Real-World Use of Granulocyte-Colony Stimulating Factor in Patients with Breast Cancer from Alberta, Canada
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Population
2.3. Study Data
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. G-CSF Use
3.3. Temporal Trends in G-CSF Use
3.4. Factors Associated with G-CSF Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, Morbidity, and Cost Associated with Febrile Neutropenia in Adult Cancer Patients. Cancer 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of Febrile Neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, X.; Gan, L.; Li, Y.; Li, H.; Cheng, Q. The Effect of Reduced RDI of Chemotherapy on the Outcome of Breast Cancer Patients. Sci. Rep. 2020, 10, 13241. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1609–e1618. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Dale, D.C.; Lyman, G.H. Chemotherapy-Induced Neutropenia. Cancer 2004, 100, 228–237. [Google Scholar] [CrossRef]
- Crespo, A.; Forbes, L.; Vu, K.; Gallo-Hershberg, D.; Enright, K.; Abdallah, M.; Febbraro, M.; Gowanlock, T.; Kennedy, K.; Lim, C.; et al. Prevention and Outpatient Management of Febrile Neutropenia in Adult Cancer Patients; Cancer Care Ontario: Toronto, ON, Canada, 2021. [Google Scholar]
- Cooper, K.L.; Madan, J.; Whyte, S.; Stevenson, M.D.; Akehurst, R.L. Granulocyte Colony-Stimulating Factors for Febrile Neutropenia Prophylaxis Following Chemotherapy: Systematic Review and Meta-Analysis. BMC Cancer 2011, 11, 404. [Google Scholar] [CrossRef]
- Henk, H.J.; Becker, L.; Tan, H.; Yu, J.; Kavati, A.; Naeim, A.; Deeter, R.; Barron, R. Comparative Effectiveness of Pegfilgrastim, Filgrastim, and Sargramostim Prophylaxis for Neutropenia-Related Hospitalization: Two US Retrospective Claims Analyses. J. Med. Econ. 2013, 16, 160–168. [Google Scholar] [CrossRef]
- Mitchell, S.; Li, X.; Woods, M.; Garcia, J.; Hebard-Massey, K.; Barron, R.; Samuel, M. Comparative Effectiveness of Granulocyte Colony-Stimulating Factors to Prevent Febrile Neutropenia and Related Complications in Cancer Patients in Clinical Practice: A Systematic Review. J. Oncol. Pharm. Pract. 2016, 22, 702–716. [Google Scholar] [CrossRef]
