Natural Blockers of PD-1/PD-L1 Interaction for the Immunotherapy of Triple-Negative Breast Cancer-Brain Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecules
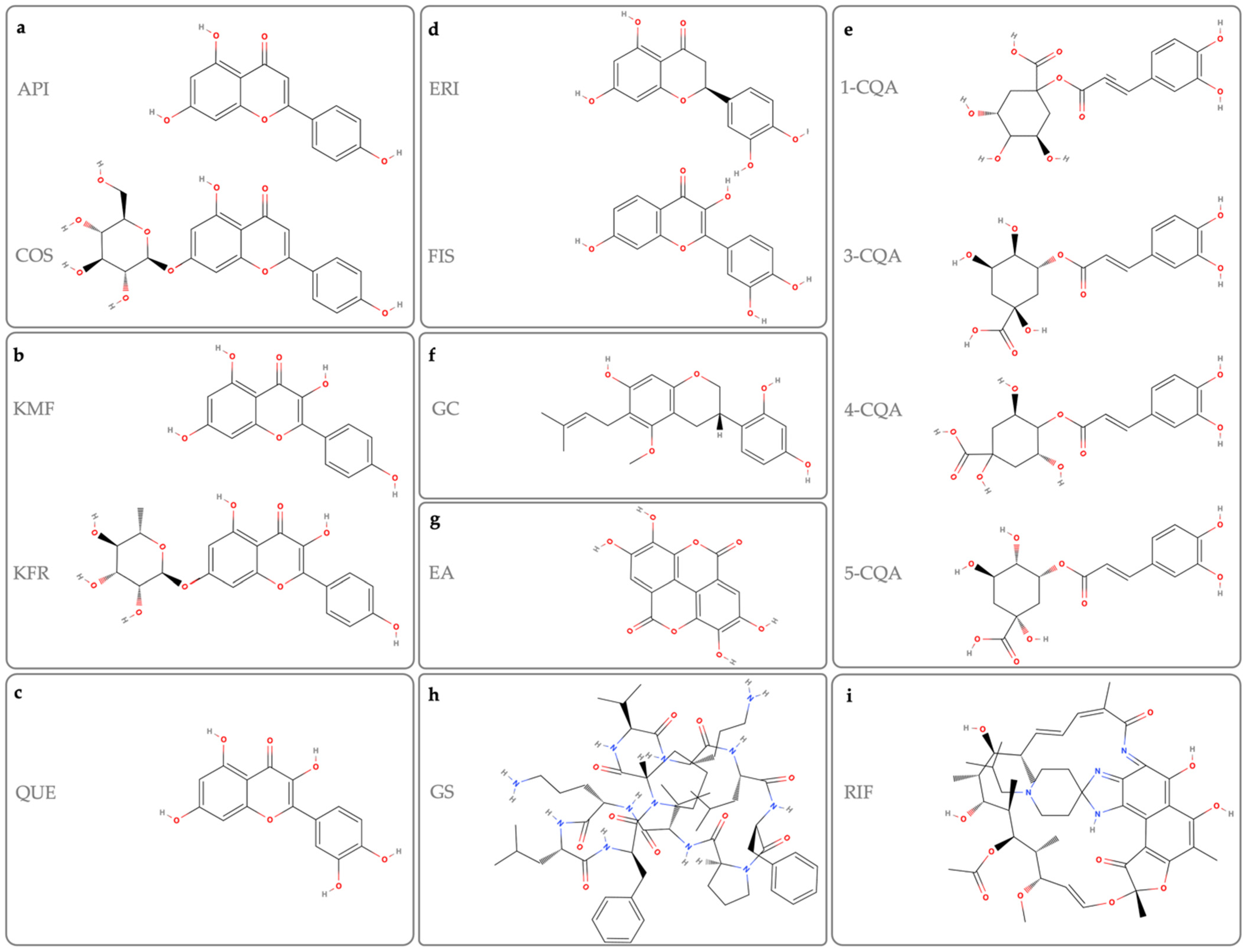
2.1. Apigenin (API) and Cosmosiin (COS)
2.2. Kaempferol and Kaempferol 7-O-Rhamnoside
2.3. Quercetin
2.4. Eriodictyol and Fisetin
2.5. Caffeoylquinic Acid
2.6. Glyasperin C
2.7. Ellagic Acid
2.8. Heterocyclic Compounds
2.9. Gramicidin S
2.10. Rifabutin (RIF)
3. Druggability of the Candidate Molecules to Brain Tumors
3.1. Physicochemical Properties
3.2. Pharmacokinetic (PK) Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.H.; Karantza, V.; Aktan, G.; Lala, M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: A systematic literature review. Breast Cancer Res. 2019, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Price, T.J.; Townsend, A.R. Druggable molecular targets for the treatment of triple negative breast cancer. J. Breast Cancer 2019, 22, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Nowsheen, S.; Maraboyina, S.; Xia, F. The role of poly (ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: A review. Cell Biosci. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Shigdar, S. Future of PD-1/PD-L1 axis modulation for the treatment of triple-negative breast cancer. Pharmacol. Res. 2022, 175, 106019. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Vetrei, C.; Passariello, M.; Froechlich, G.; Rapuano Lembo, R.; Sasso, E.; Zambrano, N.; De Lorenzo, C. Novel Combinations of Human Immunomodulatory mAbs Lacking Cardiotoxic Effects for Therapy of TNBC. Cancers 2022, 14, 121. [Google Scholar] [CrossRef]
- Passariello, M.; D’Alise, A.M.; Esposito, A.; Vetrei, C.; Froechlich, G.; Scarselli, E.; Nicosia, A.; De Lorenzo, C. Novel human anti-PD-L1 mAbs inhibit immune-independent tumor cell growth and PD-L1 associated intracellular signalling. Sci. Rep. 2019, 9, 13125. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Samarasinghe, R.M.; Shigdar, S. Triple-negative breast cancer brain metastasis: An update on druggable targets, current clinical trials, and future treatment options. Drug Discov. Today 2022, 27, 1298–1314. [Google Scholar] [CrossRef]
- Sarafraz, M.; Nakhjavani, M.; Shigdar, S.; Christo, F.C.; Rolfe, B. Modelling of mass transport and distribution of aptamer in blood-brain barrier for tumour therapy and cancer treatment. Eur. J. Pharm. Biopharm. 2022, 173, 121–131. [Google Scholar] [CrossRef]
- Deeken, J.F.; Löscher, W. The blood-brain barrier and cancer: Transporters, treatment, and Trojan horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Shah, N.; Mohammad, A.S.; Saralkar, P.; Sprowls, S.A.; Vickers, S.D.; John, D.; Tallman, R.M.; Lucke-Wold, B.P.; Jarrell, K.E.; Pinti, M. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res. 2018, 132, 47–68. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Smith, E.; Palethorpe, H.M.; Tomita, Y.; Yeo, K.; Price, T.J.; Townsend, A.R.; Hardingham, J.E. Anti-cancer effects of an optimised combination of ginsenoside Rg3 epimers on triple negative breast cancer models. Pharmaceuticals 2021, 14, 633. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Smith, E.; Yeo, K.; Palethorpe, H.M.; Tomita, Y.; Price, T.J.; Townsend, A.R.; Hardingham, J.E. Anti-angiogenic properties of ginsenoside Rg3 epimers: In vitro assessment of single and combination treatments. Cancers 2021, 13, 2223. [Google Scholar] [CrossRef]
