Impact of Sarcopenia on Patients with Localized Pancreatic Ductal Adenocarcinoma Receiving FOLFIRINOX or Gemcitabine as Adjuvant Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Method
2.1. Patients
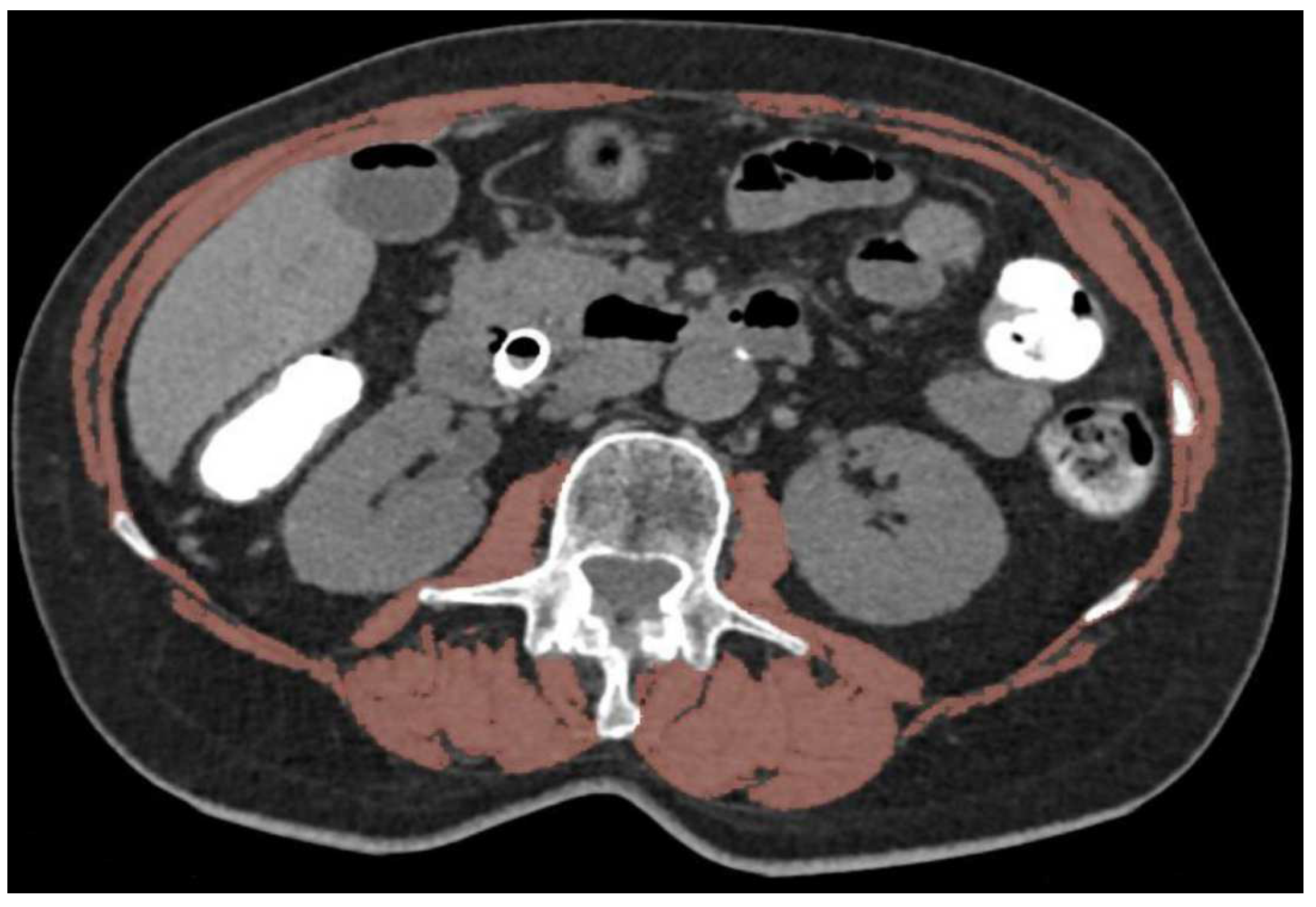
2.2. Body Composition Evaluation
2.3. Statistical Analysis
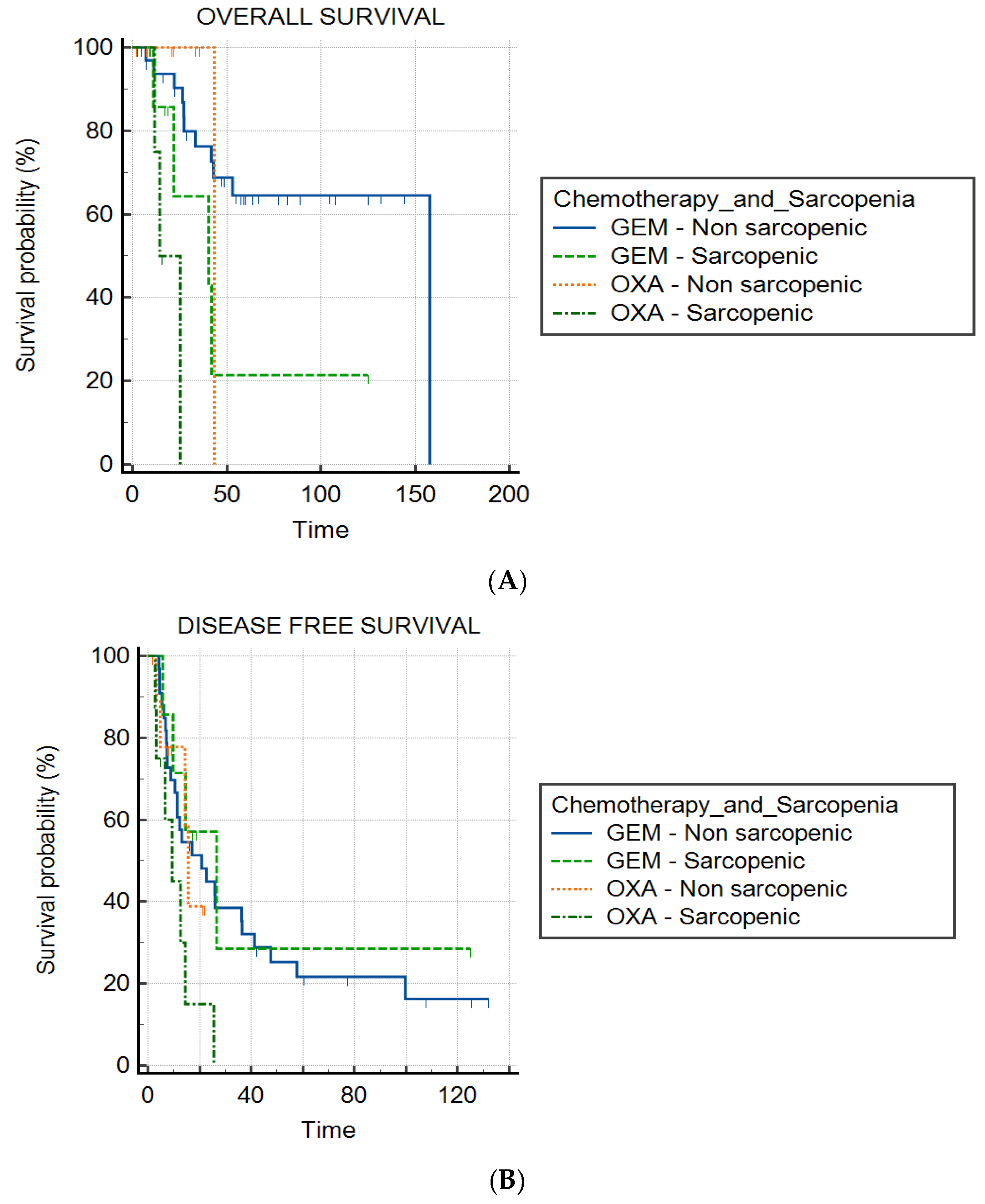
3. Results
3.1. Adjuvant Therapy
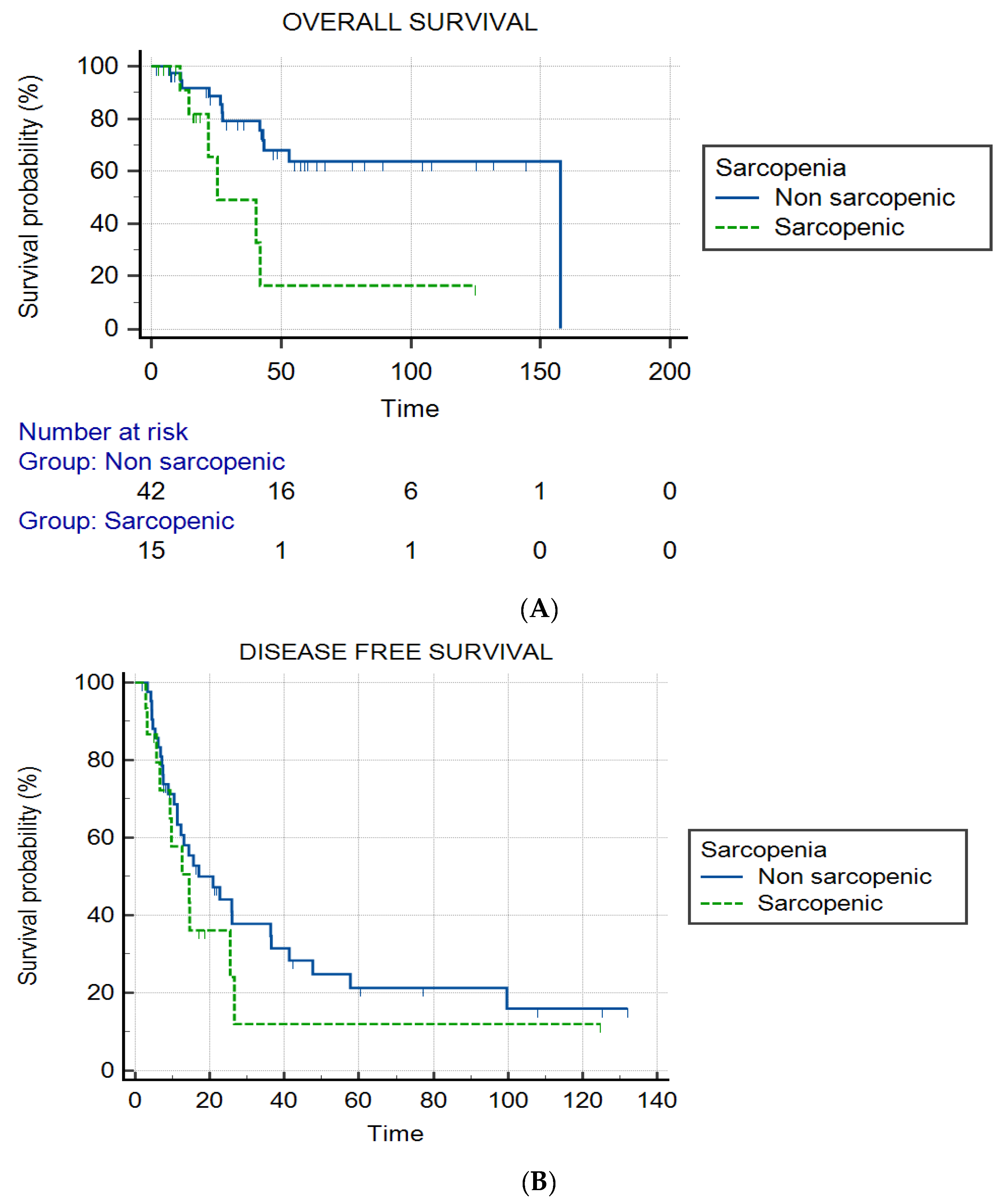
3.2. Sarcopenia
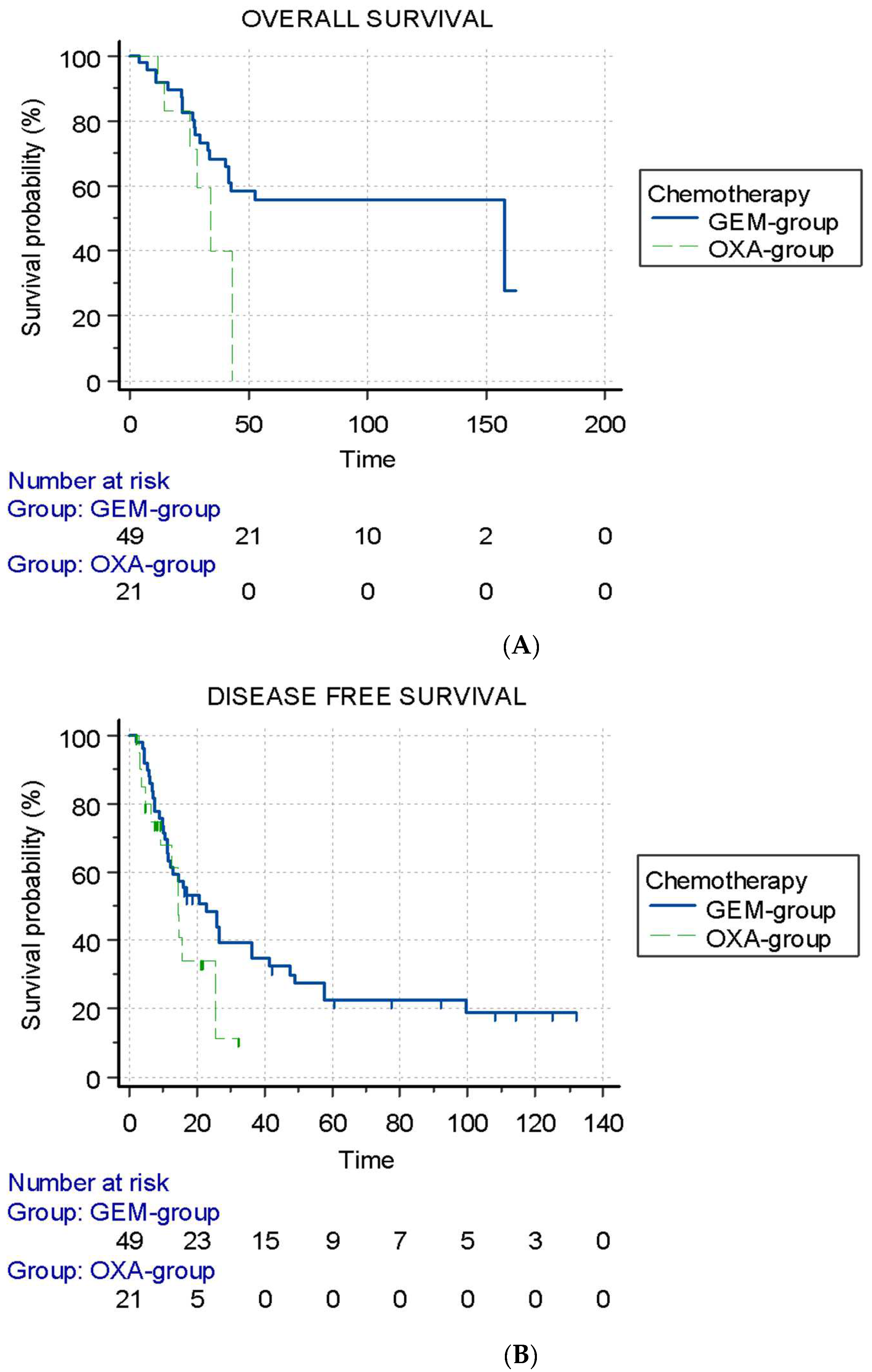
3.3. Sarcopenia and Chemotherapy Group
3.4. Tumor Status
3.5. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CT | Computed tomography |
| GEM | Gemcitabine |
| OXA | Oxaliplatin |
| DFS | Disease-free survival |
| OS | Overall survival |
| PDAC | Pancreatic ductal adenocarcinoma |
| SMI | Skeletal muscular index |
| TNM | Tumor node metastasis |
References
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, G.M.; Ladak, F.; Gallinger, S. Advances in the management of pancreatic ductal adenocarcinoma. CMAJ 2021, 193, E844–E851. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Reni, M.; Riess, H.; Pelzer, U.; O’Reilly, E.M.; Winter, J.M.; Oh, D.; Li, C.; Tortora, G.; Chang, H.; et al. APACT: Phase III, multicenter, international, openlabel, randomized trial of adjuvant nabpaclitaxel plus gemcitabine (nab-P/ G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), 4000. [Google Scholar] [CrossRef]
