UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
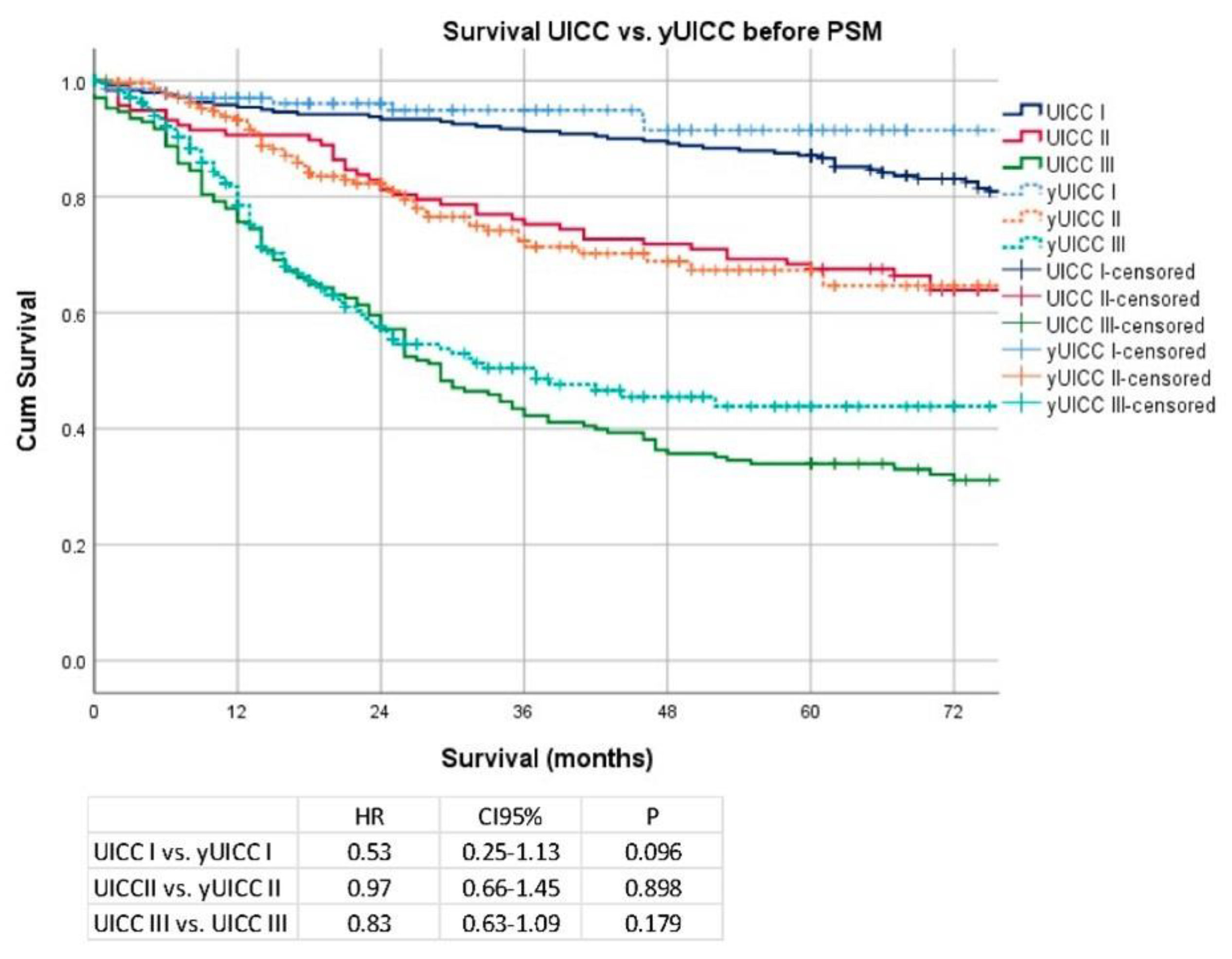
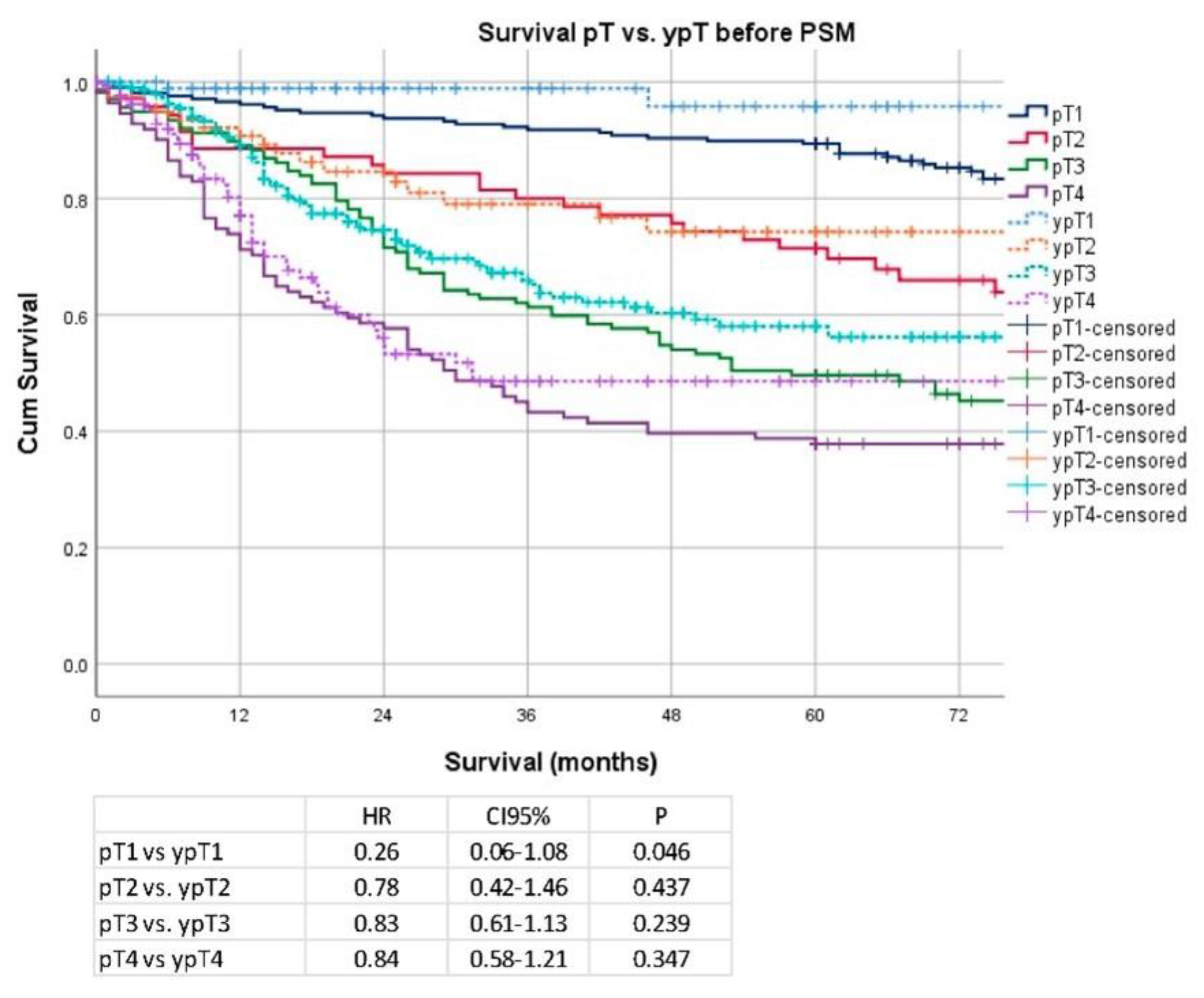
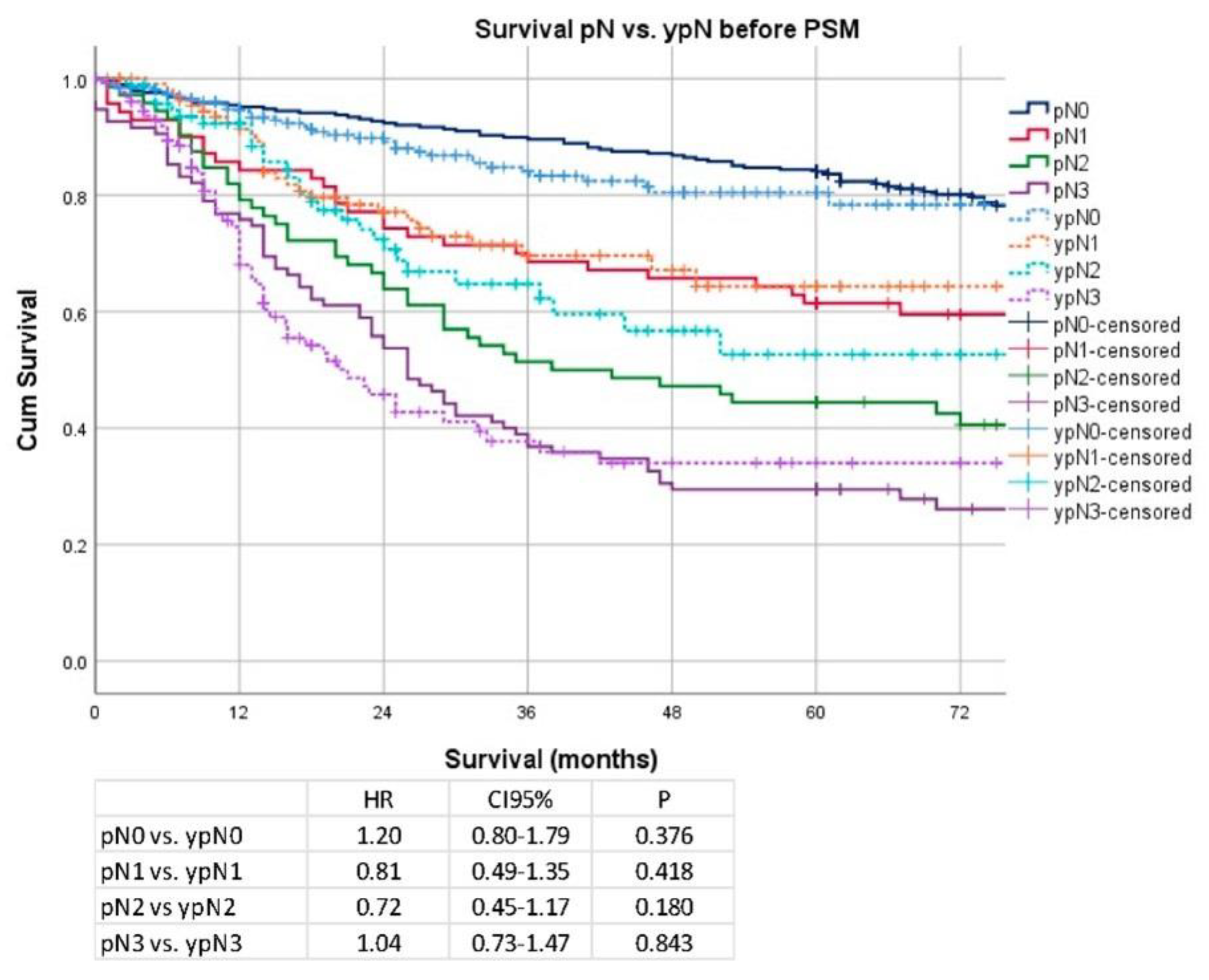
3. Results
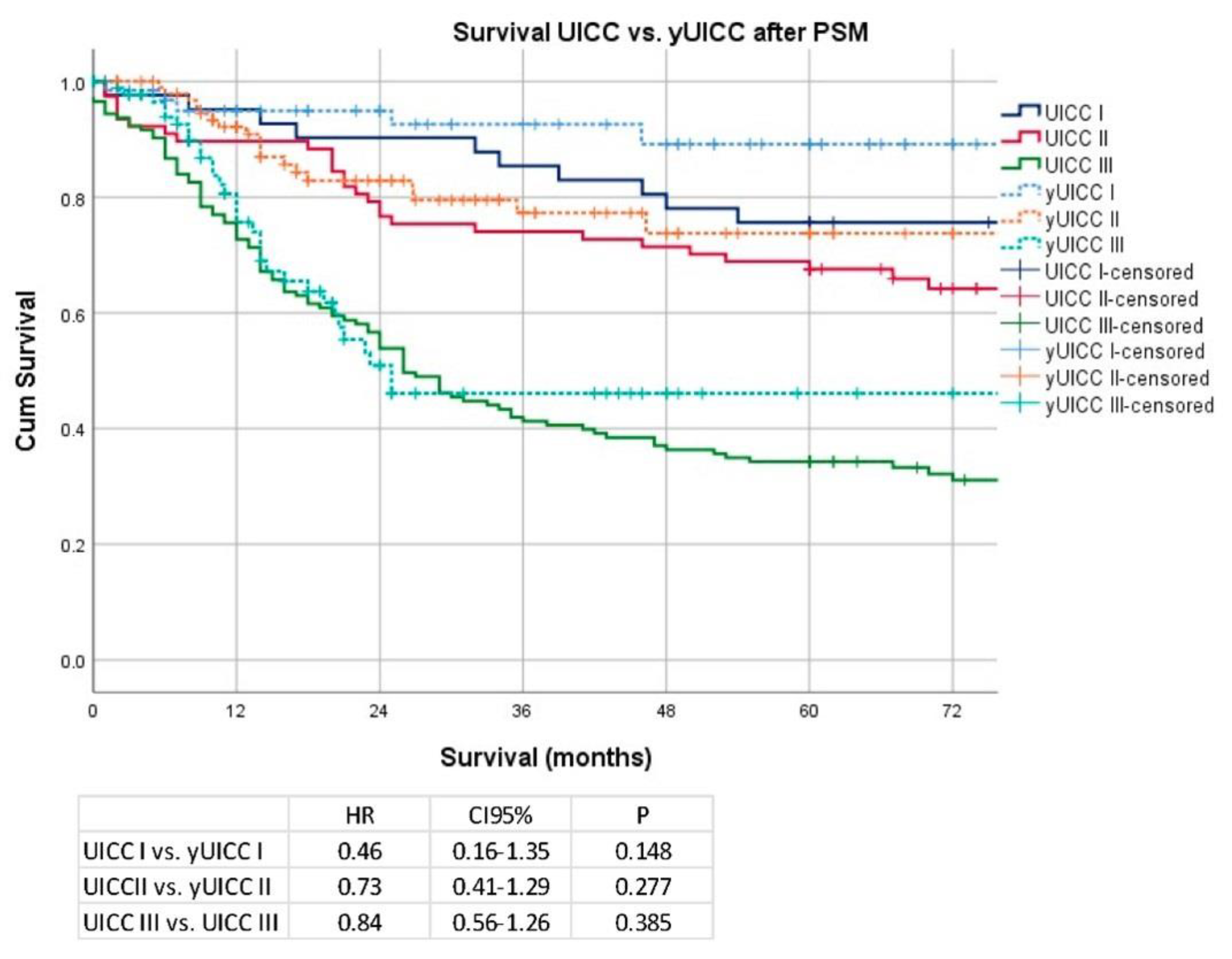
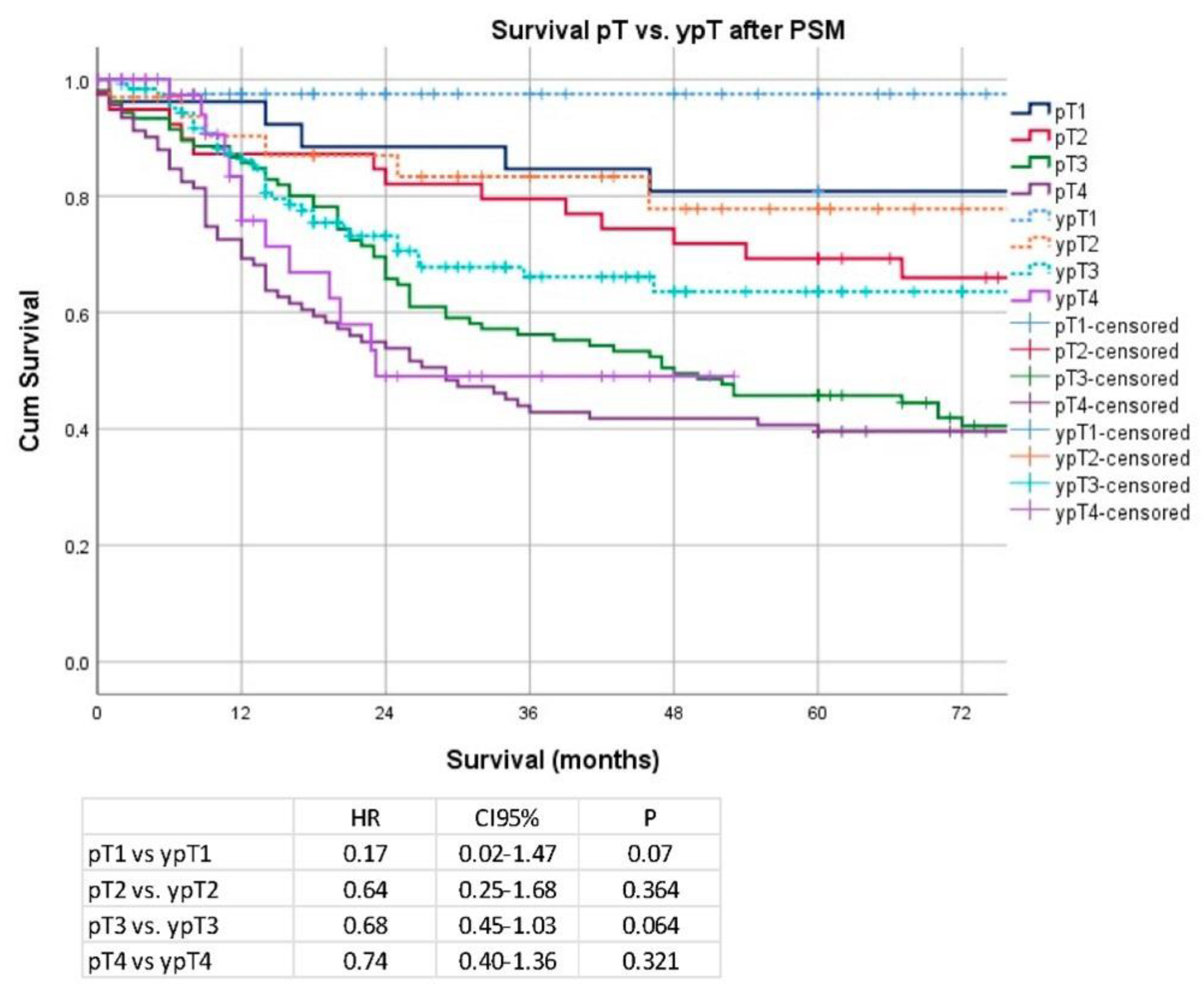
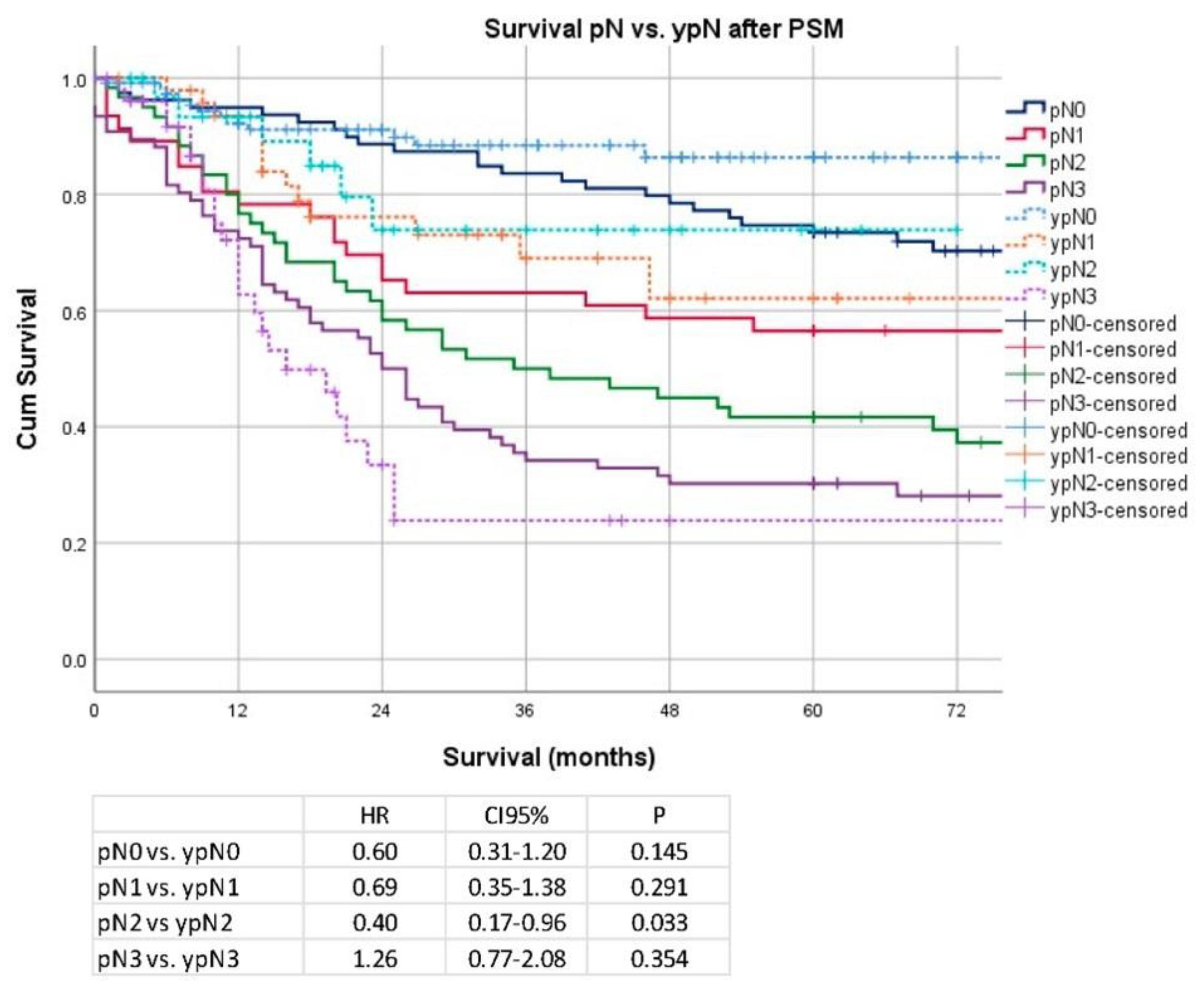
Secondary Analysis after PSM and Removal of Clinical Stage I Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guggenheim, D.E.; Shah, M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Lordick, F.; Lyros, O.; Kreuser, N.; Hölscher, A.H.; Wittekind, C. Pretherapeutic misclassification of esophageal cancer and adenocarcinoma of the esophagogastric junction: Possibilities and clinical consequences. Chirurg 2020, 91, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P.; Hulshof, M.C.C.M.; Van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Jeong, O.; Jung, M.R.; Kang, J.H.; Ryu, S.Y. Prognostic Performance of Preoperative Staging: Assessed by Using Multidetector Computed Tomography—Between the New Clinical Classification and the Pathological Classification in the Eighth American Joint Committee on Cancer Classification for Gastric Carcinoma. Ann. Surg. Oncol. 2020, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Yamada, T.; Yamamoto, K.; Komori, K.; Watanabe, H.; Hara, K.; Shimoda, Y.; Maezawa, Y.; Fujikawa, H.; Aoyama, T.; et al. Association Between Lymph Node Ratio and Survival in Patients with Pathological Stage II/III Gastric Cancer. Ann. Surg. Oncol. 2020, 27, 4235–4247. [Google Scholar] [CrossRef]
- Ikoma, N.; Estrella, J.S.; Hofstetter, W.; Das, M.P.; Minsky, B.D.; Ajani, J.A.; Fournier, K.F.; Mansfield, P.; Badgwell, B.D. Nodal Downstaging in Gastric Cancer Patients: Promising Survival if ypN0 is Achieved. Ann. Surg. Oncol. 2018, 25, 2012–2017. [Google Scholar] [CrossRef]
- Lagarde, S.M.; Kate, F.J.W.T.; Reitsma, J.B.; Busch, O.R.C.; Van Lanschot, J.J.B. Prognostic Factors in Adenocarcinoma of the Esophagus or Gastroesophageal Junction. J. Clin. Oncol. 2006, 24, 4347–4355. [Google Scholar] [CrossRef]
