Single Center Experience with a 4-Week 177Lu-PSMA-617 Treatment Interval in Patients with Metastatic Castration-Resistant Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Tracer Production
2.3. PET-Imaging
2.4. 177Lu-PSMA-617 Therapy
2.5. Image Analysis
2.6. Adverse Events and Remote Reporting via Kaiku
2.7. Statistics
3. Results
3.1. Pre-Treatment Disease Location and Tumor Burden
3.2. 177Lu-PSMA-617 Therapies
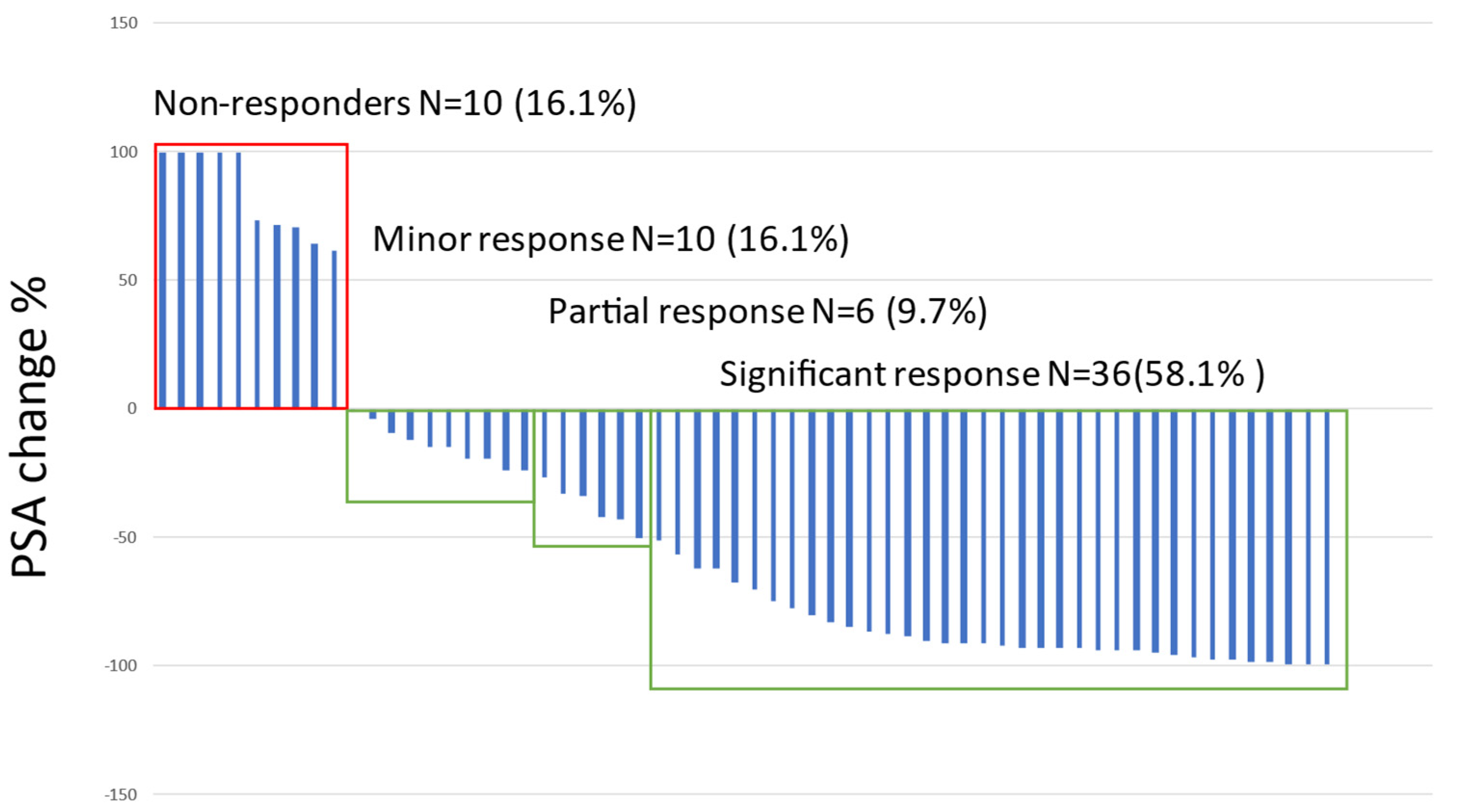
3.3. PSA and Treatment Response
3.4. Follow-Up and Survival
3.5. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Solnes, L.B.; Rowe, S.P. 177Lu-PSMA Radioligand Therapy Effectiveness in Metastatic Castration-Resistant Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Prostate 2022, 82, 826–835. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.I. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-Analysis. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM Procedure Guidelines for Radionuclide Therapy with 177Lu-Labelled PSMA-Ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Radionuclide Therapy of Metastatic Prostate Cancer. Semin. Nucl. Med. 2019, 49, 313–325. [Google Scholar] [CrossRef]
- Rahbar, K.; Boegemann, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. PSMA Targeted Radioligandtherapy in Metastatic Castration Resistant Prostate Cancer after Chemotherapy, Abiraterone and/or Enzalutamide. A Retrospective Analysis of Overall Survival. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 12–19. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Wegen, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Wei, X.; Schlenkhoff, C.; Hauser, S.; Essler, M. Overall Survival and Response Pattern of Castration-Resistant Metastatic Prostate Cancer to Multiple Cycles of Radioligand Therapy Using [177Lu]Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1448–1454. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Schlolaut, S.; Fimmers, R.; Yordanova, A.; Hirzebruch, S.; Schlenkhoff, C.; Gaertner, F.C.; Awang, Z.H.; Hauser, S.; Essler, M. Predictors of Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving [177Lu]Lu-PSMA-617 Radioligand Therapy. Oncotarget 2017, 8, 103108. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior Therapies as Prognostic Factors of Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Treated with [177Lu]Lu-PSMA-617. A WARMTH Multicenter Study (the 617 Trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Emmett, L.; Violet, J.; Zhang, A.Y.; Lawrence, N.J.; Stockler, M.; Francis, R.J.; Iravani, A.; Williams, S.; Azad, A.; et al. TheraP: A Randomized Phase 2 Trial of 177Lu-PSMA-617 Theranostic Treatment vs Cabazitaxel in Progressive Metastatic Castration-Resistant Prostate Cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019, 124, 5–13. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Ping Thang, S.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer (LuPSMA Trial): A Single-Centre, Single-Arm, Phase 2 Study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Bögemann, M.; Rahbar, K. Radioligand Therapy Using [177Lu]Lu-PSMA-617 in MCRPC: A Pre-VISION Single-Center Analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2106–2112. [Google Scholar] [CrossRef] [Green Version]
