The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. Biological Function of HER2
3. Relevance to Cancer
HER2 Expression Status in Various Cancers
4. HER2 as a Target for Therapy
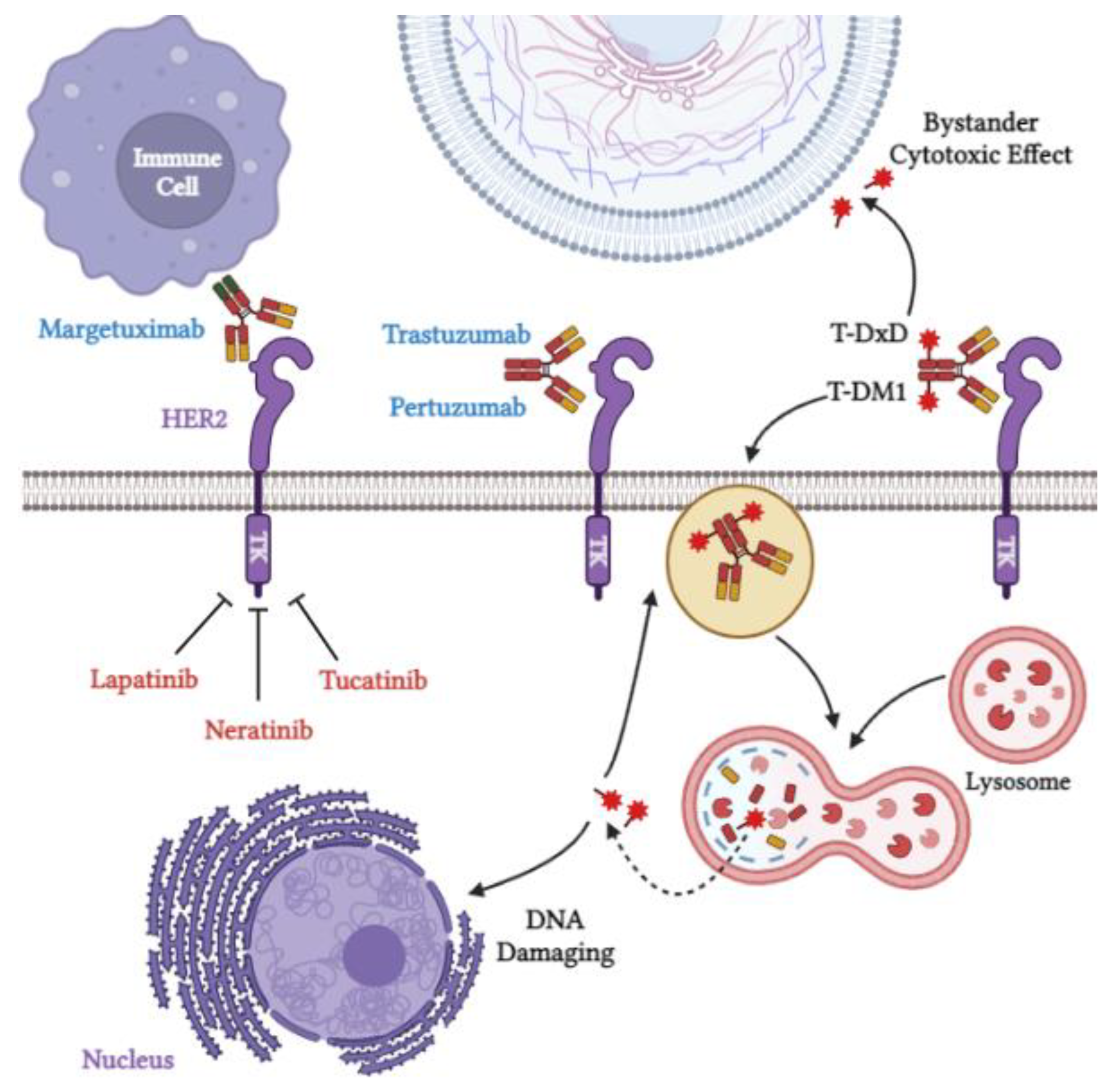
4.1. Antibody-Based Therapies
4.1.1. Trastuzumab
4.1.2. Pertuzumab
4.1.3. Margetuximab
4.2. Tyrosine Kinase Inhibitors
4.2.1. Lapatinib
4.2.2. Neratinib
4.2.3. Tucatinib
4.3. Antibody–Drug Conjugates (ADCs)
4.3.1. Trastuzumab Emtansine (T-DM1)
4.3.2. Trastuzumab Deruxtecan (T-DXd)
4.3.3. SYD985
4.4. Adoptive T-Cell Therapies
4.4.1. CAR-T-Cell Therapy
4.4.2. TCR-T-Cell Therapy
4.5. Vaccines
4.5.1. T-Cell Peptide Vaccines
- 1.
- E75:
- 2.
- GP2
- 3.
- AE37
4.5.2. B-Cell Peptide Vaccines
- 1.
- HER-Vaxx:
- 2.
- B-Vaxx
4.5.3. Dendritic Cell-Based Vaccines
5. Mechanisms of Resistance to HER2-Targeted Therapies
Tumor MicroEnvironment (TME)
6. Future Perspective and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akiyama, T.; Sudo, C.; Ogawara, H.; Toyoshima, K.; Yamamoto, T. The Product of the Human C-ErbB-2 Gene: A 185-Kilodalton Glycoprotein with Tyrosine Kinase Activity. Science 1986, 232, 1644–1646. [Google Scholar] [CrossRef]
- Stern, D.F.; Heffernan, P.A.; Weinberg, R.A. P185, a Product of the Neu Proto-Oncogene, Is a Receptorlike Protein Associated with Tyrosine Kinase Activity. Mol. Cell. Biol. 1986, 6, 1729–1740. [Google Scholar] [CrossRef]
- Fukushige, S.-I.; Matsubara, K.-I.; Yoshida, M.; Sasaki, M.; Suzuki, T.; Semba, K.; Toyoshima, K.; Yamamoto4, T. Localization of a Novel V-ErbB-Related Gene, c-ErbB-2, on Human Chromosome 17 and Its Amplification in a Gastric Cancer Cell Line. Mol. Cell. Biol. 1986, 6, 955–958. [Google Scholar]
- Yarden, Y. The EGFR Family and Its Ligands in Human Cancer: Signalling Mechanisms and Therapeutic Opportunities. Eur. J. Cancer 2001, 37, 3–8. [Google Scholar] [CrossRef]
- Nelson, E.L. HER2/Neu: An Increasingly Important Therapeutic Target. Part 1: Basic Biology & Therapeutic Armamentarium. Clin. Investig. 2014, 4, 649–671. [Google Scholar] [CrossRef]
- Piccart, M.J.; Di Leo, A.; Hamilton, A. HER2. Eur. J. Cancer 2021, 36, 1755–1761. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinić, M.; Wiedermann, U. Vaccination against Her-2/Neu, with Focus on Peptide-Based Vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef]
- Klapper, L.N.; Glathe, S.; Vaisman, N.; Hynes, N.E.; Andrews, G.C.; Sela, M.; Yarden, Y. The ErbB-2HER2 Oncoprotein of Human Carcinomas May Function Solely as a Shared Coreceptor for Multiple Stroma-Derived Growth Factors. Proc. Natl. Acad. Sci. USA 1999, 96, 4995–5000. [Google Scholar] [CrossRef]
- Chan, R.; Hardy, W.R.; Laing, M.A.; Hardy, S.E.; Muller, W.J. The Catalytic Activity of the ErbB-2 Receptor Tyrosine Kinase Is Essential for Embryonic Development. Mol. Cell. Biol. 2002, 22, 1073–1078. [Google Scholar] [CrossRef]
- Garrett, T.P.J.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The Crystal Structure of a Truncated ErbB2 Ectodomain Reveals an Active Conformation, Poised to Interact with Other ErbB Receptors. Mol. Cell 2003, 11, 495–505. [Google Scholar] [CrossRef]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Sierke, S.L.; Cheng, K.; Kim, H.-H.; Koland, J.G. Biochemical Characterization of the Protein Tyrosine Kinase Homology Domain of the ErbB3 (HER3) Receptor Protein. Biochem. J. 1997, 322, 757–763. [Google Scholar] [CrossRef]
- Press, M.F.; Cordon-Cardo, C.; Slamon, D.J. Expression of the HER-2/Neu Proto-Oncogene in Normal Human Adult and Fetal Tissues. Oncogene 1990, 5, 953–962. [Google Scholar]
- Hynes, N.E.; MacDonald, G. ErbB Receptors and Signaling Pathways in Cancer. Curr. Opin. Cell Biol. 2009, 21, 177–184. [Google Scholar] [CrossRef]
- Lee, K.F.; Simon, H.; Chen, H.; Bates, B.; Hung, M.C.; Hauser, C. Requirement for Neuregulin Receptor ErbB2 in Neural and Cardiac Development. Nature 1995, 378, 394–398. [Google Scholar] [CrossRef]
- Ménard, S.; Casalini, P.; Campiglio, M.; Pupa, S.M.; Tagliabue, E. Role of HER2/Neu in Tumor Progression and Therapy. Cell. Mol. Life Sci. 2004, 61, 2965–2978. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.P.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer HHS Public Access. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Peckys, D.B.; Hirsch, D.; Gaiser, T.; De Jonge, N. Visualisation of HER2 Homodimers in Single Cells from HER2 Overexpressing Primary Formalin Fixed Paraffin Embedded Tumour Tissue. Mol. Med. 2019, 25, 42. [Google Scholar] [CrossRef]
- Fisk, B.; Blevins, T.L.; Wharton, J.T.; Ioannides, C.G. Identification of an Immunodominant Peptide of HER-2/Neu Protooncogene Recognized by Ovarian Tumor-Specific Cytotoxic t Lymphocyte Lines. J. Exp. Med. 1995, 181, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Gheybi, E.; Salmanian, A.H.; Fooladi, A.A.I.; Salimian, J.; Hosseini, H.M.; Halabian, R.; Amani, J. Immunogenicity of Chimeric MUC1-HER2 Vaccine against Breast Cancer in Mice. Iran. J. Basic Med. Sci. 2018, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Czerniecki, B.J. Clinical Development of Immunotherapies for HER2+ Breast Cancer: A Review of HER2-Directed Monoclonal Antibodies and Beyond. Npj Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M.; AlJammaz, I. Development of the Tumor-Specific Antigen-Derived Synthetic Peptides as Potential Candidates for Targeting Breast and Other Possible Human Carcinomas. Molecules 2019, 24, 3142. [Google Scholar] [CrossRef]
- Baselga, J.; Swain, S.M. Novel Anticancer Targets: Revisiting ERBB2 and Discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Tuen Lee-Hoeflich, S.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A Central Role for HER3 in HER2-Amplified Breast Cancer: Implications for Targeted Therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef]
- Vranić, S.; Bešlija, S.; Gatalica, Z. Targeting HER2 Expression in Cancer: New Drugs and New Indications. Bosn. J. Basic Med. Sci. 2021, 21, 1–4. [Google Scholar] [CrossRef]
