-8 | DSM-III | Others | |
| Aro et al., 2005 [43] | √ | √ | √ | √ | √ | |||||||||
| Mitchell et al., 2017 [44] | √ | √ | √ | √ | √ | |||||||||
| Chang et al., 2015 [45] | √ | √ | √ | √ | √ | |||||||||
| Jacobs and Bovasso, 2000 [46] | √ | √ | √ | √ | √ | |||||||||
| Hjerl et al., 1999 [47] | √ | √ | √ | √ | √ | |||||||||
| Wakai et al., 2007 [48] | √ | √ | √ | √ | √ | |||||||||
| Yeh and Lee, 2016 (Anxiety) [49] | √ | √ | √ | √ | √ | |||||||||
| Yeh and Lee, 2016 (Depression) [49] | √ | √ | √ | √ | √ | |||||||||
| Hahn and Petitti, 1988 [50] | √ | √ | √ | √ | √ | |||||||||
| Peled et al., 2008 [51] | √ | √ | √ | √ | √ | |||||||||
3.2. Effect of the NEs on the Incidence of BC
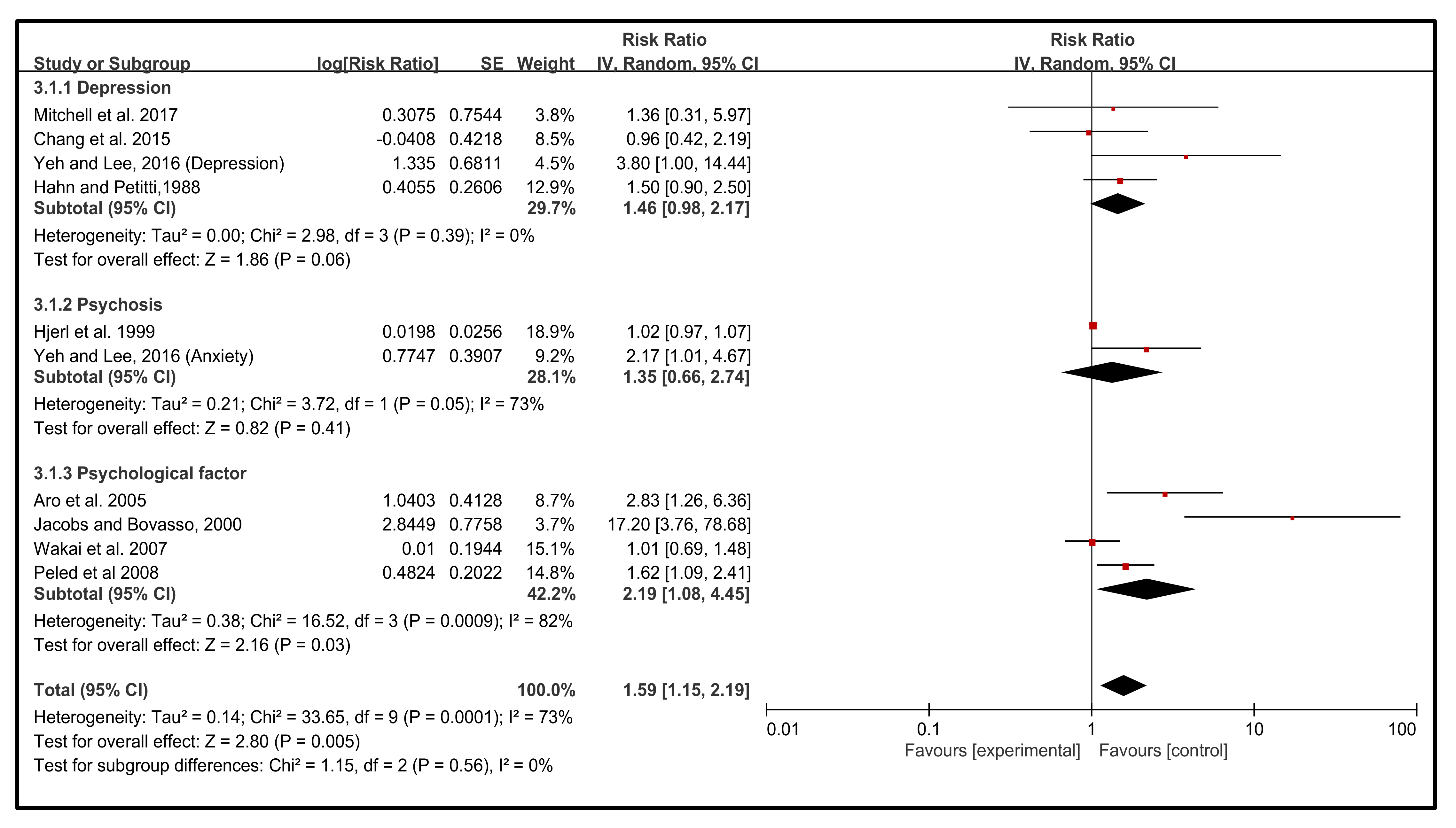
3.3. Effect of Emotion Types on the Incidence of BC
3.4. Effect of Geographical Distribution on the Incidence of BC
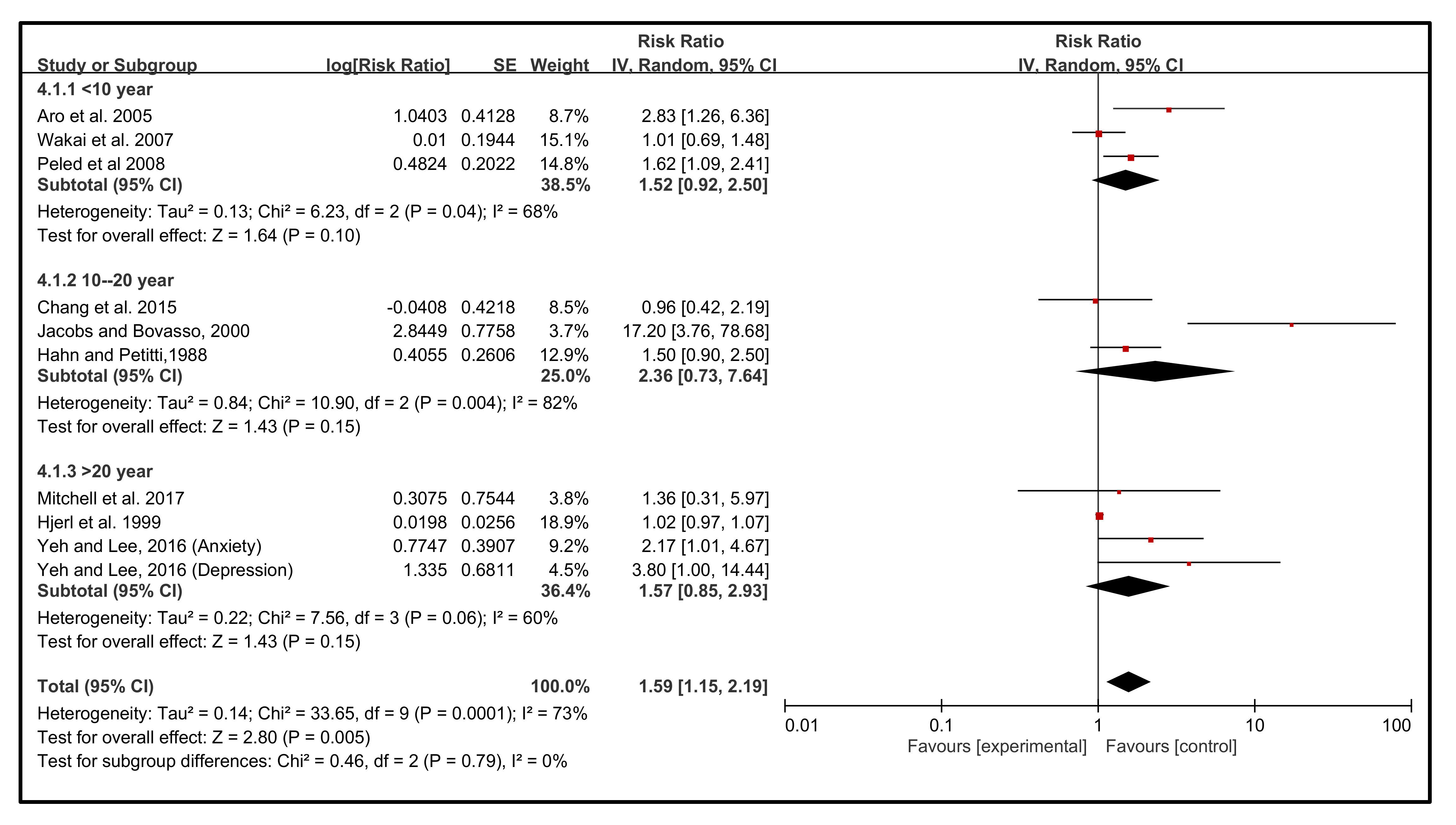
3.5. Effect of Follow-Up Years on the Incidence of BC
3.6. Effect of Diagnosis of the NEs on the Incidence of BC
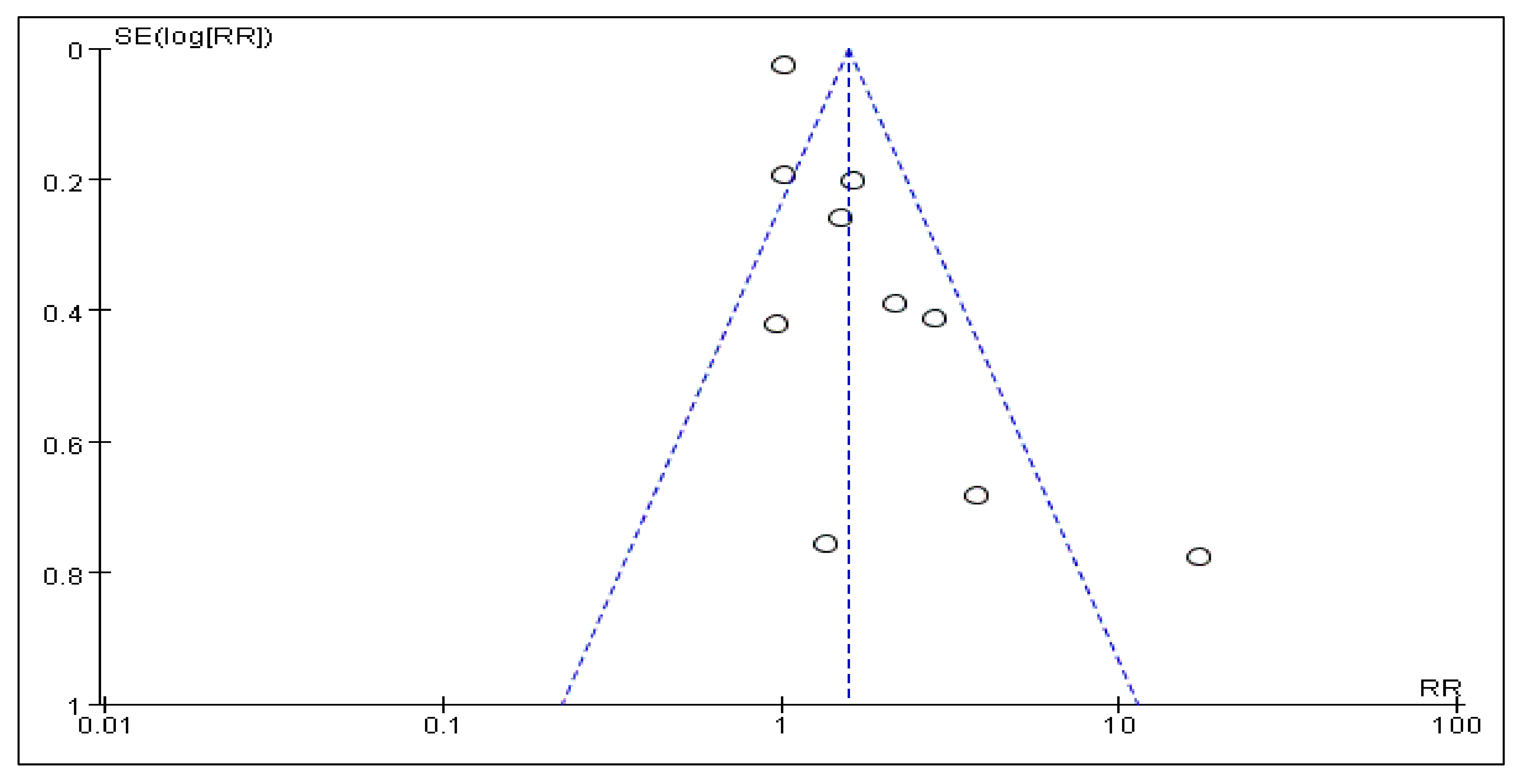
3.7. Sensitivity Analysis and Publication Bias


4. Discussion
Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganesan, K.; Xu, B. Deep frying cooking oils promote the high risk of metastases in the breast-A critical review. Food Chem. Toxicol. 2020, 144, 111648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; You, J.; Wen, Y.; Jia, L.; Gao, F.; Ganesan, K.; Chen, J. Tumorigenic risk of Angelica sinensis on ER-positive breast cancer growth through ER-induced stemness in vitro and in vivo. J. Ethnopharmacol. 2021, 280, 114415. [Google Scholar] [CrossRef] [PubMed]
- Youzhi, S.; Qianjun, C.; Pei, L.; Yi, Z.; Yanhua, H.; Xiao, Z.; Wei, M.; Lei, J.; Kumar, G.; Feizhi, M.; et al. Impact of Traditional Chinese Medicine Constitution on Breast Cancer Incidence: A Case-Control and Cross-Sectional Study. Pharmacophore 2021, 12, 46–56. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Ngan, T.T.; Nguyen, N.T.Q.; Van Minh, H.; Donnelly, M.; O’Neill, C. Effectiveness of clinical breast examination as a ’stand-alone’ screening modality: An overview of systematic reviews. BMC Cancer 2020, 20, 1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz, C.L.; Robb, K.A.; Katikireddi, S.V.; Pell, J.P.; Smith, D.J. Depressive symptoms, neuroticism, and participation in breast and cervical cancer screening: Cross-sectional and prospective evidence from UK Biobank. Psychooncology 2020, 29, 381–388. [Google Scholar] [CrossRef]