- Weycker, D.; Malin, J.; Barron, R.; Edelsberg, J.; Kartashov, A.; Oster, G. Comparative Effectiveness of Filgrastim, Pegfilgrastim, and Sargramostim as Prophylaxis Against Hospitalization for Neutropenic Complications in Patients with Cancer Receiving Chemotherapy. Am. J. Clin. Oncol. 2012, 35, 267–274. [Google Scholar] [CrossRef]
- Aapro, M.S.; Cameron, D.A.; Pettengell, R.; Bohlius, J.; Crawford, J.; Ellis, M.; Kearney, N.; Lyman, G.H.; Tjan-Heijnen, V.C.; Walewski, J.; et al. EORTC Guidelines for the Use of Granulocyte-Colony Stimulating Factor to Reduce the Incidence of Chemotherapy-Induced Febrile Neutropenia in Adult Patients with Lymphomas and Solid Tumours. Eur. J. Cancer 2006, 42, 2433–2453. [Google Scholar] [CrossRef]
- Crawford, J.; Becker, P.S.; Armitage, J.O.; Blayney, D.W.; Chavez, J.; Curtin, P.; Dinner, S.; Fynan, T.; Gojo, I.; Griffiths, E.A.; et al. Myeloid Growth Factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk Factors for Febrile Neutropenia among Patients with Cancer Receiving Chemotherapy: A Systematic Review. Crit. Rev. Oncol. Hematol. 2014, 90, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.; Koo, M.; Gill, T.; Marin, M.; Poulin–Costello, M.; Barron, R.; Mittmann, N. The Use of Granulocyte Colony–Stimulating Factors in a Canadian Outpatient Setting. Curr. Oncol. 2014, 21, 229–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mansell, K.; Bhimji, H.; Eurich, D.; Mansell, H. Potential Cost-Savings from the Use of the Biosimilars Filgrastim, Infliximab and Insulin Glargine in Canada: A Retrospective Analysis. BMC Health Serv. Res. 2019, 19, 827. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Weycker, D.; Bensink, M.; Lonshteyn, A.; Doroff, R.; Chandler, D. Use of Colony-Stimulating Factor Primary Prophylaxis and Incidence of Febrile Neutropenia from 2010 to 2016: A Longitudinal Assessment. Curr. Med. Res. Opin. 2019, 35, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, G.; Dinh, T. Understanding the Gap: A Pan-Canadian Analysis of Prescription Drug Insurance Coverage; The Conference Board of Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
