Simple Summary
Increasing awareness of thyroid cancer-related environmental risk factors is considered an essential tool for cancer prevention through risk prevention/management. Various studies have identified correlations between environmental pollutants and thyroid cancer incidence rates, and others have proposed mechanisms for thyroid cancer development. This review seeks to consolidate the known environmental risk factors contributing to thyroid carcinoma to ensure that future research endeavors may identify key focus areas. These factors have been established as contributors to the development of thyroid carcinoma and thus require further investigation to establish mechanisms by which they act and influence thyroid pathology. Identifying pathophysiology involving these environmental risk factors can allow more rapid development of hazard reduction plans, exposure remedies, and medical treatments to prevent and perhaps even reverse disease course.
Abstract
Environmental factors are established contributors to thyroid carcinomas. Due to their known ability to cause cancer, exposure to several organic and inorganic chemical toxicants and radiation from nuclear weapons, fallout, or medical radiation poses a threat to global public health. Halogenated substances like organochlorines and pesticides can interfere with thyroid function. Like phthalates and bisphenolates, polychlorinated biphenyls and their metabolites, along with polybrominated diethyl ethers, impact thyroid hormones biosynthesis, transport, binding to target organs, and impair thyroid function. A deeper understanding of environmental exposure is crucial for managing and preventing thyroid cancer. This review aims to investigate the relationship between environmental factors and the development of thyroid cancer.
1. Introduction
Environmental contaminants have been linked to several ailments, including metabolic disorders [1], allergies [2], diseases affecting male and female fertility [3], and malignancies [4], including thyroid carcinoma. Thyroid carcinoma is the most prevalent endocrine tumor [5], with enhanced diagnostic technology contributing to a recent rise in cases [6]. The highest incidence rates are found in both males and females in South Korea, while incidence rates are more significant for women than men in Eastern Asia, Australia/New Zealand, North America, Oceania, and Pacific Island nations [7]. Globally, thyroid cancer occurs predominantly in female patients compared to males, with approximately 2–4 times greater prevalence [8].
Thyroid carcinomas may originate from follicular thyroid cells or parafollicular thyroid cells. Follicular thyroid cell carcinomas progress through hypertrophy, hyperplasia, and benign (and, in some cases, eventually malignant) neoplasms. Papillary thyroid cancer (80–85%), follicular thyroid cancer (10–15%), poorly differentiated thyroid cancer (2%), and undifferentiated (anaplastic) thyroid cancer (2%) are the four subtypes of follicular cell-derived thyroid cancers [9]. These have an excellent prognosis, except for undifferentiated thyroid cancer [10].
Numerous intrinsic and extrinsic risk factors contribute to thyroid carcinogenesis. Intrinsic non-modifiable factors include biological sex, age, and familiar and hereditary conditions [11]. The ionizing radiation is considered as an established extrinsic factor, meanwhile other potential extrinsic factors are pesticides, persistent organic pollutants (POPs), endocrine-disrupting chemicals (ECDs), bisphenol A (BPA), phthalates, heavy metals, and polychlorinated biphenyls (PCBs) [12] (Figure 1).
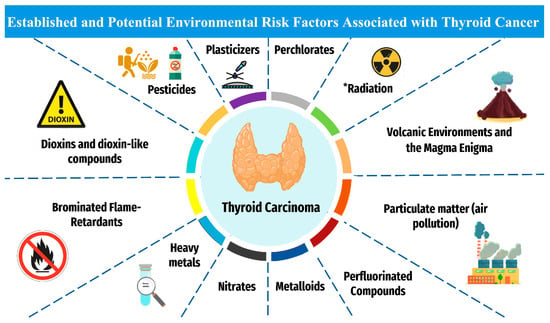
Figure 1.
The established and plausible environmental risk factors for thyroid cancer. * Established risk factor for thyroid cancer. Otherwise, other factors are associated with potential increased risk for thyroid carcinoma.
Clinically relevant thyroid carcinoma risk trends positively with age; however, the malignancy risk of such nodules paradoxically declines with age [13]. Despite the significant prevalence of nodular thyroid disease worldwide—occurring in an estimated 4.2% of individuals aged 30–59—malignancy is notably rare, with a 4.5% occurrence [14]. According to the Italian AIRTUM (Associazione Italiana dei Registri Tumori, or Italian Associations of Cancer Registries) Working Group, there was a marked rise in the incidence rates of all malignant tumors among teenagers (15–19 years old) between 1991 and 2005, particularly Hodgkin’s lymphoma and thyroid cancer. The rise in cancer incidence that was seen through the 1990s has come to an end, except for thyroid cancer in young adults [15]. Genetic susceptibility and mutations play an essential role in the predisposition to thyroid cancer. Although there is a rise in the incidence of non-medullary thyroid cancer (NMTC), tumorigenesis of this type is not yet well-developed [16]. According to numerous studies, first-degree relatives of thyroid cancer patients are 8- to 12-fold more likely to develop thyroid cancer than the general population [17,18].
Many environmental factors have not yet been sufficiently studied to adequately deduce environmental pollutants’ impact on the human endocrine system, rendering prevention strategies for such pathology more challenging [19]. This review serves to comprehensively summarize the currently identified environmental toxicants and establish their relationship to thyroid carcinoma development.
2. Materials and Methods
The association between environmental risk exposure and thyroid cancer was explored by looking for publications and studies in international databases such as PubMed, Web of Science, and Scopus.
Environmental risk factors/exposure, thyroid, cancer, pesticides, persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs), endocrine disrupting chemicals (ECDs), bisphenol A (BPA), phthalates, heavy metals, and radiation were some of the keywords applied during the search.
3. Pollutants
Endocrine-disrupting chemicals (EDCs), including industrial chemicals and pesticides, are elements with deleterious health impacts [20]. Although they are understood to be detrimental to health and correlated with thyroid carcinoma, they do not necessarily decrease thyroid hormone levels, and current studies have been unable to reveal their exact mechanism of impact on human subjects [21]. See Table 1 for a consolidated summary of the established EDCs contributing to thyroid function disruption and/or potential increased risk of carcinoma and other pathology. The suggested mechanisms of action of the “EDCs” on thyroid are summarized in Figure 2. These chemicals can impact euthyroid state by several ways, including “disturbance of thyroid hormone biosynthesis through directly targeting the sodium-iodide symporter (NIS), interplaying with thyroid hormone transporters/receptors, interfering with the hypothalamic-pituitary-thyroid axis, and disrupting multiple molecular alterations associated with thyroid pathogenesis” [22]. These findings suggest that the exposure to these chemicals as modifiable risk factors should be considered, when investigating the potential increased incidence of thyroid cancer.

Table 1.
Summary of endocrine-disrupting chemicals and their impacts on thyroid health.
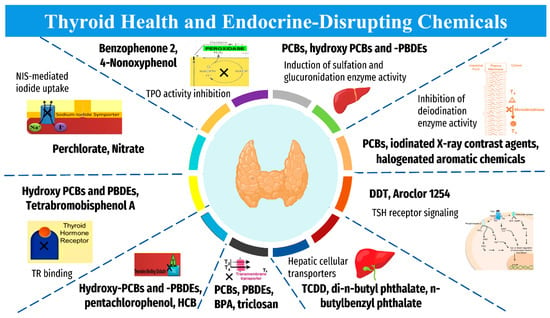
Figure 2.
Mechanism of action of “Endocrine-Disrupting Chemicals” on thyroid homeostasis. “BPA, bisphenol A; DDT, dichlorodiphenyltrichloroethane; HCB, hexachlorobenzene; PCBs, polychlorinated biphenyls; PBDEs, polybrominated diphenyl ethers; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; NIS, sodium iodide symporter; T3, triiodothyronine; T4, thyroxine; TR, thyroid hormone receptor; TBG, thyroid-binding globulin; TSH, thyroid stimulating hormone”. The collected data are based on Tang and colleagues [22].
Human studies face a considerable challenge due to lifelong chronic exposure to various substances and the significant physiological diversity in thyroid hormone levels between individuals [49]. While global and national studies provide some insight into broad trends, specific dosing is difficult to determine for each pollutant, and several confounding variables from tandem chemical exposures interfere with and challenge exposome research [50].
3.1. Pesticides
Several studies have looked into the link between pesticides and thyroid cancer. The most extensively researched organochlorine pesticide (OCP) for its ability to behave as ECD is dichlorodiphenyltrichloroethane (DDT), along with its main metabolite dichlorodiphenyldichloroethylene (p,p-DDE). Thyroid hormone synthesis exhibits developmental flaws with DDT and HCB (hexachlorobenzene) [23]. Lindane, classified as a carcinogenic OCP based on “International Agency for Research on Cancer” (IARC) [51], was positively associated with thyroid carcinogenesis in a recent study [52]. Due to their toxicity and carcinogenicity, OCPs have been prohibited in many developed nations, yet they are utilized in many developed countries [53]. Atrazine (triazine herbicide) and Malathion (an organophosphate insecticide) were linked to increased thyroid cancer risk among individuals exposed to pesticides on farms [54]. Ref The improper use of OCP led to water and soil contamination with an accumulation of residues for decades [55]. The relationship between thyroid cancer risk and pesticide exposure has been investigated in numerous epidemiological studies. Zeng et al. found that there was a statistically significant link between thyroid cancer risk and occupational exposure to biocides and pesticides among workers in comparison with harmful exposure [56]. Ref In a prospective cohort study in Iowa and North Carolina, At enrollment (1993–1997) and follow-up (1999–2005), it found that pesticide usage involving fungicide (metalaxyl) and the OCP (lindane) was linked to increased hazard ratios (HR) thyroid cancer (HR = 2.03 and HR), respectively [52]. Exposure to Malathion, an organophosphate pesticide, in the environment has been linked to an increased risk of thyroid cancer in females who work and live in agricultural areas [57]. In Norwegian research, chlordane and HCB serum OCP metabolites have been positively linked to a rising incidence of thyroid cancer [58]. While HCB breaks down into very hazardous chemicals that target thyroid hormones, DDT reduces the function of thyroid hormones more directly. OCPs are EDCs whose chemical structure resembles triiodothyronine (T3) and thyroxine (T4). OCPs can competitively bind to thyroid transport proteins, interrupting thyroid hormone signaling and transport even at low doses [53]. Carbaryl is a non-persistent carbamate pesticide that disrupts thyroid hormone by antagonizing the receptor-signaling pathway, according to an in vitro study [59]. Malathion and parathion are widely used organophosphate pesticides used to control insects in agriculture, public health, and homes and has been linked to cancer in several different organs, but the evidence in human being remains scarce [60]. The carcinogenicity of Malathion and parathion is not yet well established. However, they induced thyroid follicular cell adenoma in male rats [61]. In an in vitro study, parathion induced genotoxicity via DNA and chromosomal damage in human cells, combined with inflammation, oxidative stress, receptor-mediated modulation, and apoptosis [62]. Although parathion and malathion usage has been restricted since the eighties, several developing countries still misuse them [63].
