Population-Based Temporal Trends and Ethnic Disparity in Cervical Cancer Mortality in South Africa (1999–2018): A Join Point and Age–Period–Cohort Regression Analyses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Ethical Approval
2.3. Statistical Analysis
2.4. Annual Crude and Age-Standardised Rates
2.5. Join Point Regression Modelling
2.6. Age–Period–Cohort Modelling of Cervical Cancer Mortality
2.7. Identifiability Problem
3. Results
3.1. Trends in Cervical Cancer Mortality
3.2. Ethnic Trends of Cervical Cancer Mortality
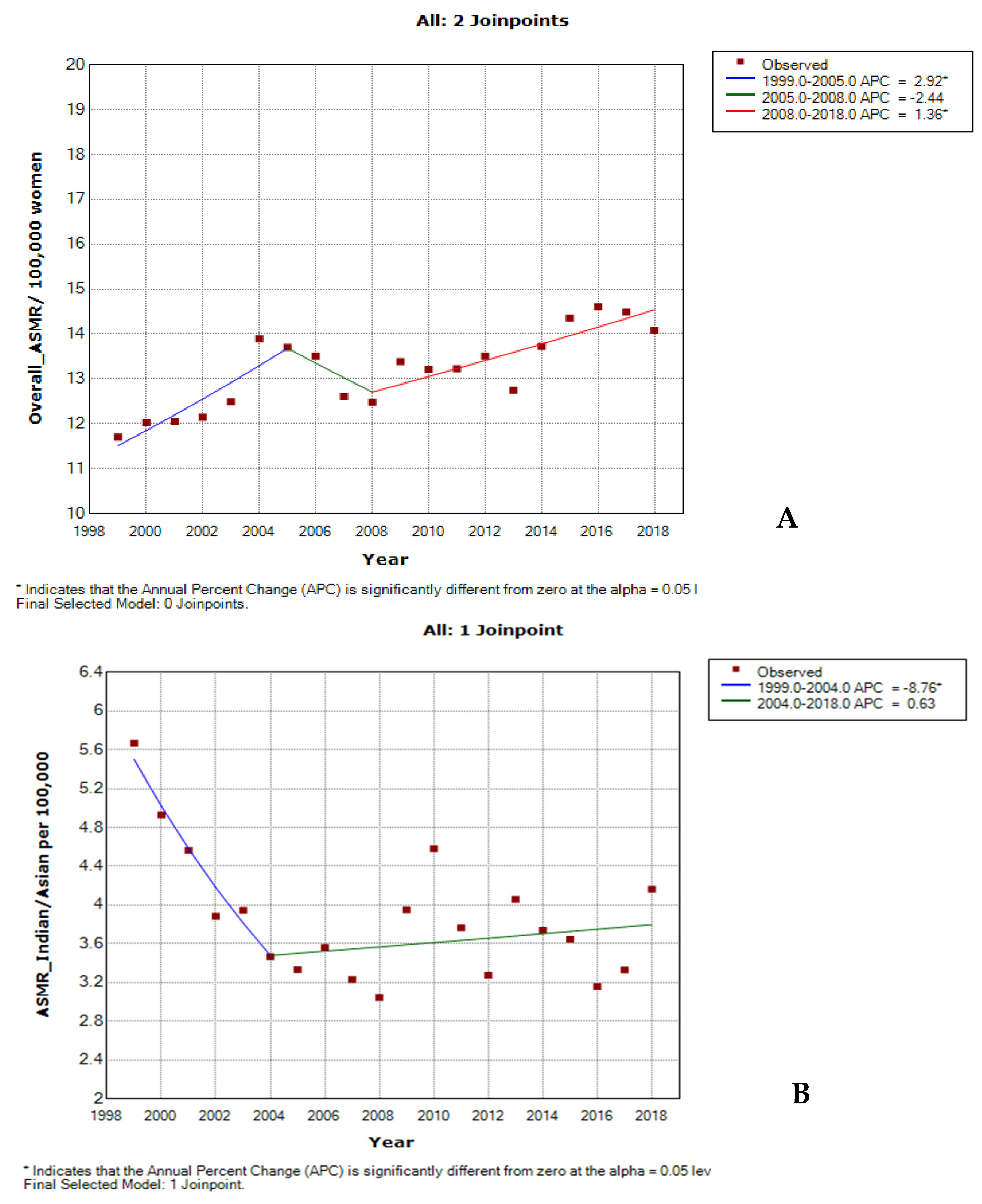
3.3. Joinpoint Trends in the Overall Age-Specific Mortality Rates for Cervical Cancer, 1999–2018
3.4. Trends in Mean Age and Age-Specific Rates of Cervical Cancer Mortality by Ethnicity