- Chan, M.Y.; Chok, K.S.H. Sarcopenia in pancreatic cancer—Effects on surgical outcomes and chemotherapy. World J. Gastrointest. Oncol. 2019, 11, 527–537. [Google Scholar] [CrossRef]
- Gruber, E.S.; Jomrich, G.; Tamandl, D.; Gnant, M.; Schindl, M.; Sahora, K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS ONE 2019, 14, e0215915. [Google Scholar] [CrossRef]
- Ninomiya, G.; Fujii, T.; Yamada, S.; Yabusaki, N.; Suzuki, K.; Iwata, N.; Kanda, M.; Hayashi, M.; Tanaka, C.; Nakayama, G.; et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int. J. Surg. 2017, 39, 45–51. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Zalite, I.O.; Zykus, R.; Gonzalez, M.F.; Saygili, F.; Pukitis, A.; Gaujoux, S.; Charnley, R.M.; Lyadov, V. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: A systematic review. Pancreatology 2015, 15, 19–24. [Google Scholar] [CrossRef]
- van Dijk, D.P.; Bakens, M.J.; Coolsen, M.M.; Rensen, S.S.; van Dam, R.M.; Bours, M.J.; Weijenberg, M.P.; Dejong, C.H.; Olde Damink, S.W. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kondrup, J.E.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.L.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Duggan, S.N.; Smyth, N.D.; O’Sullivan, M.; Feehan, S.; Ridgway, P.F.; Conlon, K.C. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr. Clin. Pract. 2014, 29, 348v–354v. [Google Scholar] [CrossRef]