- Vernimmen, D.; Gueders, M.; Pisvin, S.; Delvenne, P.; Winkler, R. Different Mechanisms Are Implicated in ERBB2 Gene Overexpression in Breast and in Other Cancers. Br. J. Cancer 2003, 89, 899. [Google Scholar] [CrossRef]
- Giles, K.M.; Barker, A.; Zhang, P.M.; Epis, M.R.; Leedman, P.J. MicroRNA Regulation of Growth Factor Receptor Signaling in Human Cancer Cells. Methods Mol. Biol. 2011, 676, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 Expression Status in Diverse Cancers: Review of Results from 37,992 Patients. Cancer Metastasis Rev. 2015, 34, 157. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Bang, Y.J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2019, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Parker, B.A.; Schwab, R.; Kurzrock, R. HER2 Aberrations in Cancer: Implications for Therapy. Cancer Treat. Rev. 2014, 40, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Wang, X.V.; Makker, V.; Luoh, S.W.; Mitchell, E.P.; Zwiebel, J.A.; Sharon, E.; Gray, R.J.; Li, S.; McShane, L.M.; et al. Ado-Trastuzumab Emtansine (T-DM1) in Patients with HER2-Amplified Tumors Excluding Breast and Gastric/Gastroesophageal Junction (GEJ) Adenocarcinomas: Results from the NCI-MATCH Trial (EAY131) Subprotocol Q. Ann. Oncol. 2019, 30, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Scholl, S.; Beuzeboc, P.; Pouillart, P. Targeting HER2 in Other Tumor Types. Ann. Oncol. 2001, 12, S81–S87. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, S.; Mani, R.; Durgapal, P.; Goyal, B.; Rajput, D.; Rao, S.; Dhar, P.; Gupta, M.; Kishore, S.; et al. Expression of Human Epidermal Growth Factor Receptor 2, Survivin, Enhancer of Zeste Homolog -2, Cyclooxygenase-2, P53 and P16 Molecular Markers in Gall Bladder Carcinoma. J. Carcinog. 2021, 20, 7. [Google Scholar] [CrossRef]
- Plum, P.S.; Gebauer, F.; Krämer, M.; Alakus, H.; Berlth, F.; Chon, S.H.; Schiffmann, L.; Zander, T.; Büttner, R.; Hölscher, A.H.; et al. HER2/Neu (ERBB2) Expression and Gene Amplification Correlates with Better Survival in Esophageal Adenocarcinoma. BMC Cancer 2019, 19, 38. [Google Scholar] [CrossRef]
- Bealy, M.A.; Abugooda, A.A.; Ahmed, R.M.E.; Khalil, N.A.R.; Elasbali, A.M.; Mohamed, G.E.Y.; Eltom, F.M.; Ahmed, H. Patterns of Immunohistochemical Expression of P53, BCL2, PTEN, and HER2/Neu Tumor Markers in Specific Breast Cancer Lesions. Evid. Based. Complement. Alternat. Med. 2022, 2022, 2026284. [Google Scholar] [CrossRef]
- Kwon, C.H.; Seo, H.I.; Kim, D.U.; Han, S.Y.; Kim, S.; Lee, S.J.; Jeon, D.Y. HER2 Status Based on Breast Cancer Guidelines as a Useful Prognostic Marker of T2 Gallbladder Cancer. Eur. J. Surg. Oncol. 2022, in press. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and Prognostic Significance of EGFR, VEGF, and HER2 Expression in Cholangiocarcinoma. Br. J. Cancer 2008, 98, 418. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, G.; Zhang, Y.; Liu, H.; Zhang, J.; Nan, P.; Tian, W. PD-L1 and HER2 Expression in Gastric Adenocarcinoma and Their Prognostic Significance. Dig. Liver Dis. 2022, 54, 1419–1427. [Google Scholar] [CrossRef]
- Sun, L.; Schroeder, M.C.; Hagemann, I.S.; Pfeifer, J.D.; Schwarz, J.K.; Grigsby, P.W.; Markovina, S.; Lin, A.J. Expression of Potential Biomarker Targets by Immunohistochemistry in Cervical Carcinomas. Int. J. Gynecol. Pathol. 2022, 41, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.M.; Cantrell, L.A.; Stoler, M.H.; Mills, A.M. HER2 Overexpression and Amplification in Uterine Carcinosarcomas with Serous Morphology. Am. J. Surg. Pathol. 2022, 46, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Li, Y.; Li, Q.; Cai, S.; Li, X.; Ma, M. HER2 Status Is Positively Associated with Vessel Invasion of Colorectal Cancer: A Retrospective Large Cohort Study. Int. J. Color. Dis. 2022, 37, 2061–2067. [Google Scholar] [CrossRef]
- Pankaj, S.; Kumari, J.; Choudhary, V.; Kumari, A.; Kumari, S.; Kumari, A.; Nazneen, S.; Madhawi, R.; Kumar, S. Prognostic Value of HER-2/Neu Gene Amplification in Epithelial Ovarian Carcinoma. J. Obstet. Gynaecol. India 2019, 69, 177. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.M.; Hitt, R.; Sebastian, P.; Carracedo, C.; Lokanatha, D.; Bourhis, J.; Temam, S.; Cupissol, D.; De Raucourt, D.; Maroudias, N.; et al. Effects of Lapatinib Monotherapy: Results of a Randomised Phase II Study in Therapy-Naive Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Br. J. Cancer 2011, 105, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.A.K.; Anil, J.; Castro, P.D.; Kemnade, J.; Suzuki, M.; Hegde, M.; Hicks, J.; Yu, W.; Sandulache, V.; Sikora, A.G. Human Epidermal Growth Factor Receptor 2 Expression in Head and Neck Squamous Cell Carcinoma: Variation within and across Primary Tumor Sites, and Implications for Antigen-Specific Immunotherapy. Head Neck 2021, 43, 1983–1994. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Sumiyoshi, S.; Sonobe, M.; Kobayashi, M.; Uehara, T.; Fujimoto, M.; Tsuruyama, T.; Date, H.; Haga, H. HER2 Status in Lung Adenocarcinoma: A Comparison of Immunohistochemistry, Fluorescence in Situ Hybridization (FISH), Dual-ISH, and Gene Mutations. Lung Cancer 2014, 85, 373–378. [Google Scholar] [CrossRef]
- Aparicio, T.; Svrcek, M.; Zaanan, A.; Beohou, E.; Laforest, A.; Afchain, P.; Mitry, E.; Taieb, J.; Di Fiore, F.; Gornet, J.M.; et al. Small Bowel Adenocarcinoma Phenotyping, a Clinicobiological Prognostic Study. Br. J. Cancer 2013, 109, 3057–3066. [Google Scholar] [CrossRef]
- Kruger, S.F.; Lohneis, A.; Abendroth, A.; Berger, A.W.; Ettrich, T.J.; Waidmann, O.; Kapp, M.; Steiner, B.; Kumbrink, J.; Reischer, A.; et al. Prognosis and Tumor Biology of Pancreatic Cancer Patients with Isolated Lung Metastases: Translational Results from the German Multicenter AIO-YMO-PAK-0515 Study. ESMO Open 2022, 7, 11. [Google Scholar] [CrossRef]
