Incidence Trends and Main Features of Gastro-Intestinal Stromal Tumours in a Mediterranean Region: A Population-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, M.T.; Clark, H.B. Gastric stromal tumors. Reappraisal of histogenesis. Am. J. Surg. Pathol. 1983, 7, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef]
- Espinosa, I.; Lee, C.H.; Kim, M.K.; Rouse, B.T.; Subramanian, S.; Montgomery, K.; Varma, S.; Corless, C.L.; Heinrich, M.C.; Smith, K.S.; et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am. J. Surg. Pathol. 2008, 32, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; McKinley, A.; Ou, W.B.; Fletcher, J.A.; et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef]
- George, S.; Wang, Q.; Heinrich, M.C.; Corless, C.L.; Zhu, M.; Butrynski, J.E.; Morgan, J.A.; Wagner, A.J.; Choy, E.; Tap, W.D.; et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: A multicenter phase II trial. J. Clin. Oncol. 2012, 30, 2401–2407. [Google Scholar] [CrossRef]
- Available online: http://www.iacr.com.fr/index.php?option=com_content&view=article&id=149:icd-o-3-2&catid=80&Itemid=545 (accessed on 12 April 2023).
- RARECARE Project. Available online: http://rarecarenet.istitutotumori.mi.it/analysis.php (accessed on 12 April 2023).
- Gatta, G.; van der Zwan, J.M.; Casali, P.G.; Siesling, S.; Dei Tos, A.P.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef]
- Padrón Municipal de Habitantes. Available online: https://econet.carm.es/inicio/-/crem/sicrem/PU_padron (accessed on 12 April 2023).
- Curado, M.P.; Edwards, B.; Shin, H.R.; Storm, H.; Ferlay, J.; Heanue, M.; Boyle, P. (Eds.) Cancer Incidence in Five Continents, Volume IX; IARC Press, International Agency for Research on Cancer: Lyon, France, 2008. [Google Scholar]
- Siesling, S.; Louwman, W.J.; Kwast, A.; Van Den Hurk, C.; O’Callaghan, M.; Rosso, S.; Zanetti, R.; Storm, H.; Comber, H.; Steliarova-Foucher, E.; et al. Uses of cancer registries for public health and clinical research in Europe: Results of the European Network of Cancer Registries survey among 161 population-based cancer registries during 2010–2012. Eur. J. Cancer 2015, 51, 1039–1049. [Google Scholar] [CrossRef]
- Galceran, J.; Ameijide, A.; Carulla, M.; Mateos, A.; Quirós, J.R.; Rojas, D.; Alemán, A.; Torrella, A.; Chico, M.; Vicente, M.; et al. Cancer incidence in Spain, 2015. Clin. Transl. Oncol. 2017, 19, 799–825. [Google Scholar] [CrossRef]
- Chirlaque, M.D.; Salmerón, D.; Galceran, J.; Ameijide, A.; Mateos, A.; Torrella, A.; Jiménez, R.; Larrañaga, N.; Marcos-Gragera, R.; Ardanaz, E.; et al. Cancer survival in adult patients in Spain. Results from nine population-based cancer registries. Clin. Transl. Oncol. 2018, 20, 201–211. [Google Scholar] [CrossRef]
- Casali, P.G.; Trama, A. Rationale of the rare cancer list: A consensus paper from the Joint Action on Rare Cancers (JARC) of the European Union (EU). ESMO Open 2020, 5, e000666. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef]
- Tiwari, R.C.; Clegg, L.X.; Zou, Z. Efficient interval estimation for age-adjusted cancer rates. Stat. Methods Med. Res. 2006, 15, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Perme, M.P.; Stare, J.; Estève, J. On Estimation in Relative Survival. Biometrics 2012, 68, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pohar Perme, M.; Estève, J.; Rachet, B. Analysing population-based cancer survival—settling the controversies. BMC Cancer 2016, 16, 933. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Lambert, P. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model; Stata Press: College Station, TX, USA, 2011. [Google Scholar]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.M.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv68–iv78. [Google Scholar] [CrossRef]
- Regulation General Data Protection Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed on 12 April 2023).