- Barnes, G.; Pathak, A.; Schwartzberg, L. G-CSF Utilization Rate and Prescribing Patterns in United States: Associations between Physician and Patient Factors and GCSF Use. Cancer Med. 2014, 3, 1477–1484. [Google Scholar] [CrossRef]
- Goyal, R.K.; Tzivelekis, S.; Rothman, K.J.; Candrilli, S.D.; Kaye, J.A. Time Trends in Utilization of G-CSF Prophylaxis and Risk of Febrile Neutropenia in a Medicare Population Receiving Adjuvant Chemotherapy for Early-Stage Breast Cancer. Supportive Care Cancer 2018, 26, 539–548. [Google Scholar] [CrossRef]
- Younis, T.; Rayson, D.; Jovanovic, S.; Skedgel, C. Cost-Effectiveness of Febrile Neutropenia Prevention with Primary versus Secondary G-CSF Prophylaxis for Adjuvant Chemotherapy in Breast Cancer: A Systematic Review. Breast. Cancer Res. Treat. 2016, 159, 425–432. [Google Scholar] [CrossRef]
- Li, E.; Mezzio, D.J.; Campbell, D.; Campbell, K.; Lyman, G.H. Primary Prophylaxis with Biosimilar Filgrastim for Patients at Intermediate Risk for Febrile Neutropenia: A Cost-Effectiveness Analysis. JCO Oncol. Pr. 2021, 17, e1235–e1245. [Google Scholar] [CrossRef]
- IMS Institute for Healthcare Informatics. Delivering on the Potential of Biosimilar Medicines; IMS Institute for Healthcare Informatics: Parsippany, NJ, USA, 2016. [Google Scholar]
- Schwartzberg, L.S.; Lal, L.S.; Balu, S.; Campbell, K.; Brekke, L.; DeLeon, A.; Elliott, C.; Korrer, S. Clinical Outcomes of Treatment with Filgrastim Versus a Filgrastim Biosimilar and Febrile Neutropenia-Associated Costs Among Patients with Nonmyeloid Cancer Undergoing Chemotherapy. J. Manag. Care Spec. Pharm. 2018, 24, 976–984. [Google Scholar] [CrossRef] [PubMed]
- IQVIA. The Impact of Biosimilar Competition in Europe; IQVIA: London, UK, 2018. [Google Scholar]
- Aapro, M.; Cornes, P.; Abraham, I. Comparative Cost-Efficiency across the European G5 Countries of Various Regimens of Filgrastim, Biosimilar Filgrastim, and Pegfilgrastim to Reduce the Incidence of Chemotherapy-Induced Febrile Neutropenia. J. Oncol. Pharm. Pract. 2012, 18, 171–179. [Google Scholar] [CrossRef]