- Ahadzadeh, A.S.; Sharif, S.P. Uncertainty and quality of life in women with breast cancer: Moderating role of coping styles. Cancer Nurs. 2018, 41, 484–490. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Q.; Ganesan, K.; Kewu, Z.; Shen, J.; Gang, F.; Luo, X.; Chen, J. The antitriple negative breast cancer efficacy of spatholobus suberectus dunn on ROS-induced noncanonical inflammasome pyroptotic pathway. Oxid. Med. Cell Longev. 2021, 2021, 5187569. [Google Scholar] [CrossRef]
- Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, J.; Chen, J. Targeting engineered nanoparticles for breast cancer therapy. Pharmaceutics 2021, 13, 1829. [Google Scholar] [CrossRef] [PubMed]
- Kugbey, N.; Meyer-Weitz, A.; Oppong Asante, K. Mental adjustment to cancer and quality of life among women living with breast cancer in Ghana. Int. J. Psychiatry Med. 2019, 54, 217–230. [Google Scholar] [CrossRef]
- Iglay, K.; Santorelli, M.L.; Hirshfield, K.M.; Williams, J.M.; Rhoads, G.G.; Lin, Y.; Demissie, K. Impact of preexisting mental illness on all-cause and breast cancer–specific mortality in elderly patients with breast cancer. J. Clin. Oncol. 2017, 35, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.-L.; Li, Q.; Gao, F.; Yang, L.; Meng, F.-J. Effects of yoga on negative emotions in patients with breast cancer: A meta-analysis of randomized controlled trials. Int. J. Nurs. Sci. 2016, 3, 299–306. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Rafiemanesh, H.; Aghamohammadi, T.; Badakhsh, M.; Amirshahi, M.; Sari, M.; Behnamfar, N.; Roudini, K. Prevalence of anxiety among breast cancer patients: A systematic review and meta-analysis. Breast Cancer 2020, 27, 166–178. [Google Scholar] [CrossRef]
- Conley, C.C.; Bishop, B.T.; Andersen, B.L. Emotions and emotion regulation in breast cancer survivorship. Healthcare 2016, 4, 56. [Google Scholar] [CrossRef]
- Díaz-García, A.; González-Robles, A.; Mor, S.; Mira, A.; Quero, S.; García-Palacios, A.; Baños, R.M.; Botella, C. Positive and Negative Affect Schedule (PANAS): Psychometric properties of the online Spanish version in a clinical sample with emotional disorders. BMC Psychiatry 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, P.; Sumo, G.; Mills, J.; Haviland, J.; Bliss, J.M. The course of anxiety and depression over 5 years of follow-up and risk factors in women with early breast cancer: Results from the UK Standardisation of Radiotherapy Trials (START). Breast 2010, 19, 84–91. [Google Scholar] [CrossRef]