- Rubió-Casadevall, J.; Borràs, J.L.; Carmona, C.; Ameijide, A.; Osca, G.; Vilardell, L.; Izquierdo, A.; Galceran, J.; Marcos-Gragera, R. Temporal trends of incidence and survival of sarcoma of digestive tract including Gastrointestinal Stromal Tumours (GIST) in two areas of the north-east of Spain in the period 1981–2005: A population-based study. Clin. Transl. Oncol. 2014, 16, 660–667. [Google Scholar] [CrossRef]
- Ressing, M.; Wardelmann, E.; Hohenberger, P.; Jakob, J.; Kasper, B.; Emrich, K.; Eberle, A.; Blettner, M.; Zeissig, S.R. Strengthening health data on a rare and heterogeneous disease: Sarcoma incidence and histological subtypes in Germany. BMC Public Health 2018, 18, 235. [Google Scholar] [CrossRef]
- Ma, G.L.; Murphy, J.D.; Martinez, M.E.; Sicklick, J.K. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: Results of a population-based study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 298–302. [Google Scholar] [CrossRef]
- Patel, N.; Benipal, B. Incidence of Gastrointestinal Stromal Tumors in the United States from 2001–2015: A United States Cancer Statistics Analysis of 50 States. Cureus 2019, 11, e4120. [Google Scholar] [CrossRef]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mucciarini, C.; Rossi, G.; Bertolini, F.; Valli, R.; Cirilli, C.; Rashid, I.; Marcheselli, L.; Luppi, G.; Federico, M. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer 2007, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Coindre, J.-M.; Ducimetière, F.; Dei Tos, A.P.; Fadda, E.; Blay, J.-Y.; Buja, A.; Fedeli, U.; Cegolon, L.; Frasson, A.; et al. Incidence of soft tissue sarcoma and beyond. Cancer 2012, 118, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Zielonk, N.; Van Eycken, E.; Sundseth, H.; Hedelin, G.; et al. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECARE net-a population-based study. Lancet. Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- Nishida, T.; Goto, O.; Raut, C.P.; Yahagi, N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016, 122, 3110–3118. [Google Scholar] [CrossRef]