- Palethorpe, H.M.; Smith, E.; Tomita, Y.; Nakhjavani, M.; Yool, A.J.; Price, T.J.; Young, J.P.; Townsend, A.R.; Hardingham, J.E. Bacopasides I and II act in synergy to inhibit the growth, migration and invasion of breast cancer cell lines. Molecules 2019, 24, 3539. [Google Scholar] [CrossRef]
- Zahir, N.V.; Nakhjavani, M.; Hajian, P.; Shirazi, F.H.; Mirzaei, H. Evaluation of silibinin effects on the viability of HepG2 (human hepatocellular liver carcinoma) and HUVEC (human umbilical vein endothelial) cell lines. Iran. J. Pharm. Res. IJPR 2018, 17, 261. [Google Scholar]
- Hu, S.C.-S.; Lee, I.T.; Yen, M.-H.; Lin, C.-C.; Lee, C.-W.; Yen, F.-L. Anti-melanoma activity of Bupleurum chinense, Bupleurum kaoi and nanoparticle formulation of their major bioactive compound saikosaponin-d. J. Ethnopharmacol. 2016, 179, 432–442. [Google Scholar] [CrossRef]
- Pellerino, A.; Bruno, F.; Bertero, L.; Bellini, E.; Beano, A.; Montemurro, F.; Valiente, M.; Rudà, R.; Soffietti, R. TMIC-01. STAT3 Expression in brain metastases from breast cancer: Correlations with different molecular subtypes and clinical outcome. Neuro-Oncology 2022, 24, vii270–vii271. [Google Scholar] [CrossRef]
- Lee, Y.; Park, H.R.; Chun, H.J.; Lee, J. Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease via mitochondrial stabilization. J. Neurosci. Res. 2015, 93, 755–765. [Google Scholar] [CrossRef]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martínez, L.; Martínez-Saez, E.; Ramón y Cajal, S. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035. [Google Scholar] [CrossRef]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kim, Y.S.; Kim, J.H.; Kim, T.I.; Li, W.; Oh, T.W.; Jeon, C.H.; Kim, S.J.; Chung, H.-S. Anticancer effect of Salvia plebeia and its active compound by improving T-cell activity via blockade of PD-1/PD-L1 interaction in humanized PD-1 mouse model. Front. Immunol. 2020, 11, 598556. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Felice, M.R.; Maugeri, A.; De Sarro, G.; Navarra, M.; Barreca, D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23, 4411. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Choi, J.G.; Li, W.; Lee, E.J.; Park, J.W.; Song, J.; Chung, H.S. Kaempferol and Its Glycoside, Kaempferol 7-O-Rhamnoside, Inhibit PD-1/PD-L1 Interaction In Vitro. Int. J. Mol. Sci. 2020, 21, 3239. [Google Scholar] [CrossRef]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264. 7 cells: In vitro assessment and a theoretical model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef]
- Chirumbolo, S. Quercetin as a potential anti-allergic drug: Which perspectives. Iran. J. Allergy Asthma Immunol. 2011, 10, 139–140. [Google Scholar]
- Park, H.-J.; Lee, C.-M.; Jung, I.D.; Lee, J.S.; Jeong, Y.-I.; Chang, J.H.; Chun, S.-H.; Kim, M.-J.; Choi, I.-W.; Ahn, S.-C. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 2009, 9, 261–267. [Google Scholar] [CrossRef]