- Vivaldi, C.; Fornaro, L.; Ugolini, C.; Niccoli, C.; Musettini, G.; Pecora, I.; Insilla, A.C.; Salani, F.; Pasquini, G.; Catanese, S.; et al. HER2 Overexpression as a Poor Prognostic Determinant in Resected Biliary Tract Cancer. Oncologist 2020, 25, 886. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Nguyen, E.; Unger, P.D.; Sherrod, A.E. Comparative Expression of Immunohistochemical Biomarkers in Cribriform and Pattern 4 Non-Cribriform Prostatic Adenocarcinoma. Exp. Mol. Pathol. 2020, 114, 104400. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Tobias-Machado, M.; Wroclawski, M.L.; Fonseca, F.L.A.; Teixeira, G.K.; Amarante, R.D.M.; Wroclawski, E.R.; Del Giglio, A. HER-2/Neu Expression in Prostate Adenocarcinoma: A Systematic Review and Meta-Analysis. J. Urol. 2010, 184, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Panvichian, R.; Tantiwetrueangdet, A.; Angkathunyakul, N.; Leelaudomlipi, S. TOP2A Amplification and Overexpression in Hepatocellular Carcinoma Tissues. Biomed Res. Int. 2015, 2015, 381602. [Google Scholar] [CrossRef] [PubMed]
- Alzeyadi, M.; Imarah, A.A.; Khayoon, S.Q.; Alhamadani, I.M. Cytogenetic Analysis of HER2 in Ovarian Cancer Patients by Fluorescence in Situ Hybridization. Eur. J. Eng. Sci. Technol. 2020, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Inman, J.L.; Kute, T.; White, W.; Pettenati, M.; Levine, E.A. Absence of HER2 Overexpression in Metastatic Malignant Melanoma. J. Surg. Oncol. 2003, 84, 82–88. [Google Scholar] [CrossRef]
- Lopes, L.F.; Bacchi, C.E. HER-2 Status in Gastrointestinal Stromal Tumor. Ann. Diagn. Pathol. 2008, 12, 283–285. [Google Scholar] [CrossRef]
- Mineo, J.F.; Bordron, A.; Baroncini, M.; Maurage, C.A.; Ramirez, C.; Siminski, R.M.; Berthou, C.; Dam Hieu, P. Low HER2-Expressing Glioblastomas Are More Often Secondary to Anaplastic Transformation of Low-Grade Glioma. J. Neurooncol. 2007, 85, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Han, J.; Zhen, L.; Zhang, T.; He, X.; Xu, E.; Li, M. HER2 Expression in Renal Cell Carcinoma Is Rare and Negatively Correlated with That in Normal Renal Tissue. Oncol. Lett. 2012, 4, 194. [Google Scholar] [CrossRef]
- Micke, P.; Hengstler, J.G.; Ros, E.; Bittinger, F.; Metz, T.; Gebhard, S.; Beeh, K.M.; Oesch, F.; Buhl, R. C-ErbB-2 Expression in Small-Cell Lung Cancer Is Associated with Poor Prognosis. Int. J. Cancer 2001, 92, 474–479. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Shah, T.; Watkins, J.; Marelli, L.; Khan, K.; Caplin, M.E. Expression of the HER-1-4 Family of Receptor Tyrosine Kinases in Neuroendocrine Tumours. Oncol. Rep. 2010, 23, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Peiper, M.; Heinecke, A.; Zurakowski, D.; Eisenberger, C.F.; Hosch, S.; Knoefel, W.T.; Izbicki, J.R. Expression of HER2/Neu Does Not Correlate with Survival in Soft Tissue Sarcoma. Onkologie 2003, 26, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Enkner, F.; Pichlhöfer, B.; Zaharie, A.T.; Krunic, M.; Holper, T.M.; Janik, S.; Moser, B.; Schlangen, K.; Neudert, B.; Walter, K.; et al. Molecular Profiling of Thymoma and Thymic Carcinoma: Genetic Differences and Potential Novel Therapeutic Targets. Pathol. Oncol. Res. 2017, 23, 551. [Google Scholar] [CrossRef]
- Mondi, M.M.; Rich, R.; Ituarte, P.; Wong, M.; Bergman, S.; Clark, O.H.; Perrier, N.D. HER2 Expression in Thyroid Tumors. Am. Surg. 2003, 69, 1100–1103. [Google Scholar] [CrossRef]
- Stankiewicz, E.; Prowse, D.M.; Ng, M.; Cuzick, J.; Mesher, D.; Hiscock, F.; Lu, Y.J.; Watkin, N.; Corbishley, C.; Lam, W.; et al. Alternative HER/PTEN/Akt Pathway Activation in HPV Positive and Negative Penile Carcinomas. PLoS ONE 2011, 6, e17517. [Google Scholar] [CrossRef]
- Cimpean, A.; Melnic, E.; Corlan, A.; Ceausu, A.; Raica, M. Heterogeneity of C Erb B family members expression is related to cell morphology and immunoprofile in pituitary adenomas. Res. Clin. Med. 2016, 1, 33–39. [Google Scholar]
- Roh, H.; Pippin, J.A.; Green, D.W.; Boswell, C.B.; Hirose, C.T.; Mokadam, N.; Drebin, J.A. HER2/Neu Antisense Targeting of Human Breast Carcinoma. Oncogene 2000, 19, 6138–6143. [Google Scholar] [CrossRef][Green Version]
- Ahmed, S.; Sami, A.; Xiang, J. HER2-Directed Therapy: Current Treatment Options for HER2-Positive Breast Cancer. Breast Cancer 2015, 22, 101–116. [Google Scholar] [CrossRef]
- Kunte, S.; Abraham, J.; Montero, A.J. Novel HER2–Targeted Therapies for HER2–Positive Metastatic Breast Cancer. Cancer 2020, 126, 4278–4288. [Google Scholar] [CrossRef]
- Nuciforo, P.; Thyparambil, S.; Aura, C.; Garrido-Castro, A.; Vilaro, M.; Peg, V.; Jimenez, J.; Vicario, R.; Cecchi, F.; Hoos, W.; et al. High HER2 Protein Levels Correlate with Increased Survival in Breast Cancer Patients Treated with Anti-HER2 Therapy. Mol. Oncol. 2016, 10, 138–147. [Google Scholar] [CrossRef]
- Schaller, G.; Bangemann, N.; Becker, C.; Bühler, H.; Opri, F.; Weitzel, H.K. Therapy of Metastatic Breast Cancer with Humanized Antibodies against the HER2 Receptor Protein. J. Cancer Res. Clin. Oncol. 1999, 125, 520–524. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal Antibody Therapy of Cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Widakowich, C.; de Castro, G.; de Azambuja, E.; Dinh, P.; Awada, A. Review: Side Effects of Approved Molecular Targeted Therapies in Solid Cancers. Oncologist 2007, 12, 1443–1455. [Google Scholar] [CrossRef]
- Vrbic, S.; Vrbic, S.; Pejcic, I.; Filipovic, S.; Kocic, B.; Vrbic, M. Current and Future Anti-HER2 Therapy in Breast Cancer Anti-HER2 Therapy in Breast Cancer. J. BUON 2013, 18, 5. [Google Scholar]
- Yao, M.; Fu, P. Advances in Anti-HER2 Therapy in Metastatic Breast Cancer. Chin. Clin. Oncol. 2018, 7, 6. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc Receptors Modulate in Vivo Cytoxicity against Tumor Targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Mohan, N.; Jiang, J.; Dokmanovic, M.; Wu, W.J. Trastuzumab-Mediated Cardiotoxicity: Current Understanding, Challenges, and Frontiers. Antib. Ther. 2018, 1, 13. [Google Scholar] [CrossRef]
