Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples and Isolation of Cell Types
2.2. Differentiation of Monocyte-Derived Macrophages (MDM) from Healthy Donors
2.3. Primary Cell Culture and Preparation of Conditioned Media for Lipid-MS
2.4. Treatment of Cells with PGI2 Analogs
2.5. Co-Cultivation of Asc-MDM and CAF
2.6. Quantification 6k-PGF1α and PGE2 by Lipid-MS
2.7. Flow Cytometric Analysis of Cell Phenotypes
2.8. Macropinocytosis Assay
2.9. cAMP Assay
2.10. Tumor Cell Migration Assay
2.11. Tumor Cell Attachment to Mesothelial Cells
2.12. VEGF-A Quantification by ELISA
2.13. Transient PTGIR Knockdown in ascTAM and Asc-MDM by RNA Interference
2.14. Immunoblotting
2.15. RT-qPCR
2.16. RNA Sequencing
2.17. Statistical Analysis
3. Results
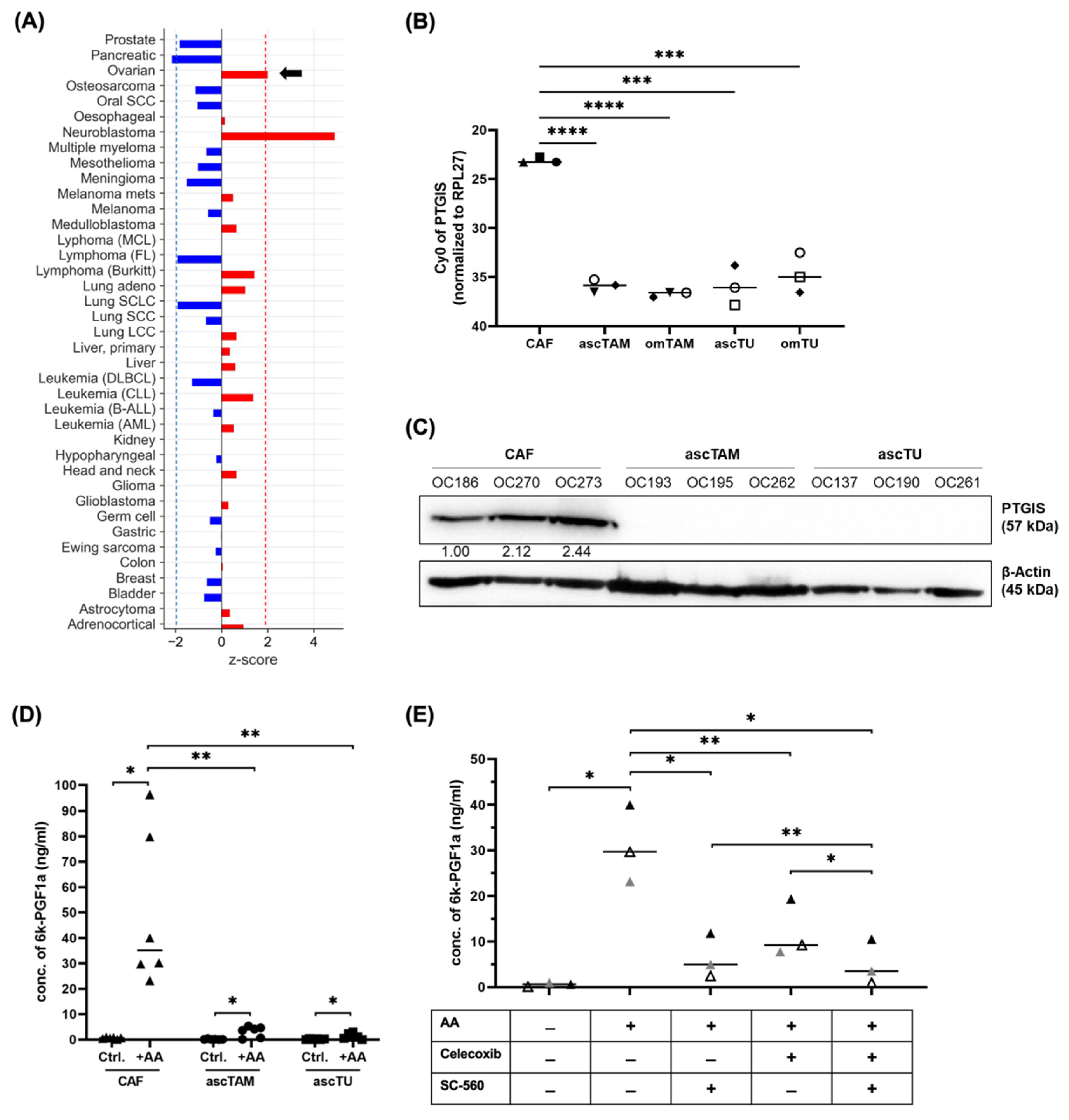
3.1. A Crucial Role for Tumor-Associated Host Cells in Lipid-Mediated Signaling
3.2. Validation of PGI2 Synthesis by Cells of the HGSC TME
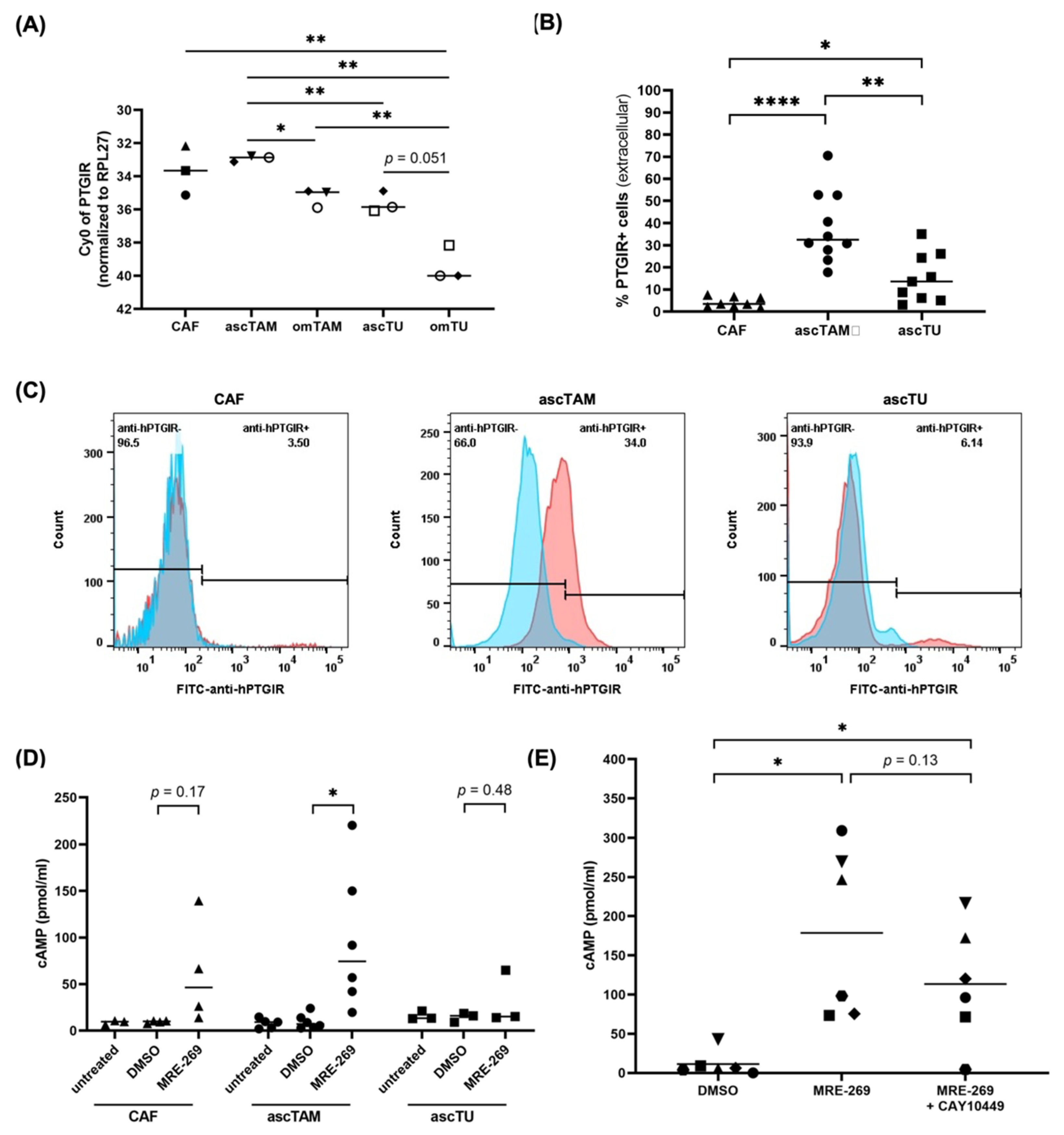
3.3. PTGIR Expression by Cells of the HGSC TME
3.4. Intracellular cAMP Accumulation by PGI2 Receptor Signaling in ascTAM
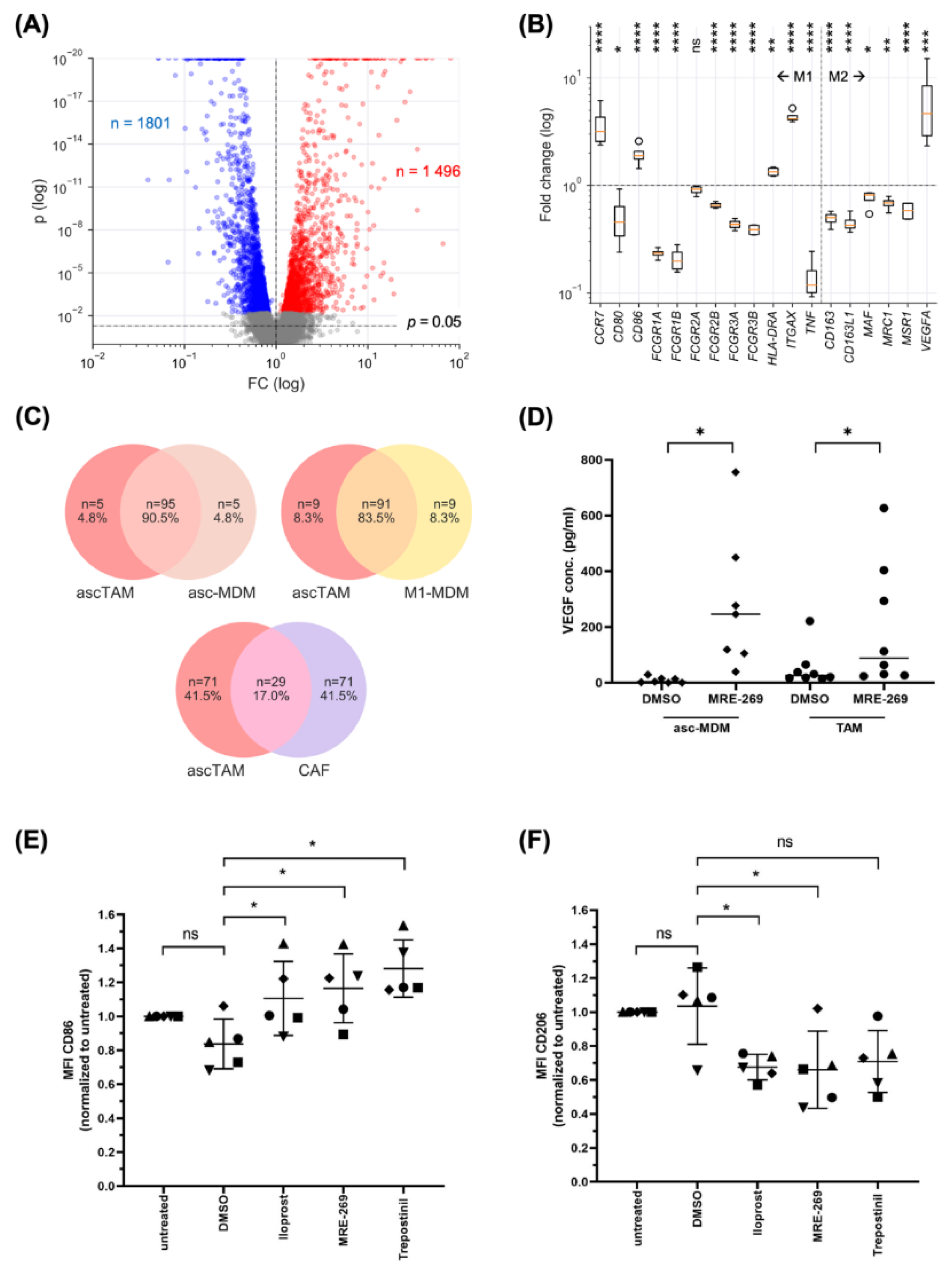
3.5. PGI2 Analogs Shift the Differentiation, Transcriptional Profile and Secretome of Macrophages towards a Pro-Tumorigenic Phenotype
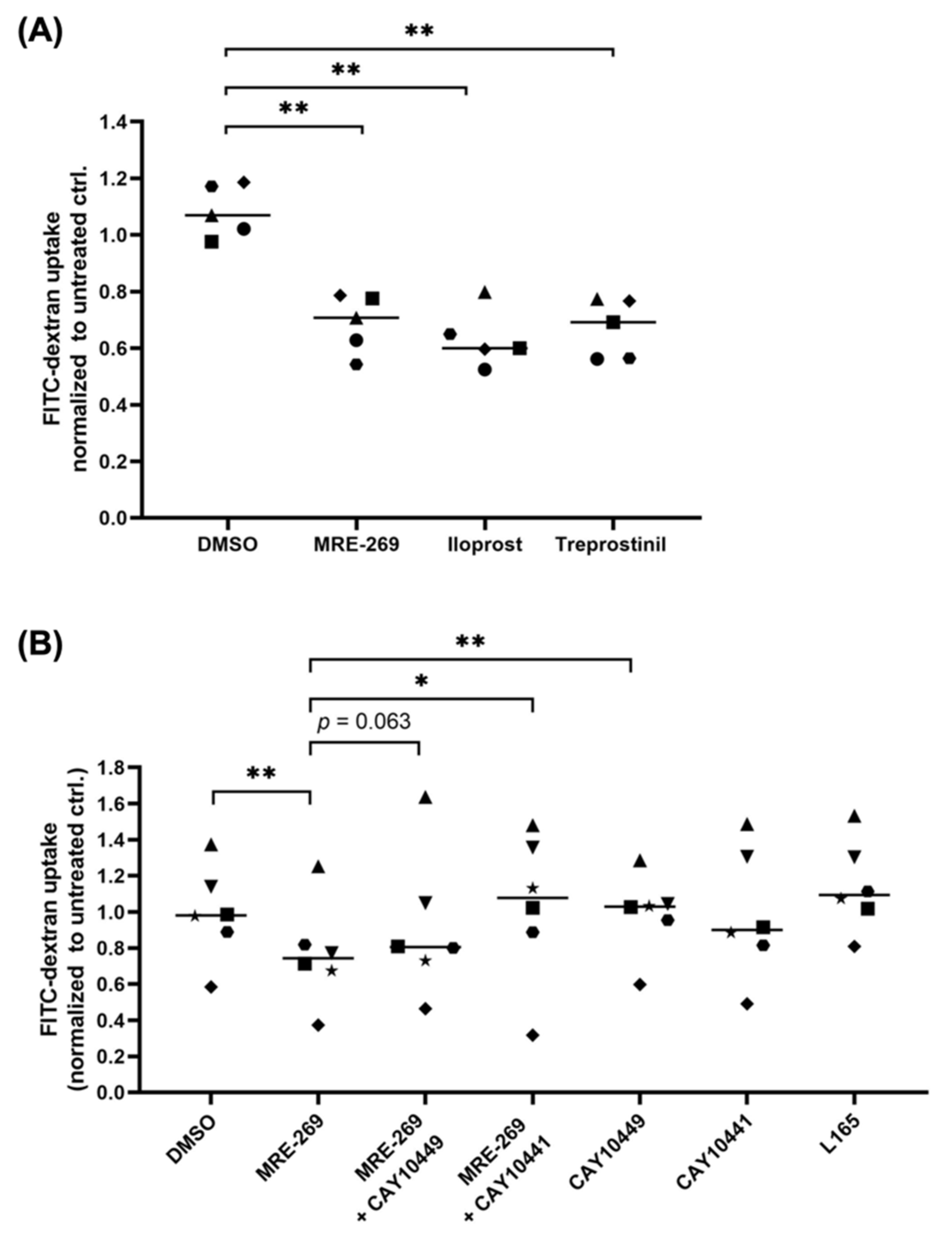
3.6. PGI2 Decreases the Phagocytic Capability of Macrophage
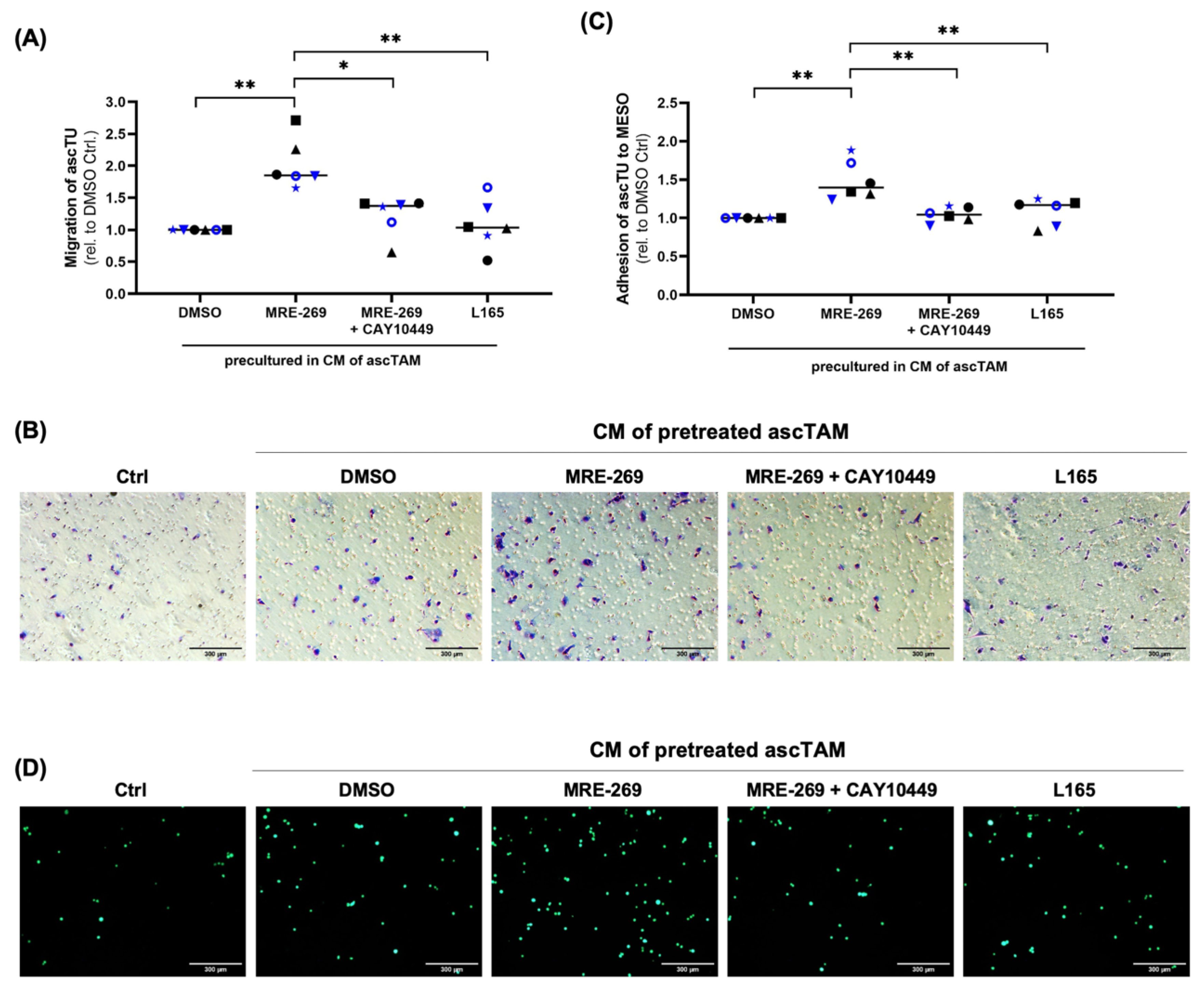
3.7. Triggering Tumor Migration and Adhesion by Factors Secreted by PGI2-Treated TAM
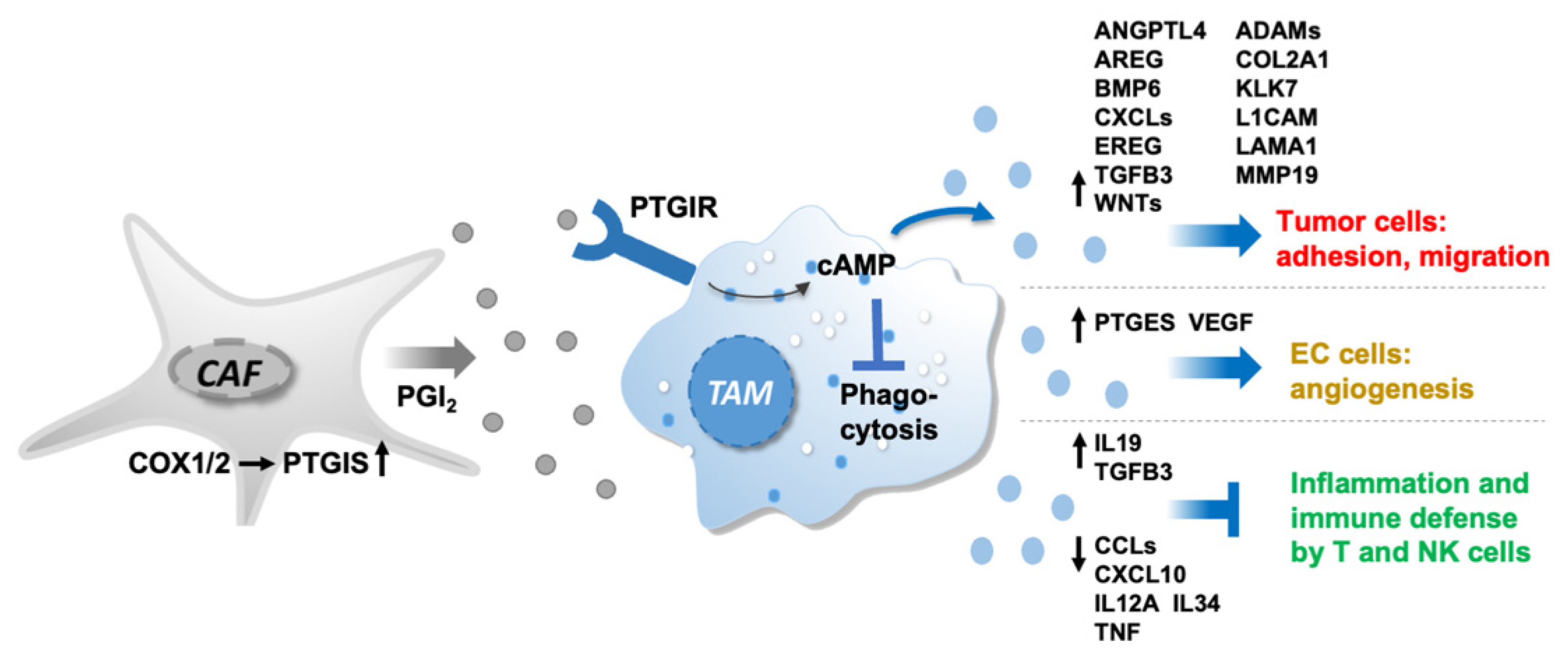
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADI | adipocytes |
| asc | ascites |
| AA | arachidonic acid |
| CAF | cancer-associated fibroblasts |
| CM | conditioned medium |
| COX1/2 | cyclooxygenase-1/-2 |
| CREB | cyclic AMP responsive element binding |
| Ctrl | control |
| ECM | extracellular matrix |
| FC | fold change |
| IBMX | phosphodiesterase (PDE) inhibitor isobutylmethylxanthine |
| HGSC | high-grade ovarian carcinoma |
| LPA | lysophosphatidic acids |
| MDM | monocyte-derived macrophages |
| MESO | mesothelial cells |
| MFI | mean fluorescence intensities |
| MS | mass spectrometry |
| NSAID | nonsteroidal anti-inflammatory drug |