- McBride, A.; Wang, W.; Campbell, K.; Balu, S.; MacDonald, K.; Abraham, I. Economic Modeling for the US of the Cost-Efficiency and Associated Expanded Treatment Access of Conversion to Biosimilar Pegfilgrastim-Bmez from Reference Pegfilgrastim. J. Med. Econ. 2020, 23, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.; Janke, A. Health Literacy and Access to Care. J. Health Commun. 2016, 21 (Suppl. 1), 43–50. [Google Scholar] [CrossRef] [PubMed]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low Health Literacy and Health Outcomes: An Updated Systematic Review. Ann. Intern. Med. 2011, 155, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Chesser, A.K.; Keene Woods, N.; Smothers, K.; Rogers, N. Health Literacy and Older Adults: A Systematic Review. Gerontol. Geriatr. Med. 2016, 2, 2333721416630492. [Google Scholar] [CrossRef] [PubMed]
- Mäenpää, J.; Varthalitis, I.; Erdkamp, F.; Trojan, A.; Krzemieniecki, K.; Lindman, H.; Bendall, K.; Vogl, F.D.; Verma, S. The Use of Granulocyte Colony Stimulating Factor (G-CSF) and Management of Chemotherapy Delivery during Adjuvant Treatment for Early-Stage Breast Cancer—Further Observations from the IMPACT Solid Study. Breast 2016, 25, 27–33. [Google Scholar] [CrossRef]
| Characteristic | Overall (n = 6662) | G-CSF Receipt | p Value | SMD | |
|---|---|---|---|---|---|
| No (n = 2861) | Yes (n = 3801) | ||||
| Age at chemotherapy initiation, y (n = 6662) | 54 (46, 61) | 54 (47, 62) | 53 (45, 61) | <0.001 ** | 0.144 † |
| <45 | 1394 (20.9%) | 518 (37.2%) | 876 (62.8%) | <0.001 ** | 0.137 † |
| 45–54 | 2139 (32.1%) | 921 (43.1%) | 1218 (56.9%) | ||
| 55–64 | 2089 (31.4%) | 926 (44.3%) | 1163 (55.7%) | ||
| ≥65 | 1040 (15.6%) | 496 (47.7%) | 544 (52.3%) | ||
| Rurality of residence (n = 6662) | 0.21 | 0.031 | |||
| Rural | 1327 (19.9%) | 590 (44.5%) | 737 (55.5%) | ||
| Urban | 5335 (80.1%) | 2271 (42.6%) | 3064 (57.4%) | ||
| Zone of residence (n = 6662) | <0.001 ** | 0.277 † | |||
| Calgary | 2717 (40.8%) | 1015 (37.4%) | 1702 (62.6%) | ||
| Central | 722 (10.8%) | 290 (40.2%) | 432 (59.8%) | ||
| Edmonton | 2283 (34.3%) | 1156 (50.6%) | 1127 (49.4%) | ||
| North | 566 (8.5%) | 282 (49.8%) | 284 (50.2%) | ||
| South | 374 (5.6%) | 118 (31.6%) | 256 (68.4%) | ||
