Efficacy and Tolerance of IMRT Boost Compared to IORT Boost in Early Breast Cancer: A German Monocenter Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgery and Radiation Treatment
2.3. Follow-Up
2.4. Statistic Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Adverse Event | IORT | IMRT | p Value (Chi-Square Test) | |
|---|---|---|---|---|
| BREAST SUBJECTIVE | PAIN | 23 (30.3%) | 29 (38.2%) | 0.355 |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 18 | 23 | ||
| 2 | 3 | 4 | ||
| 3 | 1 | 2 | ||
| 4 | 1 | 0 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 21 | 28 | ||
| Unknown | - | - | ||
| OBJECTIVE | BREAST EDEMA | 5 (6.6%) | 5 (6.6%) | 0.427 |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 2 | 2 | ||
| 2 | 3 | 3 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 3 | 5 | ||
| Unknown | - | - | ||
| FIBROSIS | 31 (40.8%) | 23 (30.3%) | 0.135 | |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 12 | 11 | ||
| 2 | 16 | 9 | ||
| 3 | 4 | 3 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 27 | 23 | ||
| Unknown | - | - | ||
| TELEANGIECTASIS | 2 (2.6%) | 2 (2.6%) | 0.503 | |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 1 | 2 | ||
| 2 | 1 | 0 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 2 | 2 | ||
| Unknown | - | - | ||
| LYMPHEDEMA (ARM) | 13 (17.1%) | 10 (13.2%) | 0.428 | |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 8 | 8 | ||
| 2 | 3 | 0 | ||
| Unknown | 2 | 2 | ||
| Relation to RT | ||||
| Yes | 11 | 10 | ||
| Unknown | 1 | 0 | ||
| RETRACTION/ | 0.300 | |||
| ATROPHY | 11 (14.5%) | 10 (13.2%) | ||
| Unknown | - | - | ||
| Grade | ||||
| 1 | 8 | 9 | ||
| 2 | 0 | 1 | ||
| 3 | 2 | 0 | ||
| 4 | 1 | 0 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 7 | 9 | ||
| Unknown | 2 | 0 | ||
| ULCER | 2 (2.6%) | 0 | 0.290 | |
| Unknown | 1 | 1 | ||
| Grade | ||||
| Unknown | 2 | 1 | ||
| Relation to RT | ||||
| Yes | 1 | - | ||
| Unknown | - | 1 | ||
| HEART SUBJECTIVE | ANGINA PECTORIS | 9 (11.8%) | 7 (9.2%) | 0.537 |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 4 | 5 | ||
| 2 | 1 | 2 | ||
| 3 | 3 | 0 | ||
| 4 | 1 | 0 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 2 | 1 | ||
| Unknown | 3 | 2 | ||
| PERICARDIAL PAIN | 7 (9.2%) | 3 (3.9%) | 0.658 | |
| Unknown | 3 | 5 | ||
| Grade | ||||
| 1 | 6 | 7 | ||
| 2 | 2 | 1 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 2 | 3 | ||
| Unknown | 2 | 3 | ||
| PALPITATION | 23 (30.3%) | 30 (39.5%) | 0.184 | |
| Unknown | - | 1 | ||
| Grade | ||||
| 1 | 18 | 15 | ||
| 2 | 4 | 10 | ||
| 3 | 1 | 4 | ||
| 4 | 0 | 2 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 5 | 3 | ||
| Unknown | 4 | 11 | ||
| DYSPNEA | 29 (38.2%) | 32 (42.1%) | 0.409 | |
| Unknown | - | 1 | ||
| Grade | ||||
| 1 | 14 | 24 | ||
| 2 | 13 | 7 | ||
| 3 | 2 | 1 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 8 | 6 | ||
| Unknown | 2 | 5 | ||
| PEDAL EDEMA | 11 (14.5%) | 21 (27.6%) | 0.165 | |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 6 | 13 | ||