- Srinivasalu, V.K.; George, A.S.; Philip, A.; Kotne, S.; Bamroo, S.; Vijaykumar, D.K.; Pillai, R.; Jose, W.M.; Pavithran, K. Effect of obesity on the toxicity profile of patients with breast cancer treated with adjuvant chemotherapy. J. Clin. Oncol. 2017, 35 (Suppl. 15), e12033. [Google Scholar] [CrossRef]
- Prado, C.M. Body composition in the promising role of CT scans. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 525–533. [Google Scholar] [CrossRef]
- Hashimoto, F.; Kakimoto, A.; Ota, N.; Ito, S.; Nishizawa, S. Automated segmentation of 2D low-dose CT images of the psoas major muscle using deep convolutional neural networks. Radiol. Phys. Technol. 2019, 12, 210–215. [Google Scholar] [CrossRef]
- Blanc-Durand, P.; Schiratti, J.B.; Schutte, K.; Jehanno, P.; Herent, P.; Pigneur, F.; Lucidarme, O.; Benaceur, Y.; Sadate, A.; Luciani, A.; et al. Abdominal musculature segmentation and surface prediction from CT using deep learning for sarcopenia assessment. Diagn. Interv. Imaging 2020, 101, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Morales-Oyarvide, V.; Rubinson, D.A.; Dunne, R.F.; Kozak, M.M.; Bui, J.L.; Yuan, C.; Qian, Z.R.; Babic, A.; Da Silva, A. Lymph node metastases in resected pancreatic ductal adenocarcinoma: Predictors of disease recurrence and survival. Br. J. Cancer 2017, 117, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Hiraoka, T.; Mizumoto, R.; Matsuno, S.; Matsumoto, Y.; Nakayama, T.; Tsunoda, T.; Suzuki, T.; Monden, M.; Saitoh, Y.; et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg. Today 1999, 29, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.J.