| om | omentum |
| OS | overall survival |
| PFS | progression-free survival |
| PGE2 | prostaglandin E2 |
| PGH2 | prostaglandin H2 |
| PGI2 | prostaglandin E2 (prostacyclin) |
| PPARβ/δ | peroxisome-proliferator-activated receptor β/δ |
| PTGER | PGE2 receptor |
| PTGIR | prostacyclin receptor |
| PTGIS | prostacyclin synthase |
| RNA-Seq | RNA sequencing |
| TAM | tumor-associated macrophages |
| TAT | tumor-associated T cells |
| TME | tumor microenvironment |
| ZO1 | zonula occludens 1 |
References
- Reinartz, S.; Lieber, S.; Pesek, J.; Brandt, D.T.; Asafova, A.; Finkernagel, F.; Watzer, B.; Nockher, W.A.; Nist, A.; Stiewe, T.; et al. Cell type-selective pathways and clinical associations of lysophosphatidic acid biosynthesis and signaling in the ovarian cancer microenvironment. Mol. Oncol. 2019, 13, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.; Finkernagel, F.; Adhikary, T.; Rohnalter, V.; Schumann, T.; Schober, Y.; Nockher, W.A.; Nist, A.; Stiewe, T.; Jansen, J.M.; et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Dietze, R.; Hammoud, M.K.; Gómez-Serrano, M.; Unger, A.; Bieringer, T.; Finkernagel, F.; Sokol, A.M.; Nist, A.; Stiewe, T.; Reinartz, S.; et al. Phosphoproteomics identify arachidonic-acid-regulated signal transduction pathways modulating macrophage functions with implications for ovarian cancer. Theranostics 2021, 11, 1377–1395. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, M.K.; Dietze, R.; Pesek, J.; Finkernagel, F.; Unger, A.; Bieringer, T.; Nist, A.; Stiewe, T.; Bhagwat, A.M.; Nockher, W.A.; et al. Arachidonic acid, a clinically adverse mediator in the ovarian cancer microenvironment, impairs JAK-STAT signaling in macrophages by perturbing lipid raft structures. Mol. Oncol. 2022, 16, 3146–3166. [Google Scholar] [CrossRef]
- Kobayashi, K.; Omori, K.; Murata, T. Role of prostaglandins in tumor microenvironment. Cancer Metastasis Rev. 2018, 37, 347–354. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Gupta, R.A.; Tan, J.; Krause, W.F.; Geraci, M.W.; Willson, T.M.; Dey, S.K.; DuBois, R.N. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 13275–13280. [Google Scholar] [CrossRef]