| 2 | 3 | 6 | ||
| 3 | 2 | 2 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 0 | 3 | ||
| Unknown | - | 1 | ||
| OBJECTIVE | PEDAL EDEMA | 11 (14.5%) | 21 (27.6%) | 0.165 |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 1 | 4 | ||
| 2 | 0 | 5 | ||
| 3 | 0 | 3 | ||
| 4 | 0 | 2 | ||
| Unknown | 10 | 7 | ||
| Relation to RT | ||||
| Yes | 0 | 3 | ||
| Unknown | - | 1 | ||
| CARDIOMEGALY | 0 | 5 (6.6%) | 0.202 | |
| Unknown | 1 | 1 | ||
| Grade | ||||
| 1 | 0 | 1 | ||
| 2 | 0 | 2 | ||
| 3 | 0 | 1 | ||
| Unknown | 1 | 1 | ||
| Relation to RT | ||||
| Yes | 0 | 2 | ||
| Unknown | - | - | ||
| CARDIAC DYSRHTYTHMIA | 4 (5.3%) | 18 (23.7%) | 0.711 | |
| Unknown | 1 | - | ||
| Grade | ||||
| 1 | 1 | 7 | ||
| 2 | 1 | 3 | ||
| 3 | 1 | 1 | ||
| 4 | 1 | 2 | ||
| Unknown | - | 5 | ||
| Relation to RT | ||||
| Yes | 1 | 1 | ||
| Unknown | 1 | 1 | ||
| MYOCARIDAL CHF | 3 (3.9%) | 4 (5.3%) | 0.214 | |
| Unknown | 1 | - | ||
| Grade | ||||
| 1 | 0 | 1 | ||
| Unknown | 2 | 3 | ||
| Relation to RT | ||||
| Yes | 0 | 1 | ||
| Unknown | - | - | ||
| MYOCARDIAL | 0.290 | |||
| ISCHEMIA | 1 (1.3%) | 2 (2.6%) | ||
| Unknown | 2 | 2 | ||
| Grade | ||||
| 1 | 0 | 0 | ||
| 2 | 0 | 0 | ||
| 3 | 0 | 0 | ||
| 4 | 1 | 1 | ||
| Unknown | - | 1 | ||
| Relation to RT | ||||
| Yes | 0 | 0 | ||
| Unknown | 1 | - | ||
| PERICARDIAL | 0.243 | |||
| DISEASE | 0 | 0 | ||
| Unknown | - | - | ||
| Grade | ||||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | - | - | ||
| Unknown | - | - | ||
| LUNG SUBJECTIVE | COUGH | 19 (25%) | 29 (38.2%) | 0.383 |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 8 | 11 | ||
| 2 | 4 | 7 | ||
| 3 | 5 | 6 | ||
| 4 | 3 | 5 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 6 | 12 | ||
| Unknown | 5 | 5 | ||
| DYSPNEA | 29 (38.2%) | 32 (42.1%) | 0.418 | |
| Unknown | - | 1 | ||
| Grade | ||||
| 1 | 15 | 24 | ||
| 2 | 13 | 7 | ||
| 3 | 2 | 1 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 9 | 6 | ||
| Unknown | 2 | 4 | ||
| CHEST PAIN | 4 (5.3%) | 7 (9.2%) | 0.427 | |
| Unknown | - | - | ||
| Grade | ||||
| 1 | 1 | 5 | ||
| 2 | 1 | 2 | ||
| 3 | 2 | 0 | ||
| Unknown | - | - | ||
| Relation to RT | ||||
| Yes | 3 | 5 | ||
| Unknown | - | - | ||
| OBJECTIVE | PULMONARY | 0.230 | ||
| FIBROSIS | 2 (2.6%) | 4 (5.3%) | ||
| Unknown | 2 | 2 | ||
| Grade | ||||
| 1 | 1 | 2 | ||
| 2 | 0 | 1 | ||
| Unknown | 1 | 1 | ||
| Relation to RT | ||||
| Yes | 1 | 4 | ||
| Unknown | - | - | ||
| LUNG FUNCTION | 16 (21.1%) | 19 (25%) | 0.448 | |
| Unknown | 1 | - | ||
| Grade | ||||
| 1 | 10 | 14 | ||
| 2 | 4 | 2 | ||
| 3 | 0 | 1 | ||
| Unknown | 2 | 2 | ||
| Relation to RT | ||||
| Yes | 6 | 5 | ||
| Unknown | - | - |
References
- Evidence-Based Guideline Breast Cancer-V4.4, © German Guideline Program in Oncology, May 2021. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/S3_Guideline_Breast_Cancer.pdf (accessed on 8 October 2022).