- Badr, H.; Milbury, K. Associations between depression, pain behaviors, and partner responses to pain in metastatic breast cancer. Pain 2011, 152, 2596–2604. [Google Scholar] [CrossRef]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Burgess, C.; Cornelius, V.; Love, S.; Graham, J.; Richards, M.; Ramirez, A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ 2005, 330, 702. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Pallant, J.F.; Rajapaksa, I. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast 2012, 21, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Taylor, S.; Adesoye, T.; Bloom, J.R. Managing psychosocial issues faced by young women with breast cancer at the time of diagnosis and during active treatment. Curr. Opin. Support. Palliat. Care 2015, 9, 279–284. [Google Scholar] [CrossRef]
- Walker, W.H., II; Kvadas, R.M.; May, L.E.; Liu, J.A.; Bumgarner, J.R.; Walton, J.C.; DeVries, A.C.; Dauchy, R.T.; Blask, D.E.; Nelson, R.J. Artificial light at night reduces anxiety-like behavior in female mice with exacerbated mammary tumor growth. Cancers 2021, 13, 4860. [Google Scholar] [CrossRef]
- Ando, N.; Iwamitsu, Y.; Kuranami, M.; Okazaki, S.; Nakatani, Y.; Yamamoto, K.; Watanabe, M.; Miyaoka, H. Predictors of psychological distress after diagnosis in breast cancer patients and patients with benign breast problems. Psychosomatics 2011, 52, 56–64. [Google Scholar] [CrossRef]
- Cohen, M. The association of cancer patients’ emotional suppression and their self-rating of psychological distress on short screening tools. Behav. Med. 2013, 39, 29–35. [Google Scholar] [CrossRef]
- Cousson-Gélie, F.; Bruchon-Schweitzer, M.; Atzeni, T.; Houede, N. Evaluation of a psychosocial intervention on social support, perceived control, coping strategies, emotional distress, and quality of life of breast cancer patients. Psychol. Rep. 2011, 108, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, Y.; He, J.; Yi, J.; Wang, Y.; Zhang, J.; Zhu, X. Emotional suppression and depressive symptoms in women newly diagnosed with early breast cancer. BMC Womens Health 2015, 15, 91. [Google Scholar] [CrossRef]
- Yang, E.V.; Glaser, R. Stress-induced immunomodulation: Implications for tumorigenesis. Brain Behav. Immun. 2003, 17, 37–40. [Google Scholar] [CrossRef]
