Single-Cell DNA Methylation Analysis in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
1.1. DNA Methylation
1.2. Single-Cell DNA Methylation
2. Single-Cell Methylome Profiling in Cancer
2.1. Single-Cell DNA Methylation in Cancer Initiation and Progression
2.2. Single-Cell DNA Methylation in Metastasis
2.3. Single-Cell DNA Methylation in Cancer Therapy
3. Single-Cell DNA Methylation Sequencing Techniques
3.1. Isolation of Single Cells
3.2. Experimental Approaches
3.3. Third-Generation Sequencing Techniques for Single-Cell DNA Methylation
4. Single-Cell DNA Methylation Bioinformatic Analyses
4.1. Preprocessing
4.1.1. Trimming
4.1.2. Genome Alignment
4.2. Normalisation
4.3. Data Sparsity
4.4. Downstream Analyses
5. Current Challenges in the Field
5.1. Bioinformatic Challenges
5.2. Experimental Challenges
5.3. Challenges in Clinical Implementation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| 5hmC | 5-hydroxymethylcytosine |
| 5mC | 5-methylcytosine |
| CGI | CpG island |
| CpG | The site of a cytosine residue adjacent to a guanine residue |
| CTC | Circulating tumour cell |
| ctDNA | Circulating tumour DNA |
| DMR | Differentially methylated region |
| DNAme | DNA methylation |
| EMT | Epithelial-mesenchymal transition |
| FACS | Fluorescence activated cell sorting |
| FFPE | Formalin-fixed paraffin-embedded |
| gDNA | Genomic DNA |
| GO | Gene ontology |
| LCM | Laser capture microdissection |
| MACS | Magnetic-activated cell sorting |
| MeDIP-seq | Methylated DNA immunoprecipitation sequencing |
| MID-RRBS | Microfluidic diffusion-based reduced representation bisulphite sequencing |
| NGS | Next generation sequencing |
| ONT | Oxford Nanopore Technologies |
| PBAT | Post-bisulphite adapter tagging |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| Q-RRBS | Quantitative reduced representation bisulphite sequencing |
| scAba-seq | Single-cell AbaSI sequencing |
| scDNAme | Single-cell DNA methylation |
| sci-MET | Single-cell combinatorial indexing for methylation analysis |
| scM&T | Single-cell DNA methylation and transcriptome sequencing |
| scMSRE-seq | Single-cell methylation sensitive restriction enzyme sequencing |
| scRNA-seq | Single-cell RNA sequencing |
| scRRBS | Single-cell reduced representation bisulphite sequencing |
| SCS | Single-cell sequencing |
| scTEM-seq | Single-cell transposable element methylation sequencing |
| scBS | Single-cell bisulphite sequencing |
| snmC-seq | Single-nucleus methylcytosine sequencing |
| t-SNE | t-distributed stochastic neighbour embedding |
| TME | Tumour microenvironment |
| UMAP | Uniform manifold approximation and projection |
| UMI | Unique molecular identifier |
References
- Wu, C.; Morris, J.R. Genes, genetics, and epigenetics: A correspondence. Science 2001, 293, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, mechanisms and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Eccles, M.R. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018, 51, 149–159. [Google Scholar] [CrossRef]
- Banerjee, R.; Smith, J.; Eccles, M.R.; Weeks, R.J.; Chatterjee, A. Epigenetic basis and targeting of cancer metastasis. Trends Cancer 2022, 8, 226–241. [Google Scholar] [CrossRef]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. 2—DNA Methylation and Cancer. In Advances in Genetics; Herceg, Z., Ushijima, T., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 70, pp. 27–56. [Google Scholar]
- Wade, P.A. Methyl CpG-binding proteins and transcriptional repression. Bioessays 2001, 23, 1131–1137. [Google Scholar] [CrossRef]
- Seisenberger, S.; Andrews, S.; Krueger, F.; Arand, J.; Walter, J.; Santos, F.; Popp, C.; Thienpont, B.; Dean, W.; Reik, W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 2012, 48, 849–862. [Google Scholar] [CrossRef]
- Walsh, C.P.; Chaillet, J.R.; Bestor, T.H. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 1998, 20, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.S. Genomic imprinting: Employing and avoiding epigenetic processes. Genes Dev. 2009, 23, 2124–2133. [Google Scholar] [CrossRef]
- Hellman, A.; Chess, A. Gene body-specific methylation on the active X chromosome. Science 2007, 315, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Shireby, G.; Dempster, E.L.; Policicchio, S.; Smith, R.G.; Pishva, E.; Chioza, B.; Davies, J.P.; Burrage, J.; Lunnon, K.; Seiler Vellame, D.; et al. DNA methylation signatures of Alzheimer’s disease neuropathology in the cortex are primarily driven by variation in non-neuronal cell-types. Nat. Commun. 2022, 13, 5620. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Subirana, I.; Sentí, M.; Pérez-Fernández, S.; de Castro Moura, M.; Esteller, M.; Marrugat, J.; Elosua, R. DNA methylation biomarkers of myocardial infarction and cardiovascular disease. Clin. Epigenet. 2021, 13, 86. [Google Scholar] [CrossRef]
