Targeting IRS-1/2 in Uveal Melanoma Inhibits In Vitro Cell Growth, Survival and Migration, and In Vivo Tumor Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture and Treatments
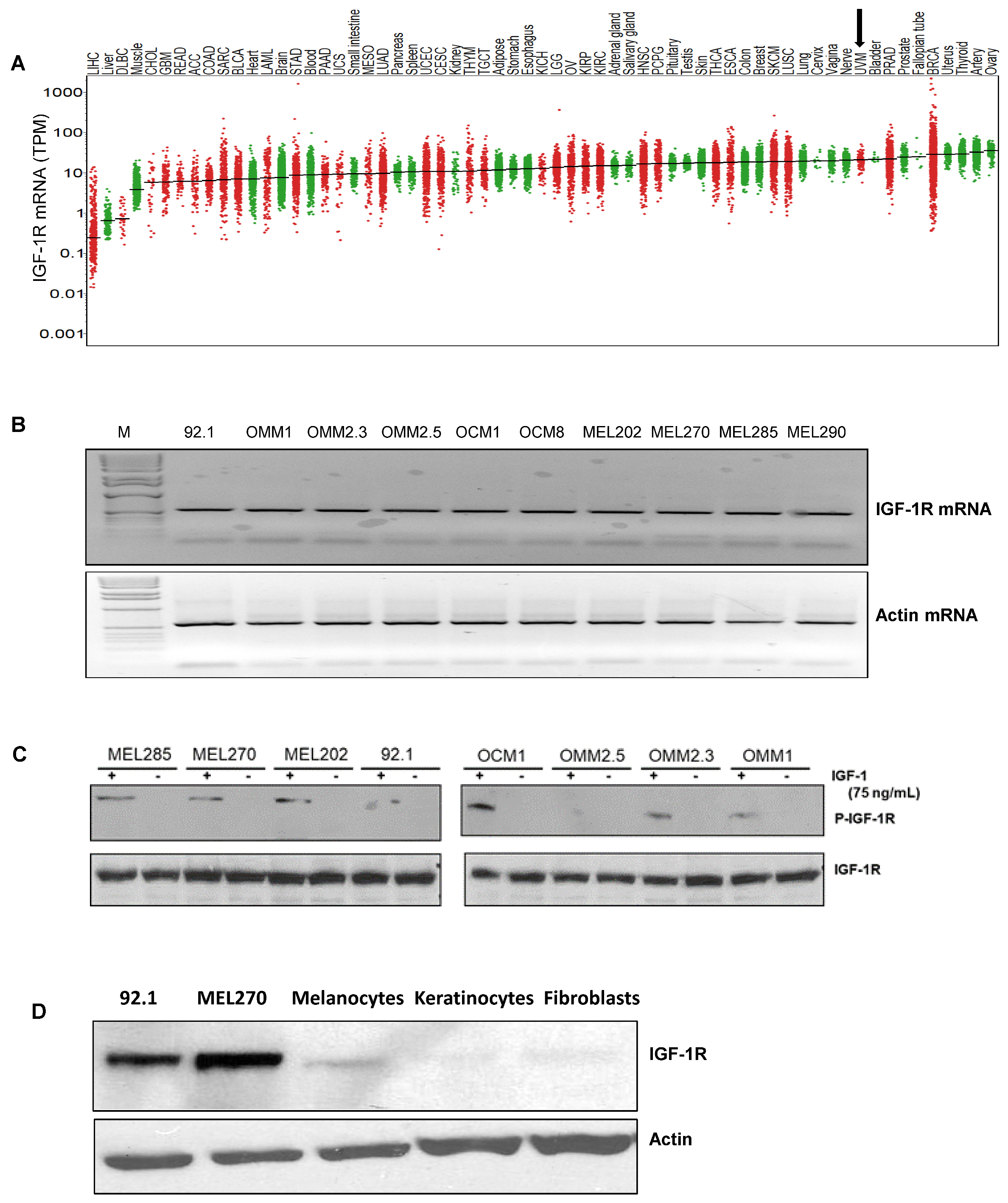
2.3. Cell Line Validation
2.4. Western Blotting
2.5. Immunohistochemical Staining for IRS-1 Expression
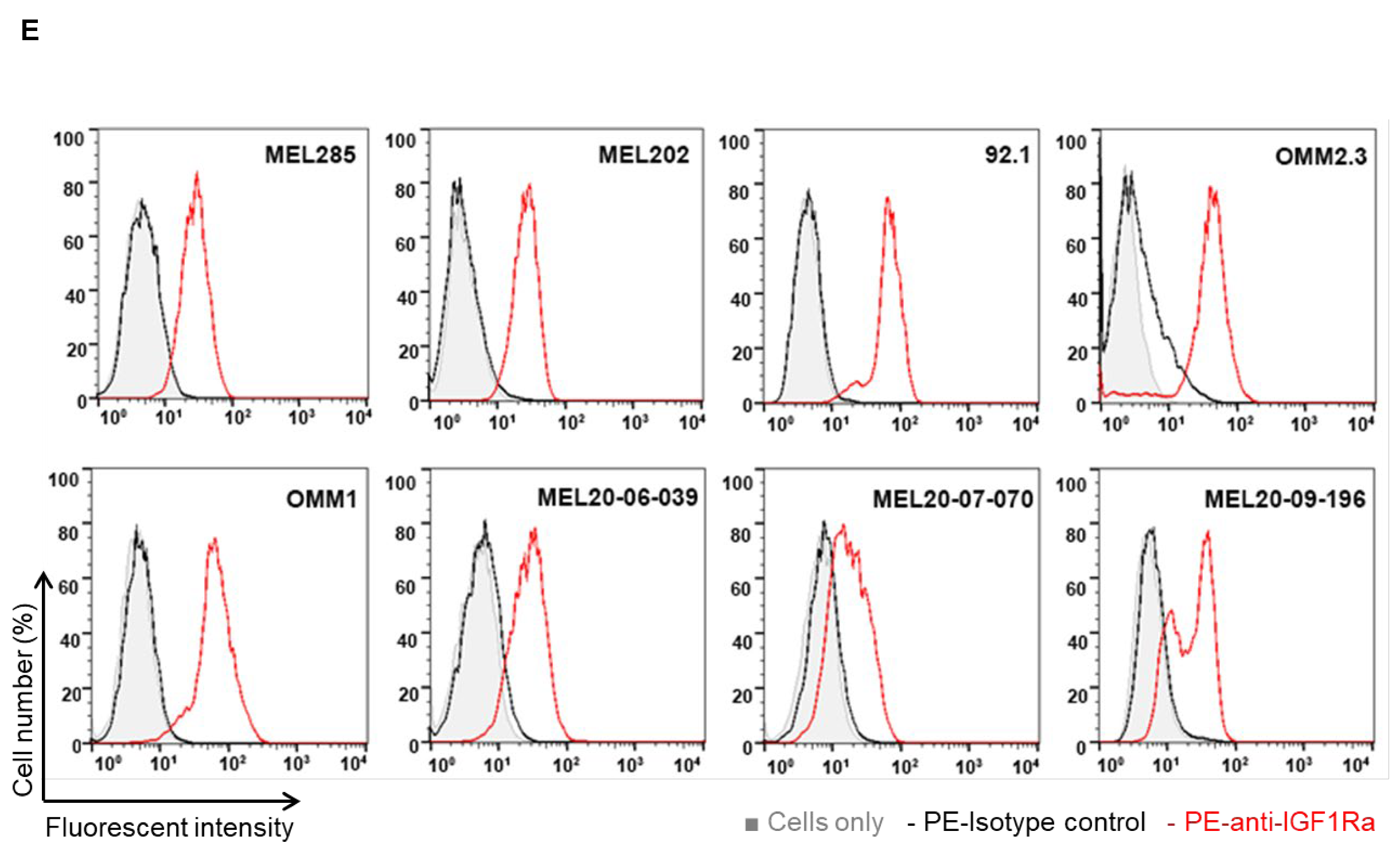
2.6. Flow Cytometry Analysis (FACS) for IGF-1R Cell Surface Expression
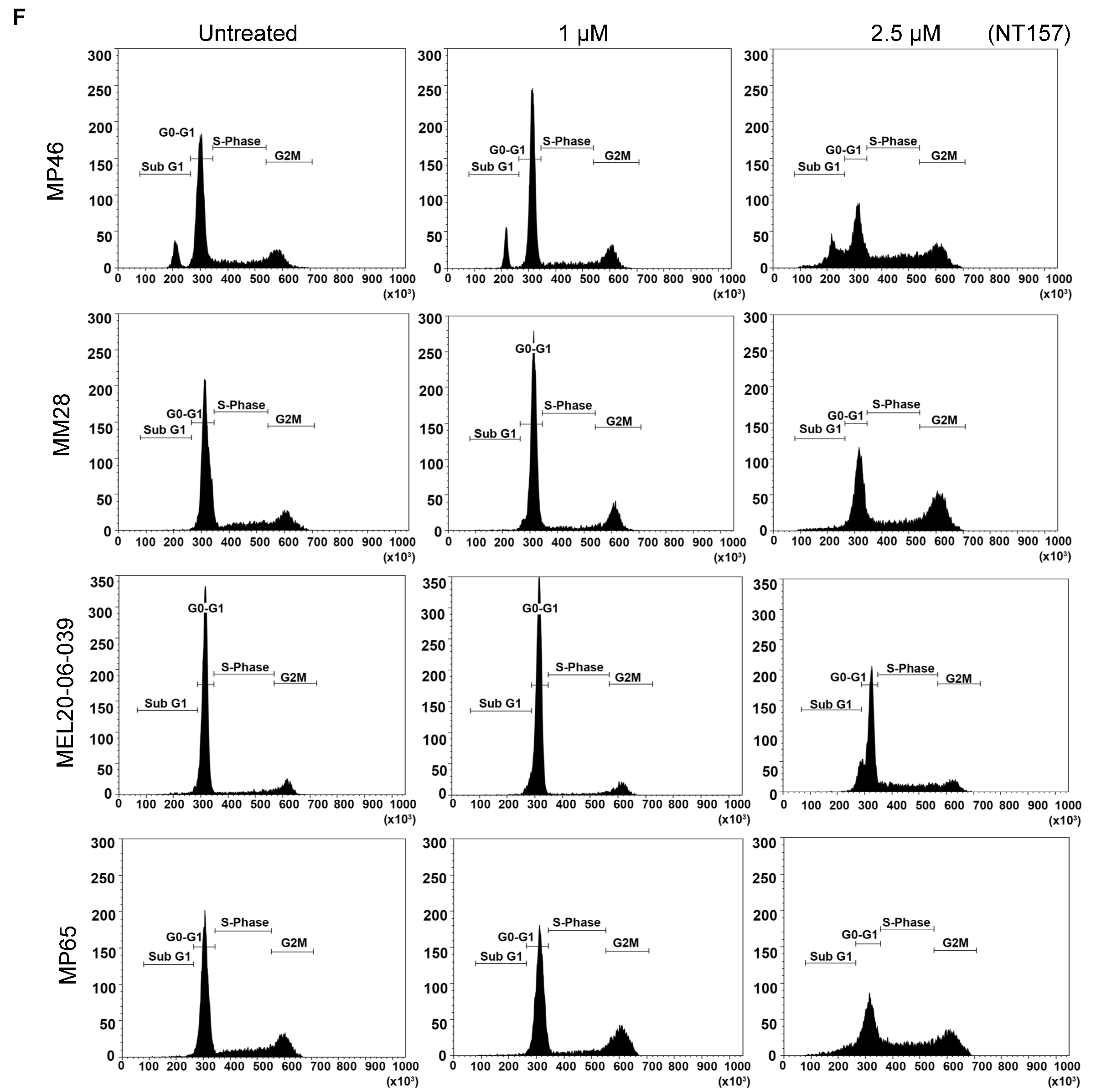
2.7. Cell Cycle Analysis Using Propidium Iodide Staining and FACS
2.8. Colony Formation Assay
2.9. Reverse Phase Protein Array (RPPA) Analysis
2.10. Chicken Egg Chorioallantoic Membrane (CAM) Tumor Xenograft Model
2.11. Cell Viability Assays
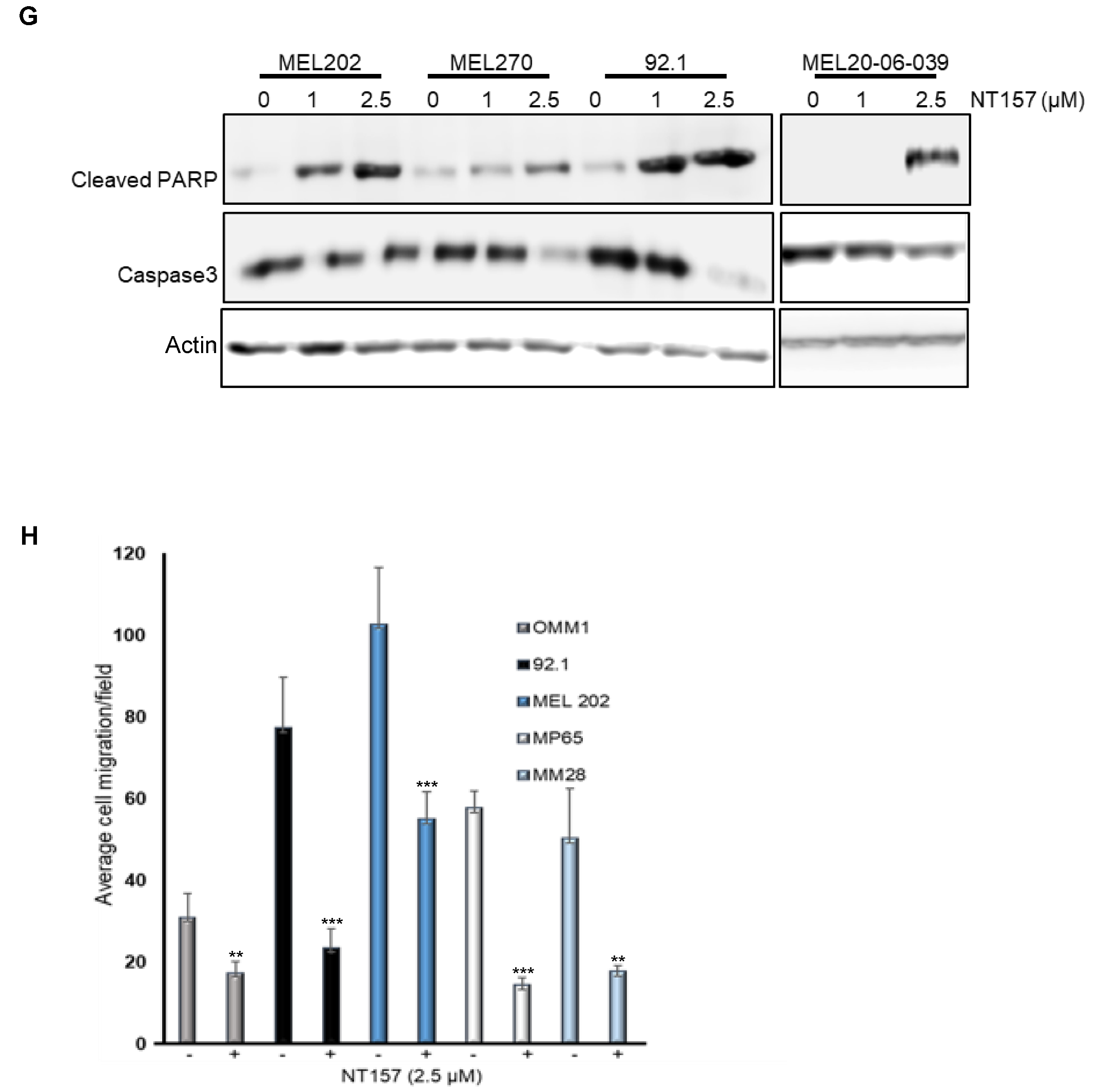
2.12. Cell Migration Assay
2.13. NT157 Treatment of SubQ UM Tumors in NSG Mice
3. Results
3.1. IGF-1R and IRS-1 Are Expressed in Uveal Melanoma
3.2. IRS-1 Inhibiton Reduces Survival, Induces Apoptosis, and Inhibits Migration of UM Cells
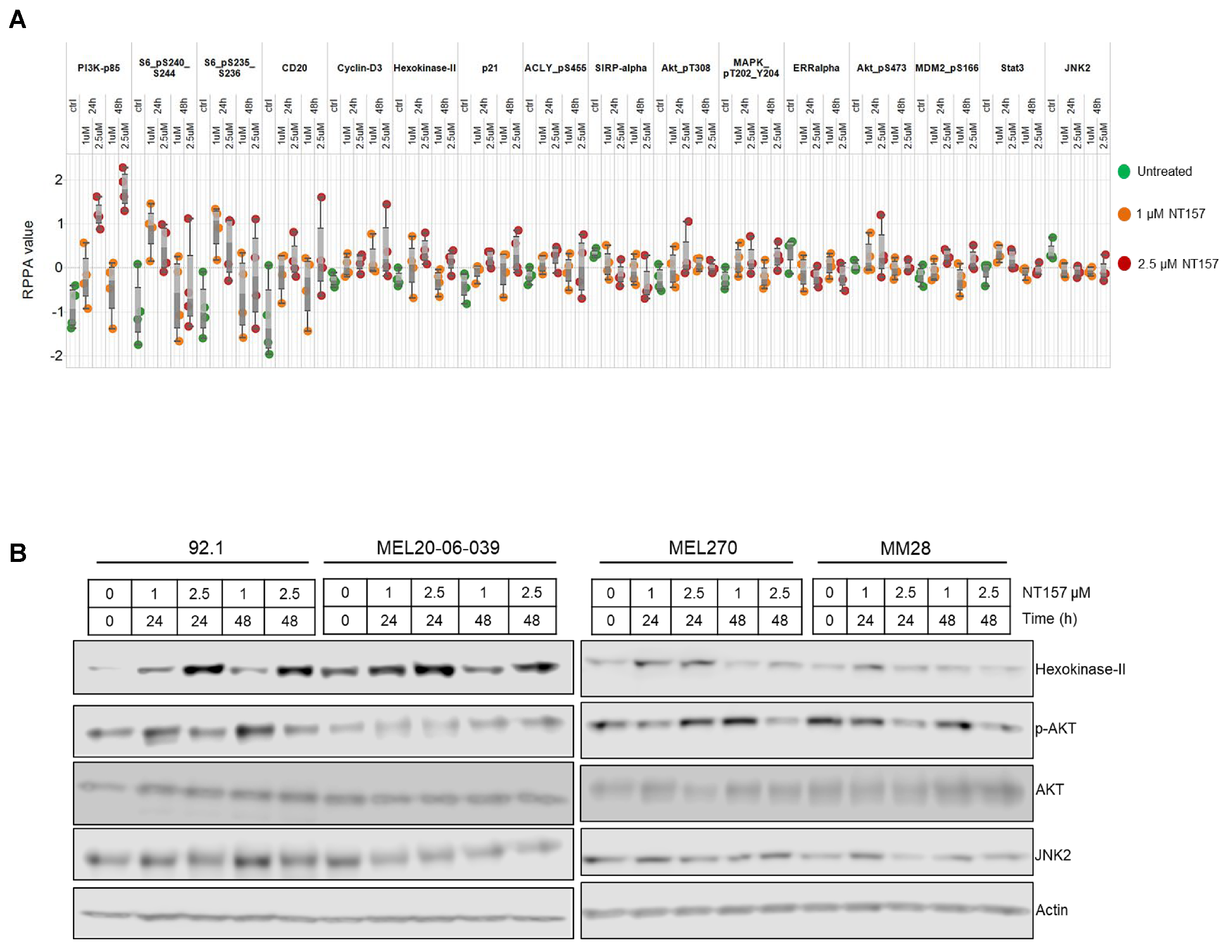
3.3. Multiple Cellular Pathways Are Modulated in Response to IRS-1 Inhibition
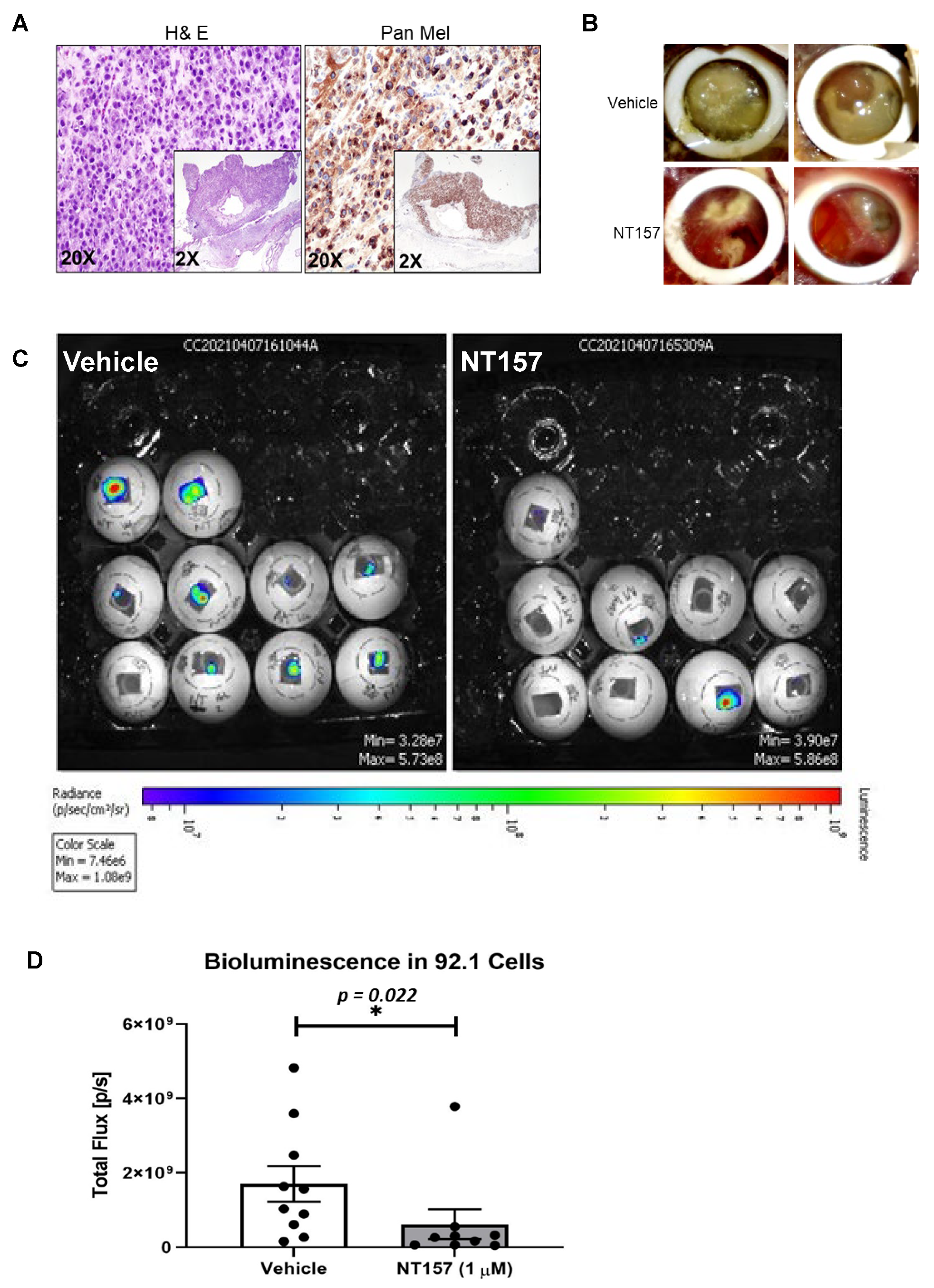
3.4. IRS-1 Targeting Reduces UM Tumor Growth in a Chicken CAM Model