- Midgett, C.; Stitham, J.; Martin, K.A.; Hwa, J. Prostacyclin receptor regulation--from transcription to trafficking. Curr. Mol. Med. 2011, 11, 517–528. [Google Scholar] [CrossRef]
- Shaul, P.W.; Kinane, B.; Farrar, M.A.; Buja, L.M.; Magness, R.R. Prostacyclin production and mediation of adenylate cyclase activity in the pulmonary artery. Alterations after prolonged hypoxia in the rat. J. Clin. Investig. 1991, 88, 447–455. [Google Scholar] [CrossRef]
- Schwaner, I.; Offermanns, S.; Spicher, K.; Seifert, R.; Schultz, G. Differential activation of Gi and GS proteins by E- and I-type prostaglandins in membranes from the human erythroleukaemia cell line, HEL. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 1995, 1265, 8–14. [Google Scholar] [CrossRef]
- Moncada, S.; Gryglewski, R.; Bunting, S.; Vane, J.R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976, 263, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Stitham, J.; Midgett, C.; Martin, K.A.; Hwa, J. Prostacyclin: An inflammatory paradox. Front. Pharmacol. 2011, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; McSharry, M.; Walker, D.; Johnson, A.; Kwak, J.; Bullock, B.; Neuwelt, A.; Poczobutt, J.M.; Sippel, T.R.; Keith, R.L.; et al. Targeted overexpression of prostacyclin synthase inhibits lung tumor progression by recruiting CD4+ T lymphocytes in tumors that express MHC class II. Oncoimmunology 2018, 7, e1423182. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.L.; Geraci, M.W. Prostacyclin in Lung Cancer. J. Thorac. Oncol. 2006, 1, 503–505. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Lee, K.-T.; Choi, Y.S.; Choi, J.-H. Iloprost, a prostacyclin analog, inhibits the invasion of ovarian cancer cells by downregulating matrix metallopeptidase-2 (MMP-2) through the IP-dependent pathway. Prostaglandins Other Lipid Mediat. 2018, 134, 47–56. [Google Scholar] [CrossRef]
- Klein, T.; Benders, J.; Roth, F.; Baudler, M.; Siegle, I.; Kömhoff, M. Expression of Prostacyclin-Synthase in Human Breast Cancer: Negative Prognostic Factor and Protection against Cell Death In Vitro. Mediat. Inflamm. 2015, 2015, 864136. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef]