- Baltrusch, H.J.F.; Gehde, E.; Titze, I.; Heinze, H.J. Early socialization and development of cancer in later life. Annal. NY Acad. Sci. 1992, 650, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Lueboonthavatchai, P. Prevalence and psychosocial factors of anxiety and depression in breast cancer patients. J. Med. Assoc. Thai. 2007, 90, 2164–2174. [Google Scholar] [PubMed]
- Chen, X.; Zheng, Y.; Zheng, W.; Gu, K.; Chen, Z.; Lu, W.; Shu, X.O. Prevalence of depression and its related factors among Chinese women with breast cancer. Acta Oncol. 2009, 48, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-F.; Jin, F.-J.; Li, N.; Guan, H.-T.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Goldstein, B.A. Not all negative emotions are equal: The role of emotional expression in online support groups for women with breast cancer. Psychooncology 2006, 15, 160–168. [Google Scholar] [CrossRef]
- Watson, M.; Homewood, J.; Haviland, J.; Bliss, J.M. Influence of psychological response on breast cancer survival: 10-year follow-up of a population-based cohort. Eur. J. Cancer 2005, 41, 1710–1714. [Google Scholar] [CrossRef]
- Groenvold, M.; Petersen, M.A.; Idler, E.; Bjorner, J.B.; Fayers, P.M.; Mouridsen, H.T. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res. Treat. 2007, 105, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Stroup, D.F. Meta-analysis of Observational Studies in Epidemiology: A proposal for reporting. JAMA 2000, 283, 2008. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.R.; Koning, H.J.D.; Schreck, M.; Henriksson, M.; Anttila, A.; Pukkala, E. Psychological risk factors of incidence of breast cancer: A prospective cohort study in Finland. Psychol. Med. 2005, 35, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.M.; Pössel, P.; Van Voorhees, B.W.; Eaton, W.W. Associations of depression status and hopelessness with breast cancer: A 24-year follow-up study. J. Health Psychol. 2016, 22, 1322–1331. [Google Scholar] [CrossRef]
- Chang, H.Y.; Keyes, K.M.; Mok, Y.; Jung, K.J.; Shin, Y.-J.; Jee, S.H. Depression as a risk factor for overall and hormone-related cancer: The Korean cancer prevention study. J. Affect. Disord. 2015, 173, 1–8. [Google Scholar] [CrossRef]
- Jacobs, J.R.; Bovasso, G.B. Early and chronic stress and their relation to breast cancer. Psychol. Med. 2000, 30, 669–678. [Google Scholar] [CrossRef]