- Fernández, J.Á.; Gómez-Ruiz, Á.J.; Olivares, V.; Ferri, B.; Frutos, M.D.; Soria, T.; Gil, P.J.; Torres, G.; Parrilla, P. Clinical and pathological features of “small” GIST (≤2 cm). What is their prognostic value? Eur. J. Surg. Oncol. 2018, 44, 580–586. [Google Scholar] [CrossRef]
- Scherübl, H. Management of early asymptomatic gastrointestinal stromal tumors of the stomach. World J. Gastrointest. Endosc. 2014, 6, 266. [Google Scholar] [CrossRef]
- Wang, M.; Xue, A.; Yuan, W.; Gao, X.; Fu, M.; Fang, Y.; Wang, L.; Shu, P.; Li, H.; Hou, Y.; et al. Clinicopathological Features and Prognosis of Small Gastric Gastrointestinal Stromal Tumors (GISTs). J. Gastrointest. Surg. 2019, 23, 2136–2143. [Google Scholar] [CrossRef]
- Søreide, K. Cancer biology of small gastrointestinal stromal tumors (<2 cm): What is the risk of malignancy? Eur. J. Surg. Oncol. 2017, 43, 1344–1349. [Google Scholar] [CrossRef]
- Kramer, K.; Knippschild, U.; Mayer, B.; Bögelspacher, K.; Spatz, H.; Henne-Bruns, D.; Agaimy, A.; Schwab, M.; Schmieder, M. Impact of age and gender on tumor related prognosis in gastrointestinal stromal tumors (GIST). BMC Cancer 2015, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Hompland, I.; Bruland, Ø.S.; Hølmebakk, T.; Poulsen, J.P.; Stoldt, S.; Hall, K.S.; Boye, K. Prediction of long-term survival in patients with metastatic gastrointestinal stromal tumor: Analysis of a large, single-institution cohort. Acta Oncol. 2017, 56, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Vehtari, A.; Riihimäki, J.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Cirilli, C.; Braconi, C.; et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012, 13, 265–274. [Google Scholar] [CrossRef]
- Güller, U.; Tarantino, I.; Cerny, T.; Schmied, B.M.; Warschkow, R. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer 2015, 15, 557. [Google Scholar] [CrossRef]