- Schuhmacher, C.; Gretschel, S.; Lordick, F.; Reichardt, P.; Hohenberger, W.; Eisenberger, C.F.; Haag, C.; Mauer, M.E.; Hasan, B.; Welch, J.; et al. Neoadjuvant Chemotherapy Compared With Surgery Alone for Locally Advanced Cancer of the Stomach and Cardia: European Organisation for Research and Treatment of Cancer Randomized Trial 40954. J. Clin. Oncol. 2010, 28, 5210–5218. [Google Scholar] [CrossRef]
- Ronellenfitsch, U.; Schwarzbach, M.; Hofheinz, R.; Kienle, P.; Kieser, M.; Slanger, T.E.; Burmeister, B.; Kelsen, D.; Niedzwiecki, D.; Schuhmacher, C.; et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: Systematic review with meta-analysis combining individual patient and aggregate data. Eur. J. Cancer 2013, 49, 3149–3158. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xing, Z.; Huang, M.; Yu, H.; Qin, Y.; Jin, Q.; Zhou, Z.; Chen, J. Nodal downstaging to ypN0 after neoadjuvant chemotherapy positively impacts on survival of cT4N+ GC/GEJ patients. J. Surg. Oncol. 2022, 126, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Friedmann, P.; Solsky, I.; Muscarella, P.; McAuliffe, J.; In, H. Providing Reliable Prognosis to Patients with Gastric Cancer in the Era of Neoadjuvant Therapies: Comparison of AJCC Staging Schemata. J. Gastric Cancer 2020, 20, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Bottcher, K.; Siewert, J.R.; Hofler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, C. The development of the TNM classification of gastric cancer. Pathol. Int. 2015, 65, 399–403. [Google Scholar] [CrossRef]
- Ajani, J.; Mansfield, P.; Crane, C.; Wu, T.; Lunagomez, S.; Lynch, P.; Janjan, N.; Feig, B.; Faust, J.; Yao, J.; et al. Paclitaxel-Based Chemoradiotherapy in Localized Gastric Carcinoma: Degree of Pathologic Response and Not Clinical Parameters Dictated Patient Outcome. J. Clin. Oncol. 2005, 23, 1237–1244. [Google Scholar] [CrossRef]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; Ajani, J.A.; Sano, T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, C.-H.; Cao, L.-L.; Li, P.; Xie, J.-W.; Wang, J.-B.; Lin, J.-X.; Chen, Q.-Y.; Lin, M.; Huang, C.