3.5. Inhibition of IRS-1 Significantly Reduces UM Tumor Growth in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horsman, D.E.; White, V.A. Cytogenetic analysis of uveal melanoma. Consistent occurrence of monosomy 3 and trisomy 8q. Cancer 1993, 71, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Lorigan, J.G.; Wallace, S.; Mavligit, G.M. The prevalence and location of metastases from ocular melanoma: Imaging study in 110 patients. AJR Am. J. Roentgenol. 1991, 157, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, G.; Huang, X.; Yu, J.; Hsieh, C.L.; Grossniklaus, H.E. In-vivo xenograft murine human uveal melanoma model develops hepatic micrometastases. Melanoma Res. 2008, 18, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Bähr, C.; Groner, B. The IGF-1 receptor and its contributions to metastatic tumor growth-novel approaches to the inhibition of IGF-1R function. Growth Factors 2005, 23, 1–14. [Google Scholar] [CrossRef]
- Economou, M.A.; All-Ericsson, C.; Bykov, V.; Girnita, L.; Bartolazzi, A.; Larsson, O.; Seregard, S. Receptors for the Liver Synthesized Growth Factors IGF-1 and HGF/SF in Uveal Melanoma: Intercorrelation and Prognostic Implications. Investig. Opthalmol. Vis. Sci. 2005, 46, 4372–4375. [Google Scholar] [CrossRef]