Furthermore, pesticides cause thyroid disruption mechanisms, including inhibiting iodine uptake, increasing clearance of thyroid hormones, interfering with iodothyronine deiodinase and thyroid peroxidase activities, interfering with cellular uptake of thyroid hormones, and modifying thyroid gene expression [64]. Consequently, alterations in thyroid hormone function could result in abnormal proliferation in the thyroid tissue, leading to oncogenesis. Multiple malignancies, including thyroid cancer, have been analyzed in various studies on other commonly used pesticides (alachlor, atrazine, chlorpyriphos, glyphosphate, imazethapyr, and metolachlor); however, they did not demonstrate a significant carcinogenic effect [23,24].
3.2. Plasticizers
Phthalates are frequently employed as plasticizers to increase materials’ malleability, flexibility, and plasticity. Phthalates can enter the human body through several methods, including ingestion, inhalation, and cutaneous exposure [27,65]. Many types of phthalates are still used in a variety of different industries, such as cosmetics, paints, food packaging, cleaning agents, and medical equipment, despite the current restriction on the use of some phthalates, such as Di-(2-Ethylhexyl) phthalate (DEHP), in children’s toys in the US and EU (tablet coatings, blood bags, tubes, drugs packaging, etc.). Phthalates do not bioaccumulate; instead, they are digested and primarily eliminated through the urine (within a few days or hours) [13,27]. The thyroid is one of the main target organs for phthalates’ capacity to disrupt the endocrine system [31]. Phthalates are particularly interesting EDCs due to continuous and long-term exposure by the entire world population. DEHP has been shown to bind to and activate the estrogen receptor (ER) and contribute to angiogenesis and tumor progression by controlling vascular endothelium growth factor (VEGF) [66]. This substance increases VEGF secretion in MELN cells (the Melbourne cells model) with continuous ER-alpha expression. Studies suggest DEHP increases the risk of developing differentiated thyroid cancer in patients with thyroid nodules [25], and DEHP metabolites in urine have been associated with papillary thyroid cancer [26]; however, establishing the precise mechanisms requires further study.
Another plasticizer that interferes with thyroid hormone is bisphenol A (BPA). BPA mimics natural hormones and interferes with their production and secretion in humans and other creatures, causing their endocrine systems to malfunction. BPA competes with endogenous estrogens to bind to membrane estrogen receptors and shares structural similarities with 17b-estradiol [29]. To date, there are few studies that have investigated the association between BPA and the risk of developing thyroid cancer in human [67]. However, according to some studies, BPA may affect thyroid function through several different mechanisms. BPA blocks the activity of human recombinant thyroid peroxidase (TPO) [68]. At the receptor level, BPA binds to the thyroid hormone receptor (TR) as a weak ligand and functions as an antagonist to T3, thereby preventing TR-mediated transcriptional activity [29,69]. In a recent study, Marotta et al. 2022 found that overweight/obese individuals subjects exposed to BPA showed high serum level of TSH [70]. This can sustain the hypothesis that BPA play role in TC development via thyroid hormones dysregulation, which in turn leads to TSH hyperstimulation and increased TC risk [70]. Fetuses and infants are especially susceptible to the consequences of BPA exposure, according to the current fetal genesis hypothesis of cancer [28].
3.3. Dioxins and Dioxin-like Compounds (Polychlorinated Biphenyls; PCBs)
Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDD) is one of the most toxic persistent organic pollutants (POPs) that are created from uncontrolled waste combustion in the presence of chlorine [71]. Dioxins are a group of chemical compounds consisting of 75 polychlorinated dibenzo-p-dioxin (PCDD) and 135 polychlorinated dibenzofurans (PCDFs) [72]. Dioxin bioaccumulates in the food chain, particularly in fat, with elimination half-lives ranging from 3 to 10 years [73]. Animals experiment and human research proved that TCDD is a potent carcinogen that can alter numerous endocrine pathways [74]. A recent study measuring PCDD/DFs using serum from a general population found that PCDD/DFs had a significant positive association with thyroid cancer development [74]. Another animal study found that TCDD exposure controls the script of an endothelial carcinogen network that leads to thyroid carcinoma [75]. Dioxins dysregulate thyroid hormone and could induce thyroid cancer through several mechanisms as binding to the protein transport of thyroid hormones [76], direct damage to the thyroid tissue, and the activation of thyroid-metabolizing enzymes [77].
The worldwide market for electronic products has been continuously growing. Improper e-waste disposal will inevitably release polychlorinated biphenyls (PCBs) and other toxicants into the environment [78]. The most common uses of PCBs include flame-retardant coatings, lubricants, elastic sealants, paints, and industrial products such as dielectric fluids in transformers and capacitors [79]. Their production was banned in most countries by the 1980s due to environmental persistence. Before their production was outlawed in the late 1970s, (PCBs) were a class of synthetic, lipophilic POPs widely utilized in industrial and consumer products for decades.
Due to PCBs’ lipophilicity, which causes them to bioaccumulate in the food chain, the consumption of large fish (such as tuna, shark, and swordfish) is the leading cause of human exposure. PCBs can cause thyroid disruption [27]. Notably, the PCBs metabolites, including sulfated, hydroxylated, and methyl sulfones, are more toxic than PCBs [80]. Although the carcinogenicity of PCBs on thyroid tissue is not well understood, a few occupational studies show an increased risk of thyroid cancer in occupational settings, such as a rise in thyroid tumor mortality rate among capacitor manufacturing workers [81]. In a recent epidemiologic study, Zhuo et al., 2022 found that specific PCB exposure (PCB-74, PCB-99, PCB-105, PCB-118) was associated with an increased risk of papillary thyroid carcinoma, particularly type PCB-118. High levels of serum PCBs were detected by gas chromatography in samples collected nine years before papillary thyroid carcinoma diagnosis among US military service members [82]. A toxicological animal study observed that PCB-118 caused a reduction in serum T4 levels in a dose-dependent manner and induced thyroid gland lesions liability in female rats [83]. According to the literature, PCBs are structurally similar to thyroid hormone and may influence thyroid function through receptor-mediated mechanisms or alter thyroid hormone metabolism [84].
Additionally, PCBs stimulate the proliferation of cancer cells through the imbalance of molecular and signal pathways [85]. Based on a recent molecular docking study, it is revealed that PCBs can dysregulate thyroid hormone by interfering with PIK3R1, RXRA, MAPK1, MAPK3, and PI3K-Akt signaling pathways. The previous intersection genes play the leading role in thyroid cancer genesis [85].
3.4. Perfluorinated Compounds
Perfluorinated compounds (PFC) have surface protective qualities and include substances like perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). PFCs are a diverse class of substances with a wide range of uses, including as additives in paper goods, stain-repellents for textiles, and in aqueous film-forming foams used to prevent electrical fires. They have been added to the Stockholm list [34] due to their stain- and oil-resistant PFC properties, which are employed in numerous consumer products and are quite persistent in the environment. An extensive investigation involving 506 workers at a PFC manufacturing plant revealed a negative correlation between PFOA and FT4, suggesting that exposure to significant PFOS may impair human thyroid function. Results of the more recent NHANES study conducted in the US revealed that men and women with high levels of PFOA and PFOS are more likely to report having thyroid disease [34]. PFC substances seem to affect how thyroid hormones are metabolized.
3.5. Brominated Flame-Retardants
Brominated flame retardants (BFRs) are frequently utilized in consumer goods such as electronics, cars, plastics, and textiles to reduce flammability [31]. Polybrominated diphenyl ethers (PBDEs) have been widely utilized as flame retardants in commercial and domestic products in the United States since the 1970s, including foam padding, textiles, electronics, cars, and airplanes [28,31]. PentaBDE, OctaBDE, and DecaBDE were used in the United States until 2004. The production of PentaBDE and OctaBDE was prohibited due to worries about toxicity and the bioaccumulation of PBDEs in blood and breast milk. The US Environmental Protection Agency published regulations in 2010 to further restrict the importation of goods containing these substances. Only one of the congeners, decabromodiphenyl ether (BDE-209), the primary congener in the DecaBDE formulation, has been studied in humans, thanks to Aschebrook-Kilfoy and colleagues [86]. The small number of cases does not imply a higher risk of thyroid cancer despite the apparent disruption of thyroid homeostasis caused by PBDEs in the environment. Kim et al. examined 36 epidemiological studies on the potential effects of BFR intake, such as impacts and changes on thyroid function [87]. The health effects of exposure to BFRs are well documented, but more epidemiological research is required, especially among children [12].
3.6. Perchlorates
Because of its well-known antithyroid properties, perchlorate has been used to diagnose and treat thyrotoxicosis. It is utilized in manufacturing weapons and pyrotechnics, and in previous years, the US drinking water supply’s perchlorate content raised concerns. The most significant pathways of exposure for the general public are through the consumption of food and water that contain perchlorates [32]. Despite the well-documented antithyroid effects of perchlorate, it is still unclear what the lowest practical level of environmental pollution is. Given the inconsistent findings of human observational research, it is still unclear whether naturally occurring quantities of perchlorate ions impact human thyroid function, particularly in neonates.
3.7. Heavy Metals
Heavy metals are substantial “environmental pollutants”, and their toxicity is a challenge of increasing significance for “evolutionary, ecological, nutritional, and environmental” causes [88]. Health effects from metals in food, water, and air have been reported [28,38,39,40,41,42,43,44,45]. While some metals, such as chromium, iron, copper, magnesium, and zinc, are necessary for life, they are toxic when they exceed their threshold limits. The toxic impacts of other metals, such as aluminum, nickel, cadmium, mercury, and lead, even though these substances do not have any biological role, are detrimental to human health [88]. Heavy metals include waste products from incinerators, combustion of gasoline or diesel fuel as essential components of particulate matter with a diameter of 10 microns or less (PM10) and fine particles that are 2.5 microns or less in diameter (PM2.5) produced from cars, trucks, and airplanes. Also include smelters, paints, insecticides, and agricultural products like disinfectants which can be absorbed through inhalation, ingestion, or even skin contact [28].
Arsenic, beryllium, cadmium, chromium, and nickel are identified as either definite or probable carcinogens according to IARC [89]. In particular, cadmium, mercury, arsenic, lead, manganese, and zinc exhibit EDCs’ capacity to disturb the hormonal system as well as they can act as carcinogens, promoting malignant transformation [35].