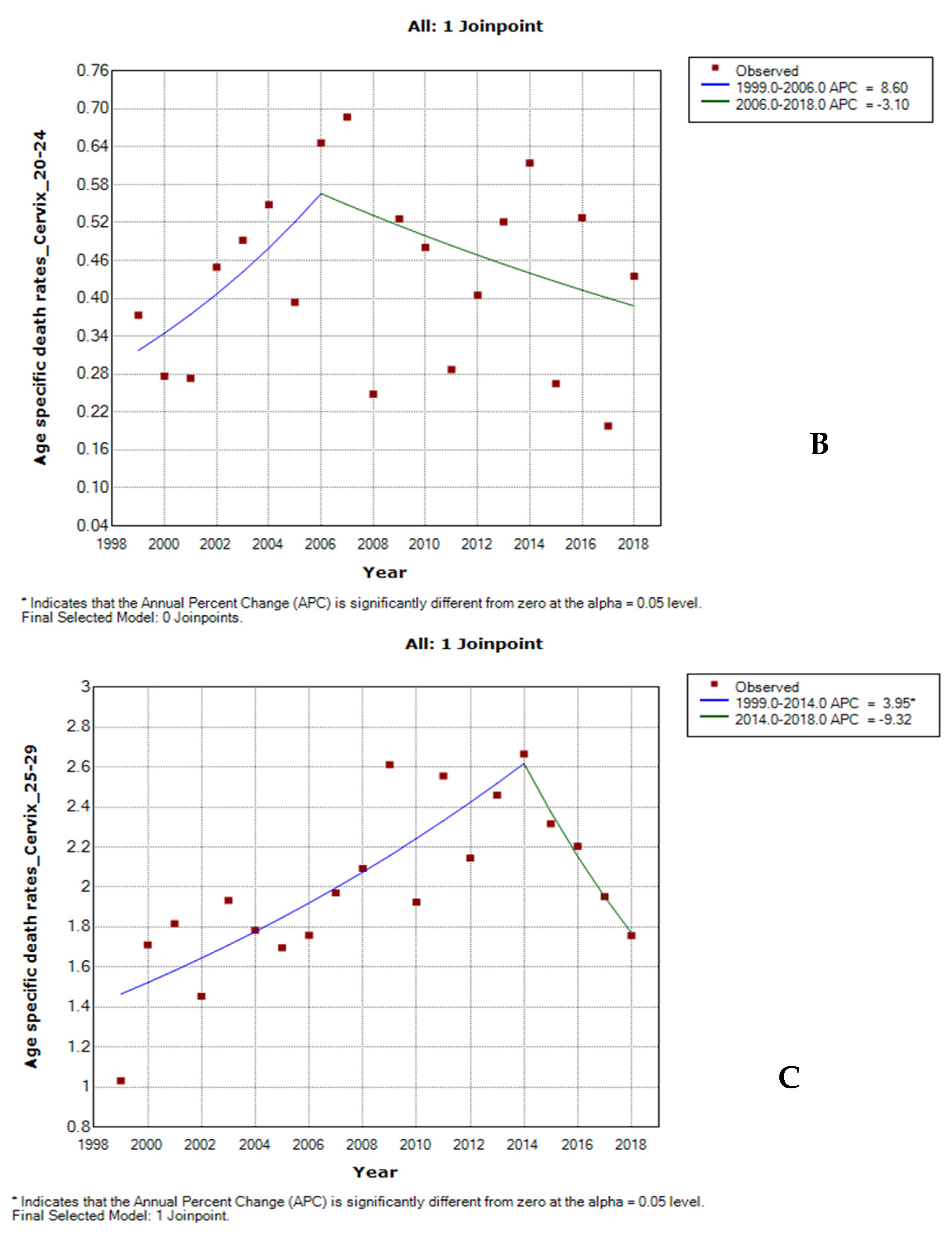
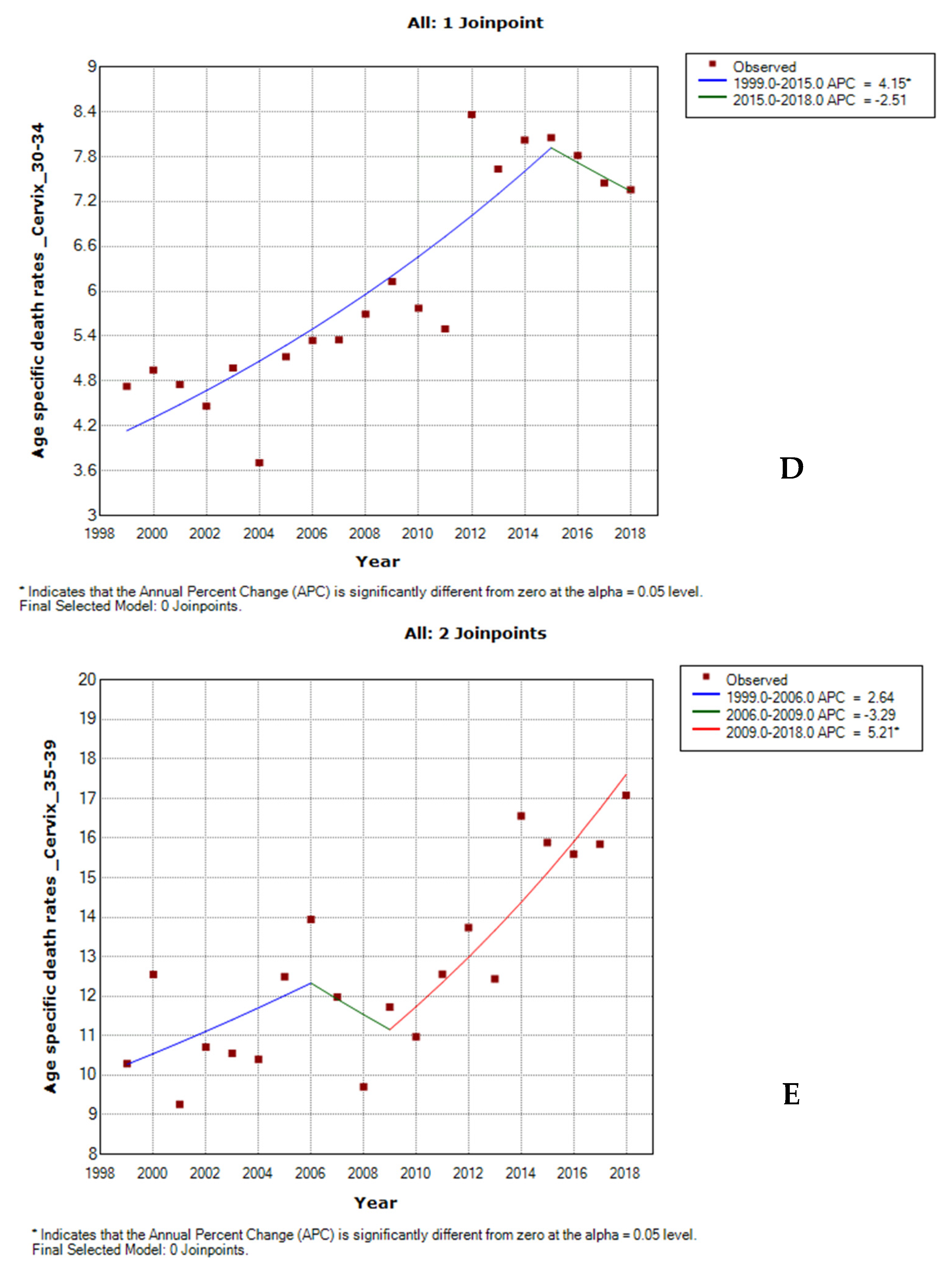
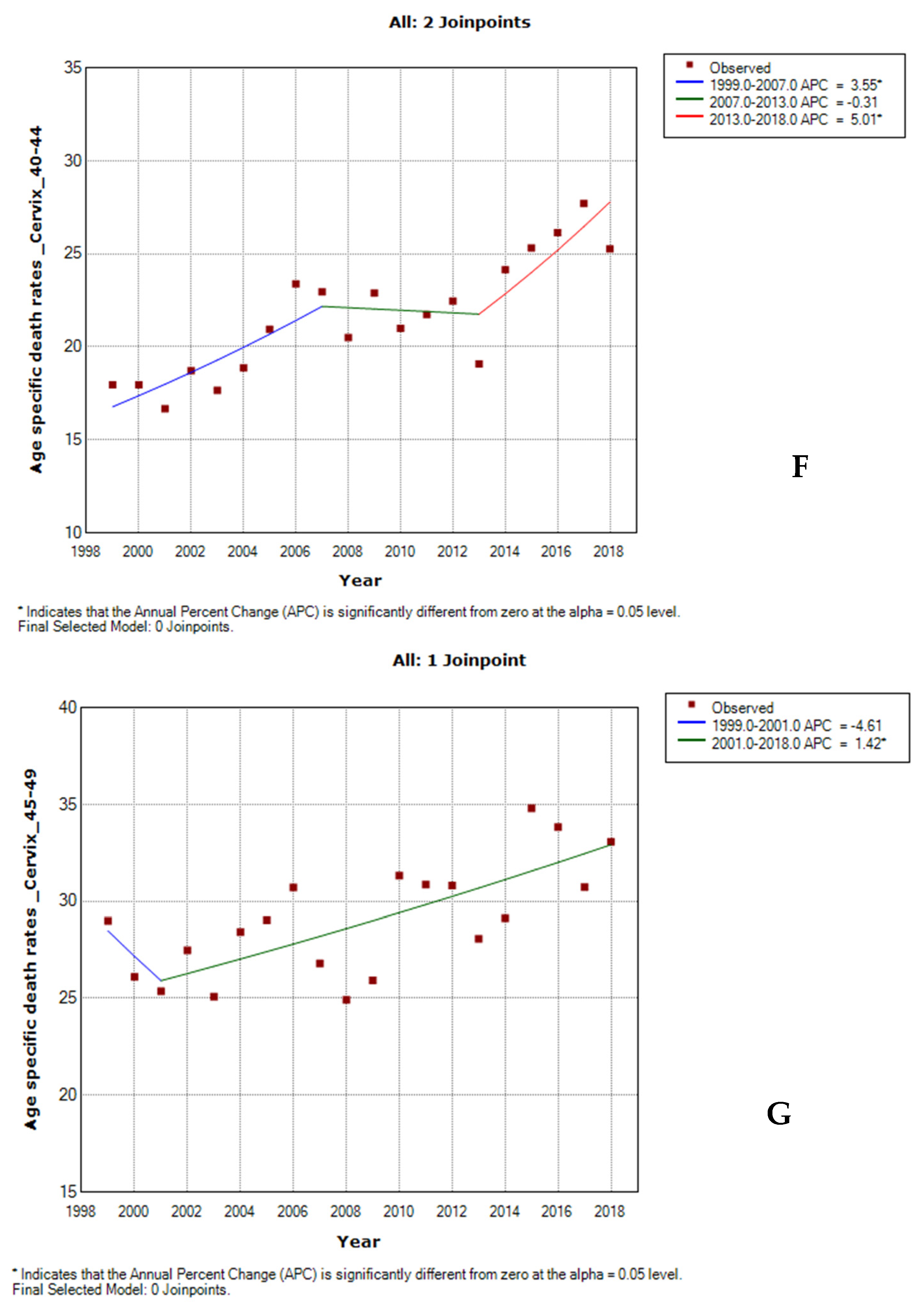
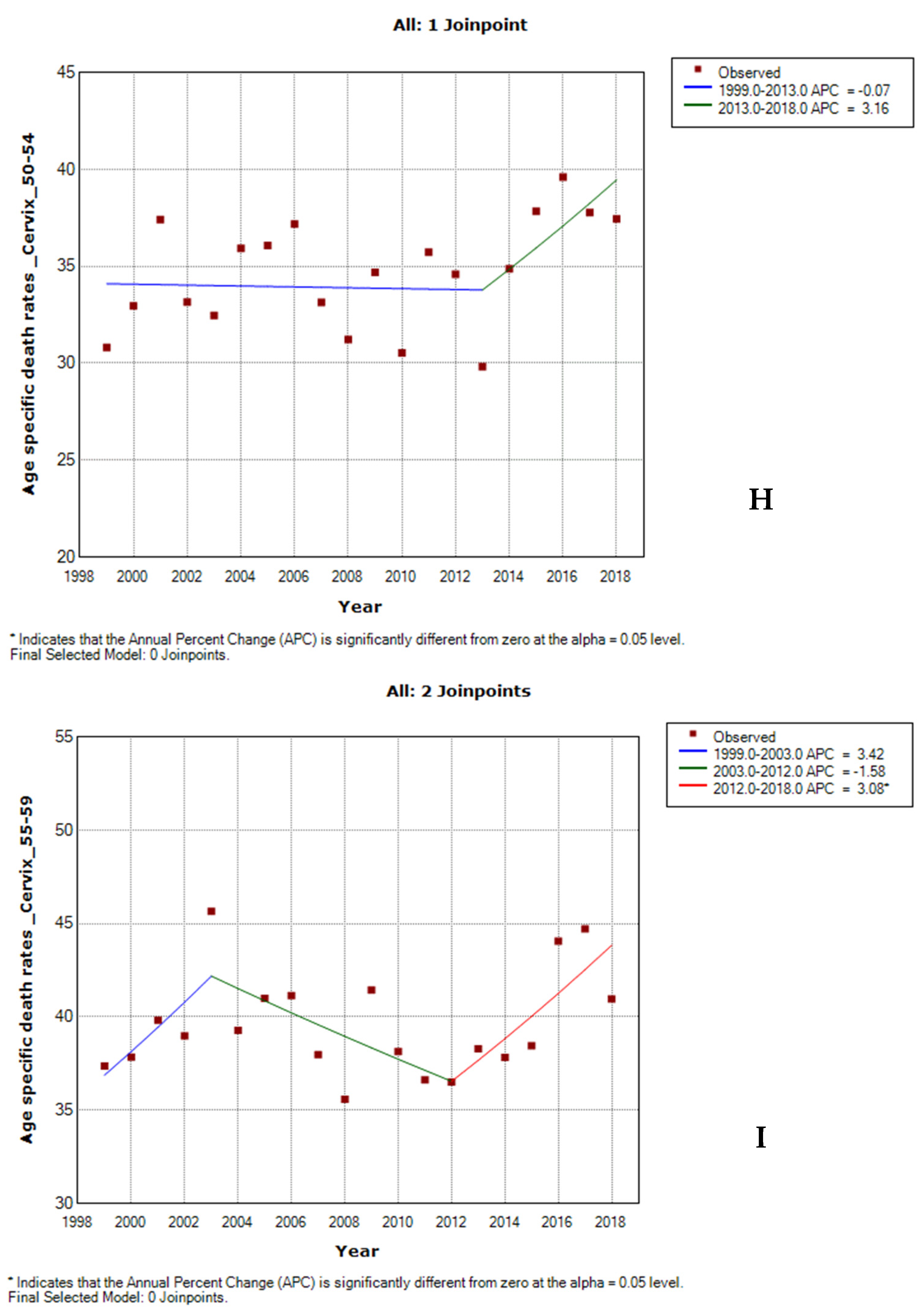
3.5. Age-Specific Death Rate from Cervical Cancer by Ethnicity
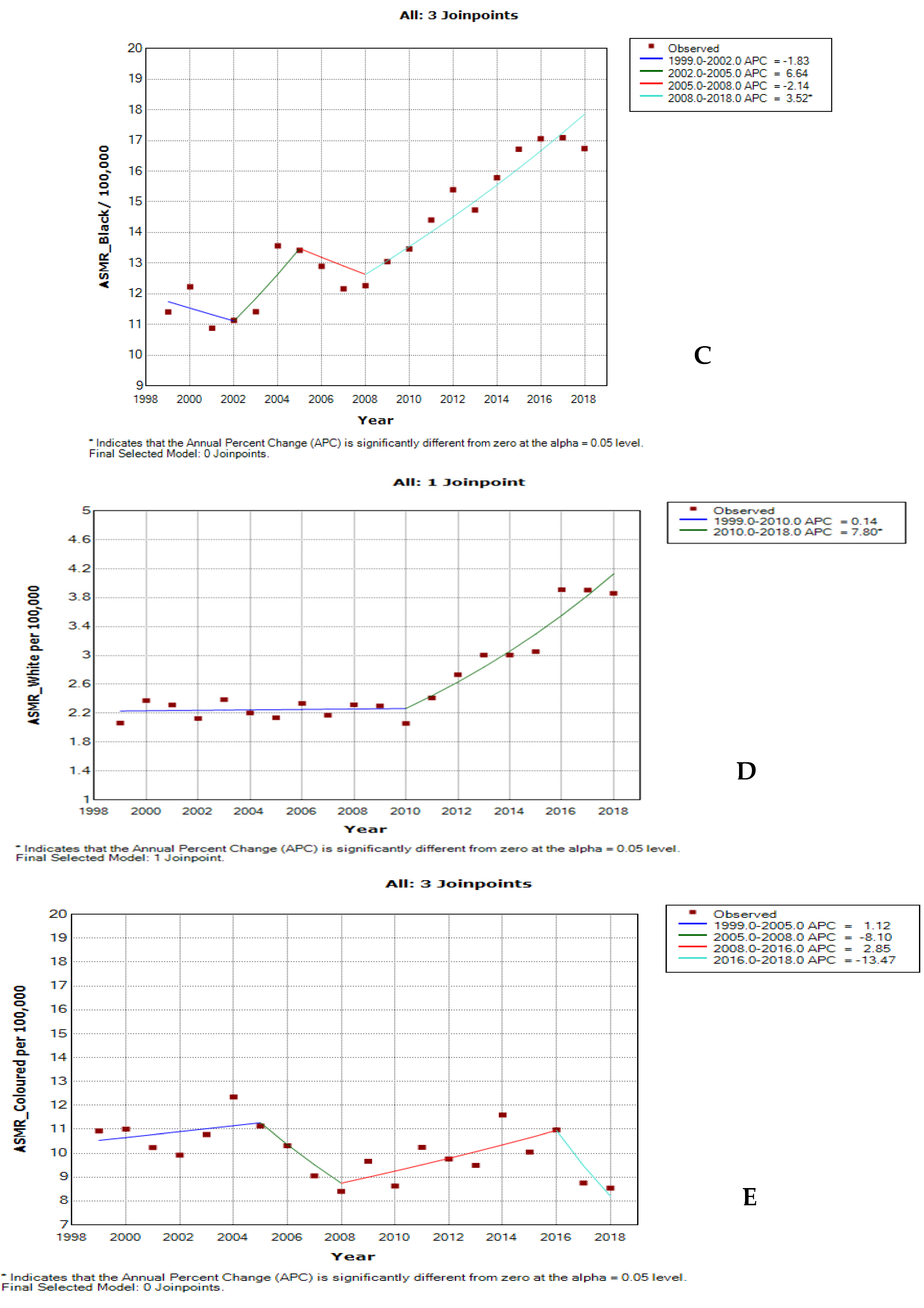
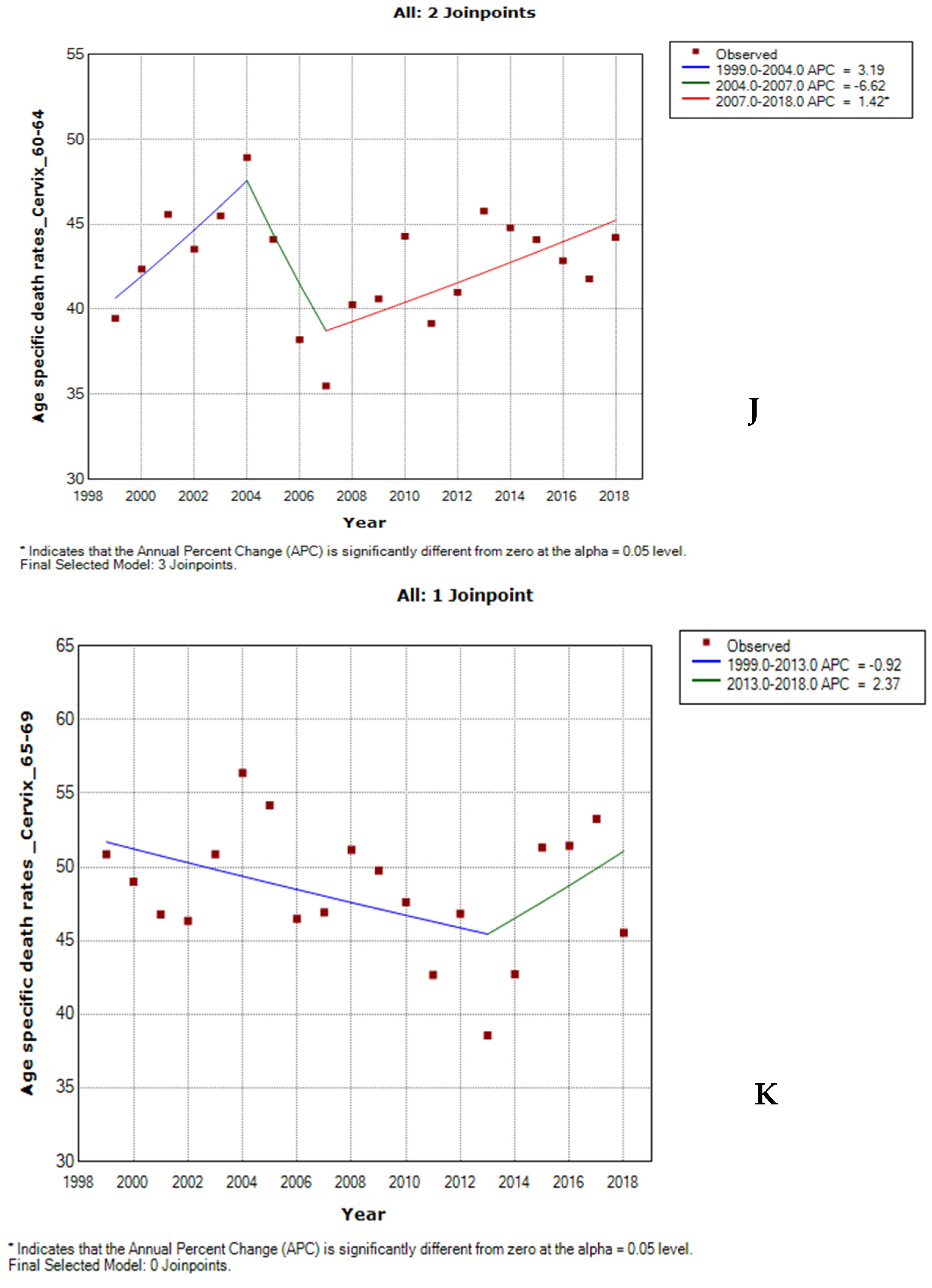
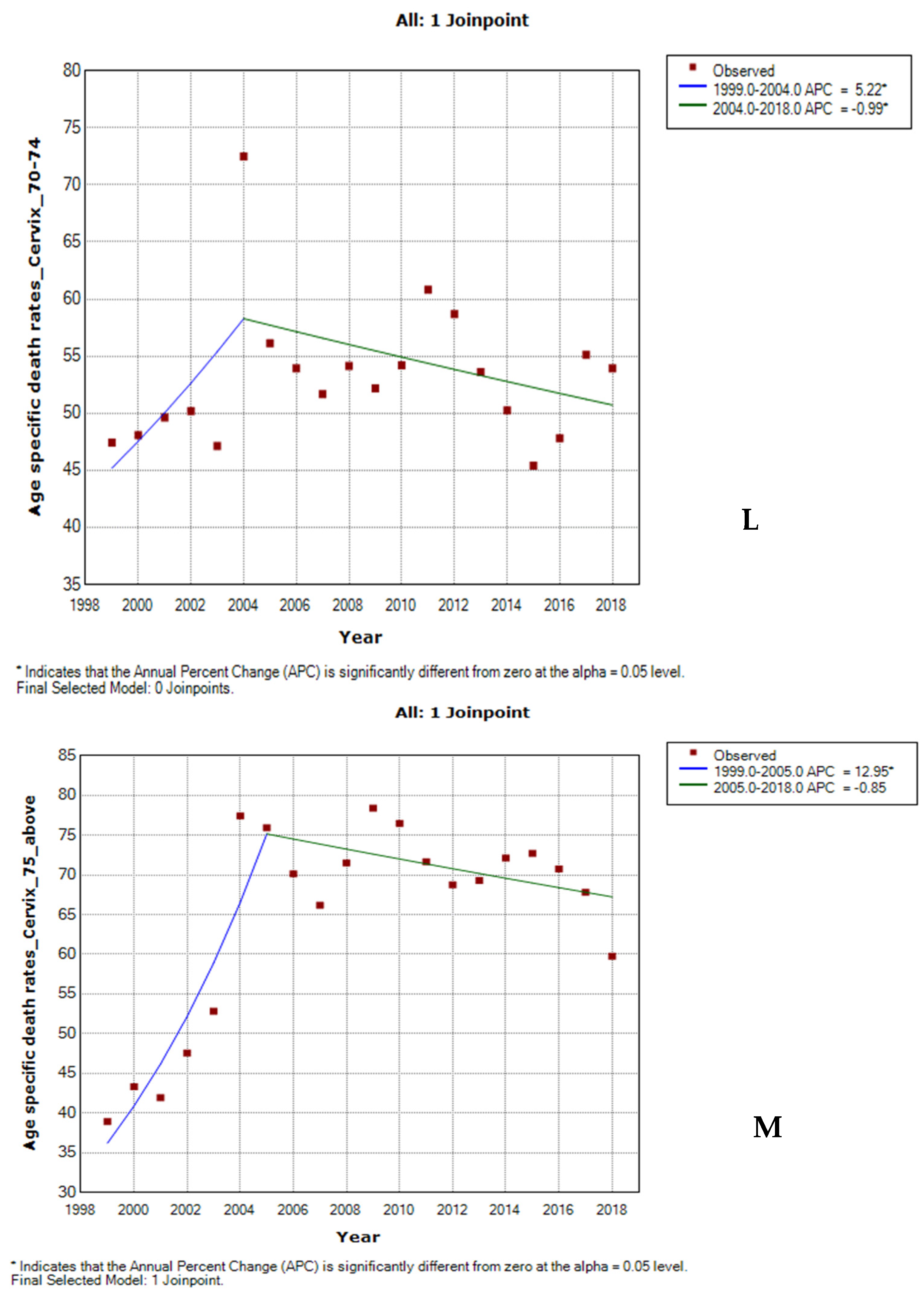
3.6. Joinpoint Trends of Age-Specific Death Rates for Cervical Cancer by Ethnicity
3.7. Age–Period–Cohort of Overall and Ethnic Trends in Cervical Cancer Mortality
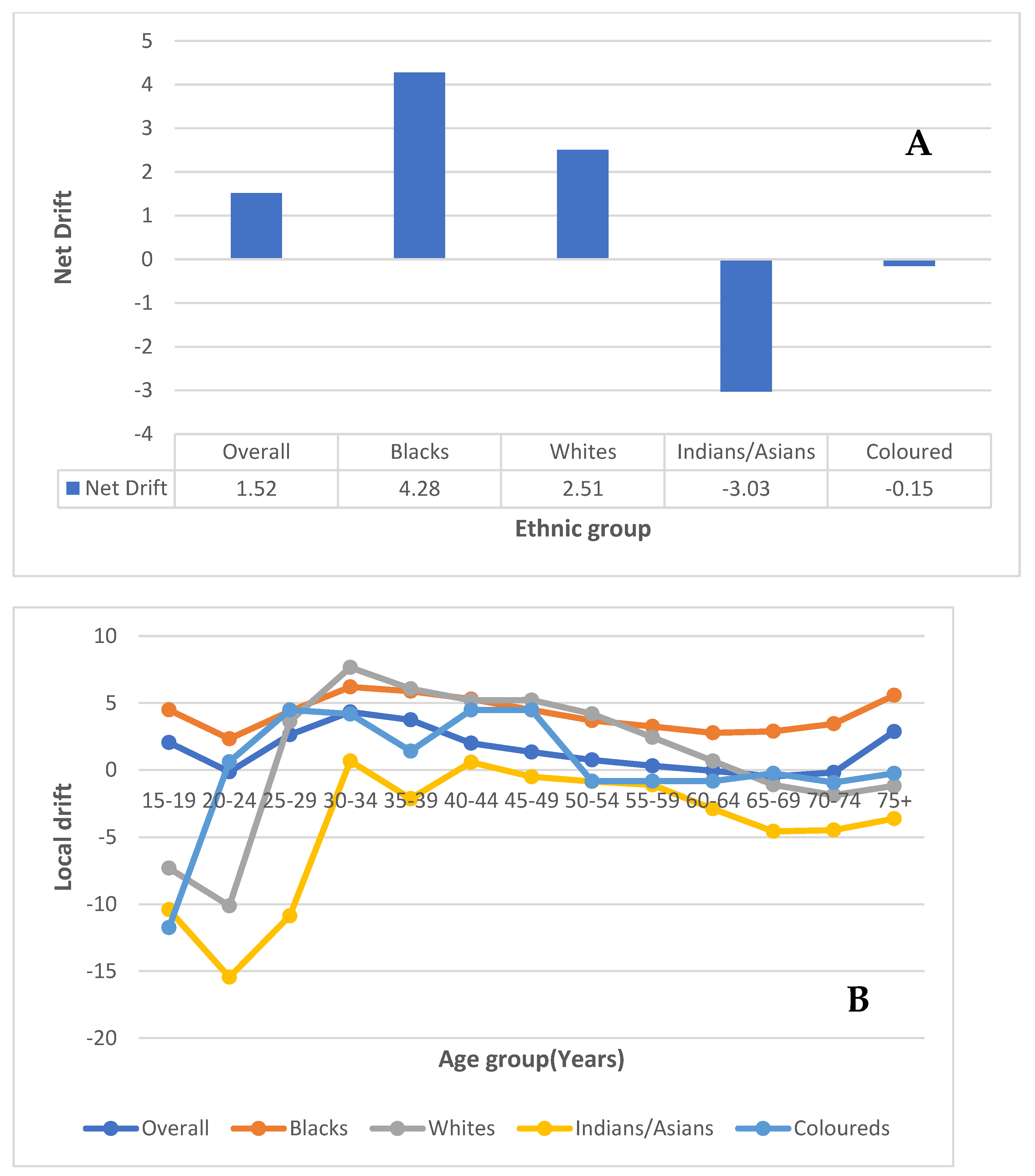
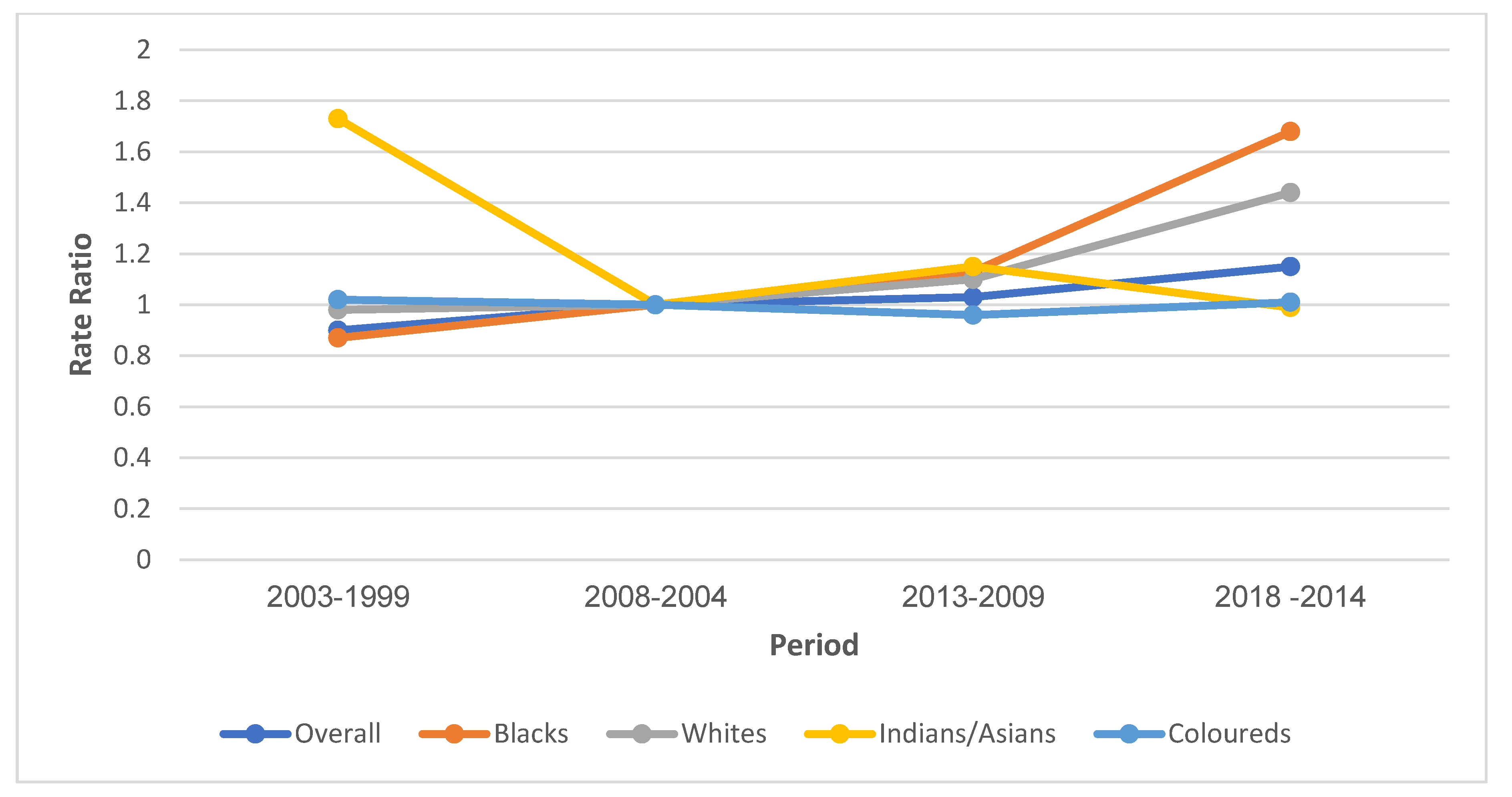
3.7.1. Local and Net Drift
3.7.2. Age Effect
3.7.3. Period Effect
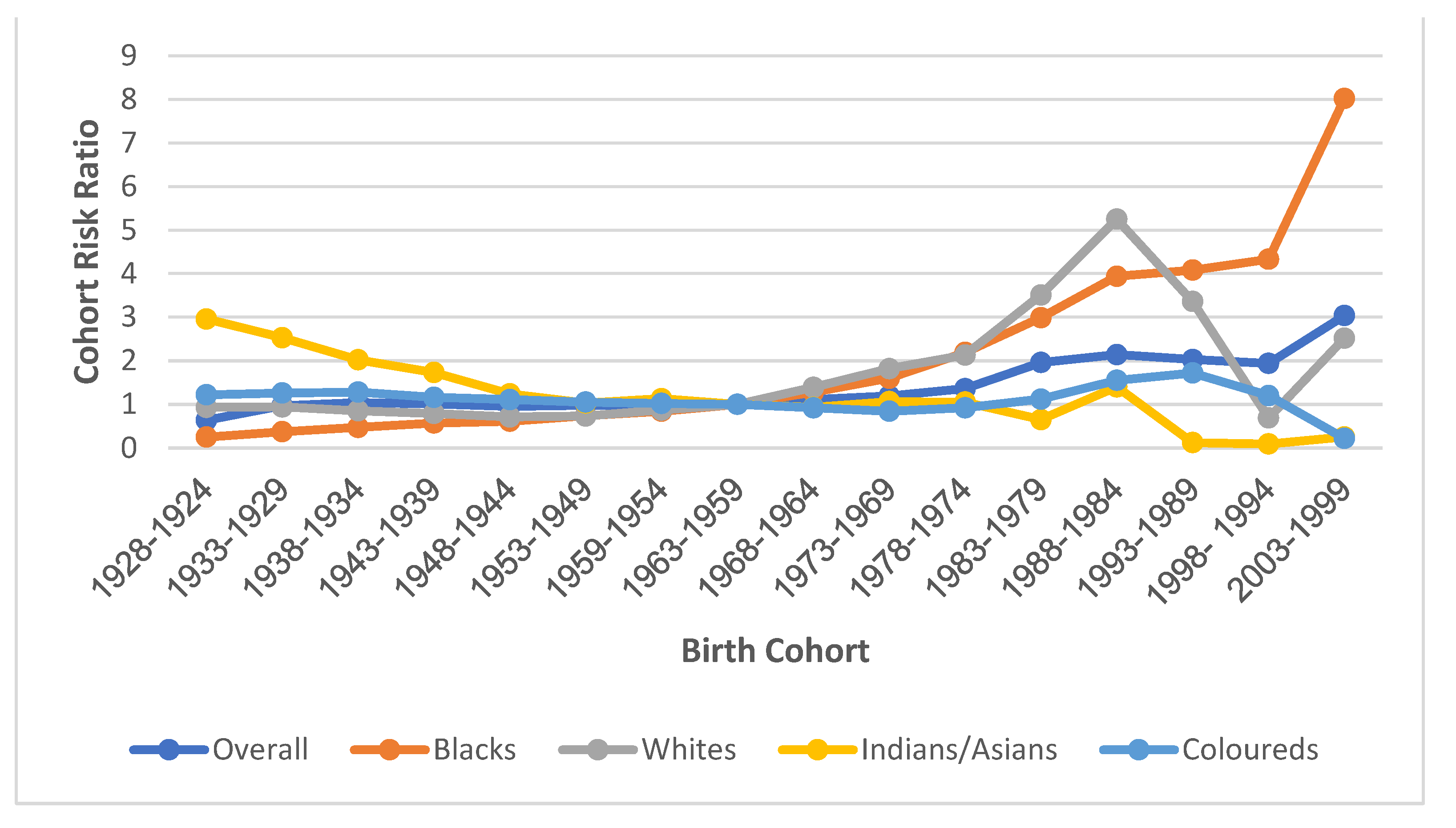
3.7.4. Cohort Effect