- Hjerl, K.; Andersen, E.W.; Keiding, N.; Sawitz, A.; Olsen, J.H.; Mortensen, P.B.; Jørgensen, T. Breast cancer risk among women with psychiatric admission with affective or neurotic disorders: A nationwide cohort study in Denmark. Br. J. Cancer 1999, 81, 907–911. [Google Scholar] [CrossRef][Green Version]
- Wakai, K.; Kojima, M.; Nishio, K.; Suzuki, S.; Niwa, Y.; Lin, Y.; Kondo, T.; Yatsuya, H.; Tamakoshi, K.; Yamamoto, A.; et al. Psychological attitudes and risk of breast cancer in Japan: A prospective study. Cancer Causes Control. 2007, 18, 259–267. [Google Scholar] [CrossRef]
- Yeh, M.L.; Lee, T.Y. A Prospective Study of the Relationship between Psychological Factors and Breast Cancer. Asia Pac. J. Oncol. Nurs. 2016, 3, 170–175. [Google Scholar] [CrossRef]
- Hahn, R.C.; Petitti, D.B. Minnesota multiphasic personality inventory-rated depression and the incidence of breast cancer. Cancer 1988, 61, 845–848. [Google Scholar] [CrossRef]
- Peled, R.; Carmil, D.; Siboni-Samocha, O.; Shoham-Vardi, I. Breast cancer, psychological distress and life events among young women. BMC Cancer 2008, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Triplett, P.T. Association of schizophrenia with the risk of breast cancer incidence: A meta-analysis. JAMA Psychiatry 2018, 75, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Carreira, H.; Williams, R.; Müller, M.; Harewood, R.; Stanway, S.; Bhaskaran, K. Associations between breast cancer survivorship and adverse mental health outcomes: A systematic review. J. Natl. Cancer Inst. 2018, 110, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Raitasalo, R.; Heliovaara, M.; Lehtinen, V.; Pukkala, E.; Teppo, L.; Maatela, J.; Aromaa, A. Elevated lung cancer risk among persons with depressed mood. Am. J. Epidemiol. 1996, 144, 1096–1103. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Guralnik, J.M.; Havlik, R.J.; Pahor, M.; Ferrucci, L.; Cerhan, J.R.; Wallace, R.B. Chronically depressed mood and cancer risk in older persons. J. Natl. Cancer Inst. 1998, 90, 1888–1893. [Google Scholar] [CrossRef]
- Lafourcade, A.; His, M.; Baglietto, L.; Boutron-Ruault, M.-C.; Dossus, L.; Rondeau, V. Factors associated with breast cancer recurrences or mortality and dynamic prediction of death using history of cancer recurrences: The French E3N cohort. BMC Cancer 2018, 18, 171. [Google Scholar] [CrossRef]