- Veronesi, U.; Marubini, E.; Mariani, L.; Galimberti, V.; Luini, A.; Veronesi, P.; Salvadori, B.; Zucali, R. Radiotherapy after breast-conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Luini, A.; Del Vecchio, M.; Greco, M.; Galimberti, V.; Merson, M.; Rilke, F.; Sacchini, V.; Saccozzi, R.; Savio, T. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N. Engl. J. Med. 1993, 328, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; McCulloch, P.B.; Levine, M.N.; Lipa, M.; Wilkinson, R.H.; Mahoney, L.J.; Basrur, V.R.; Nair, B.D.; McDermot, R.S.; Wong, C.S. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J. Natl. Cancer Inst. 1992, 84, 683–689. [Google Scholar] [CrossRef]
- Clark, R.M.; Whelan, T.; Levine, M.; Roberts, R.; Willan, A.; McCulloch, P.; Lipa, M.; Wilkinson, R.H.; Mahoney, L.J. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: An update. Ontario Clinical Oncology Group. J. Natl. Cancer Inst. 1996, 88, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Uppsala-Orebro Breast Cancer Study Group. Sector resection with or without postoperative radiotherapy for stage I breast cancer: A randomized trial. J. Natl. Cancer Inst. 1990, 82, 277–282. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Redmond, C.K.; Wolmark, N.; Wickerham, D.L.; Cronin, W.M. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N. Engl. J. Med. 1995, 333, 1456–1461. [Google Scholar] [CrossRef]
- Liljegren, G.; Holmberg, L.; Bergh, J.; Lindgren, A.; Tabár, L.; Nordgren, H.; Adami, H.O. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: A randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 2326–2333. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar]
- Bartelink, H.; Horiot, J.C.; Poortmans, P.M.; Struikmans, H.; Van den Bogaert, W.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.J.; Oei, S.B.; Wárlám-Rodenhuis, C.C.; et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3259–3265. [Google Scholar] [CrossRef]
- Bartelink, H.; Horiot, J.C.; Poortmans, P.; Struikmans, H.; Van den Bogaert, W.; Barillot, I.; Fourquet, A.; Borger, J.; Jager, J.; Hoogenraad, W.; et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N. Engl. J. Med. 2001, 345, 1378–1387. [Google Scholar] [CrossRef]