4. Discussion
4.1. Cervical Cancer Mortality Trends
4.2. Age Effect of Cervical Cancer Mortality Trends in South Africa
4.3. Cohort Effect of Cervical Cancer Mortality in South Africa
4.4. Ethnic Disparity of Cervical Cancer Trends
4.5. Strength and Limitation
5. Conclusions
Brief Policy Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.U.I.M.; Mehrotra, R.; Kwitshana, Z.M.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle—Income countries. Review 2020, 20, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical Carcinoma and Sexual Behavior: Collaborative Reanalysis of Individual Data on 15,461 Women with Cervical Carcinoma and 29,164 Women without Cervical Carcinoma from 21 Epidemiological Studies. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1060–1069. [Google Scholar] [CrossRef]
- Beral, V.; Colin, D.; Franceschi, S.; Green, J.; La Vecchia, C.; Peto, J.; Randi, G.; Herrero, R.; Hildesheim, A.; Vecchia, C.L.; et al. Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int. J. Cancer 2006, 1124, 1108–1124. [Google Scholar] [CrossRef]
- Urban, M.; Banks, E.; Egger, S.; Canfell, K.; O’Connell, D.; Beral, V.; Sitas, F. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black south african women: Case-control study. PLoS Med. 2012, 9, e1001182. [Google Scholar] [CrossRef]
- Olorunfemi, G.; Ndlovu, N.; Masukume, G.; Chikandiwa, A.; Pisa, P.T.; Singh, E. Temporal trends in the epidemiology of cervical cancer in South Africa (1994–2012). Int. J. Cancer 2018, 143, 2238–2249. [Google Scholar] [CrossRef]
- Cooper, D.; Morroni, C.; Orner, P.; Moodley, J.; Harries, J.; Cullingworth, L.; Hoffman, M. Ten years of democracy in South Africa: Documenting transformation in reproductive health policy and status. Reprod. Health Matters 2004, 12, 70–85. [Google Scholar] [CrossRef]
- Cooper, D.; Harries, J.; Moodley, J.; Constant, D.; Hodes, R.; Mathews, C.; Morroni, C.; Hoffman, M. Coming of age? Women’s sexual and reproductive health after twenty-one years of democracy in South Africa. Reprod. Health Matters 2016, 24, 79–89. [Google Scholar] [CrossRef]
- South African National Department of Health. Cervical Cancer Prevention and Control Policy; Department of Health: Pretoria, South Africa, 2017.