- Zhang, H.; Burrows, F. Targeting Multiple Signal Transduction Pathways through Inhibition of Hsp90. J. Mol. Med. 2004, 82, 488–499. [Google Scholar] [CrossRef]
- Capelan, M.; Pugliano, L.; De Azambuja, E.; Bozovic, I.; Saini, K.S.; Sotiriou, C.; Loi, S.; Piccart-Gebhart, M.J. Pertuzumab: New Hope for Patients with HER2-Positive Breast Cancer. Ann. Oncol. 2013, 24, 273–282. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Esparís-Ogando, A.; Montero, J.; Arribas, J.; Ocaña, A.; Pandiella, A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr. Pharm. Des. 2016, 22, 5887–5898. [Google Scholar] [CrossRef]
- Amiri-Kordestani, L.; Wedam, S.; Zhang, L.; Tang, S.; Tilley, A.; Ibrahim, A.; Justice, R.; Pazdur, R.; Cortazar, P. First FDA Approval of Neoadjuvant Therapy for Breast Cancer: Pertuzumab for the Treatment of Patients with HER2-Positive Breast Cancer. Clin. Cancer Res. 2014, 20, 5359–5364. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-De-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Tarantino, P.; Morganti, S.; Uliano, J.; Giugliano, F.; Crimini, E.; Curigliano, G. Margetuximab for the Treatment of HER2-Positive Metastatic Breast Cancer. Expert Opin. Biol. Ther. 2020, 21, 127–133. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.J.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA Approval Summary: Margetuximab plus Chemotherapy for Advanced or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2022, 28, 1487–1492. [Google Scholar] [CrossRef]
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-Tumor Activity and Toxicokinetics Analysis of MGAH22, an Anti-HER2 Monoclonal Antibody with Enhanced Fcg Receptor Binding Properties. Breast Cancer Res. 2011, 13, R123. [Google Scholar] [CrossRef]
- Spector, N.; Xia, W.; El-Hariry, I.; Yarden, Y.; Bacus, S. HER2 Therapy. Small Molecule HER-2 Tyrosine Kinase Inhibitors. Breast Cancer Res. 2007, 9, 205. [Google Scholar] [CrossRef]
- Wynn, C.S.; Tang, S.-C.C. Anti-HER2 Therapy in Metastatic Breast Cancer: Many Choices and Future Directions. Cancer Metastasis Rev. 2022, 41, 193–209. [Google Scholar] [CrossRef]
- Pernas, S.; Tolaney, S.M. HER2-Positive Breast Cancer: New Therapeutic Frontiers and Overcoming Resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef]
- Ryan, Q.; Ibrahim, A.; Cohen, M.H.; Johnson, J.; Ko, C.; Sridhara, R.; Justice, R.; Pazdur, R. FDA Drug Approval Summary: Lapatinib in Combination with Capecitabine for Previously Treated Metastatic Breast Cancer That Overexpresses HER-2. Oncologist 2008, 13, 1114–1119. [Google Scholar] [CrossRef]
- Schlam, I.; Swain, S.M. HER2-Positive Breast Cancer and Tyrosine Kinase Inhibitors: The Time Is Now. Npj Breast Cancer 2021, 7, 56. [Google Scholar] [CrossRef]
- Xuhong, J.-C.; Qi, X.-W.; Zhang, Y.; Jiang, J. Review Article Mechanism, Safety and Efficacy of Three Tyrosine Kinase Inhibitors Lapatinib, Neratinib and Pyrotinib in HER2-Positive Breast Cancer. Am. J. Cancer Res. 2019, 9, 2103–2119. [Google Scholar]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Shah, M.; Wedam, S.; Cheng, J.; Fiero, M.H.; Xia, H.; Li, F.; Fan, J.; Zhang, X.; Yu, J.; Song, P.; et al. FDA Approval Summary: Tucatinib for the Treatment of Patients with Advanced or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2021, 27, 1220–1226. [Google Scholar] [CrossRef]
- Lambert, J.M.; Chari, R.V.J. Ado-Trastuzumab Emtansine (T-DM1): An Antibody-Drug Conjugate (ADC) for HER2-Positive Breast Cancer. J. Med. Chem. 2014, 57, 6949–6964. [Google Scholar] [CrossRef]
- Tong, J.T.W.; Harris, P.W.R.; Brimble, M.A.; Kavianinia, I. An Insight into Fda Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Liu, B.; Lv, D.; Zhai, J.; Guan, X.; Yi, Z.; Ma, F.; Wang, N. Antibody-Drug Conjugates in HER2-Positive Breast Cancer. Chin. Med. J. 2022, 135, 261–267. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887. [Google Scholar] [CrossRef]
- Nadal-Serrano, M.; Morancho, B.; Escrivá-de-Romaní, S.; Bernadó Morales, C.; Luque, A.; Escorihuela, M.; Espinosa Bravo, M.; Peg, V.; Dijcks, F.A.; Dokter, W.H.A.; et al. The Second Generation Antibody-Drug Conjugate SYD985 Overcomes Resistances to T-DM1. Cancers 2020, 12, 670. [Google Scholar] [CrossRef]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol. Ther. Exp. 2020, 68, 2. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Guevara-Hoyer, K.; Baliu-Piqué, M.; García-Sáenz, J.Á.; Pérez-Segura, P.; Pandiella, A.; Ocaña, A. Adoptive Cell Therapy in Breast Cancer: A Current Perspective of Next-Generation Medicine. Front. Oncol. 2020, 10, 605633. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, H.; Neudorfer, J.; Gebhard, K.; Conrad, H.; Hermann, C.; Nährig, J.; Falko, F.; Weber, W.; Busch, D.H.; Peschel, C.; et al. Adoptive Transfer of Autologous, HER2-Specific, Cytotoxic T Lymphocytes for the Treatment of HER2-Overexpressing Breast Cancer. Cancer Immunol. Immunother. 2007, 57, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Hernandez, T.; de Miguel, M.; Doger, B.; Calvo, E. Adoptive Cell Therapy for Solid Tumors: Chimeric Antigen Receptor T Cells and Beyond. Curr. Opin. Pharmacol. 2021, 59, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Bear, A.S.; Fraietta, J.A.; Narayan, V.K.; O’Hara, M.; Haas, N.B. Adoptive Cellular Therapy for Solid Tumors. Am. Soc. Clin. Oncol. Educ. B. 2021, 41, 57–65. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef]
- Oved, J.H.; Barrett, D.M.; Teachey, D.T. Cellular Therapy: Immune-Related Complications. Immunol. Rev. 2019, 290, 114–126. [Google Scholar] [CrossRef]
- Wachsmann, T.L.A.; Wouters, A.K.; Remst, D.F.G.; Hagedoorn, R.S.; Meeuwsen, M.H.; van Diest, E.; Leusen, J.; Kuball, J.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Comparing CAR and TCR Engineered T Cell Performance as a Function of Tumor Cell Exposure. Oncoimmunology 2022, 11, 2033528. [Google Scholar] [CrossRef]