- Fastner, G.; Sedlmayer, F.; Merz, F.; Deutschmann, H.; Reitsamer, R.; Menzel, C.; Stierle, C.; Farmini, A.; Fischer, T.; Ciabattoni, A.; et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: Long term results of an ISIORT pooled analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 108, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Sedlmayer, F.; Reitsamer, R.; Fussl, C.; Ziegler, I.; Zehentmayr, F.; Deutschmann, H.; Kopp, P.; Fastner, G. Boost IORT in Breast Cancer: Body of evidence. Int. J. Breast Cancer 2014, 2014, 472516. [Google Scholar] [CrossRef] [PubMed]
- Cuncins-Hearn, A.; Saunders, C.; Walsh, D.; Borg, M.; Buckingham, J.; Frizelle, F.; Maddern, G. A systematic review of intraoperative radiotherapy in early breast cancer. Breast Cancer Res. Treat. 2004, 85, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Sedlmayer, F.; Reitsamer, R.; Wenz, F.; Sperk, E.; Fussl, C.; Kaiser, J.; Ziegler, I.; Zehentmayr, F.; Deutschmann, H.; Kopp, P.; et al. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat. Oncol. 2017, 12, 23. [Google Scholar] [CrossRef]
- Gatzemeier, W.; Orecchia, R.; Gatti, G.; Intra, M.; Veronesi, U. [Intraoperative radiotherapy (IORT) in treatment of breast carcinoma--a new therapeutic alternative within the scope of breast-saving therapy? Current status and future prospects. Report of experiences from the European Institute of Oncology (EIO), Mailand]. Strahlenther. Onkol. 2001, 177, 330–337. [Google Scholar]
- Lei, J.; Wang, Y.; Bi, Z.; Xue, S.; Ou, B.; Liu, K. Intraoperative radiotherapy (IORT) versus whole-breast external beam radiotherapy (EBRT) in early stage breast cancer: Results from SEER database. Jpn. J. Radiol. 2020, 38, 85–92. [Google Scholar] [CrossRef]
- Leatherman, J.; Nicholas, C.; Cusick, T.; Cooke, E.; Ablah, E.; Okut, H.; Hunt, D. Intra-operative radiation therapy versus whole breast external beam radiotherapy: A comparison of patient-reported outcomes. J. Med. 2021, 14, 170–175. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Z.; Mei, X.; Yang, Z.; Ma, J.; Chen, X.; Wang, J.; Liu, G.; Yu, X.; Guo, X. Intraoperative radiotherapy versus whole-breast external beam radiotherapy in early-stage breast cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1143. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef]