-M. The effectiveness of the 8th American Joint Committee on Cancer TNM classification in the prognosis evaluation of gastric cancer patients: A comparative study between the 7th and 8th editions. Eur. J. Surg. Oncol. EJSO 2017, 43, 2349–2356. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Q.-Y.; Parisi, A.; Ma, Y.-B.; Lin, G.-T.; Desiderio, J.; Yan, S.; Xie, J.-W.; Wang, J.-B.; Hou, J.-F.; et al. Modified ypTNM Staging Classification for Gastric Cancer after Neoadjuvant Therapy: A Multi-Institutional Study. Oncologist 2021, 26, e99–e110. [Google Scholar] [CrossRef]
- Thomaschewski, M.; Hummel, R.; Petrova, E.; Knief, J.; Wellner, U.F.; Keck, T.; Bausch, D. Impact of postoperative TNM stages after neoadjuvant therapy on prognosis of adenocarcinoma of the gastro-oesophageal junction tumours. World J. Gastroenterol. 2018, 24, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Yook, J.H.; Park, Y.-K.; Lee, J.S.; Kim, Y.-W.; Kim, J.Y.; Ryu, M.-H.; Rha, S.Y.; Chung, I.J.; Kim, I.-H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Schirren, R.; Novotny, A.; Friess, H.; Reim, D. Histopathologic Response Is a Positive Predictor of Overall Survival in Patients Undergoing Neoadjuvant/Perioperative Chemotherapy for Locally Advanced Gastric or Gastroesophageal Junction Cancers—Analysis from a Large Single Center Cohort in Germany. Cancers 2020, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
| Before PSM (All) | Surgery Only (n = 525) | CTX + Surgery (n = 624) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | <0.0001 | ||||
| Female | 187 | 35.62 | 153 | 24.52 | |
| Male | 338 | 64.38 | 471 | 75.48 | |
| Age | 65.3 ± 12.1 | 59.1 ± 11.1 | <0.0001 | ||
| <70 years | 324 | 61.71 | 510 | 81.73 | <0.0001 |
| >70 years | 201 | 38.29 | 114 | 18.27 | |
| Localization | <0.0001 | ||||
| Proximal | 232 | 44.19 | 432 | 69.23 | |
| Middle | 127 | 24.19 | 98 | 15.71 | |
| Distal | 154 | 29.33 | 74 | 11.86 | |
| Total | 12 | 2.29 | 20 | 3.21 | |
| Clinical Staging | <0.0001 | ||||
| cT1 | 140 | 26.67 | 5 | 0.80 | |
| cT2 | 159 | 30.29 | 54 | 8.65 | |
| cT3 | 214 | 40.76 | 535 | 85.74 | |
| cT4 | 12 | 2.29 | 30 | 4.81 | |