- Mabaso, M.; Makola, L.; Naidoo, I.; Mlangeni, L.L.; Jooste, S.; Simbayi, L. HIV prevalence in South Africa through gender and racial lenses: Results from the 2012 population-based national household survey. Int. J. Equity Health 2019, 18, 167. [Google Scholar] [CrossRef]
- Shisana, O.; Rehle, T.; Simbayi, L.C.; Zuma, K.; Jooste, S.; Zungu, N.; Labadarios, D.; Onoya, D. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Hum. Sci. Res. Counc. 2012, 194. [Google Scholar]
- Mbulawa, Z.Z.A.; Van Schalkwyk, C.; Hu, N.C.; Meiring, T.L.; Barnabas, S.; Dabee, S.; Jaspan, H.; Kriek, J.M.; Jaumdally, S.Z.; Muller, E.; et al. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS ONE 2018, 13, e0190166. [Google Scholar] [CrossRef] [PubMed]
- Olorunfemi, G. Trends and Determinants of the Incidence and Mortality of Cervical Cancer in South Africa (1994–2012). Master’s Thesis, University of Witswatersrand, Johanneburg, South Africa, 2017. [Google Scholar]
- Bhatla, N.; Aoki, D.; Nand, D.; Rengaswamy, S. Cancer of the cervix uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef]
- Bell, A.; Bell, A. Annals of Human Biology Age period cohort analysis: A review of what we should and shouldn’t do Age period cohort analysis: A review of what we should and shouldn’t do. Ann. Hum. Biol. 2020, 47, 208–217. [Google Scholar] [CrossRef]
- Nkengasong, J.; Gudo, E.; Macicame, I.; Maunze, X.; Amouzou, A.; Banke, K.; Dowell, S.; Jani, I. Improving birth and death data for African decision making. Lancet Glob. Health 2020, 8, e35–e36. [Google Scholar] [CrossRef] [PubMed]
- Statistics South Africa. Mid-Year Population Estimates 2015; Statistics SA: Pretoria, South Africa, 2015.
- Joubert, J.; Rao, C.; Bradshaw, D.; Vos, T.; Lopez, A.D. Evaluating the Quality of National Mortality Statistics from Civil Registration in South Africa, 1997–2007. PLoS ONE 2013, 8, e64592. [Google Scholar] [CrossRef]
- Rosenberg, P.S.; Check, D.P.; Anderson, W.F. A Web Tool for Age—Period—Cohort Analysis of Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- National Cancer Registry. Cancer in South Africa 2018. 2021. Available online: https://www.nicd.ac.za/centres/national-cancer-registry/ (accessed on 13 August 2022).
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef]
- Akinyemiju, T.F.; McDonald, J.A.; Lantz, P.M. Health care access dimensions and cervical cancer screening in South Africa: Analysis of the world health survey. BMC Public Health 2015, 15, 382. [Google Scholar] [CrossRef]
- Guo, M.; Xu, J.; Du, J. Trends in cervical cancer mortality in China from 1989 to 2018: An age-period-cohort study and Joinpoint analysis. BMC Public Health 2021, 21, 1329. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Oh, C.M.; Won, Y.J.; Lee, J.K.; Jung, K.W.; Cho, H.; Jun, J.K.; Lim, M.C.; Ki, M. Trends and age-period-cohort effects on the incidence and mortality rate of cervical cancer in Korea. Cancer Res. Treat. 2017, 49, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Piechocki, M.; Koziołek, W.; Sroka, D.; Matrejek, A.; Miziołek, P.; Saiuk, N.; Sledzik, M.; Jaworska, A.; Bereza, K.; Pluta, E.; et al. Trends in Incidence and Mortality of Gynecological and Breast Cancers in Poland (1980–2018). Clin. Epidemiol. 2022, 14, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Z.; Gao, X.; Zhang, N.; Wang, Z. The Effects of Age, Period, and Cohort on the Mortality of Cervical Cancer in Three High-Income Countries: Canada, Korea, and Italy. Biomed Res. Int. 2021, 2021, 8829122. [Google Scholar] [CrossRef]
- Pillay-van Wyk, V.; Msemburi, W.; Laubscher, R.; Dorrington, R.E.; Groenewald, P.; Glass, T.; Nojilana, B.; Joubert, J.D.; Matzopoulos, R.; Prinsloo, M.; et al. Mortality trends and differentials in South Africa from 1997 to 2012: Second National Burden of Disease Study. Lancet Glob. Health 2016, 4, e642–e653. [Google Scholar] [CrossRef]
- Mbulaiteye, S.M.; Parkin, D.M.; Rabkin, C.S. Epidemiology of AIDS-related malignancies. Hematol. Oncol. Clin. N. Am. 2003, 17, 673–696. [Google Scholar] [CrossRef]
- Simelela, N.P.; Venter, W.D.F. A brief history of South Africa’s response to AIDS. S. Afr. Med. J. 2014, 104, 249. [Google Scholar] [CrossRef]
- Reniers, G.; Blom, S.; Calvert, C.; Martin-Onraet, A.; Herbst, A.J.; Eaton, J.W.; Bor, J.; Slaymaker, E.; Li, Z.R.; Clark, S.J.; et al. Trends in the burden of HIV mortality after roll-out of antiretroviral therapy in KwaZulu-Natal, South Africa: An observational community cohort study. Lancet HIV 2017, 4, e113–e121. [Google Scholar] [CrossRef]
- Sartorius, B.; Kahn, K.; Collinson, M.A.; Sartorius, K.; Tollman, S.M. Dying in their prime: Determinants and space-time risk of adult mortality in rural South Africa. Geospat. Health 2013, 7, 237–249. [Google Scholar] [CrossRef][Green Version]