- Kawamura, K.; Komohara, Y.; Takaishi, K.; Katabuchi, H.; Takeya, M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol. Int. 2009, 59, 300–305. [Google Scholar] [CrossRef]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.-M.; Wagner, U.; Müller-Brüsselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef]
- Worzfeld, T.; Finkernagel, F.; Reinartz, S.; Konzer, A.; Adhikary, T.; Nist, A.; Stiewe, T.; Wagner, U.; Looso, M.; Graumann, J.; et al. Proteotranscriptomics Reveal Signaling Networks in the Ovarian Cancer Microenvironment. Mol. Cell Proteom. 2018, 17, 270–289. [Google Scholar] [CrossRef]
- Adhikary, T.; Wortmann, A.; Finkernagel, F.; Lieber, S.; Nist, A.; Stiewe, T.; Wagner, U.; Müller-Brüsselbach, S.; Reinartz, S.; Müller, R. Interferon signaling in ascites-associated macrophages is linked to a favorable clinical outcome in a subgroup of ovarian carcinoma patients. BMC Genom. 2017, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Y.; Wang, L.; You, H.-M.; Cheng, M.; Yang, Y.; Huang, C.; Li, J. Alternative activation of macrophages by prostacyclin synthase ameliorates alcohol induced liver injury. Lab. Investig. 2021, 101, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, D.M.; Peres, C.M.; Serezani, C.H.; Ballinger, M.N.; Carstens, J.K.; Coleman, N.; Moore, B.B.; Peebles, R.S.; Faccioli, L.H.; Peters-Golden, M. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J. Immunol. 2007, 178, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, L.; Finkernagel, F.; Jansen, J.M.; Wagner, U.; Nist, A.; Stiewe, T.; Müller-Brüsselbach, S.; Sokol, A.M.; Graumann, J.; Reinartz, S.; et al. The multicellular signalling network of ovarian cancer metastases. Clin. Transl. Med. 2021, 11, e633. [Google Scholar] [CrossRef] [PubMed]
- Steitz, A.M.; Steffes, A.; Finkernagel, F.; Unger, A.; Sommerfeld, L.; Jansen, J.M.; Wagner, U.; Graumann, J.; Müller, R.; Reinartz, S. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death Dis. 2020, 11, 249. [Google Scholar] [CrossRef]
- Pluchart, H.; Khouri, C.; Blaise, S.; Roustit, M.; Cracowski, J.-L. Targeting the Prostacyclin Pathway: Beyond Pulmonary Arterial Hypertension. Trends Pharmacol. Sci. 2017, 38, 512–523. [Google Scholar] [CrossRef]