- Frenkel, S.; Zloto, O.; Pe’er, J.; Barak, V. Insulin-like growth factor-1 as a predictive biomarker for metastatic uveal melanoma in humans. Investig. Opthalmol. Vis. Sci. 2013, 54, 490–493. [Google Scholar] [CrossRef]
- Economou, M.A.; All-Ericsson, C.; Bykov, V.; Girnita, L.; Bartolazzi, A.; Larsson, O.; Seregard, S. Receptors for the liver synthesized growth factors IGF-1 and HGF/SF in uveal melanoma: Intercorrelation and prognostic implications. Acta Ophthalmol. 2008, 86, 20–25. [Google Scholar] [CrossRef]
- Topcu-Yilmaz, P.; Kiratli, H.; Saglam, A.; Söylemezoglu, F.; Hascelik, G. Correlation of clinicopathological parameters with HGF, c-Met, EGFR, and IGF-1R expression in uveal melanoma. Melanoma Res. 2010, 20, 126–132. [Google Scholar] [CrossRef]
- All-Ericsson, C.; Girnita, L.; Seregard, S.; Bartolazzi, A.; Jager, M.J.; Larsson, O. Insulin-like growth factor-1 receptor in uveal melanoma: A predictor for metastatic disease and a potential therapeutic target. Investig. Opthalmol. Vis. Sci. 2002, 43, 1–8. [Google Scholar]
- Mattei, J.; Ballhausen, A.; Bassett, R.; Shephard, M.; Chattopadhyay, C.; Hudgens, C.; Tetzlaff, M.; Woodman, S.; Sato, T.; Patel, S.P. A phase II study of the insulin-like growth factor type I receptor inhibitor IMC-A12 in patients with metastatic uveal melanoma. Melanoma Res. 2020, 30, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Triozzi, P.L.; McKenna, K.C.; Van Meir, E.G.; Gershenwald, J.E.; Bastian, B.C.; Gutkind, J.S.; Bowcock, A.M.; Streicher, H.Z.; Patel, P.M.; et al. Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res. 2015, 28, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Mardilovich, K.; Pankratz, S.L.; Shaw, L.M. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun. Signal. 2009, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, H.; Flashner-Abramson, E.; Steiner, L.; Makedonski, K.; Song, R.; Shir, A.; Herlyn, M.; Bar-Eli, M.; Levitzki, A. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013, 73, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. Mechanisms by which IGF-I may promote cancer. Cancer Biol. Ther. 2003, 2, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef]
- Richards, J.R.; Yoo, J.H.; Shin, D.; Odelberg, S.J. Mouse models of uveal melanoma: Strengths, weaknesses, and future directions. Pigment. Cell Melanoma Res. 2020, 33, 264–278. [Google Scholar] [CrossRef]
- Kalirai, H.; Shahidipour, H.; Coupland, S.E.; Luyten, G. Use of the Chick Embryo Model in Uveal Melanoma. Ocul. Oncol. Pathol. 2015, 1, 133–140. [Google Scholar] [CrossRef]
- Burgess, B.L.; Rao, N.P.; Eskin, A.; Nelson, S.F.; McCannel, T.A. Characterization of three cell lines derived from fine needle biopsy of choroidal melanoma with metastatic outcome. Mol. Vis. 2011, 17, 607–615. [Google Scholar]
- von Euw, E.; Atefi, M.; Attar, N.; Chu, C.; Zachariah, S.; Burgess, B.L.; Mok, S.; Ng, C.; Wong, D.J.; Chmielowski, B.; et al. Antitumor effects of the investigational selective MEK inhibitor TAK733 against cutaneous and uveal melanoma cell lines. Mol. Cancer 2012, 11, 22. [Google Scholar] [CrossRef]
- De Waard-Siebinga, I.; Blom, D.J.; Griffioen, M.; Schrier, P.I.; Hoogendoorn, E.; Beverstock, G.; Danen, E.H.; Jager, M.J. Establishment and characterization of an uveal-melanoma cell line. Int. J. Cancer 1995, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.J.; Magner, J.A.; Ksander, B.R.; Dubovy, S.R. Uveal Melanoma Cell Lines: Where do they come from? (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2016, 114, T5. [Google Scholar] [PubMed]
- Ksander, B.R.; Rubsamen, P.E.; Olsen, K.R.; Cousins, S.W.; Streilein, J.W. Studies of tumor-infiltrating lymphocytes from a human choroidal melanoma. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3198–3208. [Google Scholar]
- Verbik, D.J.; Murray, T.G.; Tran, J.M.; Ksander, B.R. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int. J. Cancer 1997, 73, 470–478. [Google Scholar] [CrossRef]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar]