| cN0 | 288 | 54.86 | 104 | 16.67 | <0.0001 |
| cN1 | 237 | 45.14 | 520 | 83.33 | |
| Clinical Stage AJCC | |||||
| I | 247 | 47.05 | 0 | 0.00 | <0.0001 |
| IIA | 52 | 9.90 | 59 | 9.46 | |
| IIB | 41 | 7.81 | 100 | 16.03 | |
| III | 173 | 32.95 | 435 | 69.71 | |
| IVA | 12 | 2.29 | 30 | 4.81 | |
| Clinical Stage | |||||
| cT1/cT2 cN− | 247 | 47.05 | 0 | 0.00 | <0.0001 |
| cT3/cT4 cN0 | 41 | 7.81 | 104 | 16.67 | |
| cT1/cT2 cN+ | 52 | 9.90 | 59 | 9.46 | |
| cT3/cT4 cN+ | 185 | 35.24 | 461 | 73.88 | |
| Type of Surgery | <0.0001 | ||||
| Esophagectomy | 15 | 2.86 | 134 | 21.47 | |
| Transhiatal ext. Gastrectomy | 156 | 29.71 | 293 | 46.96 | |
| Total gastrectomy | 178 | 33.90 | 168 | 26.92 | |
| Subtotal gastrectomy | 135 | 25.71 | 28 | 4.49 | |
| Others | 41 | 7.81 | 1 | 0.16 | |
| Surgical extension | <0.0001 | ||||
| None | 308 | 58.67 | 227 | 36.38 | |
| Luminal/transhiatal | 74 | 14.10 | 251 | 40.22 | |
| Splenectomy | 17 | 3.24 | 17 | 2.72 | |
| Colon | 2 | 0.38 | 5 | 0.80 | |
| Pancreas | 3 | 0.57 | 16 | 2.56 | |
| Others | 121 | 23.05 | 108 | 17.31 | |
| Dissected LN [Median] | 25 [1–76] | 29 [5–89] | <0.0001 | ||
| ≤25 | 248 | 47.24 | 209 | 33.49 | 0.005 |
| >25 | 277 | 52.76 | 415 | 66.51 | |
| Complications | 0.57 | ||||
| None | 405 | 77.14 | 465 | 74.52 | |
| CD I/II | 71 | 13.52 | 96 | 15.38 | |
| CD III-V | 49 | 9.33 | 63 | 10.10 | |
| pT | <0.0001 | ||||
| pT0 | 3 | 0.57 | 33 | 5.29 | |
| pT1a | 97 | 18.48 | 13 | 2.08 | |
| pT1b | 107 | 20.38 | 46 | 7.37 | |
| pT2 | 70 | 13.33 | 83 | 13.30 | |
| pT3 | 138 | 26.29 | 305 | 48.88 | |
| pT4a | 98 | 18.67 | 121 | 19.39 | |
| pT4b | 12 | 2.29 | 23 | 3.69 | |
| pN | 0.004 | ||||
| pN0 | 288 | 54.86 | 273 | 43.75 | |
| pN1 | 70 | 13.33 | 119 | 19.07 | |
| pN2 | 72 | 13.71 | 99 | 15.87 | |
| pN3a | 66 | 12.57 | 95 | 15.22 | |
| pN3b | 29 | 5.52 | 38 | 6.09 | |
| UICC | <0.0001 | ||||
| UICC 0 | 0 | 0.00 | 31 | 4.97 | |
| UICC IA | 185 | 35.24 | 45 | 7.21 | |
| UICC IB | 55 | 10.48 | 63 | 10.10 | |
| UICC IIA | 61 | 11.62 | 119 | 19.07 | |
| UICC IIB | 56 | 10.67 | 113 | 18.11 | |
| UICC IIIA | 77 | 14.67 | 121 | 19.39 | |