- Mayosi, B.M.; Lawn, J.E.; van Niekerk, A.; Bradshaw, D.; Abdool Karim, S.S.; Coovadia, H.M. Health in South Africa: Changes and challenges since 2009. Lancet 2012, 380, 2029–2043. [Google Scholar] [CrossRef]
- Tanaka, S.; Palmer, M.; Katanoda, K. Trends in cervical cancer incidence and mortality of young and middle adults in Japan. Cancer Sci. 2022, 113, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.C.; Santos, D.Z.; Campos, C.S.; Vale, D.B.; Bragança, J.F.; Zeferino, L.C. Cervical cancer in women under 25 years of age and outside the screening age: Diagnosis profile and long-term outcomes. Int. J. Gynecol. Obstet. 2021, 154, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Gravdal, B.H.; Lönnberg, S.; Skare, G.B.; Sulo, G.; Bjørge, T. Cervical cancer in women under 30 years of age in Norway: A population-based cohort study. BMC Womens. Health 2021, 21, 110. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, Y.; Wen, W.; Wu, C.; Bao, P.; Wang, C.; Zhong, W.; Gao, Y.T.; Jin, F.; Xiang, Y.B.; et al. Incidence and mortality of gynaecological cancers: Secular trends in urban Shanghai, China over 40 years. Eur. J. Cancer 2016, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Vinodh, N.; Badwe, R.A.; Deo, S.V.S.; Manoharan, N.; Malik, R.; Panse, N.S.; Ramesh, C.; Shrivastava, A.; Swaminathan, R.; et al. Trends in breast and cervical cancer in India under National Cancer Registry Programme: An Age-Period-Cohort analysis. Cancer Epidemiol. 2021, 74, 101982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, E.; Yang, B.; Xiao, R.; Lu, F.; You, L.; Chen, G. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: Cervical, ovarian and uterine cancer. Gynecol. Oncol. 2021, 163, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Bakilana, A. Age at sexual debut in South Africa. Afr. J. AIDS Res. 2005, 4, 1–5. [Google Scholar] [CrossRef]
- Reddy, P.; Zuma, K.; Shisana, O.; Kim, J.; Sewpaul, R. Prevalence of tobacco use among adults in South Africa: Results from the first South African National Health and Nutrition Examination Survey. S. Afr. Med. J. 2015, 105, 648–655. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. Human Papillomavirus and Related Diseases Report; HPV Information Center: Barcelona, Spain, 2016. [Google Scholar]
- Patel, A.; Galaal, K.; Burnley, C.; Faulkner, K.; Martin-Hirsch, P.; Bland, M.J.; Leeson, S.; Beer, H.; Paranjothy, S.; Sasieni, P.; et al. Cervical cancer incidence in young women: A historical and geographic controlled UK regional population study. Br. J. Cancer 2012, 106, 1753–1759. [Google Scholar] [CrossRef]
- Tathiah, N.; Naidoo, M.; Moodley, I. Human papillomavirus (HPV) vaccination of adolescents in the South African private health sector: Lessons from the HPV demonstration project in KwaZulu-Natal. S. Afr. Med. J. 2015, 105, 954. [Google Scholar] [CrossRef]
- Bailie, R.; Selvey, C.E.; Bourne, D.; Bradshaw, D. Trends in cervical cancer mortality in South Africa. Int. J. Epidemiol. 1996, 25, 488–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akokuwebe, M.E.; Idemudia, E.S.; Lekulo, A.M.; Motlogeloa, O.W. Determinants and levels of cervical Cancer screening uptake among women of reproductive age in South Africa: Evidence from South Africa Demographic and health survey data, 2016. BMC Public Health 2021, 21, 2013. [Google Scholar] [CrossRef] [PubMed]
- Coovadia, H.; Jewkes, R.; Barron, P.; Sanders, D.; McIntyre, D. The health and health system of South Africa: Historical roots of current public health challenges. Lancet 2009, 374, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Phaswana-mafuya, N.; Peltzer, K. Breast and Cervical Cancer Screening Prevalence and Associated Factors among Women in the South African General Population. Asian Pac. J. Cancer Prev. 2018, 19, 1465–1470. [Google Scholar] [CrossRef]
- Caldwell, J.C.; Caldwell, P. The South African fertility decline. Popul. Dev. Rev. 1993, 19, 225–262. [Google Scholar] [CrossRef]
- Mkhwanazi, N. Understanding teenage pregnancy in a post-apartheid South African township. Cult. Health Sex. 2010, 12, 347–358. [Google Scholar] [CrossRef]
- Sitas, F.; Egger, S.; Bradshaw, D.; Groenewald, P.; Laubscher, R.; Kielkowski, D.; Peto, R. Differences among the coloured, white, black, and other South African populations in smoking-attributed mortality at ages 35-74 years: A case-control study of 481,640 deaths. Lancet 2013, 382, 685–693. [Google Scholar] [CrossRef]
- Reddy, P.; James, S.; Sewpaul, R.; Yach, D.; Resnicow, K.; Sifunda, S.; Mthembu, Z.; Mbewu, A. A decade of tobacco control: The South African case of politics, health policy, health promotion and behaviour change. S. Afr. Med. J. 2013, 103, 835–840. [Google Scholar] [CrossRef]
- Statistics South Africa. StatsSA Statistical Release P0302: Mid-Year Population Estimates 2020; Statistics SA: Pretoria, South Africa, 2020; pp. 1–22.