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar]
| GEM Group (n = 49) | OXA Group (n = 21) | |
|---|---|---|
| Gender | ||
| Male | 21 (43) | 12 (57) |
| Female | 28 (47) | 9 (43) |
| BMI (kg/m2) | 25 [18–31] | 24 [20–33] |
| Median age at surgery (year) | 67 [45–85] | 72 [43–81] |
| Median CA19-9 at diagnosis (UI/mL) | 214 [0–4172] | 673 [4–16000] |
| Biliary stent | 22 (44) | 7 (33,3) |
| Sarcopenia | 8 (16) | 7 (33) |
| Type of surgery | ||
| Cephalic duodenopancreatectomy | 38 (77) | 16 (77) |
| Distal pancreatectomy | 11 (23) | 5 (23) |
| Status of surgical margin | ||
| R0 | 44 (90) | 21 (100) |
| R1 | 5 (10) | 0 (0) |
| pTNM | ||
| T1 | 2 (4) | 0 |
| T2 | 12 (24) | 12 (57,1) |
| T3 | 31 (62) | 9 (42,9) |
| T4 | 5 (10) | 0 (0) |
| N0 | 9 (18) | 7 (33.3) |
| N1 | 35 (70) | 7 (33.3) |
| N2 | 6 (12) | 7 (33.3) |
| M0 | 48 (98) | 21 (100) |
| M1 | 1 (2) | 0 (0) |
| Days between CT and surgery | 20 [2–92] | 18 [3–98] |
| Days between surgery and first cycle of chemotherapy | 48 [16–414] | 55 [21–111] |
| Duration of chemotherapy (months) | 6 [2–6] | 4.5 [1–6] |
| Non-Sarcopenic (n = 43) | Sarcopenic (n = 15) | |
|---|---|---|
| Gender | ||
| Male | 20 (48) | 8 (53) |
| Female | 23 (53) | 7 (47) |