| UICC IIIB | 61 | 11.62 | 91 | 14.58 | |
| UICC IIIC | 30 | 5.71 | 41 | 6.57 | |
| UICC 0 | 0 | 0.00 | 31 | 4.97 | <0.0001 |
| UICC I | 240 | 45.71 | 108 | 17.31 | |
| UICC II | 117 | 22.29 | 232 | 37.18 | |
| UICC III | 168 | 32.00 | 253 | 40.54 | |
| Lauren type | <0.0001 | ||||
| Not classified | 144 | 27.43 | 103 | 16.51 | |
| Intestinal | 268 | 51.05 | 296 | 47.44 | |
| Diffuse | 78 | 14.86 | 142 | 22.76 | |
| Mixed | 35 | 6.67 | 82 | 13.14 | |
| Grading | 0.84 | ||||
| G1/G2 | 146 | 27.81 | 170 | 27.24 | |
| G3/G4 | 379 | 72.19 | 454 | 72.76 | |
| R | 0.06 | ||||
| R0 | 482 | 91.81 | 552 | 88.46 | |
| R1 | 43 | 8.19 | 72 | 11.54 | |
| Histopathologic Response | |||||
| Becker Ia/Ib | 169 | 27.08 | |||
| Becker II | 178 | 28.53 | |||
| Becker III | 277 | 44.39 | |||
| Univariable (All) | HR | CI 95% Lower | CI 95% Upper | p |
|---|---|---|---|---|
| nCTx | 1.04 | 0.84 | 1.28 | 0.720 |
| Gender | 0.150 | |||
| Female | 1.00 | |||
| Male | 1.17 | 0.94 | 1.46 | |
| Age | <0.0001 | |||
| <70 years | 1.00 | |||
| >70 years | 1.85 | 1.51 | 2.27 | |
| Localization | ||||
| Proximal | 1.00 | <0.0001 | ||
| Middle | 0.74 | 0.57 | 0.96 | 0.023 |
| Distal | 0.59 | 0.45 | 0.77 | 0.000 |
| Total | 1.46 | 0.84 | 2.55 | 0.184 |
| Clinical Stage AJCC | ||||
| I | 1.00 | 0.000 | ||
| IIA | 1.74 | 1.18 | 2.55 | 0.005 |
| IIB | 2.26 | 1.57 | 3.26 | 0.000 |
| III | 2.61 | 1.99 | 3.42 | 0.000 |
| IVA | 2.56 | 1.51 | 4.35 | 0.001 |
| Dissected LN | ||||
| ≤25 | 1.00 | 0.440 | ||
| >25 | 1.08 | 0.89 | 1.32 | |
| Complications | ||||
| None | 1.00 | 0.013 | ||
| CD I/II | 1.04 | 0.78 | 1.39 | 0.777 |
| CD III-V | 1.59 | 1.17 | 2.15 | 0.003 |
| UICC | ||||
| UICC 0 | 1.00 | 0.000 | ||
| UICC IA | 3.19 | 0.44 | 23.13 | 0.252 |
| UICC IB | 4.14 | 0.56 | 30.60 | 0.164 |
| UICC IIA | 6.23 | 0.86 | 45.13 | 0.070 |
| UICC IIB | 9.85 | 1.36 | 71.12 | 0.023 |
| UICC IIIA | 13.44 | 1.87 | 96.50 | 0.010 |
| UICC IIIB | 20.73 | 2.88 | 148.95 | 0.003 |
| UICC IIIC | 32.61 | 4.50 | 236.41 | 0.001 |
| Lauren type | ||||
| Not classified | 1.00 | 0.000 | ||
| Intestinal | 1.59 | 1.20 | 2.11 | 0.001 |