- Kandi, V.; Vadakedath, S. Effect of DNA Methylation in Various Diseases and the Probable Protective Role of Nutrition: A Mini-Review. Cureus 2015, 7, e309. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Bai, F.; Zhang, J.Y.; Ma, S.H.; Liu, J.; Xu, Z.D.; Zhu, H.G.; Ling, Z.Q.; Ye, D.; et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene 2013, 32, 663–669. [Google Scholar] [CrossRef]
- Haffner, M.C.; Chaux, A.; Meeker, A.K.; Esopi, D.M.; Gerber, J.; Pellakuru, L.G.; Toubaji, A.; Argani, P.; Iacobuzio-Donahue, C.; Nelson, W.G.; et al. Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget 2011, 2, 627–637. [Google Scholar] [CrossRef]
- Rodger, E.J.; Chatterjee, A.; Morison, I.M. 5-hydroxymethylcytosine: A potential therapeutic target in cancer. Epigenomics 2014, 6, 503–514. [Google Scholar] [CrossRef]
- Nguyen, A.; Yoshida, M.; Goodarzi, H.; Tavazoie, S.F. Highly variable cancer subpopulations that exhibit enhanced transcriptome variability and metastatic fitness. Nat. Commun. 2016, 7, 11246. [Google Scholar] [CrossRef]
- Kastan, M.B.; Canman, C.E.; Leonard, C.J. P53, cell cycle control and apoptosis: Implications for cancer. Cancer Metastasis Rev. 1995, 14, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, G.; Zhou, F.; Su, B.; Li, Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Couturier, C.P.; Ayyadhury, S.; Le, P.U.; Nadaf, J.; Monlong, J.; Riva, G.; Allache, R.; Baig, S.; Yan, X.; Bourgey, M.; et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 2020, 11, 3406. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Álvarez-Arenas, A.; Podolski-Renic, A.; Belmonte-Beitia, J.; Pesic, M.; Calvo, G.F. Interplay of Darwinian Selection, Lamarckian Induction and Microvesicle Transfer on Drug Resistance in Cancer. Sci. Rep. 2019, 9, 9332. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Greaves, M. Cancer: The Evolutionary Legacy; Oxford University Press on Demand: Oxford, UK, 2001. [Google Scholar]
- Gaiti, F.; Chaligne, R.; Gu, H.; Brand, R.M.; Kothen-Hill, S.; Schulman, R.C.; Grigorev, K.; Risso, D.; Kim, K.T.; Pastore, A.; et al. Epigenetic evolution and lineage histories of chronic lymphocytic leukaemia. Nature 2019, 569, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Vasantharajan, S.S.; Eccles, M.R.; Rodger, E.J.; Pattison, S.; McCall, J.L.; Gray, E.S.; Calapre, L.; Chatterjee, A. The Epigenetic landscape of Circulating tumour cells. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188514. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e114. [Google Scholar] [CrossRef] [PubMed]
- Pixberg, C.F.; Raba, K.; Müller, F.; Behrens, B.; Honisch, E.; Niederacher, D.; Neubauer, H.; Fehm, T.; Goering, W.; Schulz, W.A.; et al. Analysis of DNA methylation in single circulating tumor cells. Oncogene 2017, 36, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Hou, Y.; Zhou, X.; Li, X.; Yong, J.; Wang, Y.; Wang, W.; Yan, J.; Hu, B.; Guo, H.; et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science 2018, 362, 1060–1063. [Google Scholar] [CrossRef]
- Seymour, J.F.; Döhner, H.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. Azacitidine improves clinical outcomes in older patients with acute myeloid leukaemia with myelodysplasia-related changes compared with conventional care regimens. BMC Cancer 2017, 17, 852. [Google Scholar] [CrossRef]
- Mazloumi, Z.; Farahzadi, R.; Rafat, A.; Dizaji Asl, K.; Karimipour, M.; Montazer, M.; Movassaghpour, A.A.; Dehnad, A.; Nozad Charoudeh, H. Effect of aberrant DNA methylation on cancer stem cell properties. Exp. Mol. Pathol. 2022, 125, 104757. [Google Scholar] [CrossRef]
- Satpathy, A.T.; Granja, J.M.; Yost, K.E.; Qi, Y.; Meschi, F.; McDermott, G.P.; Olsen, B.N.; Mumbach, M.R.; Pierce, S.E.; Corces, M.R.; et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol. 2019, 37, 925–936. [Google Scholar] [CrossRef]
- Litzenburger, U.M.; Buenrostro, J.D.; Wu, B.; Shen, Y.; Sheffield, N.C.; Kathiria, A.; Greenleaf, W.J.; Chang, H.Y. Single-cell epigenomic variability reveals functional cancer heterogeneity. Genome Biol. 2017, 18, 15. [Google Scholar] [CrossRef]