- Budi, H.S.; Ahmad, F.N.; Achmad, H.; Ansari, M.J.; Mikhailova, M.V.; Suksatan, W.; Chupradit, S.; Shomali, N.; Marofi, F. Human Epidermal Growth Factor Receptor 2 (HER2)-Specific Chimeric Antigen Receptor (CAR) for Tumor Immunotherapy; Recent Progress. Stem Cell Res. Ther. 2022, 13, 40. [Google Scholar] [CrossRef]
- Ladjemi, M.Z.; Jacot, W.; Chardès, T.; Pèlegrin, A.; Navarro-Teulon, I. Anti-HER2 Vaccines: New Prospects for Breast Cancer Therapy. Cancer Immunol. Immunother. 2010, 59, 1295–1312. [Google Scholar] [CrossRef]
- Marmé, F. Immunotherapy in Breast Cancer. Oncol. Res. Treat. 2016, 39, 335–345. [Google Scholar] [CrossRef]
- Tobias, J.; Battin, C.; De Sousa Linhares, A.; Lebens, M.; Baier, K.; Ambroz, K.; Drinić, M.; Högler, S.; Inic-Kanada, A.; Garner-Spitzer, E.; et al. A New Strategy Toward B Cell-Based Cancer Vaccines by Active Immunization With Mimotopes of Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, U.; Garner-Spitzer, E.; Chao, Y.; Maglakelidze, M.; Bulat, I.; Dechaphunkul, A.; Arpornwirat, W.; Charoentum, C.; Yen, C.J.; Yau, T.C.; et al. Clinical and Immunologic Responses to a B-Cell Epitope Vaccine in Patients with HER2/Neu-Overexpressing Advanced Gastric Cancer—Results from Phase Ib Trial IMU.ACS.001. Clin. Cancer Res. 2021, 27, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Al-Shami, K.M.; Yaghan, R.J. Immunotherapy for HER2-Positive Breast Cancer: Recent Advances and Combination Therapeutic Approaches. Breast Cancer 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.D.; Payne, K.K.; Posey, A.D.; Hill, C.; Conejo-Garcia, J.; June, C.H.; Tchou, J. Immunotherapy for Breast Cancer: Current and Future Strategies HHS Public Access. Curr. Surg. Rep. 2017, 5, 31. [Google Scholar] [CrossRef]
- Wennhold, K.; Shimabukuro-Vornhagen, A.; Von Bergwelt-Baildon, M. B Cell-Based Cancer Immunotherapy. Transfus. Med. Hemotherapy 2019, 46, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Foy, T.M.; Fanger, G.R.; Hand, S.; Gerard, C.; Bruck, C.; Cheever, M.A. Designing HER2 Vaccines. Semin. Oncol. 2002, 29, 53–61. [Google Scholar] [CrossRef]
- Curigliano, G.; Spitaleri, G.; Petri, E.; Rescigno, M.; de Braud, F.; Cardillo, A.; Munzone, E.; Rocca, A.; Bonizzi, G.; Brichard, V.; et al. Breast Cancer Vaccines: A Clinical Reality or Fairy Tale? Ann. Oncol. 2006, 17, 750–762. [Google Scholar] [CrossRef]
- Keshavarz-Fathi, M.; Rezaei, N. Candidate Cancers for Vaccination. In Vaccines for Cancer Immunotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–152. [Google Scholar]
- Datta, J.; Xu, S.; Rosemblit, C.; Smith, J.B.; Cintolo, J.A.; Powell, D.J.; Czerniecki, B.J. CD4+ T-Helper Type 1 Cytokines and Trastuzumab Facilitate CD8+ T-Cell Targeting of HER2/ Neu –Expressing Cancers. Cancer Immunol. Res. 2015, 3, 455–463. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Hiller, J.P.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Peoples, G.E.; Goedegebuure, P.S.; Smith, R.; Linehan, D.C.; Yoshino, I.; Eberlein, T.J. Breast and Ovarian Cancer-Specific Cytotoxic T Lymphocytes Recognize the Same HER2/Neu-Derived Peptide. Proc. Natl. Acad. Sci. USA 1995, 92, 432–436. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Ardavanis, A.; Litton, J.K.; Shumway, N.M.; Hale, D.F.; Murray, J.L.; Perez, S.A.; Ponniah, S.; Baxevanis, C.N.; Papamichail, M.; et al. Primary Analysis of a Prospective, Randomized, Single-Blinded Phase II Trial Evaluating the HER2 Peptide GP2 Vaccine in Breast Cancer Patients to Prevent Recurrence. Oncotarget 2016, 7, 66192. [Google Scholar] [CrossRef]
- Costa, R.L.B.; Soliman, H.; Czerniecki, B.J. The Clinical Development of Vaccines for HER2+ Breast Cancer: Current Landscape and Future Perspectives. Cancer Treat. Rev. 2017, 61, 107–115. [Google Scholar] [CrossRef]
- Holmes, J.P.; Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Hueman, M.T.; Mittendorf, E.A.; Murray, J.L.; Amin, A.; Craig, D.; Von Hofe, E.; et al. Results of the First Phase I Clinical Trial of the Novel II-Key Hybrid Preventive HER-2/Neu Peptide (AE37) Vaccine. J. Clin. Oncol. 2008, 26, 3426–3433. [Google Scholar] [CrossRef]
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. J. Immunol. Res. 2017, 2017, 2680160. [Google Scholar] [CrossRef]
- Nordin, M.L.; Norpi, A.S.M.; Ng, P.Y.; Yusoff, K.; Abu, N.; Lim, K.P.; Azmi, F. HER2/Neu-Based Peptide Vaccination-Pulsed with B-Cell Epitope Induced Efficient Prophylactic and Therapeutic Antitumor Activities in TUBO Breast Cancer Mice Model. Cancers 2021, 13, 4958. [Google Scholar] [CrossRef]
- Garrett, J.T.; Rawale, S.; Allen, S.D.; Phillips, G.; Forni, G.; Morris, J.C.; Kaumaya, P.T.P. Novel Engineered Trastuzumab Conformational Epitopes Demonstrate in Vitro and in Vivo Antitumor Properties against HER-2/Neu. J. Immunol. 2007, 178, 7120–7131. [Google Scholar] [CrossRef]
- Wiedermann, U.; Davis, A.B.; Zielinski, C.C. Vaccination for the Prevention and Treatment of Breast Cancer with Special Focus on Her-2/Neu Peptide Vaccines. Breast Cancer Res. Treat. 2013, 138, 1–12. [Google Scholar] [CrossRef]
- Tobias, J.; Jasinska, J.; Baier, K.; Kundi, M.; Ede, N.; Zielinski, C.; Wiedermann, U. Enhanced and Long Term Immunogenicity of a Her-2/Neu Multi-Epitope Vaccine Conjugated to the Carrier CRM197 in Conjunction with the Adjuvant Montanide. BMC Cancer 2017, 17, 118. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mohabatkar, H.; Keyhanfar, M.; Dehkordi, A.J.; Rabbani, M. Linear and Conformational B Cell Epitope Prediction of the HER 2 ECD-Subdomain III by in Silico Methods. Asian Pac. J. Cancer Prev. 2012, 13, 3053–3059. [Google Scholar] [CrossRef]
- Wiedermann, U.; Wiltschke, C.; Jasinska, J.; Kundi, M.; Zurbriggen, R.; Garner-Spitzer, E.; Bartsch, R.; Steger, G.; Pehamberger, H.; Scheiner, O.; et al. A Virosomal Formulated Her-2/Neu Multi-Peptide Vaccine Induces Her-2/Neu-Specific Immune Responses in Patients with Metastatic Breast Cancer: A Phase i Study. Breast Cancer Res. Treat. 2010, 119, 673–683. [Google Scholar] [CrossRef]