- Lemanski, C.; Azria, D.; Thezenas, S.; Gutowski, M.; Saint-Aubert, B.; Rouanet, P.; Fenoglietto, P.; Ailleres, N.; Dubois, J.B. Intraoperative radiotherapy given as a boost for early breast cancer: Long-term clinical and cosmetic results. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1410–1415. [Google Scholar] [CrossRef]
- Ciabattoni, A.; Gregucci, F.; Fastner, G.; Cavuto, S.; Spera, A.; Drago, S.; Ziegler, I.; Mirri, M.A.; Consorti, R.; Sedlmayer, F. Correction to: IOERT versus external beam electrons for boost radiotherapy in stage I/II breast cancer: 10-year results of a phase III randomized study. Breast Cancer Res. BCR 2021, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Reitsamer, R.; Peintinger, F.; Kopp, M.; Menzel, C.; Kogelnik, H.D.; Sedlmayer, F. Local recurrence rates in breast cancer patients treated with intraoperative electron-boost radiotherapy versus postoperative external-beam electron-boost irradiation. A sequential intervention study. Strahlenther. Onkol. 2004, 180, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, M.; Kronholz, H.L.; Reinartz, G.; Schuck, A.; Brinkmann, M.; Willich, N. Reduction of lung stress in breast-IORT. Strahlenther. Onkol. 2005, 181, 15. [Google Scholar]
- Shaitelman, S.F.; Schlembach, P.J.; Arzu, I.; Ballo, M.; Bloom, E.S.; Buchholz, D.; Chronowski, G.M.; Dvorak, T.; Grade, E.; Hoffman, K.E.; et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 2015, 1, 931–941. [Google Scholar] [CrossRef]
- Kindts, I.; Laenen, A.; Depuydt, T.; Weltens, C. Tumour bed boost radiotherapy for women after breast-conserving surgery. Cochrane Database Syst. Rev. 2017, 11, CD011987. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Kronberger, C.; Moder, A.; Kopp, P.; Wallner, M.; Reitsamer, R.; Fischer, T.; Fussl, C.; Zehentmayr, F.; Sedlmayer, F.; et al. Intraoperative tumor bed boost with electrons in breast cancer of clinical stages I through III: Updated 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.C.; et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar] [CrossRef]