| BMI (kg/m2) | 24 [18–30] | 25 [20–32] |
| Median age at surgery (years) | 65 [43–85] | 73 [54–80] |
| Median CA19-9 at diagnosis (UI/mL) | 129 [3–7938] | 32 [0–16000] |
| Biliary stent | 17 (40) | 7 (47) |
| Chemotherapy group | ||
| GEM group | 32 (74) | 8 (53) |
| OXA group | 11 (26) | 7 (47) |
| Type of surgery | ||
| Cephalic duodenopancreatectomy | 33 (76) | 11 (73) |
| Distal pancreatectomy | 10 (24) | 4 (27) |
| Status of surgical margin | ||
| R0 | 40 (93) | 14 (93) |
| R1 | 3 (7) | 1 (7) |
| pTNM | ||
| T1 | 2 (5) | 0 (0) |
| T2 | 12 (29) | 8 (53) |
| T3 | 26 (60) | 7 (47) |
| T4 | 3 (7) | 0 (0) |
| N0 | 9 (21) | 5 (33) |
| N1 | 27 (62) | 7 (47) |
| N2 | 7 (17) | 3 (20) |
| M0 | 43 (100) | 15 (100) |
| M1 | 0 (0) | 0 (0) |
| Days between CT and surgery | 20 [2–98] | 19 [4–65] |
| Days between surgery and first cycle of chemotherapy | 53.5 [17–414] | 51 [31–111] |
| Duration of chemotherapy (months) | 6 [1–6] | 6 [2–6] |
| Tumor Status | Median (Months) | IQR | p | |
|---|---|---|---|---|
| Disease-free survival | T1 | 144 | 144–144 | 0.29 |
| T2 | 22.7 | 19.2–48.2 | ||
| T3 | 13 | 7.1–27.3 | ||
| T4 | 10.3 | 9.2 not reached | ||
| N0 | 66.8 | 15.3 not reached | 0.02 | |
| N1 | 43.4 | 6.5–57.7 | ||
| N2 | 27.4 | 10.2–30.4 | ||
| R0 | 25.5 | 9–48.6 | <0.01 | |
| R1 | 11.3 | 6.1–13.4 | ||
| Overall survival | T1 | 0.47 | ||
| T2 | 41.9 | 25 not reached | ||