- Maglakelidze, M.; Ryspayenva, D.; Bulat, I.; Andric, Z.; Nikolic, I.; Chawla, T.; Choudhary, V.; Venkata, G.; Radosavljevic, D.; Petrovic, Z.; et al. P-159 HERIZON: Phase 2 Part of the IMU-131 HER2/Neu Vaccine plus Chemotherapy Study Randomized in Patients with HER2/NEU Overexpressing Metastatic or Advanced Adenocarcinoma of the Stomach or Gastroesophageal Junction. Ann. Oncol. 2021, 32, S154. [Google Scholar] [CrossRef]
- A Study of IMU-131 (HER-Vaxx) in Combination With Chemotherapy or Pembrolizumab in Patients with Metastatic HER2/Neu Over-Expressing Gastric Cancer (NextHERIZON)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05311176?term=vaccine&cond=her2+cancer&draw=2&rank=76 (accessed on 23 September 2022).
- Allen, S.D.; Garrett, J.T.; Rawale, S.V.; Jones, A.L.; Phillips, G.; Forni, G.; Morris, J.C.; Oshima, R.G.; Kaumaya, P.T.P. Peptide Vaccines of the HER-2/Neu Dimerization Loop Are Effective in Inhibiting Mammary Tumor Growth in Vivo. J. Immunol. 2007, 179, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; Wesolowski, R.; Ahn, D.H.; Wu, C.; Mortazavi, A.; Lustberg, M.; Ramaswamy, B.; Fowler, J.; Wei, L.; Overholser, J.; et al. Phase i Immunotherapy Trial with Two Chimeric HER-2 B-Cell Peptide Vaccines Emulsified in Montanide ISA 720VG and Nor-MDP Adjuvant in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3495–3507. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2019, 120, 3210–3229. [Google Scholar] [CrossRef]
- Kaumaya, P.T.P.; Guo, L.; Overholser, J.; Penichet, M.L.; Bekaii-Saab, T. Immunogenicity and Antitumor Efficacy of a Novel Human PD-1 B-Cell Vaccine (PD1-Vaxx) and Combination Immunotherapy with Dual Trastuzumab/Pertuzumab-like HER-2 B-Cell Epitope Vaccines (B-Vaxx) in a Syngeneic Mouse Model. Oncoimmunology 2020, 9, 1818437. [Google Scholar] [CrossRef]
- Al-Awadhi, A.; Lee Murray, J.; Ibrahim, N.K. Developing Anti-HER2 Vaccines: Breast Cancer Experience. Int. J. Cancer 2018, 143, 2126–2132. [Google Scholar] [CrossRef]
- Gelao, L.; Criscitiello, C.; Esposito, A.; De Laurentiis, M.; Fumagalli, L.; Locatelli, M.A.; Minchella, I.; Santangelo, M.; De Placido, S.; Goldhirsch, A.; et al. Dendritic Cell-Based Vaccines: Clinical Applications in Breast Cancer. Immunotherapy 2014, 6, 349–360. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, A.U.R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Garg, A.D.; Vara Perez, M.; Schaaf, M.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Dendritic Cell-Based Anticancer Immunotherapy. Oncoimmunology 2017, 6, e1328341. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-Positive Breast Cancer: State of the Art and Future Perspectives. J. Hematol. Oncol. 2019, 12, 1–26. [Google Scholar] [CrossRef]
- Shevchenko, J.A.; Khristin, A.A.; Kurilin, V.V.; Kuznetsova, M.S.; Blinova, D.D.; Starostina, N.M.; Sidorov, S.V.; Sennikov, S.V. Autologous Dendritic Cells and Activated Cytotoxic T-cells as Combination Therapy for Breast Cancer. Oncol. Rep. 2020, 43, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Schlam, I.; Tarantino, P.; Tolaney, S.M. Overcoming Resistance to HER2-Directed Therapies in Breast Cancer. Cancers 2022, 14, 3996. [Google Scholar] [CrossRef] [PubMed]
- Elshazly, A.M.; Gewirtz, D.A. An Overview of Resistance to Human Epidermal Growth Factor Receptor 2 (Her2) Targeted Therapies in Breast Cancer. Cancer Drug Resist. 2022, 5, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Kruser, T.J.; Wheeler, D.L. Mechanisms of Resistance to HER Family Targeting Antibodies. Exp. Cell Res. 2010, 316, 1083–1100. [Google Scholar] [CrossRef] [PubMed]
- Tortora, G. Mechanisms of Resistance to HER2 Target Therapy. JNCI Monogr. 2011, 2011, 95–98. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2022, 1–26. [Google Scholar] [CrossRef]
- Blangé, D.; Stroes, C.I.; Derks, S.; Bijlsma, M.F.; van Laarhoven, H.W.M. Resistance Mechanisms to HER2-Targeted Therapy in Gastroesophageal Adenocarcinoma: A Systematic Review. Cancer Treat. Rev. 2022, 108, 102418. [Google Scholar] [CrossRef]
- Filho, O.M.; Viale, G.; Stein, S.; Trippa, L.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.M.; Waks, A.G.; et al. Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov. 2021, 11, 2474. [Google Scholar] [CrossRef]
- Adam-Artigues, A.; Arenas, E.J.; Martínez-Sabadell, A.; Brasó-Maristany, F.; Cervera, R.; Tormo, E.; Hernando, C.; Martínez, M.T.; Carbonell-Asins, J.; Simón, S.; et al. Targeting HER2-AXL Heterodimerization to Overcome Resistance to HER2 Blockade in Breast Cancer. Sci. Adv. 2022, 8, 2746. [Google Scholar] [CrossRef]
- Najor, M.; Rempert, T.H.; Bishehsari, F. Resistance to HER2-Targeted Therapies Results in Upregulation of MCL-1 and Sensitivity to Olaparib. Artic. Int. J. Sci. 2020, 9, 7–17. [Google Scholar] [CrossRef]
- Marin, A.; Al Mamun, A.; Akamatsu, H.; Ye, D.; Sudhan, D.R.; Brown, B.P.; Eli, L.; Marcelain, K.; Meiler, J.; Arteaga, C.L.; et al. Acquired Secondary HER2 Mutations Enhance HER2/MAPK Signaling and Promote Resistance to HER2 Kinase Inhibition in HER2-Mutant Breast Cancer. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Woo, S.R.; Zha, Y.; Spaapen, R.; Zheng, Y.; Corrales, L.; Spranger, S. Cancer Immunotherapy Strategies Based on Overcoming Barriers within the Tumor Microenvironment. Curr. Opin. Immunol. 2013, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Franzén, A.S.; Raftery, M.J.; Pecher, G. Implications for Immunotherapy of Breast Cancer by Understanding the Microenvironment of a Solid Tumor. Cancers 2022, 14, 3178. [Google Scholar] [CrossRef] [PubMed]
- Frankel, T.; Lanfranca, M.P.; Zou, W. The Role of Tumor Microenvironment in Cancer Immunotherapy. Adv. Exp. Med. Biol. 2017, 1036, 51–64. [Google Scholar] [CrossRef]