- Kim, S.-A.; Roh, J.-L.; Lee, S.-A.; Lee, S.-W.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Pretreatment depression as a prognostic indicator of survival and nutritional status in patients with head and neck cancer. Cancer 2015, 122, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; Hellhammer, D.H.; Pruessner, J.C.; Lupien, S.J. Self-Reported Depressive Symptoms and Stress Levels in Healthy Young Men: Associations with the Cortisol Response to Awakening. Psychosom. Med. 2003, 65, 92–99. [Google Scholar] [CrossRef]
- Sephton, S.E. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000, 92, 994–1000. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Manson, J.E.; Anderson, G.L.; Cauley, J.A.; Aragaki, A.K.; Stefanick, M.L.; Lane, D.S.; Johnson, K.C.; Wactawski-Wende, J.; Chen, C.; et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J. Natl. Cancer Inst. 2013, 105, 526–535. [Google Scholar] [CrossRef]
- Lawrence, W.R.; Kuliszewski, M.G.; Hosler, A.S.; Leinung, M.C.; Zhang, X.; Zhang, W.; Du, Z.; Schymura, M.J.; Boscoe, F.P. Association between preexisting mental illnesses and mortality among medicaid-insured women diagnosed with breast cancer. Soc. Sci. Med. 2021, 270, 113643. [Google Scholar] [CrossRef] [PubMed]
- Moschetti, I.; Cinquini, M.; Lambertini, M.; Levaggi, A.; Liberati, A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst. Rev. 2016, 2016, Cd001768. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast cancer epidemiology and risk factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Gutiérrez Hermoso, L.; Alcocer Castillejos, N.; Quiroz Friedman, P.; Peñacoba, C.; Catalá, P.; Sánchez-Román, S. Association between quality of life and positive coping strategies in breast cancer patients. Women Health 2020, 60, 1063–1069. [Google Scholar] [CrossRef]
- Zoorob, R.J.; Salemi, J.L.; Mejia de Grubb, M.C.; Modak, S.; Levine, R.S. A nationwide study of breast cancer, depression, and multimorbidity among hospitalized women and men in the United States. Breast Cancer Res. Treat. 2019, 174, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef]
- Maass, S.W.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.; de Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef]
- Jim, H.S.; Phillips, K.M.; Chait, S.; Faul, L.A.; Popa, M.A.; Lee, Y.H.; Hussin, M.G.; Jacobsen, P.B.; Small, B.J. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. 2012, 30, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.Z.; Nik-Jaafar, N.R.; Baharudin, A.; Sabki, Z.A.; Ng, C.G. Prevalence of depression in breast cancer survivors: A systematic review of observational studies. Asian Pac. J. Cancer Prev. 2013, 14, 2649–2656. [Google Scholar] [CrossRef]