Cadmium is a category I carcinogen that can build up in the thyroid, liver, pancreas, and kidneys. Cadmium levels in the thyroid are correlated with blood levels. Multinodular goiter, thyroglobulin hyposecretion, and parafollicular cell hyperplasia are common in chronic cadmium exposure [30]. Cadmium can interfere with thyroid gland function even at extremely low ambient exposures, both at the target gland and extra gland level, according to several epidemiological and experimental studies [90,91,92,93,94]. Also, it has been established that cadmium contributes to thyroid cancer and autoimmune diseases [92,95]. One central mechanism that has been suggested behind the Cadmium effect on the thyroid gland is the oxidative stress and upregulation of multiple apoptotic players, including “nuclear condensation, DNA fragmentation, Bax integration, cytochrome c release”, etc., as concluded by Buha and colleagues in their review [92].
High amounts of cadmium in thyroid tissue have been linked to more advanced thyroid cancer stages in women, according to a Korean study. This finding is of particular significance given that traditional Korean cuisine is linked to high levels of cadmium exposure [35].
Enzymes with manganese (Mn) support several metabolic pathways [96]. Manganese exposure to the general public is primarily derived from diet, though in some areas drinking water may also be a source [97]. It has been shown that abnormal thyroid hormone levels caused by high Mn exposure during pregnancy are associated with poor neurodevelopmental consequences [98]. However, a measurable rise in thyroid cancer incidence due to exposure of mice to Mn was not evident, and the available epidemiological and occupational evidence is still inconclusive [37].
Lead (Pb) has generated debate surrounding its carcinogenicity. Li and colleagues studied 96 papillary thyroid carcinomas (PTCs), 10 nodular goiters, and seven thyroid adenomas in Chinese patients with various thyroid conditions to determine the relationship between Pb and thyroid function. Serum triiodothyronine (T3), free triiodothyronine (FT3), free thyroxin (FT4), thyroid-stimulating hormone (TSH), and serum lead levels were assessed, and lead levels were substantially greater in thyroid adenoma and decreased in nodular goiter when compared to PTC (p < 0.05). Even after sex stratification, the disparity increases among women. In the PTC group, there was a negative correlation between serum lead and TSH (rs = 0.27, p < 0.05). In the PTC group, T3 was quartile-wise positively correlated with lead (rs = 0.61, p < 0.05). None of the groups showed any measurable relationships between lead and FT3 or FT4. According to the study, lead may play various etiological roles in thyroid conditions, including thyroid cancer [36].
The most prevalent form of the metal vanadium (V), which can exist in many oxidation states, is vanadium pentoxide (V2O5). Vanadium compounds are all poisonous [99]. Some authors have linked vanadium to thyroid cancer; however, no research has examined the possibility of developing thyroid illness in people or animals who have been exposed to vanadium [12,100]. In their investigation, Fallahi et al. assessed how V2O5 affected the proliferation/chemokine release of healthy thyrocytes. They found that V2O5 can induce T-helper 1 chemokines secretion (e.g., interferon and tumor necrosis factor) and enhance the impact of these chemokines in the thyroid [38].
3.8. Metalloids
Thyroid hormone synthesis and function depend on micronutrients, primarily iodine (I) and selenium (Se) [39]. Iodine and selenium have been demonstrated to impact thyroid autoimmunity [27] significantly. Iodine prophylaxis lessens thyroid diseases caused by iodine insufficiency [101].
During a modest iodine shortage, a 53% greater prevalence of spontaneous overt hypothyroidism (likely autoimmune) was seen in the Danish population. On the other hand, too much iodine is linked to the development of autoimmune thyroiditis [40]. For further information, see Section 4.1. Radioiodine 131I is used in medical diagnostic and treatments for differentiated thyroid cancer (DTC) to remove any remaining normal thyroid tissue after thyroidectomy.
In a healthy state with a regular metabolism, selenium, a trace mineral vital to human health, performs several tasks as selenoprotein [41]. The effects of selenium, whose daily requirement is between 60 and 75 g, include those on immune responses, homeostasis, cell development, and antiviral defense. Because of its role in various enzyme activities, including glutathione peroxidases, deiodinases, and thioredoxin reductases, which actively contribute to the defense against free radicals and oxidative damage, it is required for optimal thyroid function in humans [42]. Although its significance in cancer treatment is still debatable, selenium plays a role in developing thyroid carcinoma (TC) [42]. The endocrine system, especially thyroid function, may be negatively impacted by a high selenium intake [40]. A Chinese cross-sectional study in two counties of Shaanxi Province examined the connections between dietary variables, pathological thyroid diseases, and selenium levels. The prevalence of pathological thyroid disorders (subclinical hypothyroidism, hypothyroidism, AT, and enlarged thyroid) in the adequate-selenium concentration group was considerably lower than that in the low-selenium group (18.0 versus 30.5 percent; p < 0.001) [43]. Reduced odds ratios (95% confidence interval) of subclinical and clinical hypothyroidism, autoimmune thyroiditis (AT), and enlarged thyroid were found to be linked with elevated circulating selenium levels. With the help of measurements of thyroid-stimulating hormone (TSH), thyroid hormones, TPOAb and thyroglobulin antibodies (TgAb) levels, thyroid echogenicity, and thyroid hormone levels after six months of l-selenomethionine treatment, Wu et al. evaluated the true efficacy of selenium supplementation in Hashimoto’s thyroiditis [43]. They found that short-term l-selenomethionine supplementation has a limited effect on euthyroid HT’s natural course.
3.9. Nitrates
Nitrates are widespread pollutants. Nitric oxide (NO), a recognized carcinogen that is implicated in a variety of pathogenic pathways, is produced in excess when high levels of nitrates are absorbed orally. This results in hypoxia, especially in children and adolescents.
Nitrate contamination threatens thyroid functions because it can prevent the thyroid from absorbing iodide from food and drinking water [44]. Thyroid-stimulating hormone (TSH), a sensitive indicator of thyroid function, is increased as a result of compensatory thyroid hormone production being reduced by nitrate. However, a cohort study of postmenopausal women in Iowa did not find that exposure to high nitrates in the public water supply correlated to high levels of thyroid dysfunction [44]. A high TSH release has been shown to induce hypertrophy and thyroid disease, including carcinoma, in animals [102].
Consuming foods high in nitrates was not substantially linked to an increased risk of developing gastric cancer, according to a meta-analysis by Xie et al. (RR = 1.24, 95% CI = 0.89–1.72); however, people who consume more nitrites have a higher chance of developing thyroid cancer (RR = 1.52, 95% CI = 1.12–2.05) [103]. Inoue-Choi et al. found that the risk of developing thyroid cancer was 2.6 times higher in women with >10 years of exposure to public water supplies with levels surpassing 5 mg/L NO3-N than in women whose drinking water had never surpassed this level [45].
When thyroid-stimulating hormone levels were measured in an Amish community in Pennsylvania (USA), nitrate concentrations in private wells were linked to an increased prevalence of subclinical hypothyroidism in women but not men [44]. Additionally, therapeutic irradiation (131I) raises NO levels in salivary gland tissue. Numerous studies have demonstrated that NO is an intrinsic radiosensitizer; in fact, administering an inhibitor of NO production can improve the function of radio-exposed salivary glands. NO-dependent effects include genomic instability and accumulation of DNA replication errors. These effects may be seen in unirradiated cells due to signals from neighboring radiated cells. NO may quickly diffuse through the cytoplasm and plasma membranes due to its hydrophobic qualities, dissipating radiation from irradiated cells to nearby cells without the need for gap-junctional intercellular communication [46].
3.10. Air Pollution
Urban air pollution is a widely acknowledged cancer risk factor, according to the IARC of the World Health Organization (WHO), which oversaw a modification to the classification of cancer-causing chemicals [47]. The “sum of all solid and liquid particles of organic and inorganic materials suspended in air”, of which many of them are harmful, is referred to as particulate matter (PM) [104]. More people are affected by PM than other pollution [105]. Sulfate, nitrates, ammonia, sodium chloride, black carbon, mineral particles, and water vapor are the main components of PM [106]. PM10 particles, which are 10 microns in diameter or smaller, can enter the lungs and become lodged there. While particles with a diameter of 2.5 microns or smaller (PM2.5) can cross the lung barrier and enter the blood, they are far more harmful to human health. Chronic exposure to such particles increases the risk of lung cancer, as well as respiratory and cardiovascular problems [107].
According to the IARC’s 2015 position statement, almost 80% of European inhabitants are exposed to high quantities of tiny particles (PM2.5 and PM10) that have been linked to human cancer [33]. A significant prospective study found that outdoor air pollution (PM2.5, NO2, and O3) was positively linked with colorectal, kidney, and bladder cancer death [47]. In a retrospective population-based study conducted in Shanghai, China, Cong studied the impact of outdoor air pollution from waste gas emissions on cancer occurrences. More than 550,000 new cancer patients were recruited and assessed. Cancer incidence of various types, including TC, was found to have a statistically significant positive association with pollution caused by waste gas emissions [48].
4. Radiation
The association between radiation exposure and the occurrence of TC has been well documented [108,109,110,111]. The incidence and causes of death from thyroid cancer in the Life Span Study (LSS) sample in Nagasaki and Hiroshima have been investigated [112,113]. It has been established that the rise in TC is caused by increasing radiation dose and exposure time [114,115,116]. Importantly, medical radiation such as radiotherapy could increase the risk of secondary thyroid cancer in some patients, particularly children who have received radiotherapy to treat leukemia, Hodgkin lymphoma, and neuroblastoma [117].
The thyroid tissue is sensitive to radiation. Ionizing radiation proved to be carcinogenic (group 1) by IARC [118]. Its ability to deliver high amounts of energy to cells causes direct DNA distraction [119]. Recent research stated that exposure to diagnostic radiography as X-ray was linked with an increased risk of thyroid cancer, particularly thyroid microcarcinomas [120]. Additionally, childhood exposure to computerized tomography (CT) scanning was associated with a 40% increase in thyroid cancer [121]. The effects of non-ionizing radiation (NIR) on the thyroid have not yet been well developed [119]. The wide use of cell phones raises alarming concerns regarding potential harmful effects. However, some research studied the association between cell phones and thyroid cancer with conflicting outcomes [119,122,123,124]. Long-term and frequent cell phone use carries potential risks for thyroid cancer, especially among females [119]. Low-frequency electromagnetic radiation has sufficient energy to heat and vibrate molecules. Importantly, NIR can lead to oxidative stress in human cells, damaging DNA [125].
4.1. Anthropogenic Radiation
The main fission product of uranium and plutonium is iodine-131 (131I), which accounts for 3% of all fission products. It is connected to nuclear energy and diagnostic and therapeutic processes in medicine. It contributed significantly to the health risks associated with the Chernobyl disaster, open-air atomic bomb testing in the 1950s, and the initial weeks of contamination risk following the Fukushima nuclear crisis [126]. From a therapeutic perspective, radioiodine 131I is given to patients with differentiated thyroid cancer (DTC) to remove any remaining normal thyroid tissue following thyroid surgery, treat any remaining microscopic disease (adjuvant treatment), and treat macroscopic or metastatic illness [127].