- Macneil, I.A.; Burns, D.J.; Rich, B.E.; Soltani, S.M.; Kharbush, S.; Osterhaus, N.G.; Sullivan, B.F.; Hawkins, D.M.; Pietruska, J.R.; Laing, L.G. New HER2-Negative Breast Cancer Subtype Responsive to Anti-HER2 Therapy Identified. J. Cancer Res. Clin. Oncol. 2020, 146, 605–619. [Google Scholar] [CrossRef]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; McNally, K.A.; Park, J.S.; Lim, W.A. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef]
| Tumor Type | HER2 Positivity (%), Reported by Yan et al. [30] | HER2 Positivity (%), Reported by Other Studies |
|---|---|---|
| Bladder cancers | 12.4 | 16 [35] |
| Esophageal and esophagogastric junction cancers | 11.3 | 14.9 [36] |
| Breast cancers | 10.5 | 18.3 [37] |
| Gallbladder cancers | 9.8 | 11.11 [38] |
| Cholangiocarcinomas (extrahepatic) | 6.3 | 8.5 [39] |
| Gastric adenocarcinomas | 4.7 | 17.3 [40] |
| Cervical cancers | 3.9 | 1.5 [41] |
| Uterine cancers | 3 | 6 [42] |
| Testicular cancers | 2.4 | 5–8 [32] |
| Colorectal cancers | 1.8 | 2 [43] |
| Ovarian (epithelial) cancers | 1.6 | 8.16 [44] |
| Head and neck carcinomas | 1.3 | 4–19 [45,46] |
| Lung cancers (non-small cells) | 1.1 | 2.5 [47] |
| Intestinal (small) malignancies | 0.9 | 3 [48] |
| Pancreatic adenocarcinomas | 0.7 | 2 [49] |
| Cholangiocarcinomas (intrahepatic) | 0.6 | 4 [50] |
| Prostate cancers | 0.6 | 1.5 [51,52] |
| Hepatocellular carcinomas | 0.4 | 0 [53] |
| Ovarian (non-epithelial) cancers | 0.4 | 7.69 [54] |
| Melanomas | 0.1 | 0 [55] |
| Gastrointestinal stromal tumors | 0 | 0 [56] |
| Glioblastoma multiforme, high grade gliomas | 0 | 0 [57] |
| Kidney cancers | 0 | 2.3 [58] |
| Lung cancers (small-cells) | 0 | 13 [59] |
| Melanomas (uveal) | 0 | Not found |
| Neuroendocrine tumors | 0 | 0 [60] |
| Sarcomas (peritoneal, retroperitoneal) | 0 | Not found |
| Sarcomas (soft tissues) | 0 | 8 [61] |
| Thymic cancers | 0 | 0 [62] |
| Thyroid cancers | 0 | 0 [63] |
| Gliomas (low-grade) | 0 | 7 [57] |
| Oligodendrogliomas | 0 | Not found |
| Penile cancers | 0 | 0 [64] |
| Pituitary cancers | 0 | 5 [65] |
| Solitary fibrous tumors | 0 | Not found |
| Overall | 2.7 | Not found |
| NCT Number | Study Title | Study Status | Conditions | Interventions | Phases |
|---|---|---|---|---|---|
| NCT03267173 | Evaluate the Safety and Efficacy of CAR-T in the Treatment of Pancreatic Cancer. | UNKNOWN | Pancreatic Cancer | Drug: Mesothelin, PSCA, CEA, HER2, MUC1, EGFRvIII targeted and other CAR-T cell | EARLY_PHASE1 |
| NCT02547961 | Chimeric Antigen Receptor-Modified T Cells for Breast Cancer | WITHDRAWN (Project terminated due to revision of local regulations) | Breast Cancer | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1|PHASE2 |
| NCT04511871 | A Phase I Trial of CCT303-406 in Patients with Relapsed or Refractory HER2 Positive Solid Tumors | RECRUITING | Solid Tumor, Gastric Cancer, Breast Cancer, Ovarian Cancer, Sarcoma | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT04903080 | HER2-specific Chimeric Antigen Receptor (CAR) T Cells for Children with Ependymoma | RECRUITING | Ependymoma | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT00902044 | Her2 Chimeric Antigen Receptor Expressing T Cells in Advanced Sarcoma | ACTIVE_NOT_RECRUITING | Sarcoma | GENETIC: Autologous HER2-targeted CAR-T cells, DRUG: Fludarabine, DRUG: Cyclophosphamide | PHASE1 |
| NCT02713984 | A Clinical Research of CAR T Cells Targeting HER2 Positive Cancer | WITHDRAWN (Reform CAR structure due to safety consideration) | Breast Cancer, Ovarian Cancer, Lung Cancer, Gastric Cancer, Colorectal Cancer, Glioma, Pancreatic Cancer | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1, PHASE2 |
| NCT03389230 | Memory-Enriched T Cells in Treating Patients with Recurrent or Refractory Grade III-IV Glioma | RECRUITING | Glioblastoma, Malignant Glioma, Recurrent Glioma, Refractory Glioma, WHO Grade III Glioma | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT03696030 | HER2-CAR T Cells in Treating Patients with Recurrent Brain or Leptomeningeal Metastases | RECRUITING | Malignant Neoplasm, Metastatic Malignant Neoplasm in the Brain, Metastatic Malignant Neoplasm in the Leptomeninges, Breast Cancer, HER2-positive Breast Cancer | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT04650451 | Safety and Activity Study of HER2-Targeted Dual Switch CAR-T Cells (BPX-603) in Subjects with HER2-Positive Solid Tumors | RECRUITING | HER-2 Gene Amplification, HER2-positive Gastric Cancer, HER2-positive Breast Cancer, HER-2 Protein Overexpression, Solid Tumor, Adult | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT03740256 | Binary Oncolytic Adenovirus in Combination with HER2-Specific Autologous CAR VST, Advanced HER2 Positive Solid Tumors | RECRUITING | Bladder Cancer, Head and Neck Squamous Cell Carcinoma, Cancer of the Salivary Gland, Lung Cancer, Breast Cancer, Gastric Cancer, Esophageal Cancer, Colorectal Cancer, Pancreatic Adenocarcinoma, Solid Tumor | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT04995003 | HER2 Chimeric Antigen Receptor (CAR) T Cells in Combination with Checkpoint Blockade in Patients with Advanced Sarcoma | RECRUITING | Sarcoma, HER-2 Protein Overexpression | GENETIC: HER2-targeted CAR-T cells, DRUG: Pembrolizumab, DRUG: Nivolumab, DRUG: Lymphodepletion Chemotherapy | PHASE1 |
| NCT04684459 | Dual-targeting HER2 and PD-L1 CAR-T for Cancers with Pleural or Peritoneal Metastasis | RECRUITING | Peritoneal Carcinoma Metastatic, Pleural Effusion, Malignant | BIOLOGICAL: Dual-targeting HER2 and PD-L1 CAR-T cells | EARLY_PHASE1 |
| NCT00889954 | Her2 and TGFBeta Cytotoxic T Cells in Treatment of Her2 Positive Malignancy | COMPLETED | HER2 Positive Malignancies | BIOLOGICAL: TGFBeta resistant HER2/EBV-CTLs (CAR-T cells) | PHASE1 |
| NCT00924287 | Gene Therapy Using Anti-Her-2 Cells to Treat Metastatic Cancer | TERMINATED (This study was terminated after the first patient treated on study died as a result of the treatment.) | Metastatic Cancer | DRUG: HER2-targeted CAR-T cells plus IV IL-2, DRUG: Cyclophosphamide, DRUG: Fludarabine, DRUG: Mesna | PHASE1, PHASE2 |