| Neighborhood education quartile (n = 6613) | <0.001 ** | 0.114 † | |||
| Lowest | 1654 (25.0%) | 760 (45.9%) | 894 (54.1%) | ||
| Second | 1653 (25.0%) | 751 (45.4%) | 902 (54.6%) | ||
| Third | 1652 (25.0%) | 660 (40.0%) | 992 (60.0%) | ||
| Highest | 1654 (25.0%) | 666 (40.3%) | 988 (59.7%) | ||
| Neighborhood income quartile (n = 6617) | <0.001 ** | 0.109 † | |||
| Lowest | 1653 (25.0%) | 736 (44.5%) | 917 (55.5%) | ||
| Second | 1654 (25.0%) | 766 (46.3%) | 888 (53.7%) | ||
| Third | 1655 (25.0%) | 682 (41.2%) | 973 (58.8%) | ||
| Highest | 1655 (25.0%) | 655 (39.6%) | 1000 (60.4%) | ||
| Cancer stage (n = 6662) | <0.001 ** | 0.276 † | |||
| I | 1492 (22.4%) | 823 (55.2%) | 669 (44.8%) | ||
| II | 3602 (54.1%) | 1464 (40.6%) | 2138 (59.4%) | ||
| III | 1568 (23.5%) | 574 (36.6%) | 994 (63.4%) | ||
| Neutropenic risk of chemotherapy regimen (n = 6662) | <0.001 ** | 0.138 † | |||
| High | 6510 (97.7%) | 2761 (42.4%) | 3749 (57.6%) | ||
| Moderate | 152 (2.3%) | 100 (65.8%) | 52 (34.2%) | ||
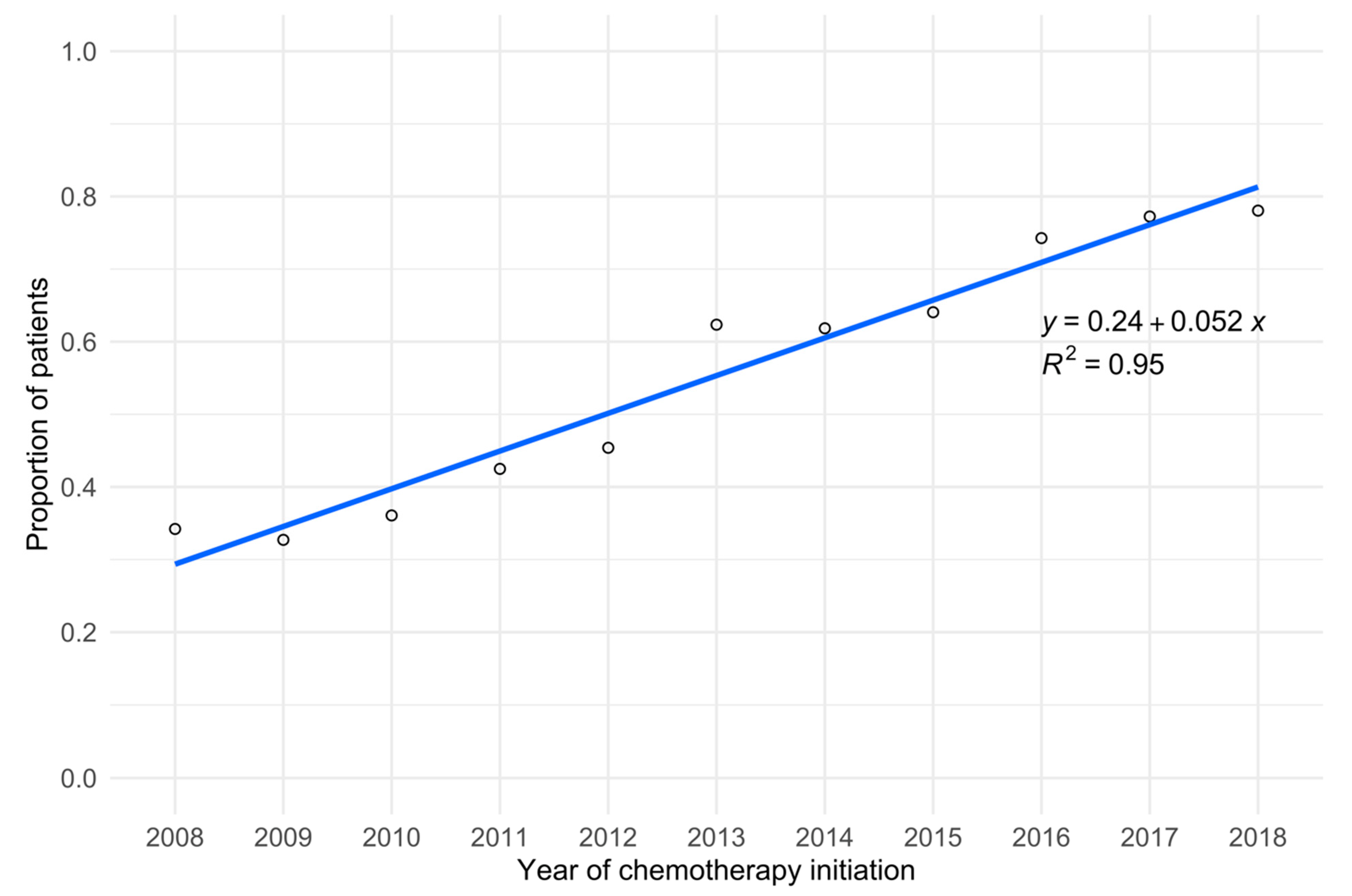
| Year of chemotherapy initiation (n = 6662) | <0.001 ** | 0.689 † | |||
| 2008 | 342 (5.1%) | 225 (65.8%) | 117 (34.2%) | ||
| 2009 | 483 (7.3%) | 325 (67.3%) | 158 (32.7%) | ||
| 2010 | 535 (8.0%) | 342 (63.9%) | 193 (36.1%) | ||
| 2011 | 652 (9.8%) | 375 (57.5%) | 277 (42.5%) | ||
| 2012 | 705 (10.6%) | 385 (54.6%) | 320 (45.4%) | ||
| 2013 | 470 (7.1%) | 177 (37.7%) | 293 (62.3%) | ||
| 2014 | 684 (10.3%) | 261 (38.2%) | 423 (61.8%) | ||
| 2015 | 843 (12.7%) | 303 (35.9%) | 540 (64.1%) | ||
| 2016 | 882 (13.2%) | 227 (25.7%) | 655 (74.3%) | ||
| 2017 | 861 (12.9%) | 196 (22.8%) | 665 (77.2%) | ||
| 2018 | 205 (3.1%) | 45 (22.0%) | 160 (78.0%) | ||
| Characteristic | Overall (n = 3694) | G-CSF Type | p Value | SMD | |
|---|---|---|---|---|---|
| Pegfilgrastim Only (n = 3477) | Filgrastim Only (n = 217) | ||||
| Age at chemotherapy initiation, y (n = 3694) | 53 (45, 61) | 53 (45, 61) | 51 (43, 58) | 0.003 ** | 0.216 † |
| <45 | 851 (23.0%) | 786 (22.6%) | 65 (30.0%) | <0.001 ** | 0.451 † |
| 45–54 | 1173 (31.8%) | 1110 (31.9%) | 63 (29.0%) | ||