- Wang, Y.; Navin, N.E. Advances and applications of single-cell sequencing technologies. Mol. Cell 2015, 58, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Zhuo, M.; Su, Z.; Duan, J.; Gao, Y.; Wang, Z.; Zong, C.; Bai, H.; Chapman, A.R.; Zhao, J.; et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc. Natl. Acad. Sci. USA 2013, 110, 21083–21088. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.G.; Adalsteinsson, V.A.; Cibulskis, K.; Choudhury, A.D.; Rosenberg, M.; Cruz-Gordillo, P.; Francis, J.M.; Zhang, C.Z.; Shalek, A.K.; Satija, R.; et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 2014, 32, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.R.C.; Zhou, L.; El-Deiry, W.S. Circulating Tumor Cells Versus Circulating Tumor DNA in Colorectal Cancer: Pros and Cons. Curr. Color. Cancer Rep. 2016, 12, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Su, Z.; Li, R.; Zhang, N.; Guo, H.; Bai, F. Single-cell DNA methylome analysis of circulating tumor cells. Chin. J. Cancer Res. 2021, 33, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Li, X.; Qin, P.Z.; Zhu, Y.; Xu, S.N.; Chen, J.P. Technical Advances in Single-Cell RNA Sequencing and Applications in Normal and Malignant Hematopoiesis. Front. Oncol. 2018, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Sheriff, F.; Cicco, R.L.D.; Pogash, T.J.; Nguyen, T.; Russo, I.H. Chapter 3-Methodology for Studying the Compartments of the Human Breast; Springer: New York, NY, USA, 2014; p. 95. [Google Scholar]
- Ahn, J.; Heo, S.; Lee, J.; Bang, D. Introduction to Single-Cell DNA Methylation Profiling Methods. Biomolecules 2021, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Farlik, M.; Sheffield, N.C.; Nuzzo, A.; Datlinger, P.; Schönegger, A.; Klughammer, J.; Bock, C. Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Rep. 2015, 10, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Guo, H.; Cao, C.; Li, X.; Hu, B.; Zhu, P.; Wu, X.; Wen, L.; Tang, F.; Huang, Y.; et al. Single-cell triple omics sequencing reveals genetic, epigenetic, and transcriptomic heterogeneity in hepatocellular carcinomas. Cell Res. 2016, 26, 304–319. [Google Scholar] [CrossRef]
- Chen, Q.-F.; Gao, H.; Pan, Q.-Y.; Wang, Y.-J.; Zhong, X.-N. Analysis at the single-cell level indicates an important role of heterogeneous global DNA methylation status on the progression of lung adenocarcinoma. Sci. Rep. 2021, 11, 23337. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, X.; Zheng, J.; Dong, D. DNA methylome profiling of circulating tumor cells in lung cancer at single base-pair resolution. Oncogene 2021, 40, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.V.; Burnard, S.M.; Roper, E.A.; Bond, D.R.; Dun, M.D.; Verrills, N.M.; Enjeti, A.K.; Lee, H.J. scTEM-seq: Single-cell analysis of transposable element methylation to link global epigenetic heterogeneity with transcriptional programs. Sci. Rep. 2022, 12, 5776. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef]
- Lu, T.; Ang, C.E.; Zhuang, X. Spatially resolved epigenomic profiling of single cells in complex tissues. Cell 2022, 185, 4448–4464.e4417. [Google Scholar] [CrossRef]
- Frommer, M.; McDonald, L.E.; Millar, D.S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy, P.L.; Paul, C.L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 1992, 89, 1827–1831. [Google Scholar] [CrossRef]
- Ma, S.; de la Fuente Revenga, M.; Sun, Z.; Sun, C.; Murphy, T.W.; Xie, H.; González-Maeso, J.; Lu, C. Cell-type-specific brain methylomes profiled via ultralow-input microfluidics. Nat. Biomed. Eng. 2018, 2, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tollefsbol, T.O. DNA methylation detection: Bisulfite genomic sequencing analysis. Methods Mol. Biol. 2011, 791, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, P.; Wu, X.; Li, X.; Wen, L.; Tang, F. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res. 2013, 23, 2126–2135. [Google Scholar] [CrossRef]
- Yoon, J.; Park, M.K.; Lee, T.Y.; Yoon, Y.J.; Shin, Y. LoMA-B: A simple and versatile lab-on-a-chip system based on single-channel bisulfite conversion for DNA methylation analysis. Lab Chip 2015, 15, 3530–3539. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, P.; Guo, F.; Li, X.; Wu, X.; Fan, X.; Wen, L.; Tang, F. Profiling DNA methylome landscapes of mammalian cells with single-cell reduced-representation bisulfite sequencing. Nat. Protoc. 2015, 10, 645–659. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Dong, S.; Liang, J.; Mao, F.; Zeng, C.; Wu, H.; Wu, J.; Cai, W.; Sun, Z.S. Q-RRBS: A quantitative reduced representation bisulfite sequencing method for single-cell methylome analyses. Epigenetics 2015, 10, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, S.A.; Lee, H.J.; Angermueller, C.; Krueger, F.; Saadeh, H.; Peat, J.; Andrews, S.R.; Stegle, O.; Reik, W.; Kelsey, G. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat. Methods 2014, 11, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Keown, C.L.; Kurihara, L.; Zhou, J.; He, Y.; Li, J.; Castanon, R.; Lucero, J.; Nery, J.R.; Sandoval, J.P.; et al. Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science 2017, 357, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Niemöller, C.; Wehrle, J.; Riba, J.; Claus, R.; Renz, N.; Rhein, J.; Bleul, S.; Stosch, J.M.; Duyster, J.; Plass, C.; et al. Bisulfite-free epigenomics and genomics of single cells through methylation-sensitive restriction. Commun. Biol. 2021, 4, 153. [Google Scholar] [CrossRef]
- Mooijman, D.; Dey, S.S.; Boisset, J.C.; Crosetto, N.; van Oudenaarden, A. Single-cell 5hmC sequencing reveals chromosome-wide cell-to-cell variability and enables lineage reconstruction. Nat. Biotechnol. 2016, 34, 852–856. [Google Scholar] [CrossRef]
- Clark, S.J.; Smallwood, S.A.; Lee, H.J.; Krueger, F.; Reik, W.; Kelsey, G. Genome-wide base-resolution mapping of DNA methylation in single cells using single-cell bisulfite sequencing (scBS-seq). Nat. Protoc. 2017, 12, 534–547. [Google Scholar] [CrossRef]
- Down, T.A.; Rakyan, V.K.; Turner, D.J.; Flicek, P.; Li, H.; Kulesha, E.; Gräf, S.; Johnson, N.; Herrero, J.; Tomazou, E.M.; et al. A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis. Nat. Biotechnol. 2008, 26, 779–785. [Google Scholar] [CrossRef]
- Mulqueen, R.M.; Pokholok, D.; Norberg, S.J.; Torkenczy, K.A.; Fields, A.J.; Sun, D.; Sinnamon, J.R.; Shendure, J.; Trapnell, C.; O'Roak, B.J.; et al. Highly scalable generation of DNA methylation profiles in single cells. Nat. Biotechnol. 2018, 36, 428–431. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef]
- Sutermaster, B.A.; Darling, E.M. Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef]
- De Marchi, T.; Braakman, R.B.; Stingl, C.; van Duijn, M.M.; Smid, M.; Foekens, J.A.; Luider, T.M.; Martens, J.W.; Umar, A. The advantage of laser-capture microdissection over whole tissue analysis in proteomic profiling studies. Proteomics 2016, 16, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-M.; Yan, Y.-Y.; Guo, Q.-R.; Ji, H.; Wang, H.; Xu, T.-T.; Makabel, B.; Pilarsky, C.; He, G.; Yu, X.-Y.; et al. Microfluidics applications for high-throughput single cell sequencing. J. Nanobiotechnol. 2021, 19, 312. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, P.L.; Salmén, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, Z.; Lu, C. Microfluidic MeDIP-seq for low-input methylomic analysis of mammary tumorigenesis in mice. Analyst 2019, 144, 1904–1915. [Google Scholar] [CrossRef]
- Hu, C.; Wu, J.; Li, P.; Zhang, Y.; Peng, Y.; Liu, R.; Du, W.; Kang, Y.; Sun, J.; Wu, J.; et al. 2cChIP-seq and 2cMeDIP-seq: The Carrier-Assisted Methods for Epigenomic Profiling of Small Cell Numbers or Single Cells. Int. J. Mol. Sci. 2022, 23, 13984. [Google Scholar] [CrossRef]
- Kashima, Y.; Sakamoto, Y.; Kaneko, K.; Seki, M.; Suzuki, Y.; Suzuki, A. Single-cell sequencing techniques from individual to multiomics analyses. Exp. Mol. Med. 2020, 52, 1419–1427. [Google Scholar] [CrossRef]
- Luo, C.; Rivkin, A.; Zhou, J.; Sandoval, J.P.; Kurihara, L.; Lucero, J.; Castanon, R.; Nery, J.R.; Pinto-Duarte, A.; Bui, B.; et al. Robust single-cell DNA methylome profiling with snmC-seq2. Nat. Commun. 2018, 9, 3824. [Google Scholar] [CrossRef]
- Hu, Y.; An, Q.; Sheu, K.; Trejo, B.; Fan, S.; Guo, Y. Single Cell Multi-Omics Technology: Methodology and Application. Front. Cell Dev. Biol. 2018, 6, 28. [Google Scholar] [CrossRef]
- Angermueller, C.; Clark, S.J.; Lee, H.J.; Macaulay, I.C.; Teng, M.J.; Hu, T.X.; Krueger, F.; Smallwood, S.; Ponting, C.P.; Voet, T.; et al. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat. Methods 2016, 13, 229–232. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, K.; An, Q.; Du, G.; Hu, G.; Xue, J.; Zhu, X.; Wang, C.-Y.; Xue, Z.; Fan, G. Simultaneous profiling of transcriptome and DNA methylome from a single cell. Genome Biol. 2016, 17, 88. [Google Scholar] [CrossRef]
- Gu, H.; Raman, A.T.; Wang, X.; Gaiti, F.; Chaligne, R.; Mohammad, A.W.; Arczewska, A.; Smith, Z.D.; Landau, D.A.; Aryee, M.J.; et al. Smart-RRBS for single-cell methylome and transcriptome analysis. Nat. Protoc. 2021, 16, 4004–4030. [Google Scholar] [CrossRef]