| NCT02442297 | T Cells Expressing HER2-specific Chimeric Antigen Receptors (CAR) for Patients with HER2-Positive CNS Tumors | RECRUITING | Brain Tumor, Recurrent, Brain Tumor, Refractory | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT04430595 | Multi-4SCAR-T Therapy Targeting Breast Cancer | RECRUITING | Breast Cancer | BIOLOGICAL: multiple 4th generation CAR-T cells targeted Her2, GD2, and CD44v6 | PHASE1, PHASE2 |
| NCT01109095 | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients with GBM | COMPLETED | Glioblastoma Multiforme (GBM) | BIOLOGICAL: HER2-targeted CAR CMV-specific CTLs (CMV-specific cytotoxic T cells) | PHASE1 |
| NCT03500991 | HER2-specific CAR T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors | RECRUITING | Central Nervous System Tumor, Pediatric, Glioma, Ependymoma, Medulloblastoma, Germ Cell Tumor, Atypical Teratoid/Rhabdoid Tumor, Primitive Neuroectodermal Tumor, Choroid Plexus Carcinoma, Pineoblastoma | BIOLOGICAL: HER2-targeted CAR-T cells | PHASE1 |
| NCT03198052 | PSCA/MUC1/TGFOI/HER2/Mesothelin/Lewis-Y/GPC3/AXL/EGFR/B7-H3/Claudin18.2-CAR-T Cells Immunotherapy Against Cancers | RECRUITING | Lung Cancer, Cancer, Immunotherapy, CAR-T Cell | BIOLOGICAL: CAR-T cells targeting PSCA, MUC1, TGFOI, HER2, Mesothelin, Lewis-Y, GPC3, AXL, EGFR, Claudin18.2, or B7-H3 | PHASE1 |
| Resistance-Causing Factor | Mechanism of Resistance | Strategies to Overcome Resistance | Resource |
|---|---|---|---|
| HER2(L755S) mutation | Activating mutation | Second-generation TKIs (neratinib) | [144,148] |
| Overexpression of HER1 and HER3 | Enhancing the affinity of HER2/HER3 and HER1/HER3 and reducing HER2 binding to neratinib | Combined anti-HER2, PI3K inhibition and TKIs (neratinib and lapatinib) | [144,146,148,149] |
| Generation of p95HER2 | A truncated form of HER2 that lacks the ECD but retains kinase activity | Combined chemotherapy (paclitaxel), trastuzumab and TKIs (lapatinib) | [144,148] |
| Overexpression of mucin 4 (MUC4) and the CD44–hyaluronan polymer complex | Masking the HER2 epitope as well as stabilizing and activating HER2 | Combined soluble TNF inhibitors, trastuzumab and TKIs (lapatinib) | [144,146,148,149] |
| Loss of PTEN | Hyperactivation of the mTOR pathway | Combined PI3K trastuzumab and pertuzumab Combination of PI3K and MEK inhibitors | [144,145,146,148,149] |
| PIK3CA mutations | Increased and unregulated activation of the PI3K pathway | T-DM1 PI3K inhibitors in combination with trastuzumab and pertuzumab Combined mTOR inhibitor (everolimus), trastuzumab and chemotherapy | [144,145,146,148,149] |
| Expression of estrogen receptors | Offering an escape from HER2 signaling inhibition insuring tumor survival | Concomitant inhibition of both ER and HER2 signaling | [144,148] |
| Overexpression of Cyclin D1 and/or CDK4/6 | Activating cell proliferation | Combination blocking of HER2 and ER and CDK4/6–cyclin D1 activation | [148,149] |
| RAS–MAPK activating mutations | Sustained activation of RAS–MAPK signaling | MEK–ERK inhibitors | [148] |
| Heterogeneous expression of HER2 | Subclone lacking the target could escape the effects of the targeted therapy and lead to tumor recurrence | Potent HER2-targeted agents (T-DXd) | [144,148,150] |
| Expressing high levels of HLA g by tumor cells | Inhibiting NK cells through the engagement of killer cell immunoglobulin-like receptors (KIRs) | Combined blockade of HLA g and PDL1/PD1 | [148] |
| Overexpression of CD47 by tumor cells | Inhibiting phagocytosis | Combination of magrolimab (mAb that targets CD47) and trastuzumab | [148] |
| Expression of CDK12- or RAC1 by tumor cells | Activating cell proliferation | Combinations of HER2 TKIs, CDK12 and RAC1 inhibitors | [148] |
| Increased activity and expression of the drug efflux pump | Reducing the cytotoxic effect of T-DM1 | Combination of T-DM1 and pump inhibitors | [144] |
| c-MET hyperactivity or amplification | Inducing HER3-mediated activation of PI3K Sustained Akt activation | c-MET inhibitors | [145,146,149] |
| Overexpression of IGF1R | Activation of HER2 Inducing degradation of p27 | IGF1R signaling inhibition | [145,146] |
| Src activation | Inhibiting PTEN | Combination of trastuzumab and Src inhibitor (dasatinib) | [145,149] |
| Suppression of the PP2A family | Sustained activation of the PI3K/AKT/mTOR pathway | Combination of EZH2 inhibitor with HER2-targeted therapy | [145] |
| Upregulation of miR-221 | Inhibiting PTEN Targeting p57 and p27 | Src inhibitors | [145] |
| AXL overexpression | Activation of PI3K/AKT and MAPK pathways in a ligand-independent manner | AXL inhibitor plus trastuzumab | [151] |
| Upregulation of MCL-1 | Inhibition of apoptosis | PARP inhibitor (olaparib) | [149,152] |
| Co-expression of HER2(T862A) and HER2(L755S) mutations | Enhancing HER2 activation and impairing TKIs sensitivity | Combined inhibition of HER2 and MEK | [153] |
| Activating mutations in TGF-β | Enhancing HER ligand shedding | Trastuzumab, pertuzumab and TGFꞵ inhibitors | [146,149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrhmoun, S.; Sennikov, S. The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions. Cancers 2022, 14, 6173. https://doi.org/10.3390/cancers14246173
Alrhmoun S, Sennikov S. The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions. Cancers. 2022; 14(24):6173. https://doi.org/10.3390/cancers14246173
Chicago/Turabian StyleAlrhmoun, Saleh, and Sergey Sennikov. 2022. "The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions" Cancers 14, no. 24: 6173. https://doi.org/10.3390/cancers14246173
APA StyleAlrhmoun, S., & Sennikov, S. (2022). The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions. Cancers, 14(24), 6173. https://doi.org/10.3390/cancers14246173