| 55–64 | 1138 (30.8%) | 1056 (30.4%) | 82 (37.8%) | ||
| ≥65 | 532 (14.4%) | 525 (15.1%) | 7 (3.2%) | ||
| Rurality of residence (n = 3694) | 0.16 | 0.103 † | |||
| Rural | 715 (19.4%) | 681 (19.6%) | 34 (15.7%) | ||
| Urban | 2979 (80.6%) | 2796 (80.4%) | 183 (84.3%) | ||
| Zone of residence (n = 3694) | <0.001 ** | 0.410 † | |||
| Calgary | 1668 (45.2%) | 1533 (44.1%) | 135 (62.2%) | ||
| Central | 416 (11.3%) | 404 (11.6%) | 12 (5.5%) | ||
| Edmonton | 1084 (29.3%) | 1041 (29.9%) | 43 (19.8%) | ||
| North | 275 (7.4%) | 257 (7.4%) | 18 (8.3%) | ||
| South | 251 (6.8%) | 242 (7.0%) | 9 (4.1%) | ||
| Neighborhood education quartile (n = 3670) | 0.02 * | 0.223 † | |||
| Lowest | 869 (23.7%) | 830 (24.0%) | 39 (18.0%) | ||
| Second | 884 (24.1%) | 840 (24.3%) | 44 (20.3%) | ||
| Third | 964 (26.3%) | 890 (25.8%) | 74 (34.1%) | ||
| Highest | 953 (26.0%) | 893 (25.9%) | 60 (27.6%) | ||
| Neighborhood income quartile (n = 3672) | 0.16 | 0.159 † | |||
| Lowest | 894 (24.3%) | 841 (24.3%) | 53 (24.4%) | ||
| Second | 868 (23.6%) | 819 (23.7%) | 49 (22.6%) | ||
| Third | 940 (25.6%) | 895 (25.9%) | 45 (20.7%) | ||
| Highest | 970 (26.4%) | 900 (26.0%) | 70 (32.3%) | ||
| Cancer stage (n = 3694) | 0.28 | 0.108 † | |||
| I | 653 (17.7%) | 606 (17.4%) | 47 (21.7%) | ||
| II | 2084 (56.4%) | 1966 (56.5%) | 118 (54.4%) | ||
| III | 957 (25.9%) | 905 (26.0%) | 52 (24.0%) | ||
| Neutropenic risk of chemotherapy regimen (n = 3694) | 0.12 | 0.104 † | |||
| High | 3643 (98.6%) | 3432 (98.7%) | 211 (97.2%) | ||
| Moderate | 51 (1.4%) | 45 (1.3%) | 6 (2.8%) | ||
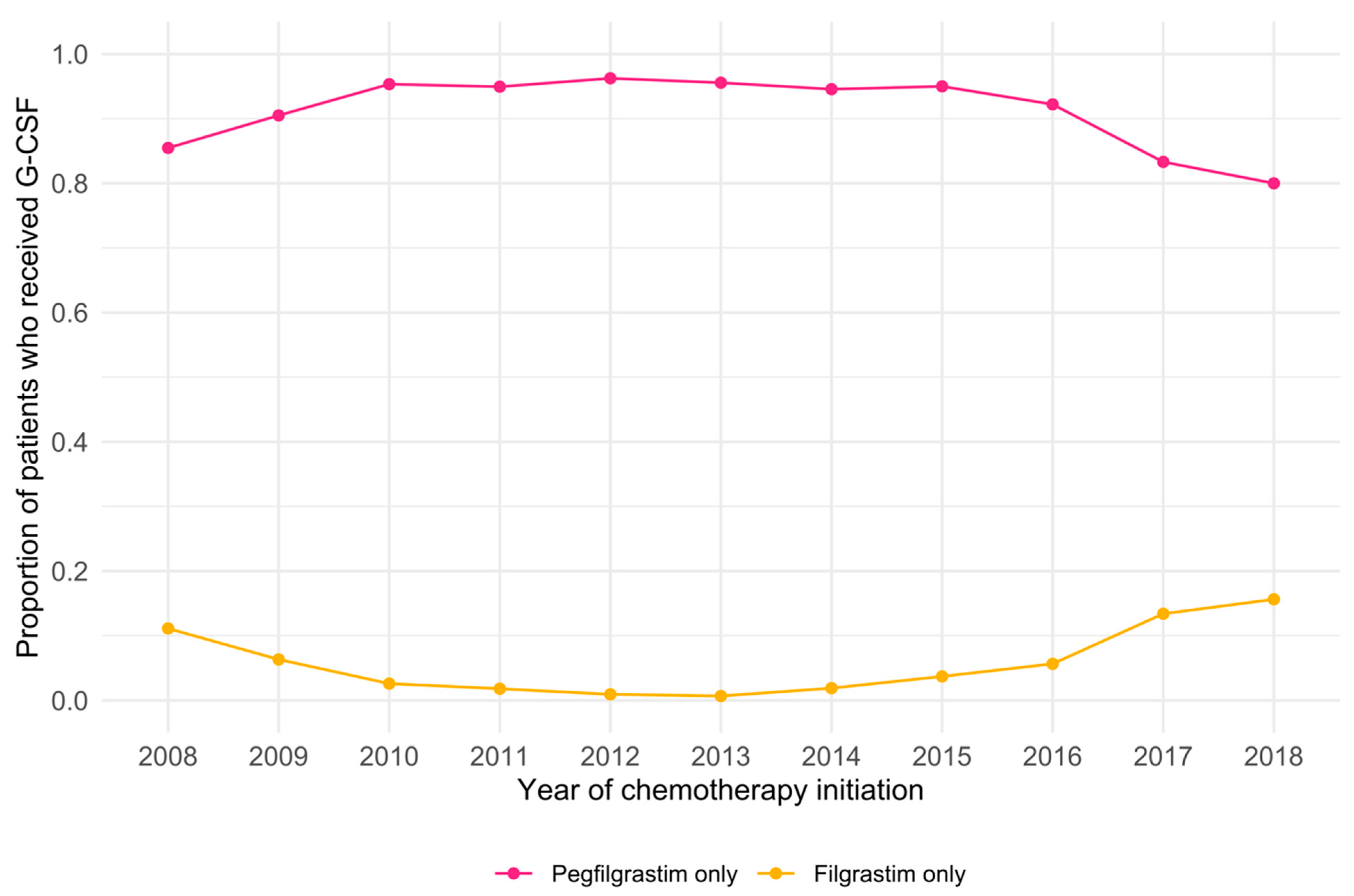
| Year of chemotherapy initiation (n = 3694) | <0.001 ** | 0.947 † | |||
| 2008 | 113 (3.1%) | 100 (2.9%) | 13 (6.0%) | ||
| 2009 | 153 (4.1%) | 143 (4.1%) | 10 (4.6%) | ||
| 2010 | 189 (5.1%) | 184 (5.3%) | 5 (2.3%) | ||
| 2011 | 268 (7.3%) | 263 (7.6%) | 5 (2.3%) | ||
| 2012 | 311 (8.4%) | 308 (8.9%) | 3 (1.4%) | ||
| 2013 | 282 (7.6%) | 280 (8.1%) | 2 (0.9%) | ||
| 2014 | 408 (11.0%) | 400 (11.5%) | 8 (3.7%) | ||
| 2015 | 533 (14.4%) | 513 (14.8%) | 20 (9.2%) | ||
| 2016 | 641 (17.4%) | 604 (17.4%) | 37 (17.1%) | ||