- Banhos Danneskiold-Samsøe, N.; Sonne, S.B.; Larsen, J.M.; Hansen, A.N.; Fjære, E.; Isidor, M.S.; Petersen, S.; Henningsen, J.; Severi, I.; Sartini, L.; et al. Overexpression of cyclooxygenase-2 in adipocytes reduces fat accumulation in inguinal white adipose tissue and hepatic steatosis in high-fat fed mice. Sci. Rep. 2019, 9, 8979. [Google Scholar] [CrossRef]
- Rohnalter, V.; Roth, K.; Finkernagel, F.; Adhikary, T.; Obert, J.; Dorzweiler, K.; Bensberg, M.; Müller-Brüsselbach, S.; Müller, R. A multi-stage process including transient polyploidization and EMT precedes the emergence of chemoresistent ovarian carcinoma cells with a dedifferentiated and pro-inflammatory secretory phenotype. Oncotarget 2015, 6, 40005–40025. [Google Scholar] [CrossRef]
- Guescini, M.; Sisti, D.; Rocchi, M.B.L.; Stocchi, L.; Stocchi, V. A new real-time PCR method to overcome significant quantitative inaccuracy due to slight amplification inhibition. BMC Bioinform. 2008, 9, 326. [Google Scholar] [CrossRef]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; et al. Ensembl 2020. Nucleic Acids Res. 2020, 48, D682–D688. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Herwig, R.; Hardt, C.; Lienhard, M.; Kamburov, A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat. Protoc. 2016, 11, 1889–1907. [Google Scholar] [CrossRef]
- Gyorffy, B.; Lánczky, A.; Szállási, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Clapp, L.H.; Abu-Hanna, J.H.J.; Patel, J.A. Diverse Pharmacology of Prostacyclin Mimetics: Implications for Pulmonary Hypertension. In Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension; Nakanishi, T., Baldwin, H.S., Fineman, J.R., Yamagishi, H., Eds.; Springer: Singapore, 2020; pp. 31–61. ISBN 978-981-15-1184-4. [Google Scholar]
- Mao, Y.; Finnemann, S.C. Regulation of phagocytosis by Rho GTPases. Small GTPases 2015, 6, 89–99. [Google Scholar] [CrossRef]
- Clayton, N.S.; Ridley, A.J. Targeting Rho GTPase Signaling Networks in Cancer. Front. Cell Dev. Biol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Schumann, T.; Adhikary, T.; Wortmann, A.; Finkernagel, F.; Lieber, S.; Schnitzer, E.; Legrand, N.; Schober, Y.; Nockher, W.A.; Toth, P.M.; et al. Deregulation of PPARβ/δ target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget 2015, 6, 13416–13433. [Google Scholar] [CrossRef]