- Das, P.M.; Singal, R. DNA Methylation and Cancer. J. Clin. Oncol. 2004, 22, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, A.; Sakata, M.; Sakuraba, K.; Goto, T.; Mizukami, H.; Saito, M.; Ishibashi, K.; Kigawa, G.; Nemoto, H.; Sanada, Y.; et al. Vimentin methylation as a marker for advanced colorectal carcinoma. Anticancer Res. 2009, 29, 279–281. [Google Scholar]
- Pott, S. Simultaneous measurement of chromatin accessibility, DNA methylation, and nucleosome phasing in single cells. eLife 2017, 6, e23203. [Google Scholar] [CrossRef]
- Clark, S.J.; Argelaguet, R.; Kapourani, C.-A.; Stubbs, T.M.; Lee, H.J.; Alda-Catalinas, C.; Krueger, F.; Sanguinetti, G.; Kelsey, G.; Marioni, J.C.; et al. scNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat. Commun. 2018, 9, 781. [Google Scholar] [CrossRef]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef]
- Feng, Z.; Fang, G.; Korlach, J.; Clark, T.; Luong, K.; Zhang, X.; Wong, W.; Schadt, E. Detecting DNA modifications from SMRT sequencing data by modeling sequence context dependence of polymerase kinetic. PLoS Comput. Biol. 2013, 9, e1002935. [Google Scholar] [CrossRef]
- Simpson, J.T.; Workman, R.E.; Zuzarte, P.C.; David, M.; Dursi, L.J.; Timp, W. Detecting DNA cytosine methylation using nanopore sequencing. Nat. Methods 2017, 14, 407–410. [Google Scholar] [CrossRef]
- Ni, P.; Xu, J.; Zhong, Z.; Zhang, J.; Huang, N.; Nie, F.; Luo, F.; Wang, J. DNA 5-methylcytosine detection and methylation phasing using PacBio circular consensus sequencing. bioRxiv 2022. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Webster, D.R.; Lee, J.H.; Travers, K.J.; Olivares, E.C.; Clark, T.A.; Korlach, J.; Turner, S.W. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat. Methods 2010, 7, 461–465. [Google Scholar] [CrossRef]
- Suzuki, Y.; Korlach, J.; Turner, S.W.; Tsukahara, T.; Taniguchi, J.; Qu, W.; Ichikawa, K.; Yoshimura, J.; Yurino, H.; Takahashi, Y.; et al. AgIn: Measuring the landscape of CpG methylation of individual repetitive elements. Bioinformatics 2016, 32, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.M.; Peluso, P.; Rowell, W.J.; Chang, P.C.; Hall, R.J.; Concepcion, G.T.; Ebler, J.; Fungtammasan, A.; Kolesnikov, A.; Olson, N.D.; et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat. Biotechnol. 2019, 37, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Tse, O.Y.O.; Jiang, P.; Cheng, S.H.; Peng, W.; Shang, H.; Wong, J.; Chan, S.L.; Poon, L.C.Y.; Leung, T.Y.; Chan, K.C.A.; et al. Genome-wide detection of cytosine methylation by single molecule real-time sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2019768118. [Google Scholar] [CrossRef] [PubMed]
- Gigante, S.; Gouil, Q.; Lucattini, A.; Keniry, A.; Beck, T.; Tinning, M.; Gordon, L.; Woodruff, C.; Speed, T.P.; Blewitt, M.E.; et al. Using long-read sequencing to detect imprinted DNA methylation. Nucleic Acids Res. 2019, 47, e46. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Huang, N.; Zhang, Z.; Wang, D.-P.; Liang, F.; Miao, Y.; Xiao, C.-L.; Luo, F.; Wang, J. DeepSignal: Detecting DNA methylation state from Nanopore sequencing reads using deep-learning. Bioinformatics 2019, 35, 4586–4595. [Google Scholar] [CrossRef] [PubMed]
- Rand, A.C.; Jain, M.; Eizenga, J.M.; Musselman-Brown, A.; Olsen, H.E.; Akeson, M.; Paten, B. Mapping DNA methylation with high-throughput nanopore sequencing. Nat. Methods 2017, 14, 411–413. [Google Scholar] [CrossRef]
- Loman, N.J.; Quick, J.; Simpson, J.T. A complete bacterial genome assembled de novo using only nanopore sequencing data. Nat. Methods 2015, 12, 733–735. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, L.; Yu, G.; Wang, D.; Xiao, C.-L.; Wang, K. Detection of DNA base modifications by deep recurrent neural network on Oxford Nanopore sequencing data. Nat. Commun. 2019, 10, 2449. [Google Scholar] [CrossRef]
- Ewing, A.D.; Smits, N.; Sanchez-Luque, F.J.; Faivre, J.; Brennan, P.M.; Richardson, S.R.; Cheetham, S.W.; Faulkner, G.J. Nanopore Sequencing Enables Comprehensive Transposable Element Epigenomic Profiling. Mol. Cell 2020, 80, 915–928.e915. [Google Scholar] [CrossRef]
- Liu, Y.; Rosikiewicz, W.; Pan, Z.; Jillette, N.; Wang, P.; Taghbalout, A.; Foox, J.; Mason, C.; Carroll, M.; Cheng, A.; et al. DNA methylation-calling tools for Oxford Nanopore sequencing: A survey and human epigenome-wide evaluation. Genome Biol. 2021, 22, 295. [Google Scholar] [CrossRef]