- Bae, J.-H.; Kim, J.-Y.; Kim, M.-J.; Chang, S.-H.; Park, Y.-S.; Son, C.-H.; Park, S.-J.; Chung, J.-S.; Lee, E.-Y.; Kim, S.-H. Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70. J. Immunother. 2010, 33, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lin, J.; Yang, Y.; Tao, L.; Li, Y.; Liu, Z.; Zhao, Q.; Diao, A. Quercetin inhibiting the PD-1/PD-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytother. Res. 2021, 35, 6441–6451. [Google Scholar] [CrossRef]
- Li, C.-W.; Lim, S.-O.; Chung, E.M.; Kim, Y.-S.; Park, A.H.; Yao, J.; Cha, J.-H.; Xia, W.; Chan, L.-C.; Kim, T. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell 2018, 33, 187–201.e110. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kim, T.I.; Kim, J.H.; Chung, H.-S. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules 2019, 24, 4062. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fu, H.; Li, C.; Deng, Z.; Chang, H. Eriodictyol inhibits breast carcinogenesis by targeting circ_0007503 and repressing PI3K/Akt pathway. Phytomedicine 2022, 102, 154159. [Google Scholar] [CrossRef] [PubMed]
- Oh, U.H.; Kim, D.-H.; Lee, J.; Han, S.-I.; Kim, J.-H. Eriodictyol induces apoptosis via regulating phosphorylation of JNK, ERK, and FAK/AKT in pancreatic cancer cells. J. Appl. Biol. Chem. 2022, 65, 83–88. [Google Scholar] [CrossRef]
- Huang, H.; He, Y.; Li, Y.; Gu, M.; Wu, M.; Ji, L. Eriodictyol suppresses the malignant progression of colorectal cancer by downregulating tissue specific transplantation antigen P35B (TSTA3) expression to restrain fucosylation. Bioengineered 2022, 13, 5551–5563. [Google Scholar] [CrossRef]
- Khozooei, S.; Lettau, K.; Barletta, F.; Jost, T.; Rebholz, S.; Veerappan, S.; Franz-Wachtel, M.; Macek, B.; Iliakis, G.; Distel, L.V. Fisetin induces DNA double-strand break and interferes with the repair of radiation-induced damage to radiosensitize triple negative breast cancer cells. J. Exp. Clin. Cancer Res. 2022, 41, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, S.; Zhang, C.; Jin, Y.; Xu, G.; Zhou, L.; Ding, G.; Pang, T.; Jia, S.; Cao, L. ZC3H13-mediated N6-methyladenosine modification of PHF10 is impaired by fisetin which inhibits the DNA damage response in pancreatic cancer. Cancer Lett. 2022, 530, 16–28. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Y.; He, T.; Wang, D.; Guo, N.; Zhang, X.; Chen, S.; Wang, H. PD-1/PD-L1 inhibitor screening of caffeoylquinic acid compounds using surface plasmon resonance spectroscopy. Anal. Biochem. 2018, 547, 52–56. [Google Scholar] [CrossRef]
- Bao, F.; Bai, H.-Y.; Wu, Z.-R.; Yang, Z.-G. Phenolic compounds from cultivated Glycyrrhiza uralensis and their PD-1/PD-L1 inhibitory activities. Nat. Prod. Res. 2021, 35, 562–569. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Lim, J.W.; Hwang, H.J.; Shin, C.S. Polyphenol compounds and anti-inflammatory activities of Korean black raspberry (Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J. Agric. Food Chem. 2012, 60, 5121–5127. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, E.; eun Lee, J.; Auh, J.H.; Choi, H.