- Kessel, K.; Seifert, R.; Schäfers, M.; Weckesser, M.; Schlack, K.; Boegemann, M.; Rahbar, K. Second Line Chemotherapy and Visceral Metastases Are Associated with Poor Survival in Patients with MCRPC Receiving 177Lu-PSMA-617. Theranostics 2019, 9, 4841–4848. [Google Scholar] [CrossRef]
- Seifert, R.; Seitzer, K.; Herrmann, K.; Kessel, K.; Schäfers, M.; Kleesiek, J.; Weckesser, M.; Boegemann, M.; Rahbar, K. Analysis of PSMA Expression and Outcome in Patients with Advanced Prostate Cancer Receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7812–7820. [Google Scholar] [CrossRef]
- Soydal, C.; Ozkan, E.; Akyurek, S.; Kucuk, N.O. Marked Response to 177Lu Prostate-Specific Membrane Antigen Treatment in Patient With Metastatic Prostate Cancer. Clin. Nucl. Med. 2016, 41, 159–160. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Rathke, H.; Giesel, F.L.; Flechsig, P.; Kopka, K.; Mier, W.; Hohenfellner, M.; Haberkorn, U.; Kratochwil, C. Repeated 177Lu-Labeled PSMA-617 Radioligand Therapy Using Treatment Activities of up to 9.3 GBq. J. Nucl. Med. 2018, 59, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Bögeman, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. Delayed Response after Repeated 177Lu-PSMA-617 Radioligand Therapy in Patients with Metastatic Castration Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 243–246. [Google Scholar] [CrossRef]
- Cardinale, J.; Martin, R.; Remde, Y.; Schäfer, M.; Hienzsch, A.; Hübner, S.; Zerges, A.M.; Marx, H.; Hesse, R.; Weber, K.; et al. Procedures for the GMP-Compliant Production and Quality Control of [18F]PSMA-1007: A next Generation Radiofluorinated Tracer for the Detection of Prostate Cancer. Pharmaceuticals 2017, 10, 77. [Google Scholar] [CrossRef]
- Vis, R.; Lavalaye, J.; van de Garde, E.M.W. GMP-Compliant 68Ga Radiolabelling in a Conventional Small-Scale Radiopharmacy: A Feasible Approach for Routine Clinical Use. EJNMMI Res. 2015, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 Radioligand Therapy and Outcome in Patients with Metastasized Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Rasul, S.; Hacker, M.; Kretschmer-Chott, E.; Leisser, A.; Grubmüller, B.; Kramer, G.; Shariat, S.; Wadsak, W.; Mitterhauser, M.; Hartenbach, M.; et al. Clinical Outcome of Standardized 177Lu-PSMA-617 Therapy in Metastatic Prostate Cancer Patients Receiving 7400 MBq Every 4 Weeks. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Ferdinandus, J.; Eppard, E.; Gaertner, F.C.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Hauser, S.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. Predictors of Response to Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with 177Lu-PSMA-617. J. Nucl. Med. 2017, 58, 312–319. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET Total Tumor Volume Predicts Outcome of Patients with Advanced Prostate Cancer Receiving [177Lu]Lu-PSMA-617 Radioligand Therapy in a Bicentric Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Kersting, D.; Seitzer, K.E.; Weber, M.; Bögemann, M.; Rahbar, K. Total Tumor Volume Reduction and Low PSMA Expression in Patients Receiving Lu-PSMA Therapy. Theranostics 2021, 11, 8143–8151. [Google Scholar] [CrossRef]
- Buteau, J.P.; Martin, A.J.; Emmett, L.; Iravani, A.; Sandhu, S.; Joshua, A.M.; Francis, R.J.; Zhang, A.Y.; Scott, A.M.; Lee, S.-T.; et al. PSMA and FDG-PET as Predictive and Prognostic Biomarkers in Patients given [177Lu]Lu-PSMA-617 versus Cabazitaxel for Metastatic Castration-Resistant Prostate Cancer (TheraP): A Biomarker Analysis from a Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2022, 23, 1389–1397. [Google Scholar] [CrossRef]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-Resistant Prostate Cancer (Figure Presented). Eur. Urol. 2019, 75, 920–926. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Fendler, W.P.; Hadaschik, B.; Herrmann, K. Prostate-Specific Membrane Antigen Targeted PET Imaging for Prostate Cancer Recurrence. Curr. Opin. Urol. 2020, 30, 635–640. [Google Scholar] [CrossRef]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine Differentiation of Prostate Cancer: A Review. Am. J. Clin. Exp. Urol. 2014, 2, 273. [Google Scholar] [PubMed]
- Parida, G.K.; Tripathy, S.; Datta Gupta, S.; Singhal, A.; Kumar, R.; Bal, C.; Shamim, S.A. Adenocarcinoma Prostate With Neuroendocrine Differentiation: Potential Utility of 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT Over 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2018, 43, 248–249. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Weckesser, M.; Roll, W.; Humberg, V.; Schlack, K.; Bögemann, M.; Bernemann, C.; Rahbar, K. Molecular Analysis of Circulating Tumor Cells of Metastatic Castration-Resistant Prostate Cancer Patients Receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7645–7655. [Google Scholar] [CrossRef]
- Zhao, S.G.; Sperger, J.M.; Schehr, J.L.; McKay, R.R.; Emamekhoo, H.; Singh, A.; Schultz, Z.D.; Bade, R.M.; Stahlfeld, C.N.; Gilsdorf, C.S.; et al. A Clinical-Grade Liquid Biomarker Detects Neuroendocrine Differentiation in Prostate Cancer. J. Clin. Investig. 2022, 132, e161858. [Google Scholar] [CrossRef]
- Stuparu, A.D.; Capri, J.R.; Meyer, C.A.L.; Le, T.M.; Evans-Axelsson, S.L.; Current, K.; Lennox, M.; Mona, C.E.; Fendler, W.P.; Calais, J.; et al. Mechanisms of Resistance to Prostate-Specific Membrane Antigen-Targeted Radioligand Therapy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2021, 62, 989–995. [Google Scholar] [CrossRef]


| Patients (n) | 62 |
| Age (y) | 71.3 (IQR 66.7–75.4) |
| Years from diagnosis (y) | 8.7 (IQR 4.3–13.2) |
| Original prostate cancer stage: | |
| Stage 1 | 4 |
| Stage 2 | 3 |
| Stage 3 | 21 |