| T3 | 43.4 | 27–158 | ||
| T4 | ||||
| N0 | 42 not reached | 0.10 | ||
| N1 | 158 | 27.5 not reached | ||
| N2 | 34.1 | 27.5–42 | ||
| R0 | 158 | 27.5 not reached | 0.04 | |
| R1 | 11 not reached |
| Grade | GEM Group | OXA Group | p | |
|---|---|---|---|---|
| Nausea, vomiting | 1 | 4 (8) | 5 (23) | 0.02 |
| 2 | 0 (0) | 6 (28) | ||
| 3 | 1 (2) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | ||
| Diarrhea | 1 | 3 (6) | 6 (28) | 0.25 |
| 2 | 2 (4) | 5 (23) | ||
| 3 | 0 (0) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | ||
| Oral mucositis | 1 | 1 (2) | 10 (47) | 0.01 |
| 2 | 1 (2) | 1 (4) | ||
| 3 | 0 (0) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | ||
| Neuropathy | 1 | 0 (0) | 6 (28) | 0.01 |
| 2 | 1 (2) | 3 (14) | ||
| 3 | 0 (0) | 4 (19) | ||
| 4 | 0 (0) | 0 (0) |
| Grade | Non-Sarcopenic | Sarcopenic | p | |
|---|---|---|---|---|
| Nausea, vomiting | 1 | 5 (12) | 2 (13) | 0.40 |
| 2 | 3 (7) | 2 (13) | ||
| 3 | 1 (2) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | ||
| Diarrhea | 1 | 7 (16) | 1 (7) | 0.23 |
| 2 | 2 (5) | 3 (20) | ||
| 3 | 0 (0) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | ||
| Oral mucositis | 1 | 5 (12) | 4 (26) | 0.72 |
| 2 | 1 (2) | 0 (0) | ||
| 3 | 0 (0) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | ||
| Neuropathy | 1 | 2 (5) | 3 (20) | 0.68 |
| 2 | 1 (2) | 2 (13) | ||
| 3 | 2 (5) | 1 (7) | ||