-K.; Lee, J.; Cho, S.; Kim, J.-H. Effects of a Rubus coreanus Miquel supplement on plasma antioxidant capacity in healthy Korean men. Nutr. Res. Pract. 2011, 5, 429–434. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, H.S.; Cho, S.G.; Shin, Y.C.; Ko, S.G. Rubus coreanus Miquel extract causes apoptosis of doxorubicin-resistant NCI/ADR-RES ovarian cancer cells via JNK phosphorylation. Mol. Med. Rep. 2016, 13, 4065–4072. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.H.; Kim, Y.S.; Kim, T.I.; Li, W.; Mun, J.-G.; Jeon, H.D.; Kee, J.-Y.; Choi, J.-G.; Chung, H.-S. Unripe black raspberry (Rubus coreanus Miquel) extract and its constitute, ellagic acid induces T cell activation and antitumor immunity by blocking PD-1/PD-L1 interaction. Foods 2020, 9, 1590. [Google Scholar] [CrossRef] [PubMed]
- Lung, J.; Hung, M.-S.; Lin, Y.-C.; Hung, C.-H.; Chen, C.-C.; Lee, K.-D.; Tsai, Y.H. Virtual Screening and In Vitro Evaluation of PD-L1 Dimer Stabilizers for Uncoupling PD-1/PD-L1 Interaction from Natural Products. Molecules 2020, 25, 5293. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, D.; Zhan, S.; Wu, W.; Xu, H.; Luo, C.; Su, H.; Feng, Y.; Shao, W.; Wan, A. Design and discovery of natural cyclopeptide skeleton based programmed death ligand 1 inhibitor as immune modulator for cancer therapy. J. Med. Chem. 2020, 63, 11286–11301. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Yoon, S.-C.; Aradhya, A.G.; Hofer, J.; Fink, M.A.; Enley, E.S.; Fisher, J.E.; Herb, M.C.; Klingos, A.; Proulx, J.T. Macrocyclic Compounds from Ansamycin Antibiotic Class as Inhibitors of PD1–PDL1 Protein–Protein Interaction. Chem. Pharm. Bull. 2018, 66, 773–778. [Google Scholar] [CrossRef]
- Faller, B.; Wohnsland, F. Physicochemical parameters as tools in drug discovery and lead optimization. In Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical, and Computational Strategies; Verlag Helvetica Chimica Acta, Postfach: Zürich, Switzerland, 2001; pp. 255–273. [Google Scholar]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRX 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Wanat, K. Biological barriers, and the influence of protein binding on the passage of drugs across them. Mol. Biol. Rep. 2020, 47, 3221–3231. [Google Scholar] [CrossRef]
- Pardridge, W.M. Recent advances in blood-brain barrier transport. Annu. Rev. Pharmacol. Toxicol. 1988, 28, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Fierer, G. Transport of tryptophan into brain from the circulating, albumin-bound pool in rats and in rabbits. J. Neurochem. 1990, 54, 971–976. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizojiri, K. Drug-protein binding and blood-brain barrier permeability. J. Pharmacol. Exp. Ther. 1999, 288, 912–918. [Google Scholar]
- Pardridge, W.M.; Sakiyama, R.; Fierer, G. Transport of propranolol and lidocaine through the rat blood-brain barrier. Primary role of globulin-bound drug. J. Clin. Investig. 1983, 71, 900–908. [Google Scholar] [CrossRef]
- Videbæk, C.; Ott, P.; Paulson, O.B.; Knudsen, G.M. Blood—Brain Barrier Transport and Protein Binding of Flumazenil and Iomazenil in the Rat: Implications for Neuroreceptor Studies. J. Cereb. Blood Flow Metab. 1999, 19, 948–955. [Google Scholar] [CrossRef]