| Diffuse | 2.42 | 1.75 | 3.33 | 0.000 |
| Mixed | 2.01 | 1.35 | 3.00 | 0.001 |
| Grading | ||||
| G1/G2 | 1.00 | 0.000 | ||
| G3/G4 | 1.58 | 1.25 | 2.00 | |
| R | 0.000 | |||
| R0 | 1.00 | |||
| R1 | 2.85 | 2.16 | 3.76 |
| Multivariable (All) | HR | CI 95% Lower | CI 95% Upper | p |
|---|---|---|---|---|
| nCTx | 1.00 | 0.78 | 1.29 | 0.98 |
| Gender | ||||
| Female | 1.00 | |||
| Male | 1.02 | 0.81 | 1.29 | 0.85 |
| Age | ||||
| <70 years | 1.00 | |||
| >70 years | 1.99 | 1.60 | 2.47 | 0.00 |
| Localization | ||||
| Proximal | 0.10 | |||
| Middle | 0.77 | 0.57 | 1.02 | 0.07 |
| Distal | 0.74 | 0.55 | 0.99 | 0.04 |
| Whole | 0.68 | 0.38 | 1.23 | 0.20 |
| Clinical Stage AJCC | ||||
| I | 0.67 | |||
| IIA | 1.02 | 0.66 | 1.58 | 0.93 |
| IIB | 1.10 | 0.70 | 1.72 | 0.69 |
| III | 0.91 | 0.62 | 1.32 | 0.61 |
| IVA | 0.75 | 0.41 | 1.39 | 0.36 |
| Dissected LN | ||||
| ≤25 | 1.00 | |||
| >25 | 0.77 | 0.62 | 0.96 | 0.02 |
| Complications | ||||
| None | 0.17 | |||
| CD-I/CD-II | 1.04 | 0.77 | 1.40 | 0.79 |
| CD-III/CD-IV | 1.36 | 0.99 | 1.86 | 0.06 |
| UICC 0 | 0.00 | |||
| UICC IA | 2.84 | 0.38 | 21.13 | 0.31 |
| UICC IB | 3.89 | 0.52 | 28.96 | 0.19 |
| UICC IIA | 5.50 | 0.76 | 40.07 | 0.09 |
| UICC IIB | 9.01 | 1.24 | 65.36 | 0.03 |
| UICC IIIA | 12.04 | 1.67 | 87.10 | 0.01 |
| UICC IIIB | 17.97 | 2.48 | 130.12 | 0.00 |
| UICC IIIC | 33.18 | 4.52 | 243.67 | 0.00 |
| Lauren histotype | ||||
| Not classified | 0.00 | |||
| Intestinal type | 1.75 | 1.28 | 2.38 | 0.00 |
| Diffuse type | 1.99 | 1.41 | 2.80 | 0.00 |
| Mixed type | 1.67 | 1.10 | 2.54 | 0.02 |
| Grading | ||||
| G1/G2 | 1.00 | |||
| G3/G4 | 1.22 | 0.92 | 1.61 | 0.17 |
| R0 | 1.00 | |||
| R1 | 1.28 | 0.94 | 1.73 | 0.12 |
| After PSM (CS-I Exclude) | Surgery Only (n = 261) | CTX + Surgery (n = 261) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | 0.30 | ||||
| Female | 79 | 30.27 | 91 | 34.87 | |
| Male | 182 | 69.73 | 170 | 65.13 | |
| Age | 66.3 ± 11.9 | 61.6 ± 11.7 | <0.0001 | ||
| <70 years | 156 | 59.77 | 174 | 66.67 | 0.12 |
| >70 years | 105 | 40.23 | 87 | 33.33 | |
| Localization | 0.31 | ||||