The effectiveness of 131I therapy depends on the tumoral thyroid tissue’s capacity to absorb iodine from the blood via the sodium/iodine transporter membrane [128]. Because tumoral tissue expresses this transporter less than healthy tissue, there may be less uptake of 131I. However, the primary goal of treatment is cell death, which is achieved through the creation of oxidative species like free radicals at the intracellular level, DNA damage, and beta radiation from 131I [128]. As expert recommendations are mainly based on data interpretation from observational retrospective research, the use of 131I to treat well-differentiated thyroid cancer is still a controversial topic [129]. According to the previous recommendations of the “American and British Thyroid Associations”, the “European and American Societies of Nuclear Medicine”, the “European Consensus Group”, and the “National Comprehensive Cancer Network”, the use of 131I was justified in high-risk, intermediate-risk, and low-risk thyroid carcinoma [127]. However, recent results of a prospective trial concluded that “a follow-up strategy that did not involve the use of radioiodine was non-inferior to an ablation strategy with radioiodine regarding the occurrence of functional, structural, and biologic events at three years” in patients with low-risk thyroid carcinoma subjected to thyroidectomy [130].
Childhood exposure to radioisotope 131I was linked to a higher risk of thyroid cancer [131]. Iodine supplementation and iodine deficiency both alter this risk. So, if radioactive iodine results from radiation accidents or during medical diagnostic and therapeutic procedures, regular iodine supplementation in iodine-deficient populations may significantly lower the risk [101]. Even though thyroid tumors can be brought on by other ionizing radiation, such as the alpha particles from plutonium-238 (238Pu) [132], the thyroid gland is susceptible to radioiodine exposure.
Children in Japan were exposed to radiation from US atomic bombings in 1945, and teenagers, in particular, exhibited an elevated risk of acquiring thyroid cancer five decades later [112,133]. The Chernobyl disaster in 1986 resulted in the most uncontrolled radioactive discharge into the environment ever seen in a civilian operation. Radiation in Belarus and Ukraine was significantly increased by 131I [134,135]. High thyroid levels, particularly in younger people, were caused by 131I contamination of fresh milk and the lack of rapid countermeasures [112]. The prevalence of thyroid peroxidase antibodies was higher in radiation-exposed children and adolescents exposed to radioactive fallout 13–15 years after the Chernobyl accident (6.4% versus 2.4% in unexposed children). The evidence of danger among exposed adults is murky since no higher risk was discovered among adult Chernobyl liquidators [126]. In Chernobyl, there was an increased incidence of thyroid cancer among exposed children [112]. Hence, thyroid cancer incidence was significantly lower in children born after the Chernobyl disaster [136]. More than 11,000 thyroid cancer cases were exposed during childhood in Ukraine, Belarus, and Russia [137]. Importantly, PTC was the most thyroid cancer type among these children [138,139]. Molecular analyses in early childhood thyroid cancer cases revealed an extremely high prevalence of genome rearrangements between the Rearranged During Transfection (RET) gene and the PTC3 gene (RET/PTC3 rearrangement) located on the same chromosome 10 [140,141]. RET/PTC oncogenic rearrangements are now identified as principal triggering mutations in radiation-related and sporadic childhood PTC [135,142]. The later event of the nuclear catastrophe at the Tokyo Electric Power Company-operated Fukushima Daiichi Nuclear Power Station, which took place in March 2011, was described by Nagataki and Takamura as having radiation effects on the thyroid. The incident discharged 120 Peta Becquerel of radioiodine into the environment, much lower than the Chernobyl disaster. Most children, including those under one year old, had thyroid radiation doses of less than 100 mSv (intervention threshold for continuous iodine delivery) since residents close to the Fukushima nuclear facility were evacuated within a short time. Of the affected children, over 280,000 have been screened, and 90 have been diagnosed with thyroid cancer to date (approximate incidence: 313 per million) [143]. Experts from Japan and international organizations, including the WHO, United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), International Commission on Radiological Protection (ICRP), International Atomic Energy Agency (IAEA), and International Agency for Research on Cancer (IARC), reviewed and examined the thyroid cancer findings in Fukushima, emphasizing that the high detection rate of thyroid cancer and benign abnormalities resulted in the higher incidence rate. However, no incidences of thyroid cancer were found in the most vulnerable population, very young children, indicating that the increase is more likely reflective of high screening rates than radiation exposure [144].
A recent meta-analysis revealed that research has not linked living close to nuclear facilities to an increased risk of thyroid cancer [143]; however, because there was significant variation among incidence studies, care must be used when interpreting the findings. Modern nuclear power stations’ radioactive releases are probably too small to be statistically detectable.
4.2. Natural Radiation
The radioactive decay of uranium, which is present in rocks and soil, results in the production of radon (Rn), an odorless, tasteless, and colorless natural gas. A component of uranium-238′s (238U) radioactive decay chain is radon-222 (222Rn). The decay cycle of thorium-232 (232Th) produces radon-220 (220Rn). Radon naturally decays into short-lived radioactive isotopes humans can breathe and drink [143]. Springs and wells that draw groundwater serve as drinking water sources in many nations [145]. Compared to surface water from reservoirs, rivers, or lakes, these water sources typically have greater radon concentrations [143]. Because radon has been linked to lung cancer, exposure to levels above 4 picocuries per liter (pCi/L) is risky. After tobacco smoke, radon is the second-leading cause of lung cancer worldwide [143]. Radon is classified as a category I carcinogen. The volcanic region of Catania Province, Sicily (Italy), has been found to have a high Rn concentration, and its population is reported to have a higher incidence of thyroid cancer than others of non-volcanic, adjacent regions. However, the authors of this study were unable to draw any conclusions about the relationship between Rn exposure and the risk of thyroid cancer [146]. An ecological study conducted in three US States (Iowa, New Jersey, and Wisconsin) revealed no connection between elevated radon levels and the prevalence of thyroid cancer in women. In fact, from 1990 to 2013, New Jersey (N = 16,906), Wisconsin (N = 7250), and Iowa (N = 4236) had the highest number of female thyroid cases documented. For each of the three states, Pearson correlation coefficients were determined between radon levels over 4 pCi/L and the age-adjusted thyroid cancer incidence rate, although none of the comparisons revealed a connection [147]. Additionally, a different Pennsylvania study found no connection between cumulative radon levels and the occurrence of thyroid cancer [148].
5. Volcanic Environments and the Magma Enigma
In 1981, an increased incidence of thyroid cancer in environments with active volcanoes came to light. Iceland and the Philippines have been noted to have higher thyroid cancer rates than otherwise similar, non-volcanic environments [149]. A 30-year retrospective study on the incidence of thyroid cancer in Iceland, which covered the years 1955–1984 and included 406 registered cases, found that the incidence was 9.5/100,000 for females and 3.4/100,000 for males, twice as high as in other Nordic regions. Thyroid cancer was similarly found in 69.4% of 72 Filipino patients with thyroidectomies due to nodular goiter, compared to 38.9% of 72 controls matched for various variables. This finding highlights the elevated risk for thyroid cancer in Filipino people. Compared to White women born in the US, the rate of TC in Filipino women born in the Philippines was 3.2 times greater. Significantly, Filipino women born in the US did not face higher risks [150].
In areas around volcanic sites, non-anthropogenic pollution of soil, water, and the environment with heavy metals has been reported, including in Sicily, Italy, which sees regular eruptions of Mt. Etna [149]. Humans in these areas have also been contaminated by non-anthropogenic pollution with heavy metals through the food chain, as shown by statistically significantly higher metal levels in urine and scalp hair than in nearby non-volcanic areas. In addition, sulfur competes with molecules containing selenium for uptake by plants, reducing selenium availability. The substantial soil acidification that occurs after volcanic eruptions, as was observed, for instance, during the eruption of Etna in 1991, has long-term adverse effects on the body, altering the redox state, impairing defense mechanisms, causing inflammation, and accelerating cancer. DNA damage is more common in people who live in volcanically active areas than in people who live in nearby control areas [151]. The hormesis effect, a biphasic dose-response relationship observed in vitro for many metals, including arsenic, cadmium, copper, and mercury provides some insight into the precise impact of certain metals at various concentrations in various human tissues. The biological effects of this phenomenon are stimulated at low doses (µM or even nM) and inhibited at more significant quantities. The exact mechanism of hormesis is unknown, but it is known that chronic exposure to various toxicants at low levels can have significant toxicological effects [152].
6. Teratogens and Developmental Hazards
Proper control of environmental pollution could have a positive impact on population health. A novel theory posits that fetal exposure to EDCs during pregnancy may lead to endocrine dysfunction later in life [153,154]. EDCs have been found in amniotic fluid [155], umbilical cord blood [29], and other body fluids [156]. Following birth, EDCs exposure could be maintained through breastfeeding [157,158], infant meals [159,160], and direct environmental interaction. Plentiful manufactured sources of EDCs lead to an estimated human exposure of 1.7–52.1 g/kg/day to phthalates, for example, and under some conditions, newborns can be exposed to up to three times as much [155].
EDCs contribute to the development of cancers through both genetic and epigenetic processes. There is growing evidence that EDCs like PCBs, BPA, and phthalates can affect the thyroid in humans. According to a Yoshinaga study, environmental exposure to hydroxylated PCBs during the first trimester of pregnancy may impact the newborn’s thyroid hormones [161]. It has been demonstrated that early exposure to some environmental chemicals with endocrine-disrupting activities, such as pesticides, may affect a newborn’s thyroid hormone state, increasing the likelihood of developing a thyroid tumor in adulthood [162].
Most oncogenic fusions detected in cancer are caused by chromosomal rearrangements brought on by medicinal and pharmacological factors. Regions with deletions and chromosomal rearrangements have been found to include weak DNA spots, which are vulnerable to several toxins [163].
Thyroid cancer is thought to be influenced by epigenetic processes, particularly abnormal DNA and microRNA methylation [164]. The post-translational alteration of histone proteins (via acetylation, methylation, phosphorylation, ubiquitylation, sumoylation, proline isomerization, and adenosine diphosphate-ribosylation) is another well-studied epigenetic change that affects gene expression [165,166]. The disruption of genetic material caused by rearrangements may result in abnormalities, the development of oncogenic fusion proteins, or gene silencing, frequently cited as the primary method of tumor suppression [165].
Point mutations in the BRAF gene (which accounts for 40% of PTC cases), the RAS gene (15% of PTC cases, primarily the follicular form), or the RET/PTC (REarranged during Transfection-RET) rearrangement are only a few examples of the genetic variables that contribute to the development of PTC (18% of PTC cases).