- de Lange, E.C.M.; Danhof, M. Considerations in the Use of Cerebrospinal Fluid Pharmacokinetics to Predict Brain Target Concentrations in the Clinical Setting. Clin. Pharmacokinet. 2002, 41, 691–703. [Google Scholar] [CrossRef]
- Pardridge, W.M. Log (BB), PS products and in silico models of drug brain penetration. Drug Discov. Today 2004, 9, 392–393. [Google Scholar] [CrossRef]
- Misra, A.; Ganesh, S.; Shahiwala, A.; Shah, S.P. Drug delivery to the central nervous system: A review. J. Pharm. Pharm. Sci. 2003, 6, 252–273. [Google Scholar]
- Pardridge, W.M.; Triguero, D.; Yang, J.; Cancilla, P.A. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. J. Pharmacol. Exp. Ther. 1990, 253, 884–891. [Google Scholar]
- Hansch, C.; Steward, A.R.; Anderson, S.M.; Bentley, D.L. Parabolic dependence of drug action upon lipophilic character as revealed by a study of hypnotics. J. Med. Chem. 1968, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A. Substituent Constants for Correlation Analysis in Chemistry and Biology; Wiley: Hoboken, NJ, USA, 1979. [Google Scholar]
- van de Waterbeemd, H.; Camenisch, G.; Folkers, G.; Chretien, J.R.; Raevsky, O.A. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drug Target. 1998, 6, 151–165. [Google Scholar] [CrossRef]
- Fichert, T.; Yazdanian, M.; Proudfoot, J.R. A structure–permeability study of small drug-like molecules. Bioorganic Med. Chem. Lett. 2003, 13, 719–722. [Google Scholar] [CrossRef]
- Levin, V.A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980, 23, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Davis, A.M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 2004, 47, 6338–6348. [Google Scholar] [CrossRef] [PubMed]
- Österberg, T.; Norinder, U. Prediction of polar surface area and drug transport processes using simple parameters and PLS statistics. J. Chem. Inf. Comput. Sci. 2000, 40, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Kelder, J.; Grootenhuis, P.D.; Bayada, D.M.; Delbressine, L.P.; Ploemen, J.-P. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res. 1999, 16, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E. In silico prediction of blood–brain barrier permeation. Drug Discov. Today 2003, 8, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.P.; Davis, A.M.; Manners, C.N. Partitioning of ionizing molecules between aqueous buffers and phospholipid vesicles. J. Pharm. Sci. 1995, 84, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Gottschlich, R.; Seelig, A. Blood-brain barrier permeation: Molecular parameters governing passive diffusion. J. Membr. Biol. 1998, 165, 201–211. [Google Scholar] [CrossRef]
- Lin, J.H.; Rodrigues, A.D. In vitro model for early studies of drug metabolism. In Pharmacokinetics Optimization in Drug Research: Biological, Physicochemical and Computational Strategies; Testa, B., Van de Waterbeemed, H.T., Folker, G., Guy, R., Eds.; Verlag Helvetica Chimica Acta, Postfach: Zürich, Switzerland, 2001; pp. 217–243. [Google Scholar]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE 2012, 7, e29647. [Google Scholar] [CrossRef]