- Loney, T.; Nagelkerke, N.J. The individualistic fallacy, ecological studies and instrumental variables: A causal interpretation. Emerg. Themes Epidemiol. 2014, 11, 18. [Google Scholar] [CrossRef]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human Papillomavirus Vaccination in South Africa: Programmatic Challenges and Opportunities for Integration with Other Adolescent Health Services? Front. Public Health 2022, 10, 799984. [Google Scholar] [CrossRef]
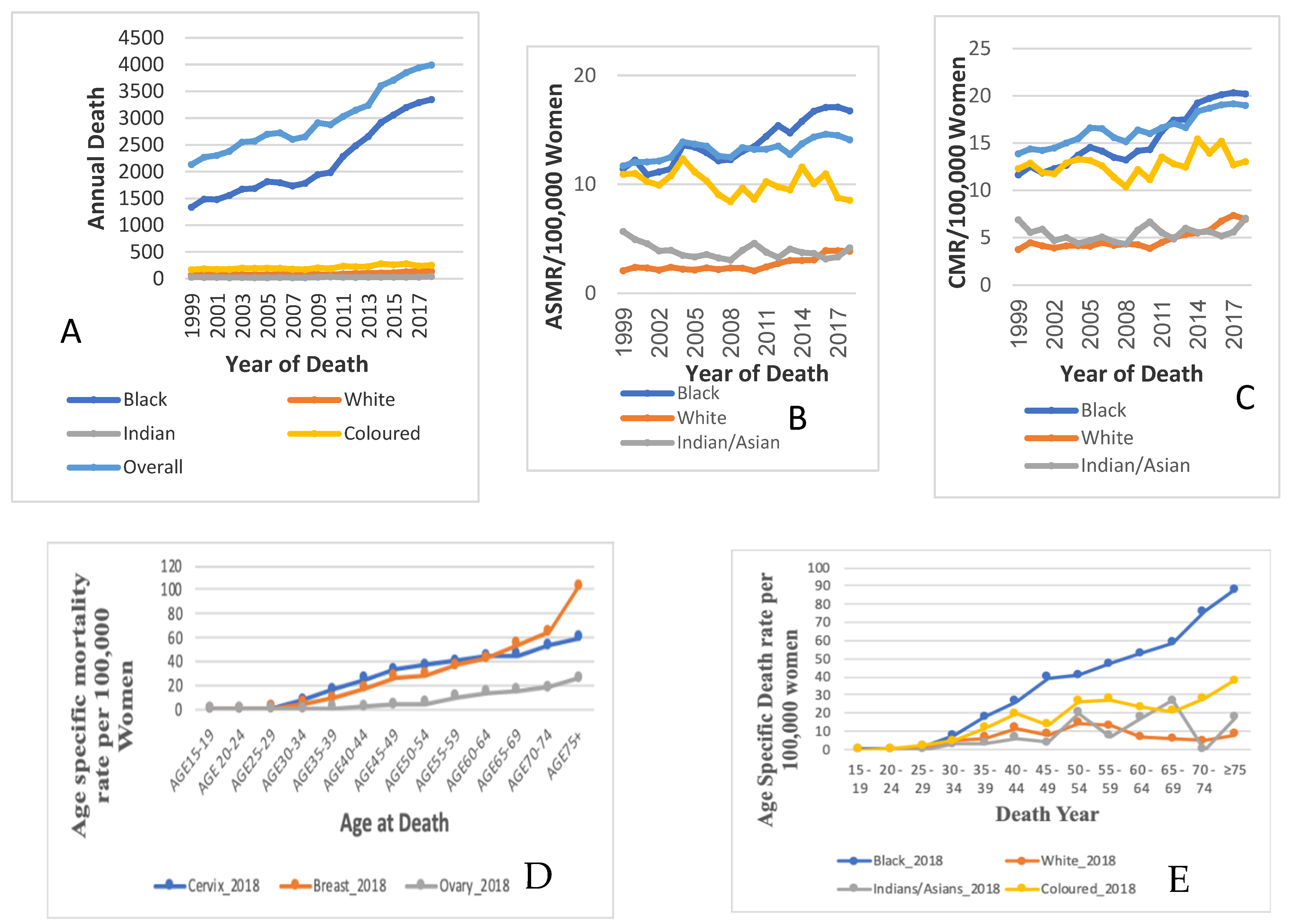
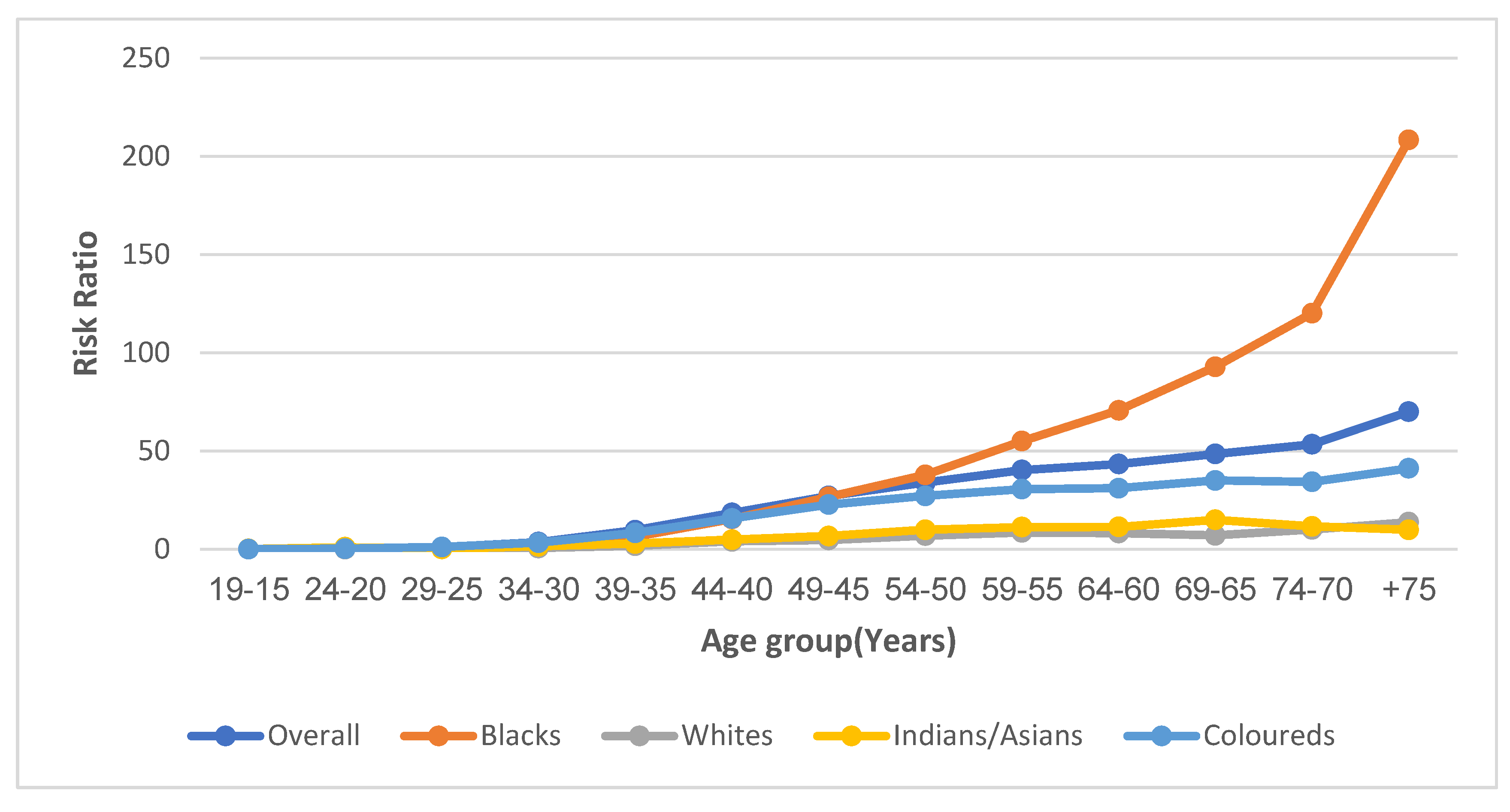
| Year | N = 59,190 | |||
|---|---|---|---|---|
| Cervix Mortality (% of Gynae & Breast) ≥15 Years | Age Mean ± SD | CMR | ASMR | |
| 1999 | 2134 (46.22) | 56.09 ± 14.25 | 13.88 | 11.7 |
| 2000 | 2270 (47.73) | 55.54 ± 14.29 | 14.38 | 12.02 |
| 2001 | 2303 (44.95) | 55.94 ± 13.85 | 14.23 | 12.05 |
| 2002 | 2378 (45.70) | 55.94 ± 14.08 | 14.49 | 12.14 |
| 2003 | 2553 (47.62) | 56.26 ± 14.40 | 15.02 | 12.49 |
| 2004 | 2569 (43.71) | 56.34 ± 14.21 | 15.48 | 13.89 |
| 2005 | 2700 (44.73) | 56.49 ± 14.27 | 16.62 | 13.7 |
| 2006 | 2725 (44.81) | 55.60 ± 14.40 | 16.54 | 13.51 |
| 2007 | 2603 (41.99) | 55.87 ± 14.56 | 15.58 | 12.6 |
| 2008 | 2651 (42.40) | 56.64 ± 14.75 | 15.15 | 12.48 |
| 2009 | 2910 (43.45) | 56.16 ± 14.88 | 16.39 | 13.38 |
| 2010 | 2877 (42.46) | 56.53 ± 14.92 | 16.01 | 13.21 |
| 2011 | 3031 (42.71) | 56.12 ± 14.88 | 16.63 | 13.22 |
| 2012 | 3150 (43.84) | 55.55 ± 14.87 | 17.07 | 13.51 |
| 2013 | 3239 (42.89) | 55.76 ± 14.89 | 16.64 | 12.74 |
| 2014 | 3604 (44.00) | 55.45 ± 14.94 | 18.37 | 13.72 |
| 2015 | 3708 (44.00) | 55.44 ± 14.91 | 18.70 | 14.35 |
| 2016 | 3852 (43.28) | 55.13 ± 14.72 | 19.06 | 14.6 |
| 2017 | 3940 (43.36) | 55.38 ± 14.66 | 19.16 | 14.49 |
| 2018 | 3993 (42.65) | 55.17 ± 14.78 | 18.98 | 14.08 |
| Cancer Type | Trends | Year Period | APC | 95% CI | p-Value | Comment | |
|---|---|---|---|---|---|---|---|
| Overall ASMR | 1 | 1999–2005 | 2.9 * | 1.1 | 4.7 | <0.001 | Significant increase |
| 2 | 2005–2008 | −2.4 | −11.9 | 8.0 | 0.6 | Non-significant decrease | |
| 3 | 2008–2018 | 1.4 * | 0.6 | 2.1 | <0.001 | Significant increase | |
| Full Range | 1999–2018 | 0.9 * | 0.6 | 1.2 | <0.001 | Significant increase | |
| Black | 1 | 1999–2002 | −1.8 | −8.9 | 5.8 | 0.6 | Non-significant decrease |
| 2 | 2002–2005 | 6.6 | −7.4 | 22.9 | 0.3 | Non-significant increase | |
| 3 | 2005–2008 | −2.1 | −15.0 | 12.7 | 0.7 | Non-significant decrease | |
| 4 | 2008–2018 | 3.5 * | 2.5 | 4.6 | <0.001 | Significant increase | |
| Full Range | 1999–2018 | 2.4 * | 1.9 | 2.9 | <0.001 | Significant increase | |
| Indian/Asian | 1 | 1999–2004 | −8.8 * | −14.7 | −2.4 | <0.001 | Significant decrease |
| 2 | 2004–2018 | 0.6 | −1.0 | 2.3 | 0.4 | Non-significant increase | |
| Full Range | 1999–2018 | −1.4 * | −2.6 | −0.2 | <0.001 | Significant decrease | |
| Coloured | |||||||
| 1 | 1999–2005 | 1.1 | −3.0 | 5.4 | 0.6 | Non-significant increase | |
| 2 | 2005–2008 | −8.1 | −29.7 | 20.1 | 0.5 | Non-significant decrease | |
| 3 | 2008–2016 | 2.8 | −0.7 | 6.5 | 0.1 | Non-significant increase | |
| 4 | 2016–2018 | −13.5 | −34.8 | 14.8 | 0.3 | Non-significant decrease | |
| Full Range | 1999–2018 | −0.7 | −1.5 | 0.1 | 0.1 | Non-significant decrease | |
| White | |||||||
| 1 | 1999–2010 | 0.1 | −1.2 | 1.4 | 0.8 | Stable | |
| 2 | 2010–2018 | 7.8 * | 5.9 | 9.7 | <0.001 | Significant increase | |
| Full Range | 1999–2018 | 3.3 * | 2.3 | 4.4 | <0.001 | Significant increase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olorunfemi, G.; Libhaber, E.; Ezechi, O.C.; Musenge, E. Population-Based Temporal Trends and Ethnic Disparity in Cervical Cancer Mortality in South Africa (1999–2018): A Join Point and Age–Period–Cohort Regression Analyses. Cancers 2022, 14, 6256. https://doi.org/10.3390/cancers14246256
Olorunfemi G, Libhaber E, Ezechi OC, Musenge E. Population-Based Temporal Trends and Ethnic Disparity in Cervical Cancer Mortality in South Africa (1999–2018): A Join Point and Age–Period–Cohort Regression Analyses. Cancers. 2022; 14(24):6256. https://doi.org/10.3390/cancers14246256
Chicago/Turabian StyleOlorunfemi, Gbenga, Elena Libhaber, Oliver Chukwujekwu Ezechi, and Eustasius Musenge. 2022. "Population-Based Temporal Trends and Ethnic Disparity in Cervical Cancer Mortality in South Africa (1999–2018): A Join Point and Age–Period–Cohort Regression Analyses" Cancers 14, no. 24: 6256. https://doi.org/10.3390/cancers14246256
APA StyleOlorunfemi, G., Libhaber, E., Ezechi, O. C., & Musenge, E. (2022). Population-Based Temporal Trends and Ethnic Disparity in Cervical Cancer Mortality in South Africa (1999–2018): A Join Point and Age–Period–Cohort Regression Analyses. Cancers, 14(24), 6256. https://doi.org/10.3390/cancers14246256