| 4 | 0 (0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortier, V.; Wei, F.; Pellat, A.; Marchese, U.; Dohan, A.; Brezault, C.; Barat, M.; Fuks, D.; Soyer, P.; Coriat, R. Impact of Sarcopenia on Patients with Localized Pancreatic Ductal Adenocarcinoma Receiving FOLFIRINOX or Gemcitabine as Adjuvant Chemotherapy. Cancers 2022, 14, 6179. https://doi.org/10.3390/cancers14246179
Mortier V, Wei F, Pellat A, Marchese U, Dohan A, Brezault C, Barat M, Fuks D, Soyer P, Coriat R. Impact of Sarcopenia on Patients with Localized Pancreatic Ductal Adenocarcinoma Receiving FOLFIRINOX or Gemcitabine as Adjuvant Chemotherapy. Cancers. 2022; 14(24):6179. https://doi.org/10.3390/cancers14246179
Chicago/Turabian StyleMortier, Victor, Felix Wei, Anna Pellat, Ugo Marchese, Anthony Dohan, Catherine Brezault, Maxime Barat, David Fuks, Philippe Soyer, and Romain Coriat. 2022. "Impact of Sarcopenia on Patients with Localized Pancreatic Ductal Adenocarcinoma Receiving FOLFIRINOX or Gemcitabine as Adjuvant Chemotherapy" Cancers 14, no. 24: 6179. https://doi.org/10.3390/cancers14246179
APA StyleMortier, V., Wei, F., Pellat, A., Marchese, U., Dohan, A., Brezault, C., Barat, M., Fuks, D., Soyer, P., & Coriat, R. (2022). Impact of Sarcopenia on Patients with Localized Pancreatic Ductal Adenocarcinoma Receiving FOLFIRINOX or Gemcitabine as Adjuvant Chemotherapy. Cancers, 14(24), 6179. https://doi.org/10.3390/cancers14246179