- Ding, S.-M.; Zhang, Z.-H.; Song, J.; Cheng, X.-D.; Jiang, J.; Jia, X.-B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327. [Google Scholar] [CrossRef]
- Wan, L.; Guo, C.; Yu, Q.; Li, Y.; Wang, X.; Wang, X.; Chen, C. Quantitative determination of apigenin and its metabolism in rat plasma after intravenous bolus administration by HPLC coupled with tandem mass spectrometry. J. Chromatogr. B 2007, 855, 286–289. [Google Scholar] [CrossRef]
- Kim, M.T.; Sedykh, A.; Chakravarti, S.K.; Saiakhov, R.D.; Zhu, H. Critical evaluation of human oral bioavailability for pharmaceutical drugs by using various cheminformatics approaches. Pharm. Res. 2014, 31, 1002–1014. [Google Scholar] [CrossRef]
- Gradolatto, A.; Basly, J.-P.; Berges, R.; Teyssier, C.; Chagnon, M.-C.; Siess, M.-H.; Canivenc-Lavier, M.-C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005, 33, 49–54. [Google Scholar] [CrossRef]
- Chen, Z.; Tu, M.; Sun, S.; Kong, S.; Wang, Y.; Ye, J.; Li, L.; Zeng, S.; Jiang, H. The exposure of luteolin is much lower than that of apigenin in oral administration of Flos Chrysanthemi extract to rats. Drug Metab. Pharmacokinet. 2012, 27, 162–168. [Google Scholar] [CrossRef]
- Zhang, X.-f.; Han, R.-m.; Sun, X.-r.; Li, G.-y.; Yang, Q.-f.; Li, Q.; Gai, W.; Zhang, M.; Chen, L.; Yang, G. The effect of the skeleton structure of flavanone and flavonoid on interaction with transferrin. Bioorganic Med. Chem. Lett. 2013, 23, 6677–6681. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood–brain barrier cell and Caco-2 cell models. Toxicol. Vitr. 2014, 28, 388–396. [Google Scholar] [CrossRef] [PubMed]
- DeRango-Adem, E.F.; Blay, J. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Zabela, V.; Sampath, C.; Oufir, M.; Moradi-Afrapoli, F.; Butterweck, V.; Hamburger, M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia 2016, 115, 189–197. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhu, L.; Zhao, M.; Shi, J.; Li, Y.; Yu, J.; Jiang, H.; Wu, J.; Tong, Y.; Liu, Y.; et al. In Vivo Exposure of Kaempferol Is Driven by Phase II Metabolic Enzymes and Efflux Transporters. AAPS J. 2016, 18, 1289–1299. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Choi, J.-S. Enhanced bioavailability of etoposide after oral or intravenous administration of etoposide with kaempferol in rats. Arch. Pharmacal Res. 2009, 32, 133–138. [Google Scholar] [CrossRef]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Yang, L.-L.; Xiao, N.; Li, X.-W.; Fan, Y.; Alolga, R.N.; Sun, X.-Y.; Wang, S.-L.; Li, P.; Qi, L.-W. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci. Rep. 2016, 6, 35460. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Yáñez, J.A.; Remsberg, C.M.; Miranda, N.D.; Vega-Villa, K.R.; Andrews, P.K.; Davies, N.M. Pharmacokinetics of selected chiral flavonoids: Hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm. Drug Dispos. 2008, 29, 63–82. [Google Scholar] [CrossRef]
- Touil, Y.S.; Auzeil, N.; Boulinguez, F.; Saighi, H.; Regazzetti, A.; Scherman, D.; Chabot, G.G. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochem. Pharmacol. 2011, 82, 1731–1739. [Google Scholar] [CrossRef]