- Villanueva, H.; Sikora, A.G. The Chicken Embryo Chorioallantoic Membrane (CAM): A Versatile Tool for the Study of Patient-Derived Xenografts. Methods Mol. Biol. 2022, 2471, 209–220. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; El-Naggar, A.K.; Williams, M.D.; Clayman, G.L. Small molecule c-MET inhibitor PHA665752: Effect on cell growth and motility in papillary thyroid carcinoma. Head Neck 2008, 30, 991–1000. [Google Scholar] [CrossRef]
- Abdel-Wahab, R.; Shehata, S.; Hassan, M.M.; Habra, M.A.; Eskandari, G.; Tinkey, P.T.; Mitchell, J.; Lee, J.S.; Amin, H.M.; Kaseb, A.O. Type I insulin-like growth factor as a liver reserve assessment tool in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 131–142. [Google Scholar] [CrossRef]
- Maki, R.G. Small is beautiful: Insulin-like growth factors and their role in growth, development, and cancer. J. Clin. Oncol. 2010, 28, 4985–4995. [Google Scholar] [CrossRef]
- Awh, C.C.; Wilson, M.W. Symptomatic Liver Metastasis Prompting Diagnosis of Uveal Melanoma. Ocul. Oncol. Pathol. 2020, 6, 164–167. [Google Scholar] [CrossRef]