- van der Graaf, W.T.A.; Tielen, R.; Bonenkamp, J.J.; Lemmens, V.; Verhoeven, R.H.A.; de Wilt, J.H.W. Nationwide trends in the incidence and outcome of patients with gastrointestinal stromal tumour in the imatinib era. Br. J. Surg. 2018, 105, 1020–1027. [Google Scholar] [CrossRef]
- Goldstein, D.; Lee, C.; Tracey, E.; Cook-Yarborough, C.; Lord, S. Validating innovation: A population-based study of gastrointestinal stromal tumors (GIST) to estimate the survival benefit of imatinib. J. Clin. Oncol. 2011, 29, 10060. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Liu, S.; Guan, W. Comparative clinical features and short-term outcomes of gastric and small intestinal gastrointestinal stromal tumours: A retrospective study. Sci. Rep. 2019, 9, 10033. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, M.; Henne-Bruns, D.; Mayer, B.; Knippschild, U.; Rolke, C.; Schwab, M.; Kramer, K. Comparison of Different Risk Classification Systems in 558 Patients with Gastrointestinal Stromal Tumors after R0-Resection. Front. Pharmacol. 2016, 7, 504. [Google Scholar] [CrossRef]
- Rutkowski, P.; Skoczylas, J.; Wisniewski, P. Is the surgical margin in gastrointestinal stromal tumors different? Visc. Med. 2018, 34, 347–352. [Google Scholar] [CrossRef]
- Nishida, T.; Hølmebakk, T.; Raut, C.P.; Rutkowski, P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann. Surg. Oncol. 2019, 26, 1669–1675. [Google Scholar] [CrossRef]
- Vallilas, C.; Sarantis, P.; Kyriazoglou, A.; Koustas, E.; Theocharis, S.; Papavassiliou, A.G.; Karamouzis, M.V. Gastrointestinal stromal tumors (GISTS): Novel therapeutic strategies with immunotherapy and small molecules. Int. J. Mol. Sci. 2021, 22, 493. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Gray, R.; Chen, A.; Li, S.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Mitchell, E.P.; John Iafrate, A.; Sklar, J.; et al. The molecular analysis for therapy choice (NCI-MATCH) trial: Lessons for genomic trial design. J. Natl. Cancer Inst. 2020, 112, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
| Cases | ||||