- Huang, M.-C.; Hsueh, T.Y.; Cheng, Y.-Y.; Lin, L.-C.; Tsai, T.-H. Pharmacokinetics and biliary excretion of fisetin in rats. J. Agric. Food Chem. 2018, 66, 6300–6307. [Google Scholar] [CrossRef]
- Su, D.; Huang, J.; Song, Y.; Feng, Y. Comparative pharmacokinetics and tissue distribution study of mono-, and di-caffeoylquinic acids isomers of Ainsliaea fragrans Champ by a fast UHPLC–MS/MS method. Fitoterapia 2014, 99, 139–152. [Google Scholar] [CrossRef]
- Lei, F.; Xing, D.-M.; Xiang, L.; Zhao, Y.-N.; Wang, W.; Zhang, L.-J.; Du, L.-J. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chromatogr. B 2003, 796, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Teel, R.W.; Martin, R.M. Disposition of the plant phenol ellagic acid in the mouse following oral administration by gavage. Xenobiotica 1988, 18, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.C.; Huang, M.-T.; Chang, R.L.; Sayer, J.M.; Jerina, D.M.; Conney, A.H. Disposition of the naturally occurring antimutagenic plant phenol, ellagic acid, and its synthetic derivatives, 3-O-decylellagic acid and 3,3′-di-O-methylellagic acid in mice. Carcinogenesis 1986, 7, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ma, X.; Du, Y.; Feng, J. Understanding Patterns of Brain Metastasis in Triple-Negative Breast Cancer and Exploring Potential Therapeutic Targets. OncoTargets Ther. 2021, 14, 589–607. [Google Scholar] [CrossRef]
- Nader-Marta, G.; Martins-Branco, D.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Danielli, L.; Lambertini, M.; Kotecki, N.; Awada, A.; de Azambuja, E. Efficacy of tyrosine kinase inhibitors for the treatment of patients with HER2-positive breast cancer with brain metastases: A systematic review and meta-analysis. ESMO Open 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Ravisankar, S.; Agah, S.; Kim, H.; Talcott, S.; Wu, C.; Awika, J. Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chem. 2019, 279, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Houghton, P.; Xiang, D.; Duan, W.; Shigdar, S. Truncation and mutation of a transferrin receptor aptamer enhances binding affinity. Nucleic Acid Ther. 2016, 26, 348–354. [Google Scholar] [CrossRef]
- Macdonald, J.; Henri, J.; Goodman, L.; Xiang, D.; Duan, W.; Shigdar, S. Development of a bifunctional aptamer targeting the transferrin receptor and epithelial cell adhesion molecule (EpCAM) for the treatment of brain cancer metastases. ACS Chem. Neurosci. 2017, 8, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Denoyer, D.; Henri, J.; Jamieson, A.; Burvenich, I.J.; Pouliot, N.; Shigdar, S. Bifunctional aptamer–doxorubicin conjugate crosses the blood–brain barrier and selectively delivers its payload to EpCAM-positive tumor cells. Nucleic Acid Ther. 2020, 30, 117–128. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, Y.H.; Lennox, K.A.; Behlke, M.A.; Davidson, B.L. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther. Nucleic Acids 2013, 2, e67. [Google Scholar] [CrossRef]