- Sokolenko, E.A.; Berchner-Pfannschmidt, U.; Ting, S.C.; Schmid, K.W.; Bechrakis, N.E.; Seitz, B.; Tsimpaki, T.; Kraemer, M.M.; Fiorentzis, M. Optimisation of the Chicken Chorioallantoic Membrane Assay in Uveal Melanoma Research. Pharmaceutics 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Winnay, J.; Kondo, T.; Bronson, R.T.; Guimaraes, A.R.; Alemán, J.O.; Luo, J.; Stephanopoulos, G.; Weissleder, R.; Cantley, L.C.; et al. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res. 2010, 70, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ueki, K.; Kondo, T.; Kahn, C.R. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell. Biol. 2004, 24, 5434–5446. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.W.; Wei, A.Z.; Maniar, A.B.; Carvajal, R.D. Tebentafusp in advanced uveal melanoma: Proof of principle for the efficacy of T-cell receptor therapeutics and bispecifics in solid tumors. Expert Opin. Biol. Ther. 2022, 22, 997–1004. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]


| NT157 Treatment | |||||
|---|---|---|---|---|---|
 | |||||
| Percent Gated Cells in subG1 Phase |  | NT157 (µM) | 0 | 1 | 2.5 |
| MP46 | 8.37 | 8.26 | 13.86 | ||
| MP65 | 1.53 | 1.88 | 7.64 | ||
| MM28 | 0.79 | 1.56 | 3.64 | ||