| Stage 4 | 33 |
| Gleason score at diagnosis: | |
| 6 | 8 |
| 7 (3 + 4) | 9 |
| 7 (4 + 3) | 9 |
| 8 | 15 |
| 9 | 18 |
| 10 | 1 |
| Tumor Burden and Tumor Lesion Activities | |
|---|---|
| Parameter | Median (IQR) |
| Metabolic tumor volume, all lesions (MTVtotal, cm3) | 413.9 (IQR 68.9–1067.7) |
| MTV in bone metastases (cm3) | 296.8 (IQR 47.7–965.1) |
| MTV in all lymph node metastases (cm3) | 37.1 (IQR 14.8–112.9) |
| MTV in pelvic lymph node metastases (cm3) | 22.9 (IQR 6.9–49.3) |
| MTV in para-aortic lymph node metastases (cm3) | 35.3 (IQR 9.1–66.3) |
| MTV in thoracic lymph nodes | 14.0 (4.1–41.1) |
| MTV in lung metastases (cm3) | 1.7 (IQR 1.6–9.8) |
| MTV in liver metastases (cm3) | 507.0 (IQR 204–622.6) |
| MTV in prostate/prostatic fossa (cm3) | 17.9 (IQR 5.2–45.1) |
| Total tumor lesion activity (TLAtotal, cm3*SUVmax) | 2786.5 (IQR 386.4–7977.6) |
| TLA in bone metastases | 1725.4 (IQR 336.5–6699.2) |
| TLA in lymph node metastases | 254.7 (IQR 336.5–6699.2) |
| TLA in pelvic lymph node metastases | 191.9 (IQR 37.3–473.2) |
| TLA in para-aortic lymph node metastases | 256.1 (IQR 38.3–736.9) |
| TLA in thoracic lymph nodes | 52.3 (IQR 16.9–294.1) |
| TLA in lung metastases | 6.7 (IQR 3.2–13.3) |
| TLA in liver metastases | 4401.2 (IQR 2878.4–4589.7) |
| TLA in prostate/prostatic fossa | 92.0 (IQR 7.9–17.6) |
| SUVmax in bone metastases | 24.3 (IQR 9.7–42.3) |
| SUVmax in pelvic metastases | 21.8 (IQR 9.2–32.0) |
| SUVmax in para-aortic metastases | 19.7 (IQR 9–36.9) |
| SUVmax in thoracic metastases | 9.5 (IQR 6.6.0–9.8) |
| SUVmax in lung metastases | 3.0 (IQR 1.0–1.5) |
| SUVmax in liver metastases | 16.8 (IQR 15.3–24.9) |
| SUVmax in prostate/prostatic fossa | 12.8 (IQR 7.9–17.6) |
| Overall Survival (OS) | ||
|---|---|---|
| Parameter | HR (95% CI) | p-Value |
| Years from diagnosis | 0.85 (0.68–1.06) | 0.144 |
| PSA prior Lu-treatment | 1.16 (1.03–1.30) | 0.018 |
| PSA velocity | 1.13 (0.98–1.31) | 0.102 |
| PSA doubling time | 1.09 (0.84–1.40) | 0.523 |
| Gleason score | 1.03 (0.80–1.34) | 0.801 |
| Number of Lu-PSMA treatments | 0.78 (0.63–0.97) | 0.028 |
| MTVtotal | 1.24 (1.07–1.43) | 0.003 |
| TLAtotal | 1.17 (1.03–1.32) | 0.017 |
| Bone MTV | 1.15 (1.01–1.30) | 0.031 |
| Bone TLA | 1.11 (0.99–1.23) | 0.075 |
| Bone SUVmax | 0.94 (0.74–1.20) | 0.609 |
| Lymph node MTV total | 1.26 (1.05–1.51) | 0.013 |
| Lymph node TLA total | 1.17 (1.01–1.36) | 0.032 |
| Pelvic lymph node MTV | 1.13 (0.92–1.37) | 0.242 |
| Pelvic lymph node TLA | 1.09 (0.92–1.29) | 0.325 |
| Pelvic lymph node SUVmax | 1.13 (0.80–1.60) | 0.488 |
| Para-aortic lymph node MTV | 1.61 (1.21–2.12) | 0.0009 |
| Para-aortic lymph node TLA | 1.37 (1.10–1.70) | 0.004 |
| Para-aortic lymph node SUVmax | 1.26 (0.89–1.77) | 0.195 |
| Thoracic lymph node MTV | 1.15 (0.95–1.40) | 0.144 |
| Thoracic lymph node TLA | 1.08 (0.93–1.26) | 0.298 |
| Thoracic lymph node SUVmax | 0.92 (0.63–1.35) | 0.678 |
| Liver MTV | 1.43 (0.82–2.51) | 0.210 |