Some environmental factors, such as benzene, found in cigarette smoke/car exhaust, diethylnitrosamine, present in pesticides, cigarette smoke, cured meat, and cancer chemotherapy, can significantly increase “fragile site breakages” with subsequent increase in the thyroid cancer risk [149,167].
7. Conclusions
This review explored how environmental risk factors may lead to an increased risk of thyroid cancer. Experimental studies have shown that several chemical groups may adversely disrupt thyroid function; however, only the impacts of environmental PCB levels have been thoroughly researched in epidemiological studies. For most substances, the association of exposure with the risk of thyroid cancer has not been thoroughly investigated, and the findings are not always consistent. There is strong evidence that PCBs impair thyroid function, and research from other halogenated substances, BPA, certain metals, metalloids, and phthalates suggests that these chemicals may also disrupt thyroid function and thus poses a risk for tumor promotion.
Based on the published data, we can hypothesize that thyroid cancer may result from the simultaneous coexistence of several conditions, including the genetic background of the individuals, environmental risk factors exposure, and nutritional factors, among others. Excessive nitrate uptake from drinking water increases nitrite production, causing hypoxia, especially in children, and from an excess of NO, a carcinogenic compound. Salivary glands are exposed to radiation via natural and therapeutic means, such as by dental X-ray examination. Hazardous geographic environments, such as volcanic zones and areas exposed to air pollution, lead to a lack of selenium or iodine. If any of these processes coincide with thyroid radiation exposure, markedly elevated NO concentrations in the body enhance the carcinogenic effect of radiation.
Unavoidable lifetime human exposure to a combination of these environmental toxins raises serious questions about their ability to cause thyroid cancer, mainly if exposure occurs during delicate developing stages. The development and severity of thyroid cancer in children and adults may be determined partly by fetal exposure to environmental effects during pregnancy. Further research should also focus on preventing overdiagnosis and overtreatment, both of which pose potential health concerns to individuals, along with excessive financial burden for patients in some countries.
Future studies should focus on determining safe levels of exposure to persistent organic pollutants following rigorous protocols as recommended by Zhang et al. [168]. This includes accounting for potential confounders, such as occupation, lifestyle, or exposure to several substances that may increase the risk of thyroid cancer. More rigorous evidence-based research and an integrated interdisciplinary approach, including “improved analytical tools and exposome databases, along with individual exposure measurements and effect-directed analysis”, according to Tang et al. [22], are required to understand better the mechanisms that lead to thyroid cancer and provide a complete picture of actual environmental exposures, and ultimately, to discover the specific environmental factors that increase the chance of disease occurrence in humans.
Author Contributions
Conceptualization, E.K. (Eva Kruger) and E.A.T.; methodology, E.K. (Eva Kruger), S.A.S. and E.A.T.; software, E.K. (Eva Kruger), S.A.S., E.A.T. and M.S.F.; validation, M.H.H., S.A.S., A.W., M.S.F. and E.K. (Emad Kandil); formal analysis, E.K. (Eva Kruger), S.A.S. and E.A.T.; investigation, E.K. (Eva Kruger), M.H.H., S.A.S. and E.A.T.; resources, E.K. (Eva Kruger), E.A.T., S.A.S. and E.K. (Emad Kandil); data curation, E.K. (Eva Kruger), S.A.S. and E.A.T.; writing—original draft preparation, E.K. (Eva Kruger), S.A.S. and E.A.T.; writing—review and editing, E.K. (Eva Kruger), S.A.S., A.W., M.S.F. and E.K. (Emad Kandil); visualization, E.K. (Eva Kruger), M.H.H., S.A.S., E.A.T. and M.S.F.; supervision, M.S.F. and E.K. (Emad kandil); project administration, E.A.T. and E.K. (Emad Kandil); funding acquisition, E.K. (Emad Kandil). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Loula Burton (Tulane University School of Medicine) for providing language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Le Magueresse-Battistoni, B.; Vidal, H.; Naville, D. Environmental Pollutants and Metabolic Disorders: The Multi-Exposure Scenario of Life. Front. Endocrinol. 2018, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Eberlein-Koenig, B.; Behrendt, H. Environmental pollution and allergy. Ann. Allergy Asthma Immunol. 2001, 87, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.O.; Calogero, A.E.; Giacone, F.; Fiore, M.; Barchitta, M.; Agodi, A.; Ferrante, M. B(a)P adduct levels and fertility: A cross-sectional study in a Sicilian population. Mol. Med. Rep. 2017, 15, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Pope, C.A. Lung cancer and air pollution. Environ. Health Perspect. 1995, 103, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.A. Endocrine tumors: Evaluation of the thyroid nodule. Curr. Opin. Oncol. 2003, 15, 66–70. [Google Scholar] [CrossRef]
- Marcello, M.A.; Malandrino, P.; Almeida, J.; Martins, M.B.; Cunha, L.L.; Bufalo, N.E.; Pellegriti, G.; Ward, L.S. The influence of the environment on the development of thyroid tumors: A new appraisal. Endocrine-Related Cancer 2014, 21, T235–T254. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Prim. 2015, 1, 15077. [Google Scholar] [CrossRef]
- Panchangam, R.B.; Puthenveetil, P.; Mayilvaganan, S. Prognostic Impact of Focal Poorly Differentiated Areas in Follicular Differentiated Thyroid Cancer: Is It a Distinct Entity from Poorly Differentiated Thyroid Cancer? Indian J. Surg. Oncol. 2021, 13, 157–163. [Google Scholar] [CrossRef]
- Berinde, G.M.; Socaciu, A.I.; Socaciu, M.A.; Cozma, A.; Rajnoveanu, A.G.; Petre, G.E.; Piciu, D. Thyroid Cancer Diagnostics Related to Occupational and Environmental Risk Factors: An Integrated Risk Assessment Approach. Diagnostics 2022, 12, 318. [Google Scholar] [CrossRef]
- Fiore, M.; Conti, G.O.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M.A.; Ferrante, M. Role of Emerging Environmental Risk Factors in Thyroid Cancer: A Brief Review. Int. J. Environ. Res. Public Health 2019, 16, 1185. [Google Scholar] [CrossRef]
- Kwong, N.; Medici, M.; Angell, T.E.; Liu, X.; Marqusee, E.; Cibas, E.S.; Krane, J.F.; Barletta, J.A.; Kim, M.I.; Larsen, P.R.; et al. The Influence of Patient Age on Thyroid Nodule Formation, Multinodularity, and Thyroid Cancer Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.M.; Wheeler, M.H. Thyroid Nodules: Rational Management. World J. Surg. 2000, 24, 934–941. [Google Scholar] [CrossRef]
- Lise, M.; Franceschi, S.; Buzzoni, C.; Zambon, P.; Falcini, F.; Crocetti, E.; Serraino, D.; Iachetta, F.; Zanetti, R.; Vercelli, M.; et al. Changes in the Incidence of Thyroid Cancer Between 1991 and 2005 in Italy: A Geographical Analysis. Thyroid 2012, 22, 27–34. [Google Scholar] [CrossRef]
- Kamani, T.; Charkhchi, P.; Zahedi, A.; Akbari, M.R. Genetic susceptibility to hereditary non-medullary thyroid cancer. Hered. Cancer Clin. Pract. 2022, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hemminki, K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int. J. Cancer 2001, 92, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Tallini, G.; Romeo, G. Genetic Predisposition to Familial Nonmedullary Thyroid Cancer: An Update of Molecular Findings and State-of-the-Art Studies. J. Oncol. 2010, 2010, 385206. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Overview of air pollution and endocrine disorders. Int. J. Gen. Med. 2018, 11, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Jugan, M.-L.; Levi, Y.; Blondeau, J.-P. Endocrine disruptors and thyroid hormone physiology. Biochem. Pharmacol. 2010, 79, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, J.; Zhou, Q.; Xu, S.; Cai, Z.; Jiang, G. Thyroid Cancer “Epidemic”: A Socio-Environmental Health Problem Needs Collaborative Efforts. Environ. Sci. Technol. 2020, 54, 3725–3727. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.; Kim, J.H.; Song, H.S. Persistent organic pollutants, pesticides, and the risk of thyroid cancer: Systematic review and meta-analysis. Eur. J. Cancer Prev. 2019, 28, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.; Moase, C.; Chan, P. A Review of Pesticide Exposure and Cancer Incidence in the Agricultural Health Study Cohort. Environ. Health Perspect. 2010, 118, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Russo, G.; Gambardella, C.; Grasso, M.; La Sala, D.; Chiofalo, M.G.; D’Anna, R.; Puzziello, A.; Docimo, G.; Masone, S.; et al. Human exposure to bisphenol AF and diethylhexylphthalate increases susceptibility to develop differentiated thyroid cancer in patients with thyroid nodules. Chemosphere 2019, 218, 885–894. [Google Scholar] [CrossRef]
- Miao, H.; Liu, X.; Li, J.; Zhang, L.; Zhao, Y.; Liu, S.; Ni, S.; Wu, Y. Associations of urinary phthalate metabolites with risk of papillary thyroid cancer. Chemosphere 2020, 241, 125093. [Google Scholar] [CrossRef]
- Meeker, J.D. Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 2010, 66, 236–241. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, F.; Zhang, J.; Hao, L.; Jiang, J.; Dang, L.; Mei, D.; Fan, S.; Yu, Y.; Jiang, L. Bisphenol A and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway. Arch. Biochem. Biophys. 2017, 633, 29–39. [Google Scholar] [CrossRef]
- Saleh, H.N.; Panahande, M.; Yousefi, M.; Asghari, F.B.; Conti, G.O.; Talaee, E.; Mohammadi, A.A. Carcinogenic and Non-carcinogenic Risk Assessment of Heavy Metals in Groundwater Wells in Neyshabur Plain, Iran. Biol. Trace Element Res. 2018, 190, 251–261. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, W.; Xu, Q.; Li, X.; Zhou, L.; Ye, L. Di(2-ethylhexyl) phthalate (DEHP) and thyroid: Biological mechanisms of interference and possible clinical implications. Environ. Sci. Pollut. Res. 2021, 29, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.A.; Shields, B.M.; He, X.; Pearce, E.N.; Braverman, L.E.; Sturley, R.; Vaidya, B. Effect of perchlorate and thiocyanate exposure on thyroid function of pregnant women from South-West England: A cohort study. Thyroid. Res. 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The Effects of Metals as Endocrine Disruptors. J. Toxicol. Environ. Health Part B 2009, 12, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef]
- Chung, H.-K.; Nam, J.S.; Ahn, C.W.; Lee, Y.S.; Kim, K.R. Some Elements in Thyroid Tissue are Associated with More Advanced Stage of Thyroid Cancer in Korean Women. Biol. Trace Elem. Res. 2016, 171, 54–62. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, J.; Jin, L.; Yang, F.; Wang, J.; Wang, O.; Gao, Y. Correlation between serum lead and thyroid diseases: Papillary thyroid carcinoma, nodular goiter, and thyroid adenoma. Int. J. Environ. Health Res. 2017, 27, 409–419. [Google Scholar] [CrossRef]