- Amarasinghe, S.L.; Su, S.; Dong, X.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Kingan, S.B.; Heaton, H.; Cudini, J.; Lambert, C.C.; Baybayan, P.; Galvin, B.D.; Durbin, R.; Korlach, J.; Lawniczak, M.K.N. A High-Quality De novo Genome Assembly from a Single Mosquito Using PacBio Sequencing. Genes 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Heavens, D.; Chooneea, D.; Giolai, M.; Cuber, P.; Aanstad, P.; Martin, S.; Alston, M.; Misra, R.; Clark, M.D.; Leggett, R.M. How low can you go? Driving down the DNA input requirements for nanopore sequencing. bioRxiv 2021. [Google Scholar] [CrossRef]
- Iqbal, W.; Zhou, W. Computational methods for single-cell DNA methylomes. Genom. Proteom. Bioinform. 2022. [Google Scholar] [CrossRef]
- Chen, G.; Ning, B.; Shi, T. Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front. Genet. 2019, 10, 317. [Google Scholar] [CrossRef]
- Schultz, M.D.; He, Y.; Whitaker, J.W.; Hariharan, M.; Mukamel, E.A.; Leung, D.; Rajagopal, N.; Nery, J.R.; Urich, M.A.; Chen, H.; et al. Human body epigenome maps reveal noncanonical DNA methylation variation. Nature 2015, 523, 212–216. [Google Scholar] [CrossRef]
- Kapourani, C.A.; Sanguinetti, G. Melissa: Bayesian clustering and imputation of single-cell methylomes. Genome Biol. 2019, 20, 61. [Google Scholar] [CrossRef]
- de Souza, C.P.E.; Andronescu, M.; Masud, T.; Kabeer, F.; Biele, J.; Laks, E.; Lai, D.; Ye, P.; Brimhall, J.; Wang, B.; et al. Epiclomal: Probabilistic clustering of sparse single-cell DNA methylation data. PLoS Comput. Biol. 2020, 16, e1008270. [Google Scholar] [CrossRef]
- Shahryary, Y.; Hazarika, R.R.; Johannes, F. MethylStar: A fast and robust pre-processing pipeline for bulk or single-cell whole-genome bisulfite sequencing data. BMC Genom. 2020, 21, 479. [Google Scholar] [CrossRef]
- Uzun, Y.; Yu, W.; Chen, C.; Tan, K. SINBAD: A flexible tool for single cell DNA methylation data. bioRxiv 2021. [Google Scholar] [CrossRef]
- Danese, A.; Richter, M.L.; Chaichoompu, K.; Fischer, D.S.; Theis, F.J.; Colomé-Tatché, M. EpiScanpy: Integrated single-cell epigenomic analysis. Nat. Commun. 2021, 12, 5228. [Google Scholar] [CrossRef] [PubMed]
- Kapourani, C.-A.; Argelaguet, R.; Sanguinetti, G.; Vallejos, C.A. scMET: Bayesian modeling of DNA methylation heterogeneity at single-cell resolution. Genome Biol. 2021, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zou, J.; Tang, J.; Liang, L.; Cao, X.; Fan, S. scMelody: An Enhanced Consensus-Based Clustering Model for Single-Cell Methylation Data by Reconstructing Cell-to-Cell Similarity. Front. Bioeng. Biotechnol. 2022, 10, 842019. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Kang, H.; Xiong, Z.; Ma, Y.; Jin, T.; Gong, Z.; Yi, L.; Zhang, M.; Wu, S.; Wang, G.; et al. scMethBank: A database for single-cell whole genome DNA methylation maps. Nucleic Acids Res. 2022, 50, D380–D386. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhu, R.; Yang, H.; Lu, Q.; Wang, W.; Song, L.; Sun, X.; Zhang, G.; Li, S.; Yang, J.; et al. Assessing the Impact of Data Preprocessing on Analyzing Next Generation Sequencing Data. Front. Bioeng. Biotechnol. 2020, 8, 817. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Krueger, F.; James, F.; Ewels, P.; Afyounian, E.; Schuster-Boeckler, B. FelixKrueger/TrimGalore: v0.6.7. Available online: https://doi.org/10.5281/zenodo.5127899 (accessed on 25 July 2022).
- Krueger, F. Mispriming in PBAT Libraries Causes Methylation Bias and Poor Mapping Efficiencies. Available online: https://sequencing.qcfail.com/articles/mispriming-in-pbat-libraries-causes-methylation-bias-and-poor-mapping-efficiencies/ (accessed on 22 July 2022).
- Krueger, F.; Andrews, S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 2011, 27, 1571–1572. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Krueger, F. Soft-Clipping of Reads May Add Potentially Unwanted Alignments to Repetitive Regions. Available online: https://sequencing.qcfail.com/articles/soft-clipping-of-reads-may-add-potentially-unwanted-alignments-to-repetitive-regions/ (accessed on 22 July 2022).
- Tran, H.; Porter, J.; Sun, M.A.; Xie, H.; Zhang, L. Objective and comprehensive evaluation of bisulfite short read mapping tools. Adv. Bioinform. 2014, 2014, 472045. [Google Scholar] [CrossRef]
- Wu, P.; Gao, Y.; Guo, W.; Zhu, P. Using local alignment to enhance single-cell bisulfite sequencing data efficiency. Bioinformatics 2019, 35, 3273–3278. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, J.; Tian, W.; Luo, C.; Bartlett, A.; Aldridge, A.; Lucero, J.; Osteen, J.K.; Nery, J.R.; Chen, H.; et al. DNA Methylation Atlas of the Mouse Brain at Single-Cell Resolution. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ng, H.K.; Drautz-Moses, D.I.; Schuster, S.C.; Beck, S.; Kim, C.; Chambers, J.C.; Loh, M. Systematic evaluation of library preparation methods and sequencing platforms for high-throughput whole genome bisulfite sequencing. Sci. Rep. 2019, 9, 10383. [Google Scholar] [CrossRef] [PubMed]