- Adhikary, T.; Wortmann, A.; Schumann, T.; Finkernagel, F.; Lieber, S.; Roth, K.; Toth, P.M.; Diederich, W.E.; Nist, A.; Stiewe, T.; et al. The transcriptional PPARβ/δ network in human macrophages defines a unique agonist-induced activation state. Nucleic Acids Res. 2015, 43, 5033–5051. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.V.; Skrinska, V.A.; Chisolm, G.M.; Hesse, B.L. Stability of prostacyclin in human and rabbit whole blood and plasma. Thromb. Res. 1986, 43, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Daikoku, T.; Wang, D.; Tranguch, S.; Morrow, J.D.; Orsulic, S.; DuBois, R.N.; Dey, S.K. Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer. Cancer Res. 2005, 65, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Beeghly-Fadiel, A.; Wilson, A.J.; Keene, S.; El Ramahi, M.; Xu, S.; Marnett, L.J.; Fadare, O.; Crispens, M.A.; Khabele, D. Differential cyclooxygenase expression levels and survival associations in type I and type II ovarian tumors. J. Ovarian Res. 2018, 11, 17. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med. Res. Rev. 2019, 39, 114–145. [Google Scholar] [CrossRef]
- Zhang, D.; Bai, B.; Xi, Y.; Wang, T.; Zhao, Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016, 142, 368–377. [Google Scholar] [CrossRef]
- Ammundsen, H.B.; Faber, M.T.; Jensen, A.; Høgdall, E.; Blaakaer, J.; Høgdall, C.; Kjaer, S.K. Use of analgesic drugs and risk of ovarian cancer: Results from a Danish case-control study. Acta Obstet. Gynecol. Scand. 2012, 91, 1094–1102. [Google Scholar] [CrossRef]
- Trabert, B.; Ness, R.B.; Lo-Ciganic, W.-H.; Murphy, M.A.; Goode, E.L.; Poole, E.M.; Brinton, L.A.; Webb, P.M.; Nagle, C.M.; Jordan, S.J.; et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: A pooled analysis in the Ovarian Cancer Association Consortium. J. Natl. Cancer Inst. 2014, 106, djt431. [Google Scholar] [CrossRef]
- Barnard, M.E.; Poole, E.M.; Curhan, G.C.; Eliassen, A.H.; Rosner, B.A.; Terry, K.L.; Tworoger, S.S. Association of Analgesic Use with Risk of Ovarian Cancer in the Nurses’ Health Studies. JAMA Oncol. 2018, 4, 1675–1682. [Google Scholar] [CrossRef]