|---|---|---|---|---|
| Total | Men | Women | p-Value | |
| N (%) | N (%) | N (%) | ||
| Total 2001–2015 | 171 (100%) | 93 (54.4%) | 78 (45.6%) | 0.032 |
| Period | ||||
| 2001–2005 | 33 (19.3%) | 19 (20.4%) | 14 (18.0%) | 0.792 |
| 2006–2010 | 40 (23.4%) | 20 (21.5%) | 20 (25.6%) | |
| 2011–2015 | 98 (57.3%) | 54 (58.1%) | 44 (56.4%) | |
| Age groups, years | ||||
| 15–39 | 8 (4.7%) | 5 (5.4%) | 3 (3.9%) | 0.176 |
| 40–64 | 69 (40.3%) | 43 (46.2%) | 26 (33.3%) | |
| 65 and older | 94 (55.0%) | 45 (48.4%) | 49 (62.8%) | |
| Age, years | ||||
| Minimum age | 24.5 | 24.5 | 32.0 | |
| Maximum age | 89.2 | 88.5 | 89.2 | |
| Mean age | 65.0 | 63.4 | 66.9 | 0.100 |
| 95% confidence interval for mean age | 62.9–67.1 | 60.4–66.5 | 64.1–69.6 | |
| Median age | 67.5 | 64.6 | 69.0 | 0.194 |
| Primary site of tumour (CIE-O) | ||||
| Oesophagus | 3 (1.8%) | 2 (2.1%) | 1 (1.3%) | 0.088 |
| Stomach | 90 (52.6%) | 45 (48.4%) | 45 (57.7%) | |
| Small bowel | 58 (33.9%) | 34 (36.6%) | 24 (30.8%) | |
| Colon and rectum | 6 (3.5%) | 6 (6.4%) | 0 | |
| Ill-defined intra-abdominal site | 13 (7.6%) | 5 (5.4%) | 8 (10.2%) | |
| Peritoneum | 1 (0.6%) | 1 (1.1%) | 0 | |
| Death risk level | ||||
| Very high | 31 (18.1%) | 19 (20.4%) | 12 (15.4%) | 0.707 |
| High | 77 (45.0%) | 41 (44.1%) | 36 (46.1%) | |
| Intermediate | 22 (12.9%) | 10 (10.8%) | 12 (15.4%) | |
| Low | 26 (15.2%) | 14 (15.0%) | 12 (15.4%) | |
| Very low | 15 (8.8%) | 9 (9.7%) | 6 (7.7%) | |
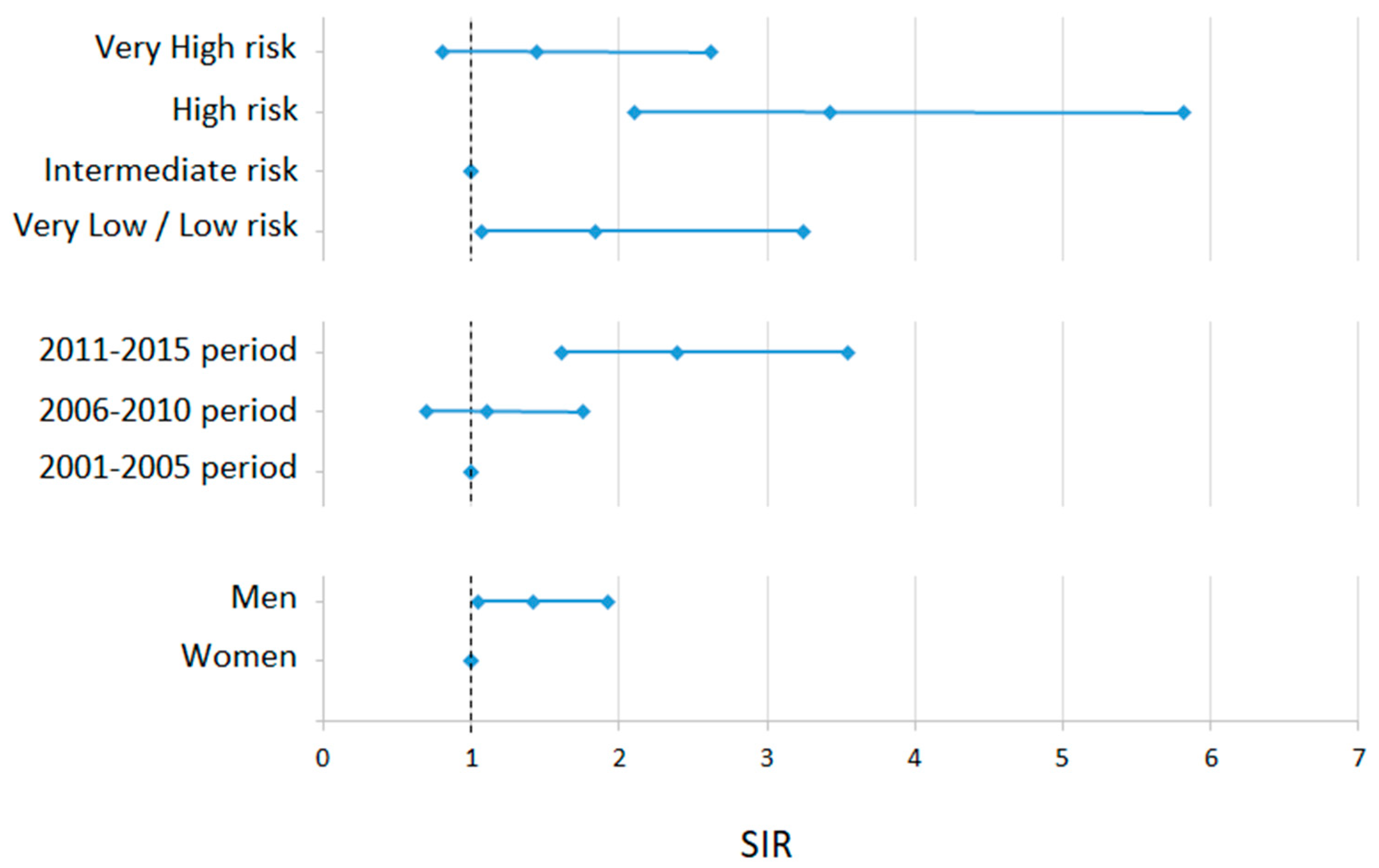
| Crude Rate | Adjusted Rate to EU76 (95% CI) | Adjusted Rate to EU13 (95% CI) | Adjusted Rate to World Population (95% CI) | |
|---|---|---|---|---|
| Sex: | ||||
| Total | 0.82 | 0.75 (0.64–0.88) | 1.05 (0.90–1.22) | 0.53 (0.45–0.62) |
| Men | 0.88 | 0.87 (0.69–1.07) | 1.22 (0.97–1.50) | 0.60 (0.48–0.75) |
| Women | 0.76 | 0.65 (0.51–0.82) | 0.92 (0.73–1.15) | 0.46 (0.36–0.59) |
| Period: | ||||
| 2001–2005 | 0.52 | 0.54 (0.37–0.77) | 0.69 (0.47–0.97) | 0.40 (0.27–0.57) |