- Assem, F.L.; Holmes, P.; Levy, L.S. The Mutagenicity and Carcinogenicity of Inorganic Manganese Compounds: A Synthesis of The Evidence. J. Toxicol. Environ. Health Part B 2011, 14, 537–570. [Google Scholar] [CrossRef]
- Fallahi, P.; Foddis, R.; Elia, G.; Ragusa, F.; Patrizio, A.; Benvenga, S.; Cristaudo, A.; Antonelli, A.; Ferrari, S.M. Vanadium pentoxide induces the secretion of CXCL9 and CXCL10 chemokines in thyroid cells. Oncol. Rep. 2018, 39, 2422–2426. [Google Scholar] [CrossRef]
- Triggiani, V.; Tafaro, E.; Giagulli, V.A.; Sabbà, C.; Resta, F.; Licchelli, B.; Guastamacchia, E. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocrine Metab. Immune Disord.-Drug Targets 2009, 9, 277–294. [Google Scholar] [CrossRef]
- Winther, K.H.; Bonnema, S.J.; Cold, F.; Debrabant, B.; Nybo, M.; Cold, S.; Hegedüs, L. Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur. J. Endocrinol. 2015, 172, 657–667. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Rothman, K.J. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Jones, R.R.; Anderson, K.E.; Cantor, K.P.; Cerhan, J.R.; Krasner, S.; Robien, K.; Weyer, P.J.; Ward, M.H. Nitrate and nitrite ingestion and risk of ovarian cancer among postmenopausal women in Iowa. Int. J. Cancer 2014, 137, 173–182. [Google Scholar] [CrossRef]
- Drozd, V.M.; Branovan, I.; Shiglik, N.; Biko, J.; Reiners, C. Thyroid Cancer Induction: Nitrates as Independent Risk Factors or Risk Modulators after Radiation Exposure, with a Focus on the Chernobyl Accident. Eur. Thyroid J. 2018, 7, 67–74. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope, C.A.; Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient Air Pollution and Cancer Mortality in the Cancer Prevention Study II. Environ. Health Perspect. 2017, 125, 087013. [Google Scholar] [CrossRef]
- Cong, X. Air pollution from industrial waste gas emissions is associated with cancer incidences in Shanghai, China. Environ. Sci. Pollut. Res. 2018, 25, 13067–13078. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef]
- Walker, D.I.; Valvi, D.; Rothman, N.; Lan, Q.; Miller, G.W.; Jones, D.P. The Metabolome: A Key Measure for Exposome Research in Epidemiology. Curr. Epidemiol. Rep. 2019, 6, 93–103. [Google Scholar] [CrossRef]
- Samet, J.M.; Chiu, W.A.; Cogliano, V.; Jinot, J.; Kriebel, D.; Lunn, R.M.; Beland, F.A.; Bero, L.; Browne, P.; Fritschi, L.; et al. The IARC Monographs: Updated Procedures for Modern and Transparent Evidence Synthesis in Cancer Hazard Identification. Gynecol. Oncol. 2019, 112, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lerro, C.C.; Freeman, L.E.B.; DellaValle, C.T.; Andreotti, G.; Hofmann, J.N.; Koutros, S.; Parks, C.G.; Shrestha, S.; Alavanja, M.C.; Blair, A.; et al. Pesticide exposure and incident thyroid cancer among male pesticide applicators in agricultural health study. Environ. Int. 2020, 146, 106187. [Google Scholar] [CrossRef] [PubMed]
- Deziel, N.C.; Warren, J.L.; Huang, H.; Zhou, H.; Sjodin, A.; Zhang, Y. Exposure to polychlorinated biphenyls and organochlorine pesticides and thyroid cancer in connecticut women. Environ. Res. 2020, 192, 110333. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.E.B.; Rusiecki, J.A.; Hoppin, J.; Lubin, J.H.; Koutros, S.; Andreotti, G.; Zahm, S.H.; Hines, C.J.; Coble, J.B.; Barone-Adesi, F.; et al. Atrazine and Cancer Incidence Among Pesticide Applicators in the Agricultural Health Study (1994–2007). Environ. Health Perspect. 2011, 119, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Lerro, C.; Lavoué, J.; Huang, H.; Siemiatycki, J.; Zhao, N.; Ma, S.; Deziel, N.C.; Friesen, M.C.; Udelsman, R.; et al. Occupational exposure to pesticides and other biocides and risk of thyroid cancer. Occup. Environ. Med. 2017, 74, 502–510. [Google Scholar] [CrossRef]
- Lerro, C.C.; Koutros, S.; Andreotti, G.; Friesen, M.C.; Alavanja, M.C.; Blair, A.; Hoppin, J.A.; Sandler, D.P.; Lubin, J.H.; Ma, X.; et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2015, 72, 736–744. [Google Scholar] [CrossRef]
- Lerro, C.C.; Jones, R.R.; Langseth, H.; Grimsrud, T.K.; Engel, L.S.; Sjödin, A.; Choo-Wosoba, H.; Albert, P.; Ward, M.H. A nested case-control study of polychlorinated biphenyls, organochlorine pesticides, and thyroid cancer in the Janus Serum Bank cohort. Environ. Res. 2018, 165, 125–132. [Google Scholar] [CrossRef]
- Sun, H.; Shen, O.-X.; Xu, X.-L.; Song, L.; Wang, X.-R. Carbaryl, 1-naphthol and 2-naphthol inhibit the beta-1 thyroid hormone receptor-mediated transcription in vitro. Toxicology 2008, 249, 238–242. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Cabello, G.; Valenzuela, M.; Vilaxa, A.; Durán, V.; Rudolph, I.; Hrepic, N.; Calaf, G. A rat mammary tumor model induced by the organophosphorous pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ. Health Perspect. 2001, 109, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Toledano, D.S.; Estrada-Muñiz, E.; Vega, L. Genotoxicity of the organophosphate pesticide malathion and its metabolite dimethylthiophosphate in human cells in vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2020, 856–857, 503233. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, E.L.D.; da Silva, J.D.S.; Nakamura, T.C.; Diniz, P.H.G.D.; Oliveira, U.R.; de Souza, J.R. Distribution of organochlorine, organophosphates, carbamate, thiocarbamate, pyrethroids, and strobilurins in surface sediments of the Rio de Ondas watershed by GC-MS. J. Environ. Sci. Health Part B 2021, 56, 357–369. [Google Scholar] [CrossRef]
- Zoeller, R.T. Environmental Chemicals Impacting the Thyroid: Targets and Consequences. Thyroid 2007, 17, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Muscogiuri, G.; Piscitelli, P. Environment and Health: Not Only Cancer. Int. J. Environ. Res. Public Health 2016, 13, 724. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 2015, 36, 699–703. [Google Scholar] [CrossRef]
- Alsen, M.; Sinclair, C.; Cooke, P.; Ziadkhanpour, K.; Genden, E.; van Gerwen, M. Endocrine Disrupting Chemicals and Thyroid Cancer: An Overview. Toxics 2021, 9, 14. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Geng, X.; Ji, X.; Zhang, X.; Yue, H.; Li, G.; Sang, N. Bisphenol A Analogs Induce Cellular Dysfunction in Human Trophoblast Cells in a Thyroid Hormone Receptor-Dependent Manner: In Silico and In Vitro Analyses. Environ. Sci. Technol. 2022, 56, 8384–8394. [Google Scholar] [CrossRef]
- Marotta, V.; Grumetto, L.; Neri, I.; Russo, G.; Tortora, A.; Izzo, G.; Panariello, I.; Rocco, D.; Pezzullo, L.; Vitale, M. Exposure to Bisphenol A increases malignancy risk of thyroid nodules in overweight/obese patients. Environ. Pollut. 2022, 316, 120478. [Google Scholar] [CrossRef]
- Fiedler, H. Dioxins and Furans (PCDD/PCDF). In Persistent Organic Pollutants; Springer: Berlin/Heidelberg, Germany, 2006; pp. 123–201. [Google Scholar] [CrossRef]
- Safe, S. Polychlorinated Biphenyls (PCBs), Dibenzo-p-Dioxins (PCDDs), Dibenzofurans (PCDFs), and Related Compounds: Environmental and Mechanistic Considerations Which Support the Development of Toxic Equivalency Factors (TEFs). Crit. Rev. Toxicol. 1990, 21, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Birnbaum, L.; Ryan, J.J.; Constable, J.D. Dioxins: An overview. Environ. Res. 2006, 101, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, Y.; Kang, Y.; Jung, K.; Jee, S. The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 8745. [Google Scholar] [CrossRef] [PubMed]
- Reale, C.; Russo, F.; Credendino, S.C.; Cuomo, D.; De Vita, G.; Mallardo, M.; Pennino, F.; Porreca, I.; Triassi, M.; De Felice, M.; et al. A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis. Int. J. Environ. Res. Public Health 2019, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Lans, M.C.; Spiertz, C.; Brouwer, A.; Koeman, J.H. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994, 270, 129–136. [Google Scholar] [CrossRef]
- Brouwer, A.; Morse, D.C.; Lans, M.C.; Gerlienke Schuur, A.; Murk, A.J.; Klasson-Wehler, E.; Bergman, Å.; Visser, T.J. Interactions of Persistent Environmental Organohalogens With the Thyroid Hormone System: Mechanisms and Possible Consequences for Animal and Human Health. Toxicol. Ind. Health 1998, 14, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, C.-T.; Chen, S.-J.; Yan, X.; Guo, M.-N.; Wang, M.-H.; Yu, Y.-J.; Yang, Z.-Y.; Mai, B.-X. Disruption of thyroid hormone (TH) levels and TH-regulated gene expression by polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and hydroxylated PCBs in e-waste recycling workers. Environ. Int. 2017, 102, 138–144. [Google Scholar] [CrossRef]
- Erickson, M.D.; Ii, R.G.K. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 2010, 18, 135–151. [Google Scholar] [CrossRef]
- Li, Y.; Bako, C.M.; Saktrakulkla, P.; Lehmler, H.-J.; Hornbuckle, K.C.; Schnoor, J.L. Interconversion between methoxylated, hydroxylated and sulfated metabolites of PCB 3 in whole poplar plants. Sci. Total Environ. 2021, 785, 147341. [Google Scholar] [CrossRef]
- Mallin, K.; McCann, K.; D’Aloisio, A.; Freels, S.; Piorkowski, J.; Dimos, J.; Persky, V. Cohort Mortality Study of Capacitor Manufacturing Workers, 1944–2000. J. Occup. Environ. Med. 2004, 46, 565–576. [Google Scholar] [CrossRef]
- Zhuo, H.; Huang, H.; Sjodin, A.; Jin, L.; Ma, S.; Denic-Roberts, H.; Warren, J.L.; Jones, R.; Davis, M.; Sun, P.; et al. A nested case-control study of serum polychlorinated biphenyls and papillary thyroid cancer risk among U.S. military service members. Environ. Res. 2022, 212, 113367. [Google Scholar] [CrossRef] [PubMed]
- Program, N.T. Toxicology and carcinogenesis studies of 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) (CAS No. 31508-00-6) in female harlan Sprague-Dawley rats (gavage studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2010, 559, 1–174. [Google Scholar]
- Brouwer, A.; Longnecker, M.P.; Birnbaum, L.; Cogliano, J.; Kostyniak, P.; Moore, J.; Schantz, S.; Winneke, G. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ. Health Perspect. 1999, 107, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, H.; He, Q.; Liu, J.; Liu, H.; Li, Y.; Chen, X. Network Toxicology Guided Mechanism Study on the Association between Thyroid Function and Exposures to Polychlorinated Biphenyls Mixture. BioMed Res. Int. 2022, 2022, 2394398. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; DellaValle, C.T.; Purdue, M.; Kim, C.; Zhang, Y.; Sjodin, A.; Ward, M.H. Polybrominated Diphenyl Ethers and Thyroid Cancer Risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial Cohort. Am. J. Epidemiol. 2015, 181, 883–888. [Google Scholar] [CrossRef]
- Kim, Y.R.; Harden, F.A.; Toms, L.-M.L.; Norman, R.E. Health consequences of exposure to brominated flame retardants: A systematic review. Chemosphere 2014, 106, 1–19. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- WHO. International Agency for Research on Cancer. Available online: https://www.iarc.who.int/ (accessed on 12 December 2022).