- Merritt, M.A.; Rice, M.S.; Barnard, M.E.; Hankinson, S.E.; Matulonis, U.A.; Poole, E.M.; Tworoger, S.S. Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): A cohort study. Lancet Oncol. 2018, 19, 1107–1116. [Google Scholar] [CrossRef]
- Wield, A.M.; Walsh, C.S.; Rimel, B.J.; Cass, I.; Karlan, B.Y.; Li, A.J. Aspirin use correlates with survival in women with clear cell ovarian cancer. Gynecol. Oncol. Rep. 2018, 25, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Gubbala, V.B.; Jytosana, N.; Trinh, V.Q.; Maurer, H.C.; Naeem, R.F.; Lytle, N.K.; Ma, Z.; Zhao, S.; Lin, W.; Han, H.; et al. Eicosanoids in the pancreatic tumor microenvironment—A multicellular, multifaceted progression. Gastro Hep Adv. 2022, 1, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.; Shiwen, X. Role of prostaglandins in fibroblast activation and fibrosis. J. Cell Commun. Signal. 2010, 4, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, e99911. [Google Scholar] [CrossRef] [PubMed]
- Rynne-Vidal, A.; Au-Yeung, C.L.; Jiménez-Heffernan, J.A.; Pérez-Lozano, M.L.; Cremades-Jimeno, L.; Bárcena, C.; Cristóbal-García, I.; Fernández-Chacón, C.; Yeung, T.L.; Mok, S.C.; et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J. Pathol. 2017, 242, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.-C.; Reynolds, J.V.; O’Byrne, K.J.; Pidgeon, G.P. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim. Biophys. Acta 2010, 1805, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Kamio, K.; Liu, X.; Sugiura, H.; Togo, S.; Kobayashi, T.; Kawasaki, S.; Wang, X.; Mao, L.; Ahn, Y.; Hogaboam, C.; et al. Prostacyclin analogs inhibit fibroblast contraction of collagen gels through the cAMP-PKA pathway. Am. J. Respir. Cell Mol. Biol. 2007, 37, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef]
- Clark, K.; MacKenzie, K.F.; Petkevicius, K.; Kristariyanto, Y.; Zhang, J.; Choi, H.G.; Peggie, M.; Plater, L.; Pedrioli, P.G.A.; McIver, E.; et al. Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc. Natl. Acad. Sci. USA 2012, 109, 16986–16991. [Google Scholar] [CrossRef]