| Proximal | 143 | 54.79 | 152 | 58.24 | |
| Middle | 55 | 21.07 | 52 | 19.92 | |
| Distal | 56 | 21.46 | 44 | 16.86 | |
| Total | 7 | 2.68 | 13 | 4.98 | |
| Clinical Staging | 0.67 | ||||
| cT1 | 6 | 2.30 | 4 | 1.53 | |
| cT2 | 39 | 14.94 | 32 | 12.26 | |
| cT3 | 204 | 78.16 | 215 | 82.38 | |
| cT4 | 12 | 4.60 | 10 | 3.83 | |
| cN0 | 38 | 14.56 | 53 | 20.31 | 0.11 |
| cN1 | 223 | 85.44 | 208 | 79.69 | |
| Clinical Stage AJCC | 0.38 | ||||
| IIA | 45 | 17.24 | 36 | 13.79 | |
| IIB | 38 | 14.56 | 51 | 19.54 | |
| III | 166 | 63.60 | 164 | 62.84 | |
| IVA | 12 | 4.60 | 10 | 3.83 | |
| Clinical Stage | 0.177 | ||||
| cT3/cT4 cN0 | 38 | 14.56 | 53 | 20.31 | |
| cT1/cT2 cN+ | 45 | 17.24 | 36 | 13.79 | |
| cT3/cT4 cN+ | 178 | 68.20 | 172 | 65.90 | |
| Type of Surgery | <0.0001 | ||||
| Esophagectomy | 13 | 4.98 | 45 | 17.24 | |
| Transhiatal ext. Gastrectomy | 115 | 44.06 | 104 | 39.85 | |
| Total gastrectomy | 95 | 36.40 | 93 | 35.63 | |
| Subtotal gastrectomy | 30 | 11.49 | 18 | 6.90 | |
| Others | 8 | 3.07 | 1 | 0.38 | |
| Surgical extension | <0.0001 | ||||
| None | 118 | 45.21 | 120 | 45.98 | |
| Luminal/transhiatal | 44 | 16.86 | 86 | 32.95 | |
| Splenectomy | 13 | 4.98 | 6 | 2.30 | |
| Colon | 2 | 0.77 | 4 | 1.53 | |
| Pancreas | 2 | 0.77 | 7 | 2.68 | |
| Others | 82 | 31.42 | 38 | 14.56 | |
| Dissected LN [Median] | 28 [1–76] | 28 [6–70] | 0.79 | ||
| ≤25 | 95 | 36.40 | 94 | 36.02 | 1.00 |
| >25 | 166 | 63.60 | 167 | 63.98 | |
| Complications | 0.35 | ||||
| None | 208 | 36.40 | 194 | 74.33 | |
| CD I/II | 29 | 63.60 | 37 | 14.18 | |
| CD III-V | 24 | 9.20 | 30 | 11.49 | |
| pT | <0.0001 | ||||
| pT0 | 1 | 0.38 | 14 | 5.36 | |
| pT1a | 5 | 1.92 | 9 | 3.45 | |
| pT1b | 20 | 7.66 | 19 | 7.28 | |
| pT2 | 39 | 14.94 | 35 | 13.41 | |
| pT3 | 106 | 40.61 | 130 | 49.81 | |
| pT4a | 78 | 29.89 | 46 | 17.62 | |
| pT4b | 12 | 4.60 | 8 | 3.07 | |
| pN | 0.001 | ||||
| pN0 | 79 | 30.27 | 120 | 45.98 | |
| pN1 | 46 | 17.62 | 52 | 19.92 | |
| pN2 | 60 | 22.99 | 35 | 13.41 | |
| pN3a | 52 | 19.92 | 39 | 14.94 | |
| pN3b | 24 | 9.20 | 15 | 5.75 | |
| UICC | <0.0001 | ||||
| UICC 0 | 0 | 0.00 | 14 | 5.36 | |