- Camorani, S.; Crescenzi, E.; Fedele, M.; Cerchia, L. Oligonucleotide aptamers against tyrosine kinase receptors: Prospect for anticancer applications. Biochim. Biophys. Acta 2018, 1869, 263–277. [Google Scholar] [CrossRef]
- Perisano, C.; Vitiello, R.; Sgambato, A.; Greco, T.; Cianni, L.; Ragonesi, G.; Malara, T.; Maccauro, G.; Martini, M. Evaluation of PD1 and PD-L1 expression in high-grade sarcomas of the limbs in the adults: Possible implications of immunotherapy. J. Biol. Regul. Homeost. Agents 2020, 34, 289–294. [Google Scholar]
| Candidate | Inhibiting PD-1/PD-L1 | Interaction with PD-L1 | EC50—T Cell Activity | In Vivo Studies | |||
|---|---|---|---|---|---|---|---|
| Potency | IC50 | KD | Binding Score | Dose | Reduced TG 1 | ||
| SPE 2 | 42% (50 mg/mL) | 27 µg/mL | 100 mg/mL 300 mg/mL | 45% 78% | |||
| SPE-EA 3 | 63% (50 mg/mL) | 1 µg/mL | |||||
| API 4 | |||||||
| COS 5 | 85 µM | −6.2 kcal/mol | |||||
| KMF 6 | 8 µM | −5.4 kcal/mol | 16 µM | ||||
| KOR 7 | 156 µM | −5.6 kcal/mol | |||||
| QUE 8 | 80% (5 µM) | 0.2 µM | 4.53 µM | 60 mg/mL | |||
| TVE 9 | 26 µM | ||||||
| ERI 10 | 0.04 µM | ||||||
| FIS 11 | 0.04 µM | ||||||
| CQA 12 | 0.17 µM | ||||||
| GC 13 | 65% (100 µM) | ||||||
| RCE 14 | 84 µg/mL | 56 µg/mL | 50 mg/mL 100 mg/mL | 67% 74% | |||
| EA 15 | 23 µg/mL | ||||||
| GS-d 16 | 95% (20 µM) | 1.42 µM | |||||
| Rifabutin | 25 µM | ||||||
| Candidate | Formula | MW 1 | O+N 2 | LogP | PSA 3 | pKa a. 4 | pKa b. 5 | Charge 6 | H acc. 7 | H don. 8 | R bond 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| API | C15H10O5 | 270.2369 | 5 | 2.71 | 86.99 | 6.57 | −5.4 | −1 | 5 | 3 | 1 |
| COS | C21H20O10 | 432.3775 | 10 | 0.44 | 166.14 | 7.3 | −3 | 0 | 10 | 6 | 4 |
| KMF | C15H10O6 | 286.2363 | 6 | 2.56 | 107.22 | 6.38 | −3.9 | −1 | 6 | 4 | 1 |
| KOR | C21H20O10 | 432.3775 | 10 | 1.24 | 166.14 | 7.08 | −3.6 | 0 | 10 | 6 | 3 |
| QUE | C15H10O7 | 302.2357 | 7 | 1.48 | 127.45 | 6.38 | −4 | −1 | 7 | 5 | 1 |
| ERI | C15H12O6 | 288.255 | 6 | 2.53 | 107.22 | 7.85 | −5 | 0 | 6 | 4 | 1 |
| FIS | C15H10O6 | 286.2363 | 6 | 1.81 | 107.22 | 6.32 | −3.9 | −1 | 6 | 4 | 1 |
| 1-CQA | C16H18O9 | 354.3087 | 9 | −0.4 | 211 | 3.22 | −3.2 | −1 | 8 | 6 | 5 |
| 3-CQA | C16H18O9 | 354.3087 | 9 | −0.27 | 164.75 | 3.33 | −3.2 | −1 | 8 | 6 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhjavani, M.; Shigdar, S. Natural Blockers of PD-1/PD-L1 Interaction for the Immunotherapy of Triple-Negative Breast Cancer-Brain Metastasis. Cancers 2022, 14, 6258. https://doi.org/10.3390/cancers14246258
Nakhjavani M, Shigdar S. Natural Blockers of PD-1/PD-L1 Interaction for the Immunotherapy of Triple-Negative Breast Cancer-Brain Metastasis. Cancers. 2022; 14(24):6258. https://doi.org/10.3390/cancers14246258
Chicago/Turabian StyleNakhjavani, Maryam, and Sarah Shigdar. 2022. "Natural Blockers of PD-1/PD-L1 Interaction for the Immunotherapy of Triple-Negative Breast Cancer-Brain Metastasis" Cancers 14, no. 24: 6258. https://doi.org/10.3390/cancers14246258
APA StyleNakhjavani, M., & Shigdar, S. (2022). Natural Blockers of PD-1/PD-L1 Interaction for the Immunotherapy of Triple-Negative Breast Cancer-Brain Metastasis. Cancers, 14(24), 6258. https://doi.org/10.3390/cancers14246258