- Zamanighomi, M.; Lin, Z.; Daley, T.; Chen, X.; Duren, Z.; Schep, A.; Greenleaf, W.J.; Wong, W.H. Unsupervised clustering and epigenetic classification of single cells. Nat. Commun. 2018, 9, 2410. [Google Scholar] [CrossRef]
- Bravo González-Blas, C.; Minnoye, L.; Papasokrati, D.; Aibar, S.; Hulselmans, G.; Christiaens, V.; Davie, K.; Wouters, J.; Aerts, S. cisTopic: Cis-regulatory topic modeling on single-cell ATAC-seq data. Nat. Methods 2019, 16, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Uzun, Y.; Wu, H.; Tan, K. Predictive modeling of single-cell DNA methylome data enhances integration with transcriptome data. Genome Res. 2021, 31, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.J.; Taylor, M.G.; Pearce, F.G.; Kennedy, M.A. Allelic Dropout During Polymerase Chain Reaction due to G-Quadruplex Structures and DNA Methylation Is Widespread at Imprinted Human Loci. G3 2017, 7, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Angermueller, C.; Lee, H.J.; Reik, W.; Stegle, O. DeepCpG: Accurate prediction of single-cell DNA methylation states using deep learning. Genome Biol. 2017, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, P.; Lu, Y.; Wu, F.X. Identifying cell types from single-cell data based on similarities and dissimilarities between cells. BMC Bioinform. 2021, 22, 255. [Google Scholar] [CrossRef]
- Maksimovic, J.; Oshlack, A.; Phipson, B. Gene set enrichment analysis for genome-wide DNA methylation data. Genome Biol. 2021, 22, 173. [Google Scholar] [CrossRef]
- Yuan, G.C.; Cai, L.; Elowitz, M.; Enver, T.; Fan, G.; Guo, G.; Irizarry, R.; Kharchenko, P.; Kim, J.; Orkin, S.; et al. Challenges and emerging directions in single-cell analysis. Genome Biol. 2017, 18, 84. [Google Scholar] [CrossRef]
- Žurauskienė, J.; Yau, C. pcaReduce: Hierarchical clustering of single cell transcriptional profiles. BMC Bioinform. 2016, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, V.Y.; Kirschner, K.; Schaub, M.T.; Andrews, T.; Yiu, A.; Chandra, T.; Natarajan, K.N.; Reik, W.; Barahona, M.; Green, A.R.; et al. SC3: Consensus clustering of single-cell RNA-seq data. Nat. Methods 2017, 14, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Ocone, A.; Haghverdi, L.; Mueller, N.S.; Theis, F.J. Reconstructing gene regulatory dynamics from high-dimensional single-cell snapshot data. Bioinformatics 2015, 31, i89–i96. [Google Scholar] [CrossRef]
- McGinnis, C.S.; Murrow, L.M.; Gartner, Z.J. DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019, 8, 329–337.e324. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Kaczorowski, D.; Valdes-Mora, F.; Nordon, R.E.; Neild, A.; Farbehi, N.; Bartonicek, N.; Gallego-Ortega, D. Droplet-based single cell RNAseq tools: A practical guide. Lab Chip 2019, 19, 1706–1727. [Google Scholar] [CrossRef]
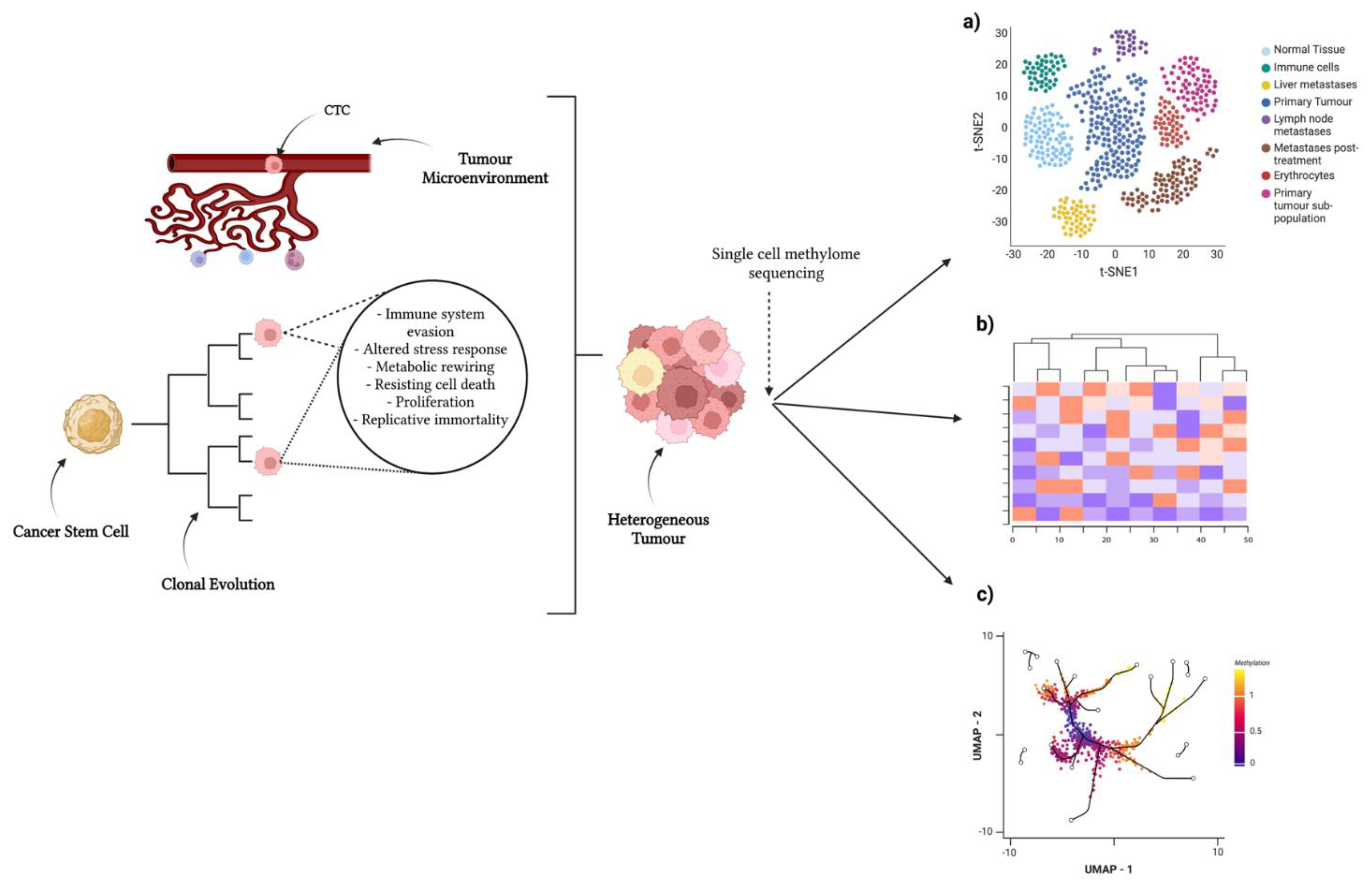
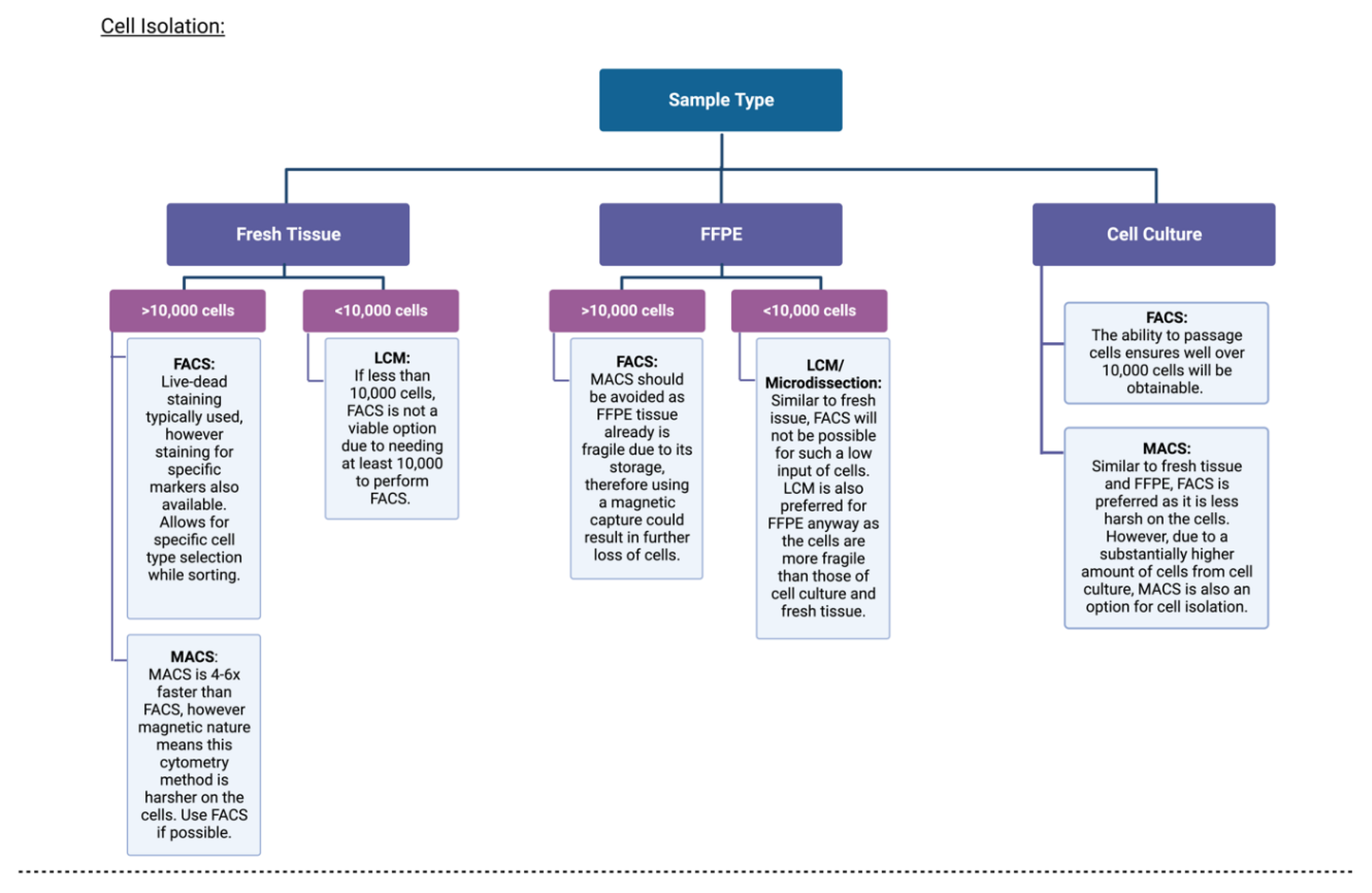
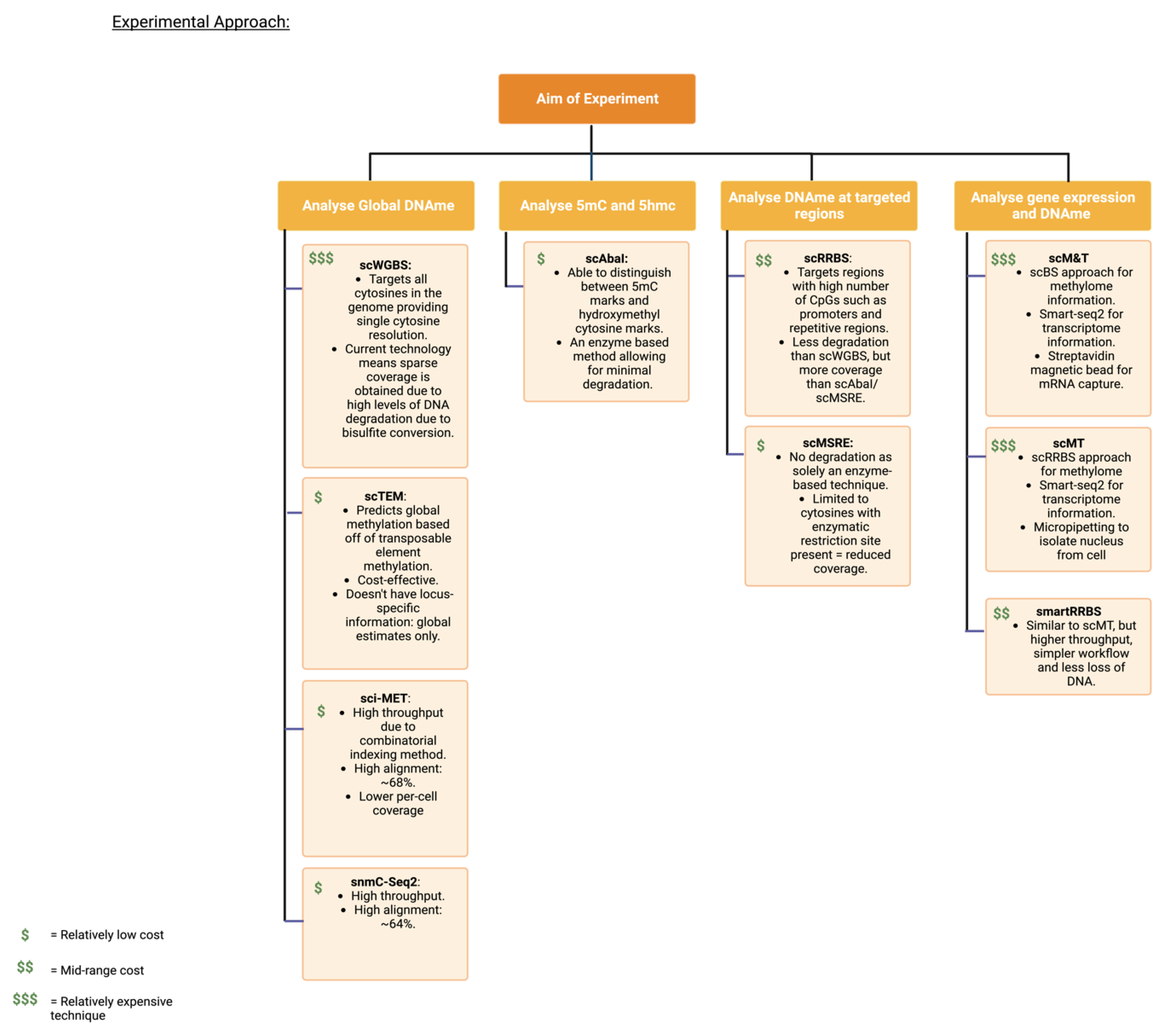
| Cancer Type | Genome-Wide or Gene-Specific | Findings | Year Published | References |
|---|---|---|---|---|
| HL60 (acute promyelocytic leukemia cell line) and K562 (erythroleukemia-derived cell line) | Genome-wide | First implementation of single-cell WGBS | 2015 | [51] |
| Hepatocellular carcinoma | Genome-wide | Identification of subpopulations within tumour | 2016 | [52] |
| Metastatic breast cancer (mBC) and metastatic castration-resistant prostate cancer (mCRPC) | CDH1 and miR200 promoters. | CTCs from same patient displayed heterogeneous methylation patterns. Different methylation patterns at these promoters in mCRPC vs. mBC CTCs suggesting differentially regulated miR200 loops in these two tumour entities. | 2017 | [37] |
| Colorectal cancer | Genome-wide | Sub-lineages identified in patients found metastases at multiple sites had a common origin | 2018 | [38] |
| Chronic Lymphocytic Leukaemia | Genome-wide | Subpopulations preferentially expelled from lymph nodes after treatment | 2019 | [33] |
| Lung Adenocarcinoma | Genome-wide | Global methylation heterogeneity amongst tumours associated with the progression of LAC | 2021 | [53] |
| Lung Cancer | Genome-wide | Unique CTC DNA methylation signature distinguished it from the primary tumour | 2021 | [54] |