- Curcic, M.; Janković, S.; Jacevic, V.; Stankovic, S.; Vucinic, S.; Durgo, K.; Bulat, Z.; Antonijevic, B. Combined effects of cadmium and decabrominated diphenyl ether on thyroid hormones in rats. Arch. Ind. Hyg. Toxicol. 2012, 63, 255–262. [Google Scholar] [CrossRef]
- Jancic, S.A.; Stosic, B.Z. Cadmium Effects on the Thyroid Gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar] [CrossRef]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef]
- Nugegoda, D.; Kibria, G. Effects of environmental chemicals on fish thyroid function: Implications for fisheries and aquaculture in Australia. Gen. Comp. Endocrinol. 2017, 244, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Salama, A.F.; El Nimr, T.M.; El Gamal, D.M. Effects of phytate on thyroid gland of rats intoxicated with cadmium. Toxicol. Ind. Health 2013, 31, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, Y.; Chen, Y.; Chen, C.; Han, B.; Li, Q.; Zhu, C.; Xia, F.; Zhai, H.; Wang, N.; et al. Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ. Pollut. 2017, 230, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Culotta, V.C.; Daly, M.J. Manganese Complexes: Diverse Metabolic Routes to Oxidative Stress Resistance in Prokaryotes and Yeast. Antioxid. Redox Signal. 2013, 19, 933–944. [Google Scholar] [CrossRef]
- Sachse, B.; Kolbaum, A.E.; Ziegenhagen, R.; Andres, S.; Berg, K.; Dusemund, B.; Hirsch-Ernst, K.I.; Kappenstein, O.; Müller, F.; Röhl, C.; et al. Dietary Manganese Exposure in the Adult Population in Germany—What Does it Mean in Relation to Health Risks? Mol. Nutr. Food Res. 2019, 63, e1900065. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.C.; Castañeda, J.P.; Liljedahl, E.R.; Mora, A.M.; Menezes-Filho, J.A.; Smith, D.R.; Mergler, D.; Reich, B.; Giffin, A.; Hoppin, J.A.; et al. Exposure to common-use pesticides, manganese, lead, and thyroid function among pregnant women from the Infants’ Environmental Health (ISA) study, Costa Rica. Sci. Total. Environ. 2021, 810, 151288. [Google Scholar] [CrossRef]
- Luz, A.L.; Wu, X.; Tokar, E.J. Toxicology of inorganic carcinogens. Adv. Mol. Toxicol. 2018, 12, 1–46. [Google Scholar]
- Nettore, I.C.; Colao, A.; Macchia, P.E. Nutritional and Environmental Factors in Thyroid Carcinogenesis. Int. J. Environ. Res. Public Health 2018, 15, 1735. [Google Scholar] [CrossRef]
- Winder, M.; Kosztyła, Z.; Boral, A.; Kocełak, P.; Chudek, J. The Impact of Iodine Concentration Disorders on Health and Cancer. Nutrients 2022, 14, 2209. [Google Scholar] [CrossRef]
- Foster, J.R.; Tinwell, H.; Melching-Kollmuss, S. A review of species differences in the control of, and response to, chemical-induced thyroid hormone perturbations leading to thyroid cancer. Arch. Toxicol. 2021, 95, 807–836. [Google Scholar] [CrossRef]
- Xie, L.; Mo, M.; Jia, H.-X.; Liang, F.; Yuan, J.; Zhu, J. Association between dietary nitrate and nitrite intake and site-specific cancer risk: Evidence from observational studies. Oncotarget 2016, 7, 56915–56932. [Google Scholar] [CrossRef]
- Rai, P.K. Multifaceted health impacts of particulate matter (PM) and its management: An overview. Environ. Skept. Crit. 2015, 4, 1–26. [Google Scholar]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmospheric Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Zhang, L.; Ou, C.; Magana-Arachchi, D.; Vithanage, M.; Vanka, K.S.; Palanisami, T.; Masakorala, K.; Wijesekara, H.; Yan, Y.; Bolan, N.; et al. Indoor Particulate Matter in Urban Households: Sources, Pathways, Characteristics, Health Effects, and Exposure Mitigation. Int. J. Environ. Res. Public Health 2021, 18, 11055. [Google Scholar] [CrossRef] [PubMed]
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, M.S.; Hunter, R.P. Health Outcomes of Exposure to Biological and Chemical Components of Inhalable and Respirable Particulate Matter. Int. J. Environ. Res. Public Health 2016, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Seaberg, R.M.; Eski, S.; Freeman, J.L. Influence of Previous Radiation Exposure on Pathologic Features and Clinical Outcome in Patients With Thyroid Cancer. Arch. Otolaryngol.-Head Neck Surg. 2009, 135, 355–359. [Google Scholar] [CrossRef]
- Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 911. [Google Scholar] [CrossRef]
- Iglesias, M.L.; Schmidt, A.; Al Ghuzlan, A.; Lacroix, L.; de Vathaire, F.; Chevillard, S.; Schlumberger, M. Radiation exposure and thyroid cancer: A review. Arq. Bras. Endocrinol. Metabol. 2017, 61, 180–187. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hayashi, K.; Scherb, H. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine 2019, 98, e17165. [Google Scholar] [CrossRef]
- Suzuki, K.; Saenko, V.; Yamashita, S.; Mitsutake, N. Radiation-Induced Thyroid Cancers: Overview of Molecular Signatures. Cancers 2019, 11, 1290. [Google Scholar] [CrossRef]
- Little, M.P.; Brenner, A.V.; Grant, E.J.; Sugiyama, H.; Preston, D.L.; Sakata, R.; Cologne, J.; Velazquez-Kronen, R.; Utada, M.; Mabuchi, K.; et al. Age effects on radiation response: Summary of a recent symposium and future perspectives. Int. J. Radiat. Biol. 2022, 98, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.V.; Tronko, M.; Hatch, M.; Bogdanova, T.; Oliynik, V.A.; Lubin, J.H.; Zablotska, L.B.; Tereschenko, V.P.; McConnell, R.J.; Zamotaeva, G.A.; et al. I-131 Dose Response for Incident Thyroid Cancers in Ukraine Related to the Chornobyl Accident. Environ. Health Perspect. 2011, 119, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.K.; Gorski, A.; Tsyb, A.F.; Maksioutov, M.; Tumanov, K.; Vlasov, O.K. Radiation-epidemiological studies of thyroid cancer incidence among children and adolescents in the Bryansk oblast of Russia after the Chernobyl accident (1991–2001 follow-up period). Radiat. Environ. Biophys. 2006, 45, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Takamura, N.; Ohtsuru, A.; Suzuki, S. Radiation Exposure and Thyroid Cancer Risk After the Fukushima Nuclear Power Plant Accident in Comparison with the Chernobyl Accident. Radiat. Prot. Dosim. 2016, 171, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Fiz, F.; Bottoni, G.; De Luca, C.; Massollo, M.; Catrambone, U.; Foppiani, L.; Muraca, M.; Garaventa, A.; Trimboli, P. Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable? Cancers 2021, 13, 4692. [Google Scholar] [CrossRef]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Deziel, N.C.; Huang, H.; Zhao, N.; Ma, S.; Ni, X.; Udelsman, R.; Zhang, Y. Genetic susceptibility may modify the association between cell phone use and thyroid cancer: A population-based case-control study in Connecticut. Environ. Res. 2019, 182, 109013. [Google Scholar] [CrossRef]
- Sandler, J.E.; Huang, H.; Zhao, N.; Wu, W.; Liu, F.; Ma, S.; Udelsman, R.; Zhang, Y. Germline Variants in DNA Repair Genes, Diagnostic Radiation, and Risk of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 285–294. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef]
- Benson, V.S.; Pirie, K.; Schüz, J.; Reeves, G.K.; Beral, V.; Green, J. Mobile phone use and risk of brain neoplasms and other cancers: Prospective study. Int. J. Epidemiol. 2013, 42, 792–802. [Google Scholar] [CrossRef]
- Altekruse, S.; Das, A.; Cho, H.; Petkov, V.; Yu, M. Do US thyroid cancer incidence rates increase with socioeconomic status among people with health insurance? An observational study using SEER population-based data. BMJ Open 2015, 5, e009843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.; Deziel, N.C.; Huang, H.; Chen, Y.; Ni, X.; Ma, S.; Udelsman, R.; Zhang, Y. Cell phone use and risk of thyroid cancer: A population-based case–control study in Connecticut. Ann. Epidemiol. 2018, 29, 39–45. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn. Biol. Med. 2016, 35, 303–304. [Google Scholar] [CrossRef]
- Weiss, W. Chernobyl thyroid cancer: 30 years of follow-up overview. Radiat. Prot. Dosim. 2018, 182, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Estorch, M.; Mitjavila, M.; Muros, M.A.; Caballero, E. Radioiodine treatment of differentiated thyroid cancer related to guidelines and scientific literature. Rev. Española De Med. Nucl. E Imagen Mol. 2019, 38, 195–203. [Google Scholar] [CrossRef]
- De la Vieja, A.; Riesco-Eizaguirre, G. Radio-Iodide Treatment: From Molecular Aspects to the Clinical View. Cancers 2021, 13, 995. [Google Scholar] [CrossRef]
- TuttleMD, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Fuehrer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470. [Google Scholar] [CrossRef]