- Krebber, A.M.; Buffart, L.M.; Kleijn, G.; Riepma, I.C.; de Bree, R.; Leemans, C.R.; Becker, A.; Brug, J.; van Straten, A.; Cuijpers, P.; et al. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 2014, 23, 121–130. [Google Scholar] [CrossRef]
- Walker, J.; Holm Hansen, C.; Martin, P.; Sawhney, A.; Thekkumpurath, P.; Beale, C.; Symeonides, S.; Wall, L.; Murray, G.; Sharpe, M. Prevalence of depression in adults with cancer: A systematic review. Ann. Oncol. 2013, 24, 895–900. [Google Scholar] [CrossRef]
- Eskelinen, M.; Korhonen, R.; Selander, T.; Ollonen, P. Beck depression inventory as a predictor of long-term outcome among patients admitted to the breast cancer diagnosis unit: A 25-year cohort study in Finland. Anticancer Res. 2017, 37, 819–824. [Google Scholar] [CrossRef]
- Phillips, K.A.; Osborne, R.H.; Giles, G.G.; Dite, G.S.; Apicella, C.; Hopper, J.L.; Milne, R.L. Psychosocial factors and survival of young women with breast cancer: A population-based prospective cohort study. J. Clin. Oncol. 2008, 26, 4666–4671. [Google Scholar] [CrossRef]
- Iwamitsu, Y.; Shimoda, K.; Abe, H.; Tani, T.; Okawa, M.; Buck, R. The relation between negative emotional suppression and emotional distress in breast cancer diagnosis and treatment. Health Commun. 2005, 18, 201–215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Ganesan, K.; Liu, X.; Ye, Q.; Cheung, Y.; Liu, D.; Zhong, S.; Chen, J. Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer. Cancers 2022, 14, 475. https://doi.org/10.3390/cancers14030475
Xu C, Ganesan K, Liu X, Ye Q, Cheung Y, Liu D, Zhong S, Chen J. Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer. Cancers. 2022; 14(3):475. https://doi.org/10.3390/cancers14030475
Chicago/Turabian StyleXu, Cong, Kumar Ganesan, Xiaoyan Liu, Qiaobo Ye, Yuenshan Cheung, Dan Liu, Shaowen Zhong, and Jianping Chen. 2022. "Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer" Cancers 14, no. 3: 475. https://doi.org/10.3390/cancers14030475
APA StyleXu, C., Ganesan, K., Liu, X., Ye, Q., Cheung, Y., Liu, D., Zhong, S., & Chen, J. (2022). Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer. Cancers, 14(3), 475. https://doi.org/10.3390/cancers14030475