| 2006–2010 | 0.55 | 0.52 (0.37–0.72) | 0.72 (0.51–0.99) | 0.37 (0.26–0.53) |
| 2011–2015 | 1.33 | 1.15 (0.92–1.41) | 1.66 (1.35–2.03) | 0.79 (0.63–0.98) |
| Risk level: | ||||
| Very high | 0.15 | 0.14 (0.10–0.20) | 0.20 (0.13–0.28) | 0.10 (0.07–0.14) |
| High | 0.37 | 0.35 (0.27–0.43) | 0.47 (0.37–0.59) | 0.24 (0.19–0.31) |
| Intermediate | 0.10 | 0.09 (0.06–0.15) | 0.13 (0.08–0.20) | 0.07 (0.04–0.11) |
| Low/Very low | 0.19 | 0.16 (0.11–0.22) | 0.24 (0.17–0.38) | 0.11 (0.08–0.16) |
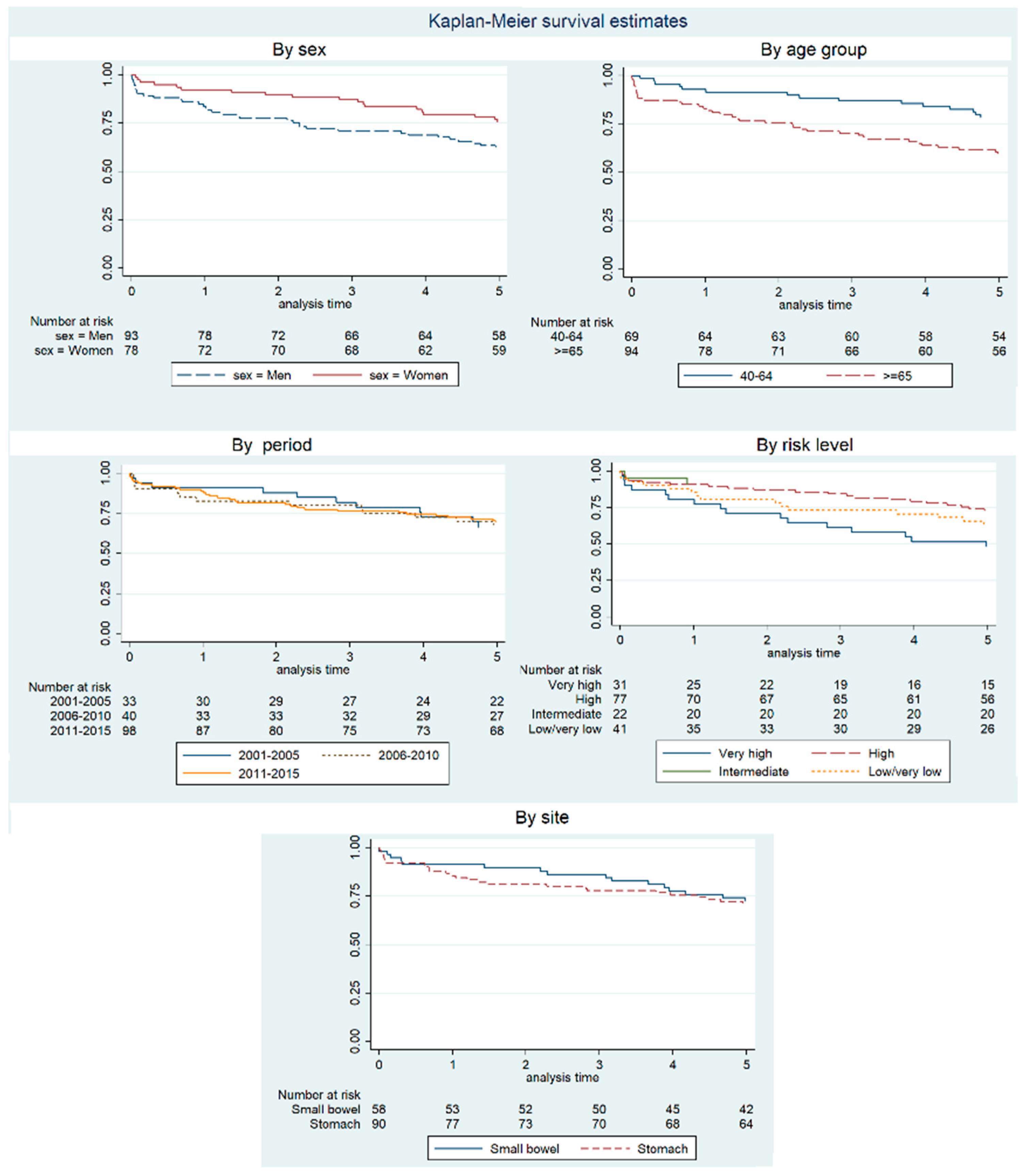
| Net Survival (%). 95% CI | Observed Survival at 5 Years (%). 95% CI | |||
|---|---|---|---|---|
| 1 Year | 3 Years | 5 Years | ||
| Sex | ||||
| Total | 89.45 (84.45–94.46) | 82.74 (76.16–89.32) | 76.97 (68.16–85.55) | 68.42 (60.88–74.81) |
| Men | 85.79 (78.19–93.39) | 75.68 (65.75–85.62) | 71.44 (60.10–82.79) | 62.37 (51.70–71.32) |
| Women | 93.34 (87.37–99.31) | 91.21 (83.10–99.32) | 83.70 (73.01–94.40) | 75.64 (64.51–83.71) |
| Period | ||||
| 2001–2005 | 91.26 (81.57–100.00) | 84.92 (71.12–98.72) | 72.31 (55.25–89.38) | 66.67 (47.94–79.96) |
| 2006–2010 | 84.06 (72.27–95.84) | 84.24 (71.46–97.02) | 79.32 (62.72–95.92) | 67.50 (50.70–79.66) |
| 2011–2015 | 90.74 (84.37–97.12) | 80.86 (71.71–90.02) | 77.71 (67.17–88.26) | 69.39 (59.23–77.49) |
| Age, years | ||||
| From 15 to 39 | * | * | 87.84 (66.31–100.00) | 87.50 (38.70–98.14) |
| From 40 to 64 | 93.03 (86.93–99.12) | 88.10 (80.11–96.09) | 80.08 (70.15–90.00) | 78.26 (66.56–86.28) |
| More than 64 | 85.85 (78.04–93.66) | 77.35 (66.95–87.76) | 73.76 (61.43–86.08) | 59.57 (48.95–68.69) |
| Risk level | ||||
| Very high | 81.72 (67.88–95.55) | 63.82 (45.92–81.71) | 52.66 (33.81–71.50) | 48.39 (30.18–64.41) |
| High | 92.03 (85.58–98.49) | 89.12 (80.40–97.83) | 81.37 (70.02–92.71) | 72.73 (61.31–81.28) |
| Intermediate | 92.64 (80.64–100.00) | * | * | 90.91 (68.30–97.65) |
| Low/very low | 87.42 (76.45–98.40) | 76.58 (62.02–91.13) | 71.83 (55.07–88.58) | 65.74 (55.97–73.85) |
| Variables | HR | 95% CI | p-Value | |