- Leboulleux, S.; Bournaud, C.; Chougnet, C.N.; Zerdoud, S.; Al Ghuzlan, A.; Catargi, B.; Cao, C.D.; Kelly, A.; Barge, M.-L.; Lacroix, L.; et al. Thyroidectomy without Radioiodine in Patients with Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 923–932. [Google Scholar] [CrossRef]
- Cardis, E.; Kesminiene, A.; Ivanov, V.; Malakhova, I.; Shibata, Y.; Khrouch, V.; Drozdovitch, V.; Maceika, E.; Zvonova, I.; Vlassov, O.; et al. Risk of Thyroid Cancer After Exposure to 131I in Childhood. J. Natl. Cancer Inst. 2005, 97, 724–732. [Google Scholar] [CrossRef]
- Schlumberger, M.; Chevillard, S.; Ory, K.; Dupuy, C.; Le Guen, B.; De Vathaire, F. Thyroid cancer following exposure to ionising radiation. Cancer Radiother. 2011, 15, 394–399. [Google Scholar] [CrossRef]
- Hatch, M.; Cardis, E. Somatic health effects of Chernobyl: 30 years on. Eur. J. Epidemiol. 2017, 32, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Radiation carcinogenesis: Lessons from Chernobyl. Oncogene 2008, 27 (Suppl. S2), S9–S18. [Google Scholar] [CrossRef]
- Saenko, V.; Ivanov, V.; Tsyb, A.; Bogdanova, T.; Tronko, M.; Demidchik, Y.; Yamashita, S. The Chernobyl Accident and its Consequences. Clin. Oncol. 2011, 23, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, Y.E.; Saenko, V.A.; Yamashita, S. Childhood thyroid cancer in Belarus, Russia, and Ukraine after Chernobyl and at present. Arq. Bras. Endocrinol. Metabol. 2007, 51, 748–762. [Google Scholar] [CrossRef] [PubMed]
- McCall, C. Chernobyl disaster 30 years on: Lessons not learned. Lancet 2016, 387, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.K.; Kashcheev, V.; Chekin, S.Y.; Maksioutov, M.; Tumanov, K.; Vlasov, O.K.; Shchukina, N.; Tsyb, A.F. Radiation-epidemiological studies of thyroid cancer incidence in Russia after the Chernobyl accident (estimation of radiation risks, 1991-2008 follow-up period). Radiat. Prot. Dosim. 2012, 151, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.; Vaisman, F.; Tronko, M. Clinical Presentation and Clinical Outcomes in Chernobyl-related Paediatric Thyroid Cancers: What Do We Know Now? What Can We Expect in the Future? Clin. Oncol. 2011, 23, 268–275. [Google Scholar] [CrossRef]
- Klugbauer, S.; Lengfelder, E.; Demidchik, E.P.; Rabes, H.M. High prevalence of RET rearrangement in thyroid tumors of children from Belarus after the Chernobyl reactor accident. Oncogene 1995, 11, 2459–2467. [Google Scholar]
- Fugazzola, L.; Pilotti, S.; Pinchera, A.; Vorontsova, T.V.; Mondellini, P.; Bongarzone, I.; Greco, A.; Astakhova, L.; Butti, M.G.; Demidchik, E.P. Oncogenic rearrangements of the RET proto-oncogene in papillary thyroid carcinomas from children exposed to the Chernobyl nuclear accident. Cancer Res. 1995, 55, 5617–5620. [Google Scholar]
- Santoro, M.; Carlomagno, F. Oncogenic rearrangements driving ionizing radiation–associated human cancer. J. Clin. Investig. 2013, 123, 4566–4568. [Google Scholar] [CrossRef]
- Keramati, H.; Ghorbani, R.; Fakhri, Y.; Khaneghah, A.M.; Conti, G.O.; Ferrante, M.; Ghaderpoori, M.; Taghavi, M.; Baninameh, Z.; Bay, A.; et al. Radon 222 in drinking water resources of Iran: A systematic review, meta-analysis and probabilistic risk assessment (Monte Carlo simulation). Food Chem. Toxicol. 2019, 115, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.A.; Thomas, G.A.; Yamashita, S. Meeting report: The 5th International expert symposium in Fukushima on radiation and health. Environ. Health 2017, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Kapembo, M.L.; Mukeba, F.B.; Sivalingam, P.; Mukoko, J.B.; Bokolo, M.K.; Mulaji, C.K.; Mpiana, P.T.; Poté, J.W. Survey of water supply and assessment of groundwater quality in the suburban communes of Selembao and Kimbanseke, Kinshasa in Democratic Republic of the Congo. Sustain. Water Resour. Manag. 2021, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; De Vathaire, F.; Scollo, C.; Attard, M.; Giordano, C.; Arena, S.; Dardanoni, G.; Frasca, F.; Malandrino, P.; Vermiglio, F.; et al. Papillary Thyroid Cancer Incidence in the Volcanic Area of Sicily. Gynecol. Oncol. 2009, 101, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Oakland, C.; Meliker, J.R. County-Level Radon and Incidence of Female Thyroid Cancer in Iowa, New Jersey, and Wisconsin, USA. Toxics 2018, 6, 17. [Google Scholar] [CrossRef]
- Goyal, N.; Camacho, F.; Mangano, J.; Goldenberg, D. Evaluating for a geospatial relationship between radon levels and thyroid Cancer in Pennsylvania. Laryngoscope 2014, 125, E45–E49. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Ronchi, A.; Minoia, C.; Cataldo, D.; Regalbuto, C.; Giordano, C.; Attard, M.; Squatrito, S.; Trimarchi, F.; et al. Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 2015, 53, 471–479. [Google Scholar] [CrossRef]
- Nguyen, M.-L.T.; Hu, J.; Mph, K.G.H.; Daza, E.J.; Cullen, M.R.; Orloff, L.A.; Palaniappan, L.P. Thyroid cancer mortality is higher in Filipinos in the United States: An analysis using national mortality records from 2003 through 2012. Cancer 2017, 123, 4860–4867. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Arruda, M.S.C.; Garcia, P.V. Evidence of DNA damage in humans inhabiting a volcanically active environment: A useful tool for biomonitoring. Environ. Int. 2012, 49, 51–56. [Google Scholar] [CrossRef]
- Calabrese, E.; Iavicoli, I.; Calabrese, V. Hormesis: Its impact on medicine and health. Hum. Exp. Toxicol. 2012, 32, 120–152. [Google Scholar] [CrossRef]
- Csaba, G. The Faulty Perinatal Hormonal Imprinting as Functional Teratogen. Curr. Pediatr. Rev. 2016, 12, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Calkins, K.; Devaskar, S.U. Fetal Origins of Adult Disease. Curr. Probl. Pediatric Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arguelles, D.; Campioli, E.; Culty, M.; Zirkin, B.; Papadopoulos, V. Fetal origin of endocrine dysfunction in the adult: The phthalate model. J. Steroid Biochem. Mol. Biol. 2013, 137, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Schantz, S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenetics 2018, 4, dvy022. [Google Scholar] [CrossRef]
- Brajenović, N.; Karačonji, I.B.; Jurič, A. Levels of polychlorinated biphenyls in human milk samples in European countries. Arh. Hig. Rada Toksikol. 2018, 69, 135–153. [Google Scholar] [CrossRef]
- Björklund, K.L.; Vahter, M.; Palm, B.; Grandér, M.; Lignell, S.; Berglund, M. Metals and trace element concentrations in breast milk of first time healthy mothers: A biological monitoring study. Environ. Health 2012, 11, 92. [Google Scholar] [CrossRef]
- Spungen, J.H.; MacMahon, S.; Leigh, J.; Flannery, B.; Kim, G.; Chirtel, S.; Smegal, D. Estimated US infant exposures to 3-MCPD esters and glycidyl esters from consumption of infant formula. Food Addit. Contam. Part A 2018, 35, 1085–1092. [Google Scholar] [CrossRef]
- Li, L.; Arnot, J.A.; Wania, F. Revisiting the Contributions of Far- and Near-Field Routes to Aggregate Human Exposure to Polychlorinated Biphenyls (PCBs). Environ. Sci. Technol. 2018, 52, 6974–6984. [Google Scholar] [CrossRef]
- Hisada, A.; Shimodaira, K.; Okai, T.; Watanabe, K.; Takemori, H.; Takasuga, T.; Koyama, M.; Watanabe, N.; Suzuki, E.; Shirakawa, M.; et al. Associations between levels of hydroxylated PCBs and PCBs in serum of pregnant women and blood thyroid hormone levels and body size of neonates. Int. J. Hyg. Environ. Health 2014, 217, 546–553. [Google Scholar] [CrossRef]
- Freire, C.; Lopez-Espinosa, M.-J.; Fernandez, M.F.; Molina-Molina, J.-M.; Prada, R.; Olea, N. Prenatal exposure to organochlorine pesticides and TSH status in newborns from Southern Spain. Sci. Total. Environ. 2011, 409, 3281–3287. [Google Scholar] [CrossRef]
- Hasty, P.; Montagna, C. Chromosomal Rearrangements in Cancer: Detection and potential causal mechanisms. Mol. Cell. Oncol. 2014, 1, e29904. [Google Scholar] [CrossRef] [PubMed]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cheng, S.-Y. Epigenetic Modifications: Novel Therapeutic Approach for Thyroid Cancer. Endocrinol. Metab. 2017, 32, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Zarkesh, M.; Zadeh-Vakili, A.; Azizi, F.; Foroughi, F.; Akhavan, M.M.; Hedayati, M. Altered Epigenetic Mechanisms in Thyroid Cancer Subtypes. Mol. Diagn. Ther. 2017, 22, 41–56. [Google Scholar] [CrossRef]
- Dillon, L.W.; Lehman, C.E.; Wang, Y.-H. The Role of Fragile Sites in Sporadic Papillary Thyroid Carcinoma. J. Thyroid Res. 2012, 2012, 927683. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Liu, Y.P.; Miao, S.S.; Liu, X.D.; Ma, S.M.; Qu, Z.Y. Exposure to persistent organic pollutants and thyroid cancer risk: A study protocol of systematic review and meta-analysis. BMJ Open 2021, 11, e048451. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).