| Liver TLA | 1.28 (0.78–2.10) | 0.336 |
| liver SUVmax | 1.19 (0.32–4.44) | 0.802 |
| Lung MTV | 0.70 (0.38–1.29) | 0.255 |
| Lung TLA | 0.72 (0.37–1.43) | 0.350 |
| Lung SUVmax | 0.35 (0.10–1.23) | 0.101 |
| PSA Progression-Free Survival (PFS) | ||
|---|---|---|
| Parameter | HR (95% CI) | p-Value |
| Years from diagnosis | 0.96 (0.81–1.14) | 0.629 |
| Pre-treatment PSA | 1.12 (1.02–1.22) | 0.014 |
| PSA velocity | 1.25 (1.09–1.43) | 0.001 |
| PSA doubling time | 0.98 (0.78–1.22) | 0.839 |
| Gleason score | 0.94 (0.78–1.14) | 0.518 |
| Number of Lu-PSMA treatments | 0.79 (0.67–0.94) | 0.006 |
| MTVtotal | 1.13 (1.00–1.26) | 0.037 |
| TLAtotal | 1.08 (0.98–1.18) | 0.119 |
| Bone MTV | 1.10 (0.99–1.21) | 0.069 |
| Bone TLA | 1.07 (0.98–1.16) | 0.157 |
| Bone SUVmax | 0.92 (0.75–1.13) | 0.438 |
| Lymph node MTV | 1.05 (0.92–1.20) | 0.443 |
| Lymph node TLA | 1.04 (0.94–1.15) | 0.481 |
| Pelvic lymph node MTV | 1.05 (0.90–1.23) | 0.558 |
| Pelvic lymph node TLA | 1.04 (0.91–1.18) | 0.556 |
| Pelvic lymph node SUVmax | 1.20 (0.89–1.61) | 0.229 |
| Para-aortic lymph node MTV | 1.07 (0.86–1.32) | 0.541 |
| Para-aortic lymph node TLA | 1.04 (0.89–1.21) | 0.656 |
| Para-aortic lymph node SUVmax | 1.07 (0.79–1.46) | 0.648 |
| Thoracic lymph node MTV | 1.02 (0.88–1.19) | 0.763 |
| Thoracic lymph node TLA | 1.00 (0.89–1.14) | 0.936 |
| Thoracic lymph node SUVmax | 0.88 (0.63–1.21) | 0.425 |
| Liver MTV | 1.38 (0.82–2.32) | 0.223 |
| Liver TLA | 1.27 (0.79–2.06) | 0.328 |
| Liver SUVmax | 1.11 (0.32–3.88) | 0.870 |
| Lung MTV | 0.78 (0.51–1.21) | 0.271 |
| Lung TLA | 0.82 (0.50–1.34) | 0.419 |
| Lung SUVmax | 0.85 (0.44–1.64) | 0.621 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemppainen, J.; Kangasmäki, A.; Malaspina, S.; Pape, B.; Jalomäki, J.; Kairemo, K.; Kononen, J.; Joensuu, T. Single Center Experience with a 4-Week 177Lu-PSMA-617 Treatment Interval in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers 2022, 14, 6155. https://doi.org/10.3390/cancers14246155
Kemppainen J, Kangasmäki A, Malaspina S, Pape B, Jalomäki J, Kairemo K, Kononen J, Joensuu T. Single Center Experience with a 4-Week 177Lu-PSMA-617 Treatment Interval in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers. 2022; 14(24):6155. https://doi.org/10.3390/cancers14246155
Chicago/Turabian StyleKemppainen, Jukka, Aki Kangasmäki, Simona Malaspina, Bernd Pape, Jarno Jalomäki, Kalevi Kairemo, Juha Kononen, and Timo Joensuu. 2022. "Single Center Experience with a 4-Week 177Lu-PSMA-617 Treatment Interval in Patients with Metastatic Castration-Resistant Prostate Cancer" Cancers 14, no. 24: 6155. https://doi.org/10.3390/cancers14246155
APA StyleKemppainen, J., Kangasmäki, A., Malaspina, S., Pape, B., Jalomäki, J., Kairemo, K., Kononen, J., & Joensuu, T. (2022). Single Center Experience with a 4-Week 177Lu-PSMA-617 Treatment Interval in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers, 14(24), 6155. https://doi.org/10.3390/cancers14246155