- Avni, D.; Ernst, O.; Philosoph, A.; Zor, T. Role of CREB in modulation of TNFalpha and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Mol. Immunol. 2010, 47, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Luan, B.; Yoon, Y.-S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Liou, J.-Y.; Wu, K.K. Prostacyclin protects vascular integrity via PPAR/14-3-3 pathway. Prostaglandins Other Lipid Mediat. 2015, 118-119, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hertz, R.; Berman, I.; Keppler, D.; Bar-Tana, J. Activation of gene transcription by prostacyclin analogues is mediated by the peroxisome-proliferators-activated receptor (PPAR). Eur. J. Biochem. 1996, 235, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X.; Li, X.; Wu, X.; Tang, M.; Wang, X. Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol. Rep. 2018, 39, 818–826. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Xie, H.; Yuan, J.; Jiang, X.-Y.; Yong, J.-H.; Zeng, D.; Dou, Y.-Y.; Xiao, S.-S. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol. Ther. 2019, 20, 956–966. [Google Scholar] [CrossRef]
- Na, Y.-R.; Yoon, Y.-N.; Son, D.-I.; Seok, S.-H. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerfeld, L.; Knuth, I.; Finkernagel, F.; Pesek, J.; Nockher, W.A.; Jansen, J.M.; Wagner, U.; Nist, A.; Stiewe, T.; Müller-Brüsselbach, S.; et al. Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment. Cancers 2022, 14, 6154. https://doi.org/10.3390/cancers14246154
Sommerfeld L, Knuth I, Finkernagel F, Pesek J, Nockher WA, Jansen JM, Wagner U, Nist A, Stiewe T, Müller-Brüsselbach S, et al. Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment. Cancers. 2022; 14(24):6154. https://doi.org/10.3390/cancers14246154
Chicago/Turabian StyleSommerfeld, Leah, Isabel Knuth, Florian Finkernagel, Jelena Pesek, Wolfgang A. Nockher, Julia M. Jansen, Uwe Wagner, Andrea Nist, Thorsten Stiewe, Sabine Müller-Brüsselbach, and et al. 2022. "Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment" Cancers 14, no. 24: 6154. https://doi.org/10.3390/cancers14246154
APA StyleSommerfeld, L., Knuth, I., Finkernagel, F., Pesek, J., Nockher, W. A., Jansen, J. M., Wagner, U., Nist, A., Stiewe, T., Müller-Brüsselbach, S., Müller, R., & Reinartz, S. (2022). Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment. Cancers, 14(24), 6154. https://doi.org/10.3390/cancers14246154