|---|---|---|---|---|
| Sex | Women | Ref. | ||
| Men | 1.71 | (0.92–3.20) | 0.091 | |
| Age group, years | 15–39 | Ref. | ||
| 40–64 | 3.07 | (0.38–24.68) | 0.292 | |
| 65 and more | 5.80 | (0.73–46.05) | 0.096 | |
| Period | 2001–2005 | Ref. | ||
| 2006–2010 | 0.82 | (0.37–1.86) | 0.643 | |
| 2011–2015 | 0.65 | (0.33–1.28) | 0.212 | |
| Risk level | Very low/low | 1.38 | (0.56–3.39) | 0.482 |
| Intermediate | 0.18 | (0.01–5.69) | 0.333 | |
| High | Ref. | |||
| Very high | 2.30 | (1.01–5.22) | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaamonde-Martín, R.J.; Ballesta-Ruiz, M.; Sánchez-Gil, A.; Fernández, J.Á.; Martínez-Barba, E.; Martínez-García, J.; Gatta, G.; Chirlaque-López, M.D. Incidence Trends and Main Features of Gastro-Intestinal Stromal Tumours in a Mediterranean Region: A Population-Based Study. Cancers 2023, 15, 2994. https://doi.org/10.3390/cancers15112994
Vaamonde-Martín RJ, Ballesta-Ruiz M, Sánchez-Gil A, Fernández JÁ, Martínez-Barba E, Martínez-García J, Gatta G, Chirlaque-López MD. Incidence Trends and Main Features of Gastro-Intestinal Stromal Tumours in a Mediterranean Region: A Population-Based Study. Cancers. 2023; 15(11):2994. https://doi.org/10.3390/cancers15112994
Chicago/Turabian StyleVaamonde-Martín, Ricardo J., Mónica Ballesta-Ruiz, Antonia Sánchez-Gil, Juan Ángel Fernández, Enrique Martínez-Barba, Jerónimo Martínez-García, Gemma Gatta, and María D. Chirlaque-López. 2023. "Incidence Trends and Main Features of Gastro-Intestinal Stromal Tumours in a Mediterranean Region: A Population-Based Study" Cancers 15, no. 11: 2994. https://doi.org/10.3390/cancers15112994
APA StyleVaamonde-Martín, R. J., Ballesta-Ruiz, M., Sánchez-Gil, A., Fernández, J. Á., Martínez-Barba, E., Martínez-García, J., Gatta, G., & Chirlaque-López, M. D. (2023). Incidence Trends and Main Features of Gastro-Intestinal Stromal Tumours in a Mediterranean Region: A Population-Based Study. Cancers, 15(11), 2994. https://doi.org/10.3390/cancers15112994