- Brouwers, P.J.A.M.; van Werkhoven, E.; Bartelink, H.; Fourquet, A.; Lemanski, C.; van Loon, J.; Maduro, J.H.; Russell, N.S.; Scheijmans, L.J.E.E.; Schinagl, D.A.X.; et al. Predictors for poor cosmetic outcome in patients with early stage breast cancer treated with breast conserving therapy: Results of the Young boost trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 128, 434–441. [Google Scholar] [CrossRef]
- Fastner, G.; Reitsamer, R.; Urbański, B.; Kopp, P.; Murawa, D.; Adamczyk, B.; Karzcewska, A.; Milecki, P.; Hager, E.; Reiland, J.; et al. Toxicity and cosmetic outcome after hypofractionated whole breast irradiation and boost-IOERT in early stage breast cancer (HIOB): First results of a prospective multicenter trial (NCT01343459). Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 146, 136–142. [Google Scholar] [CrossRef]
- Piotrowski, I.; Kulcenty, K.; Wichtowski, M.; Murawa, D.; Suchorska, W. Intraoperative Radiotherapy of Breast Cancer and Its Biological Effects. Breast Care 2017, 12, 109–113. [Google Scholar] [CrossRef]
- Singla, R.; King, S.; Albuquerque, K.; Creech, S.; Dogan, N. Simultaneous-integrated boost intensity-modulated radiation therapy (SIB-IMRT) in the treatment of early-stage left-sided breast carcinoma. Med. Dosim. Off. J. Am. Assoc. Med. Dosim. 2006, 31, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Li, X.A.; Earl, M.A.; Sarfaraz, M.; Kiggundu, E. Simultaneous integrated boost for breast cancer using IMRT: A radiobiological and treatment planning study. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1513–1522. [Google Scholar] [CrossRef] [PubMed]



| IORT-Boost | IMRT-Boost | Primary Tumor Stage | Postoperative Tumor Stage | p Value | |
|---|---|---|---|---|---|
| Group size | 76 | 76 | |||
| Age average (range) [y] | 53.50 (29–68) | 67.00 (41–90) | |||
| Histology | 0.691 | ||||
| IDC/NST *** | 61 (80.3%) (IDC) | 59 (77.6%) (NST) | |||
| ILC | 4 (5.3%) | 12 (15.8%) | |||
| mixed | 5 (6.6%) | 1 (1.3%) | |||
| other (tubular, mucinous, medullary) | 4 (5.3%) | 4 (5.3%) | |||
| atypical medullary | 2 (2.6%) | 0 | |||
| Primary Tumor stage | 0.006 ** | ||||
| pT1 | 68 (89.5%) | 53 (69.7%) | |||
| pT2 | 7 (9.2%) | 14 (18.4%) | |||
| pT3–4 | 0 | 0 | |||
| cT1b | 0 | 2 (2.6%) | |||
| cT2 | 0 | 7 (9.2%) | |||
| (m)pT1c | 1 (1.3%) | 0 | |||
| ∑ T1–2 | 76 | 76 | |||
| Grading | 0.348 | ||||
| G1 | 22 (28.9%) | 18 (23.7%) | |||
| G2 | 39 (51.3%) | 36 (47.4%) | |||
| G3 | 14 (18.4%) | 22 (28.9%) | |||