| UICC IA | 18 | 6.90 | 21 | 8.05 | |
| UICC IB | 23 | 8.81 | 28 | 10.73 | |
| UICC IIA | 40 | 15.33 | 51 | 19.54 | |
| UICC IIB | 37 | 14.18 | 52 | 19.92 | |
| UICC IIIA | 68 | 26.05 | 41 | 15.71 | |
| UICC IIIB | 49 | 18.77 | 37 | 14.18 | |
| UICC IIIC | 26 | 9.96 | 17 | 6.51 | |
| UICC 0 | 0 | 0.00 | 14 | 5.36 | <0.0001 |
| UICC I | 41 | 15.71 | 49 | 18.77 | |
| UICC II | 77 | 29.50 | 103 | 39.46 | |
| UICC III | 143 | 54.79 | 95 | 36.40 | |
| Lauren type | 0.94 | ||||
| Not classified | 41 | 15.71 | 42 | 16.09 | |
| Intestinal | 125 | 47.89 | 118 | 45.21 | |
| Diffuse | 63 | 24.14 | 66 | 25.29 | |
| Mixed | 32 | 12.26 | 35 | 13.41 | |
| Grading | 0.03 | ||||
| G1/G2 | 45 | 17.24 | 67 | 25.67 | |
| G3/G4 | 216 | 82.76 | 194 | 74.33 | |
| R | 0.79 | ||||
| R0 | 227 | 86.97 | 230 | 88.12 | |
| R1 | 34 | 13.03 | 31 | 11.88 | |
| Histopathologic Response | |||||
| Becker Ia/Ib | 77 | 29.50 | |||
| Becker II | 67 | 25.67 | |||
| Becker III | 117 | 44.83 | |||
| yUICC I | yUICC II | yUICC III | p < 0.001 | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Responder | 45 | 17.24 | 23 | 8.81 | 9 | 3.45 | |
| Non-responder | 18 | 6.90 | 80 | 30.65 | 86 | 32.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimpel, R.; Novotny, A.; Slotta-Huspenina, J.; Langer, R.; Friess, H.; Reim, D. UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer. Cancers 2022, 14, 6169. https://doi.org/10.3390/cancers14246169
Dimpel R, Novotny A, Slotta-Huspenina J, Langer R, Friess H, Reim D. UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer. Cancers. 2022; 14(24):6169. https://doi.org/10.3390/cancers14246169
Chicago/Turabian StyleDimpel, Rebekka, Alexander Novotny, Julia Slotta-Huspenina, Rupert Langer, Helmut Friess, and Daniel Reim. 2022. "UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer" Cancers 14, no. 24: 6169. https://doi.org/10.3390/cancers14246169
APA StyleDimpel, R., Novotny, A., Slotta-Huspenina, J., Langer, R., Friess, H., & Reim, D. (2022). UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer. Cancers, 14(24), 6169. https://doi.org/10.3390/cancers14246169