| 6 Cancer Types: Prostate, Colon, Small cell lung, Lung Adenocarcinoma, Breast, and Gastric | Genome-wide | Potential to identify tumours of origin for CTC based on methylome profiles. Report diverse evolutionary histories of CTCs | 2021 | [47] |
| KG1a Acute Myeloid Leukaemia | Transposable elements: SINE Alu | TEs as a surrogate for predicting single-cell global DNA methylation. Method has greater alignment and costs 3-fold less than scBS-seq | 2022 | [55] |
| Method | Key Features | Year of First Study | References |
|---|---|---|---|
| Single-cell reduced representation bisulphite sequencing (scRRBS) | Bias in regions with high CpG density, limited coverage in regions with low CpG density | 2013 | [69] |
| Cost-effective | |||
| Single-cell bisulphite sequencing (scBS-seq) | Single base resolution High cost | 2014 | [73] |
| DNA degradation. | |||
| Quantitative RRBS (Q-RRBS) | Incorporated UMIs for PCR-duplicate removal | 2015 | [71] |
| Single-cell AbaSI sequencing (scAba-seq) | Low false-positive rate | 2016 | [74] |
| Distinguishes between 5hmC and 5mC | |||
| No chemical degradation | |||
| Single nucleus methylcytosine sequencing (snmC-seq2) | Reaction occurs within the nucleus Single-strand library preparation | 2018 | [75] |
| Single-cell combinatorial indexing for methylation analysis (sci-MET) | Lower coverage but higher throughput relative to other methods | 2018 | [59] |
| Microfluidic diffusion based RRBS (MID-RRBS) | Diffusion-based reagent exchange allows for minimal loss of DNA. Microfluidic device allows for multiple cells to be done in parallel. | 2018 | [62] |
| Single-cell methylation-sensitive restriction enzyme sequencing (scMSRE) | Analysis limited to methylation at restriction sites No chemical degradation scCGI | 2021 | [76] |
| Single-cell transposable element sequencing (scTEM-seq) | Uses transposable elements as surrogates to predict single-cell global methylation | 2022 | [55] |
| Analysis Category | Name of Pipeline and Year Published | Environment | Features |
|---|---|---|---|
| Preprocessing | MethylPy (2015) [109] | Python | Processes raw reads through to methylation state. Combines data from adjacent cytosine for dealing with low coverage data |
| Imputation | MELISA (2019) [110] | R | Uses information from neighbouring CpGs and from neighbouring cells with similar CpG patterns to predict missing CpG methylation states. Also uses Bayesian clustering to identify subsets of cells based on epigenetic state |
| Imputation | Epiclomal (2020) [111] | R and Python | Simultaneously clusters sparse single-cell DNAme data and imputes missing values |
| Preprocessing | MethylStar (2020) [112] | Python | Contains a “quick run” option that streamlines all preprocessing steps, including trimming, alignment, removal of duplicates, and methylation calling |
| Overall Analysis | SINBAD (2021) [113] | R | Contains 5 modules consisting of pre-processing, mapping, methylation, dimensionality, and gene signature profiling |
| Downstream Analyses | EpiScanpy (2021) [114] | R | A scRNA-seq workflow adapted for sc-ATAC and sc-DNAme analyses |
| Preprocessing | scMET (2021) [115] | R | Hierarchical Bayesian model designed to overcome data sparsity. Also performs differential methylation and variability analyses |
| Downstream Analyses | scMelody (2022) [116] | R and Python | Consensus-based clustering model that takes into account distance relationships between cells to improve the identification of subpopulations |
| Downstream Analyses | scMethBank (2022) [117] | Online | Provides curated metadata of 8000+ samples of different cell types and states. Provides online tools for simple and practical downstream analyses such as lollipop plots, DMR annotation and enrichment analysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, H.; Lee, H.; Gupta, I.; Rodger, E.J.; Chatterjee, A. Single-Cell DNA Methylation Analysis in Cancer. Cancers 2022, 14, 6171. https://doi.org/10.3390/cancers14246171
O’Neill H, Lee H, Gupta I, Rodger EJ, Chatterjee A. Single-Cell DNA Methylation Analysis in Cancer. Cancers. 2022; 14(24):6171. https://doi.org/10.3390/cancers14246171
Chicago/Turabian StyleO’Neill, Hannah, Heather Lee, Ishaan Gupta, Euan J. Rodger, and Aniruddha Chatterjee. 2022. "Single-Cell DNA Methylation Analysis in Cancer" Cancers 14, no. 24: 6171. https://doi.org/10.3390/cancers14246171
APA StyleO’Neill, H., Lee, H., Gupta, I., Rodger, E. J., & Chatterjee, A. (2022). Single-Cell DNA Methylation Analysis in Cancer. Cancers, 14(24), 6171. https://doi.org/10.3390/cancers14246171