| missing | 1 (1.3%) | 0 | |||
| Chemotherapy | 0.708 | ||||
| yes | 20 (26.3%) | 18 (23.7%) | |||
| no | 56 (73.7%) | 58 (76.3%) | |||
| Neoadjuvant Chemotherapy | 0.002 ** | ||||
| yes | 0 | 9 (11.8%) | |||
| cT2 | ypT0 | ||||
| cT2 | ypT0 | ||||
| cT2 | ypT0 | ||||
| cT2 | ypT1 | ||||
| cT1b | ypT1b | ||||
| cT2 | ypt1c | ||||
| cT2 | ypTis | ||||
| cT2 | ypT0 | ||||
| cT1b | ypT1c | ||||
| no | 76 (100%) | 67 (88.2%) | |||
| Endocrine therapy | 0.182 | ||||
| yes | 61 (80.3%) | 67 (88.2%) | |||
| no | 15 (19.7%) | 9 (11.8%) |
| Receptor Status | Frequency | Percent (%) |
|---|---|---|
| ER+, PR+, Her2 neu 0 | 9 | 5.9 |
| ER+, PR+, c-erbB2 2+ | 1 | 0.7 |
| ER+, PR−, c-erbB2− | 5 | 3.3 |
| ER−, PR+, c-erbB2− | 1 | 0.7 |
| ER−, PR−, c-erbB2 3+ | 1 | 0.7 |
| ER−, PR− | 2 | 1.3 |
| ER+, PR− | 1 | 0.7 |
| ER+, PR+ | 1 | 0.7 |
| ER−, PR−, Her2 neu − | 4 | 2.6 |
| ER−, PR−, Her2 neu + | 1 | 0.7 |
| ER+, PR+, Her2 neu 1+ | 9 | 5.9 |
| ER−, PR−, Her2 neu 3+ | 5 | 3.3 |
| ER+, PR−, Her2 neu 2+ | 1 | 0.7 |
| ER+, PR+, Her2 neu 3+ | 2 | 1.3 |
| ER+, PR+, Her2 neu − | 78 | 51.3 |
| ER+, PR−, Her2 neu 3+ | 1 | 0.7 |
| ER+, PR−, Her2 neu 1+ | 4 | 2.6 |
| ER+, PR−, Her2 neu − | 9 | 5.9 |
| ER+, PR+, Her2 neu 2+ | 5 | 3.3 |
| ER+, PR+, c-erbB2+ | 4 | 2.6 |
| ER+, PR−, c-erbB2+ | 1 | 0.7 |
| ER−, PR−, c-erbB2− | 6 | 3.9 |
| ER−, PR+, c-erbB2− | 1 | 0.7 |
| ∑ | 152 | 100 |
| Influencing Factor | Univariate p Value/HR/Cl 95% | Multivariate p Value | HR/Cl 95% |
|---|---|---|---|
| age | 0.764/1.009/0.949–1.074 | 0.592 | 0.992/0.927–1.062 |
| grading | 0.122/2.067/0.805–5.311 | 3.066/0.953–9.866 | |
| HR-status | 0.690/1.025/0.905–1.161 | 0.999/0.882–1.132 | |
| pathological T stage | 0.912/0.967/0.540–1.731 | 1.545/0.581–4.111 | |
| histology | 0.311/1.378/0.778–2.441 | 1.563/0.739–3.081 | |
| chemotherapy | 0.849/0.860/0.178–4.150 | 0.300/0.041–2.210 | |
| neoadjuvant chemotherapy | 0.297/3.775/0.440–32.374 | 17.295/0.160–1874.099 | |
| antihormonal therapy | 0.730/0.752/0.155–3.638 | 1.313/0.184–9.354 | |
| resection status | 0.586/1.582/0.369–6.793 | 0.868/0.116–6.490 | |
| LAW | 0.525/0.048/0.000–12,334,080.6 | 0.000/0.000 |
| Subjective Cosmetic Result | IORT | IMRT | p Value (Chi-Square-Test) |
|---|---|---|---|
| 1 | 40 (52.6%) | 17 (22.4%) | 0.315 |