| 2017 | 643 (17.4%) | 554 (15.9%) | 89 (41.0%) | ||
| 2018 | 153 (4.1%) | 128 (3.7%) | 25 (11.5%) | ||
| G-CSF as primary prophylaxis for first chemotherapy cycle (n = 3694) | <0.001 ** | 0.251 † | |||
| No | 3233 (87.5%) | 3062 (88.1%) | 171 (78.8%) | ||
| Yes | 461 (12.5%) | 415 (11.9%) | 46 (21.2%) | ||
| Variable | OR for Receiving G-CSF (95% CI) | p Value |
|---|---|---|
| Age at chemotherapy initiation, y | <0.001 a** | |
| <45 | Reference | |
| 45–54 | 0.84 (0.72–0.98) | |
| 55–64 | 0.76 (0.65–0.89) | |
| ≥65 | 0.57 (0.48–0.68) | |
| Rurality of residence | 0.84 | |
| Rural | Reference | |
| Urban | 1.01 (0.83–1.23) | |
| Zone of residence | <0.001 ** | |
| Calgary | Reference | |
| Central | 0.97 (0.78–1.22) | |
| Edmonton | 0.59 (0.52–0.67) | |
| North | 0.59 (0.46–0.75) | |
| South | 1.12 (0.87–1.46) | |
| Neighborhood education quartile | 0.004 a** | |
| Lowest | Reference | |
| Second | 1.07 (0.91–1.26) | |
| Third | 1.28 (1.07–1.53) | |
| Highest | 1.29 (1.06–1.57) | |
| Neighborhood income quartile | 0.12 a | |
| Lowest | Reference | |
| Second | 0.88 (0.75–1.03) | |
| Third | 1.06 (0.90–1.26) | |
| Highest | 1.09 (0.91–1.31) | |
| Cancer stage | <0.001 a** | |
| I | Reference | |
| II | 1.93 (1.69–2.21) | |
| III | 2.60 (2.22–3.05) | |
| Neutropenic risk of chemotherapy regimen | 0.009 ** | |
| High | Reference | |
| Moderate | 0.57 (0.39–0.82) | |
| Year of chemotherapy initiation | <0.001 a** | |
| 2008 | Reference | |
| 2009 | 0.90 (0.67–1.22) | |
| 2010 | 1.15 (0.85–1.55) | |
| 2011 | 1.48 (1.11–1.97) | |
| 2012 | 1.68 (1.27–2.22) | |
| 2013 | 3.01 (2.23–4.09) | |
| 2014 | 3.24 (2.44–4.32) | |
| 2015 | 3.71 (2.82–4.91) | |
| 2016 | 6.37 (4.80–8.49) | |
| 2017 | 7.61 (5.71–10.19) | |
| 2018 | 8.55 (5.67–13.10) |
| Variable | OR for Receiving Filgrastim Only vs. Pegfilgrastim Only (95% CI) | p Value |
|---|---|---|
| Age at treatment initiation, y | 0.001 a** | |
| <45 | Reference | |
| 45–54 | 0.75 (0.52–1.09) | |
| 55–64 | 0.98 (0.69–1.40) | |
| ≥65 | 0.15 (0.06–0.32) | |
| Rurality of residence | 0.77 | |
| Rural | Reference | |
| Urban | 0.93 (0.57–1.56) | |
| Zone of residence | <0.001 ** | |
| Calgary | Reference | |