| MEL20-06-039 | 4.32 | 5.71 | 7.25 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chattopadhyay, C.; Bhattacharya, R.; Roszik, J.; Khan, F.S.; Wells, G.A.; Villanueva, H.; Qin, Y.; Bhattacharya, R.; Patel, S.P.; Grimm, E.A. Targeting IRS-1/2 in Uveal Melanoma Inhibits In Vitro Cell Growth, Survival and Migration, and In Vivo Tumor Growth. Cancers 2022, 14, 6247. https://doi.org/10.3390/cancers14246247
Chattopadhyay C, Bhattacharya R, Roszik J, Khan FS, Wells GA, Villanueva H, Qin Y, Bhattacharya R, Patel SP, Grimm EA. Targeting IRS-1/2 in Uveal Melanoma Inhibits In Vitro Cell Growth, Survival and Migration, and In Vivo Tumor Growth. Cancers. 2022; 14(24):6247. https://doi.org/10.3390/cancers14246247
Chicago/Turabian StyleChattopadhyay, Chandrani, Rajat Bhattacharya, Jason Roszik, Fatima S. Khan, Gabrielle A. Wells, Hugo Villanueva, Yong Qin, Rishav Bhattacharya, Sapna P. Patel, and Elizabeth A. Grimm. 2022. "Targeting IRS-1/2 in Uveal Melanoma Inhibits In Vitro Cell Growth, Survival and Migration, and In Vivo Tumor Growth" Cancers 14, no. 24: 6247. https://doi.org/10.3390/cancers14246247
APA StyleChattopadhyay, C., Bhattacharya, R., Roszik, J., Khan, F. S., Wells, G. A., Villanueva, H., Qin, Y., Bhattacharya, R., Patel, S. P., & Grimm, E. A. (2022). Targeting IRS-1/2 in Uveal Melanoma Inhibits In Vitro Cell Growth, Survival and Migration, and In Vivo Tumor Growth. Cancers, 14(24), 6247. https://doi.org/10.3390/cancers14246247