| 2 | 23 (30.3%) | 14 (18.4%) | |
| 3 | 5 (6.6%) | 0 | |
| 4 | 1 (1.3%) | 0 | |
| 5 | 0 | 0 | |
| 6 | 0 | 0 | |
| Missing | 7 (9.2%) | 45 (59.2 %) |
| Objective Cosmetic Result | IORT | IMRT | p Value (Chi-Square-Test) |
|---|---|---|---|
| 1 | 22 (28.9%) | 17 (22.4%) | 0.719 |
| 2 | 24 (31.6%) | 13 (17.1%) | |
| 3 | 4 (5.3%) | 2 (2.6%) | |
| 4 | 0 | 0 | |
| 5 | 0 | 0 | |
| 6 | 0 | 0 | |
| Missing | 26 (34.2%) | 44 (57.9%) |
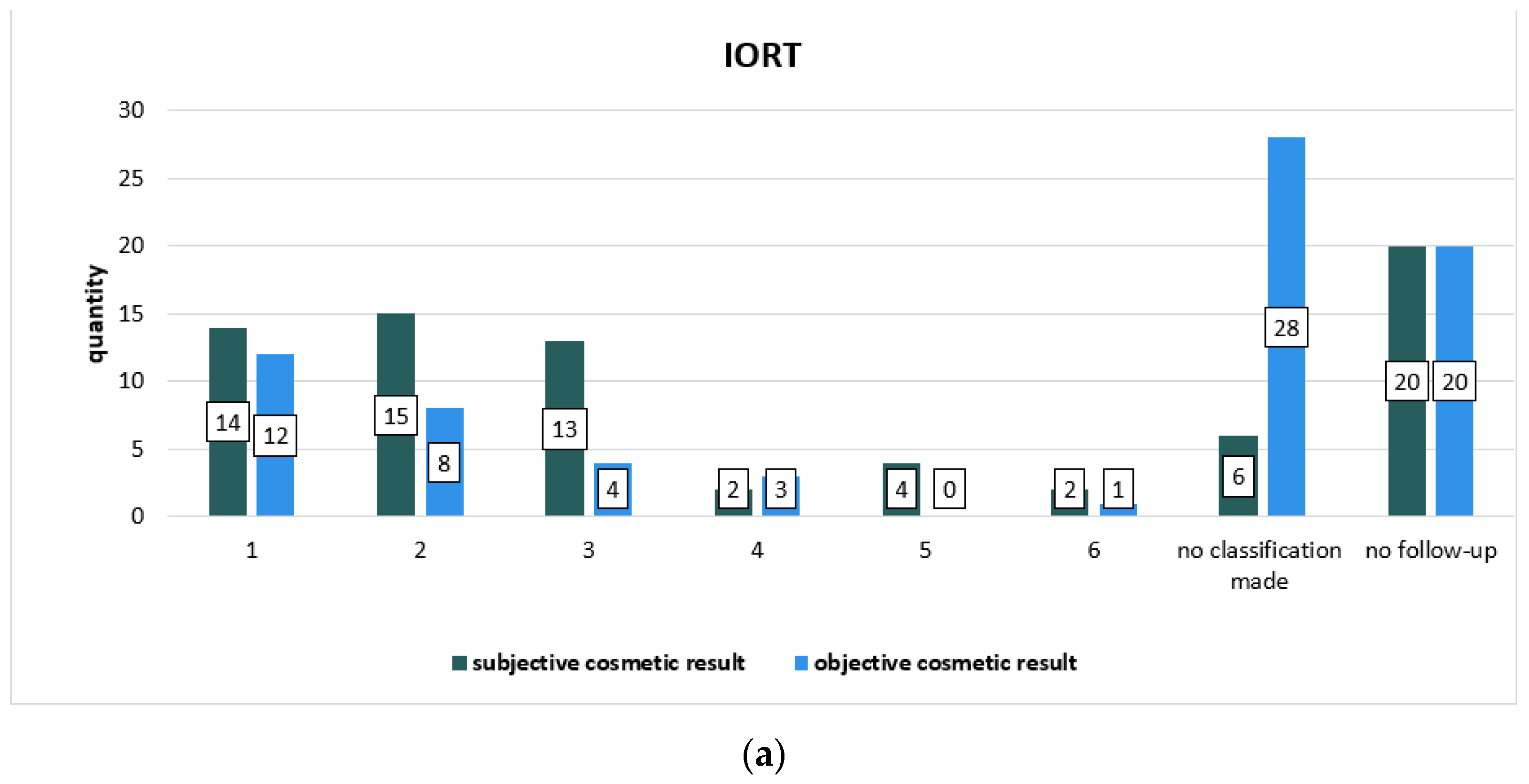
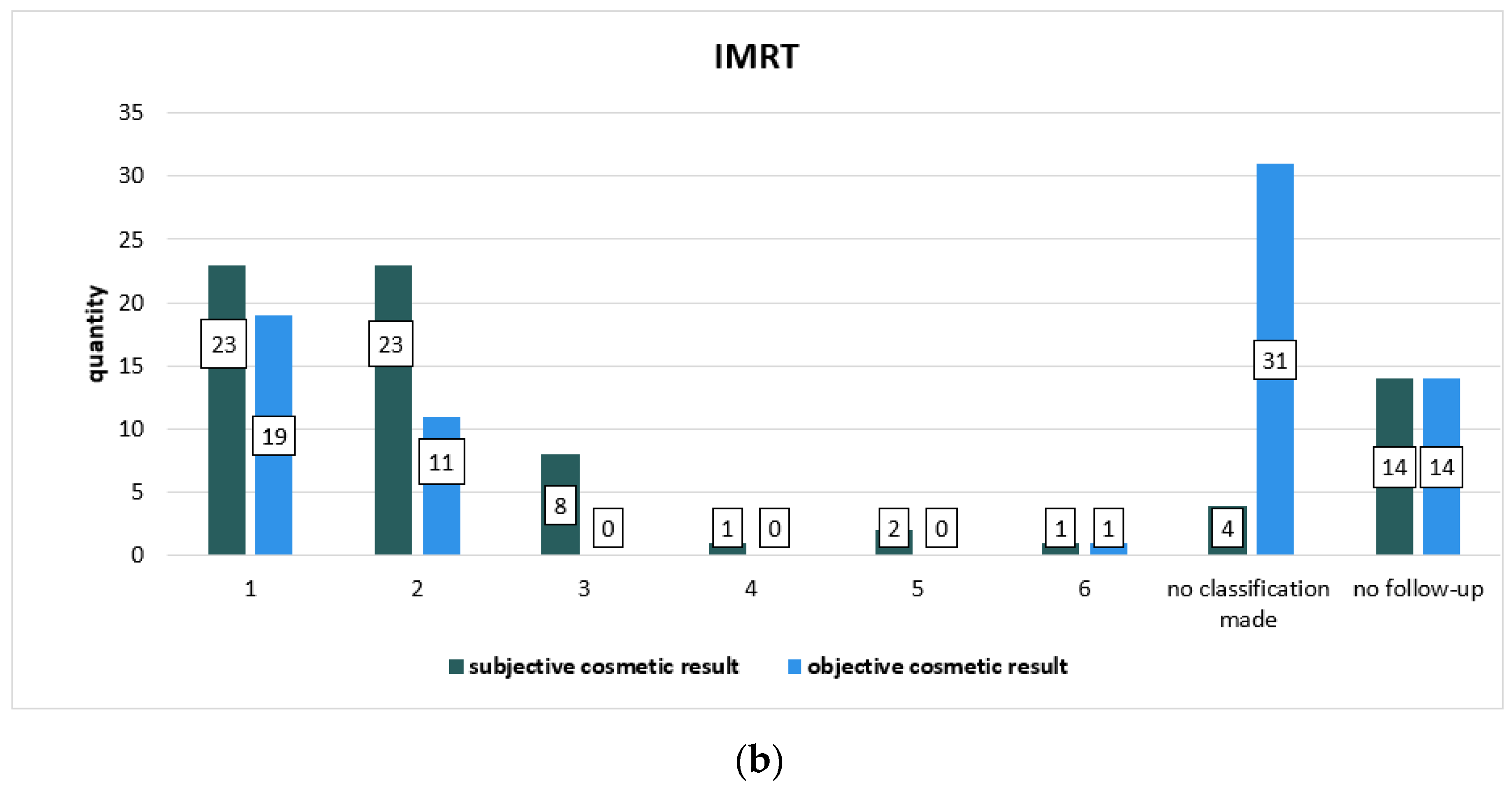
| Subjective Cosmetic Result | IORT | IMRT | p Value (Chi-Square-Test) |
|---|---|---|---|
| 1 | 14 (18.4%) | 23 (30.3%) | 0.345 |
| 2 | 15 (19.7%) | 23 (30.3%) | |
| 3 | 13 (17.1%) | 8 (10.5%) | |
| 4 | 2 (2.6%) | 1 (1.3%) | |
| 5 | 4 (5.3%) | 2 (2.6%) | |
| 6 | 2 (2.6%) | 1 (1.3%) | |
| No classification made | 6 (7.9%) | 4 (5.3%) | |
| No follow-up | 20 (26.3%) | 14 (18.4%) |
| Objective Cosmetic Result | IORT | IMRT | p Value (Chi-Square-Test) |
|---|---|---|---|
| 1 | 12 (15.8%) | 19 (25.0%) | 0.114 |
| 2 | 8 (10.5%) | 11 (14.5%) | |
| 3 | 4 (5.3%) | 0 | |
| 4 | 3 (3.9%) | 0 | |
| 5 | 0 | 0 | |
| 6 | 1 (1.3%) | 1 (1.3%) | |
| No classification made | 28 (36.8%) | 31 (40.8%) | |
| No follow-up | 20 (26.3%) | 14 (18.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, L.; Tio, J.; Eich, H.T.; Reinartz, G. Efficacy and Tolerance of IMRT Boost Compared to IORT Boost in Early Breast Cancer: A German Monocenter Study. Cancers 2022, 14, 6196. https://doi.org/10.3390/cancers14246196
Schumacher L, Tio J, Eich HT, Reinartz G. Efficacy and Tolerance of IMRT Boost Compared to IORT Boost in Early Breast Cancer: A German Monocenter Study. Cancers. 2022; 14(24):6196. https://doi.org/10.3390/cancers14246196
Chicago/Turabian StyleSchumacher, Luisa, Joke Tio, Hans Theodor Eich, and Gabriele Reinartz. 2022. "Efficacy and Tolerance of IMRT Boost Compared to IORT Boost in Early Breast Cancer: A German Monocenter Study" Cancers 14, no. 24: 6196. https://doi.org/10.3390/cancers14246196
APA StyleSchumacher, L., Tio, J., Eich, H. T., & Reinartz, G. (2022). Efficacy and Tolerance of IMRT Boost Compared to IORT Boost in Early Breast Cancer: A German Monocenter Study. Cancers, 14(24), 6196. https://doi.org/10.3390/cancers14246196