| Central | 0.40 (0.19–0.78) | |
| Edmonton | 0.42 (0.28–0.61) | |
| North | 0.75 (0.40–1.35) | |
| South | 0.46 (0.21–0.91) | |
| Neighborhood education quartile | 0.33 a | |
| Lowest | Reference | |
| Second | 1.30 (0.79–2.14) | |
| Third | 1.82 (1.09–3.08) | |
| Highest | 1.33 (0.75–2.35) | |
| Neighborhood income quartile | 0.67 a | |
| Lowest | Reference | |
| Second | 0.78 (0.50–1.22) | |
| Third | 0.59 (0.36–0.95) | |
| Highest | 0.91 (0.56–1.47) | |
| Cancer stage | 0.73 a | |
| I | Reference | |
| II | 0.85 (0.59–1.23) | |
| III | 0.95 (0.62–1.48) | |
| Neutropenic risk of chemotherapy regimen | <0.001 ** | |
| High | Reference | |
| Moderate | 2.75 (0.95–6.90) | |
| Year of chemotherapy initiation | <0.001 a** | |
| 2008 | Reference | |
| 2009 | 0.53 (0.21–1.29) | |
| 2010 | 0.21 (0.06–0.59) | |
| 2011 | 0.14 (0.04–0.40) | |
| 2012 | 0.08 (0.02–0.28) | |
| 2013 | 0.06 (0.01–0.24) | |
| 2014 | 0.17 (0.06–0.44) | |
| 2015 | 0.36 (0.17–0.81) | |
| 2016 | 0.58 (0.29–1.25) | |
| 2017 | 1.54 (0.80–3.18) | |
| 2018 | 1.81 (0.84–4.09) | |
| G-CSF as primary prophylaxis for first chemotherapy cycle | 0.02 * | |
| No | Reference | |
| Yes | 1.44 (0.98–2.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, P.Q.; Newcomer, B.J.; Cheung, W.Y. Real-World Use of Granulocyte-Colony Stimulating Factor in Patients with Breast Cancer from Alberta, Canada. Cancers 2022, 14, 6197. https://doi.org/10.3390/cancers14246197
Ding PQ, Newcomer BJ, Cheung WY. Real-World Use of Granulocyte-Colony Stimulating Factor in Patients with Breast Cancer from Alberta, Canada. Cancers. 2022; 14(24):6197. https://doi.org/10.3390/cancers14246197
Chicago/Turabian StyleDing, Philip Q., Brandt J. Newcomer, and Winson Y. Cheung. 2022. "Real-World Use of Granulocyte-Colony Stimulating Factor in Patients with Breast Cancer from Alberta, Canada" Cancers 14, no. 24: 6197. https://doi.org/10.3390/cancers14246197
APA StyleDing, P. Q., Newcomer, B. J., & Cheung, W. Y. (2022). Real-World Use of Granulocyte-Colony Stimulating Factor in Patients with Breast Cancer from Alberta, Canada. Cancers, 14(24), 6197. https://doi.org/10.3390/cancers14246197






