Genetically Modified Circulating Levels of Advanced Glycation End-Products and Their Soluble Receptor (AGEs-RAGE Axis) with Risk and Mortality of Breast Cancer
Simple Summary
Abstract
1. Background
2. Methods
2.1. Study Population and Data Collection
2.2. Measurement of Plasma AGEs and sRAGE Levels
2.3. Selection of SNPs and DNA Genotyping
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Association of Plasma Levels of AGEs-RAGE Axis with Risks of Breast Cancer
3.3. Subgroup Analysis
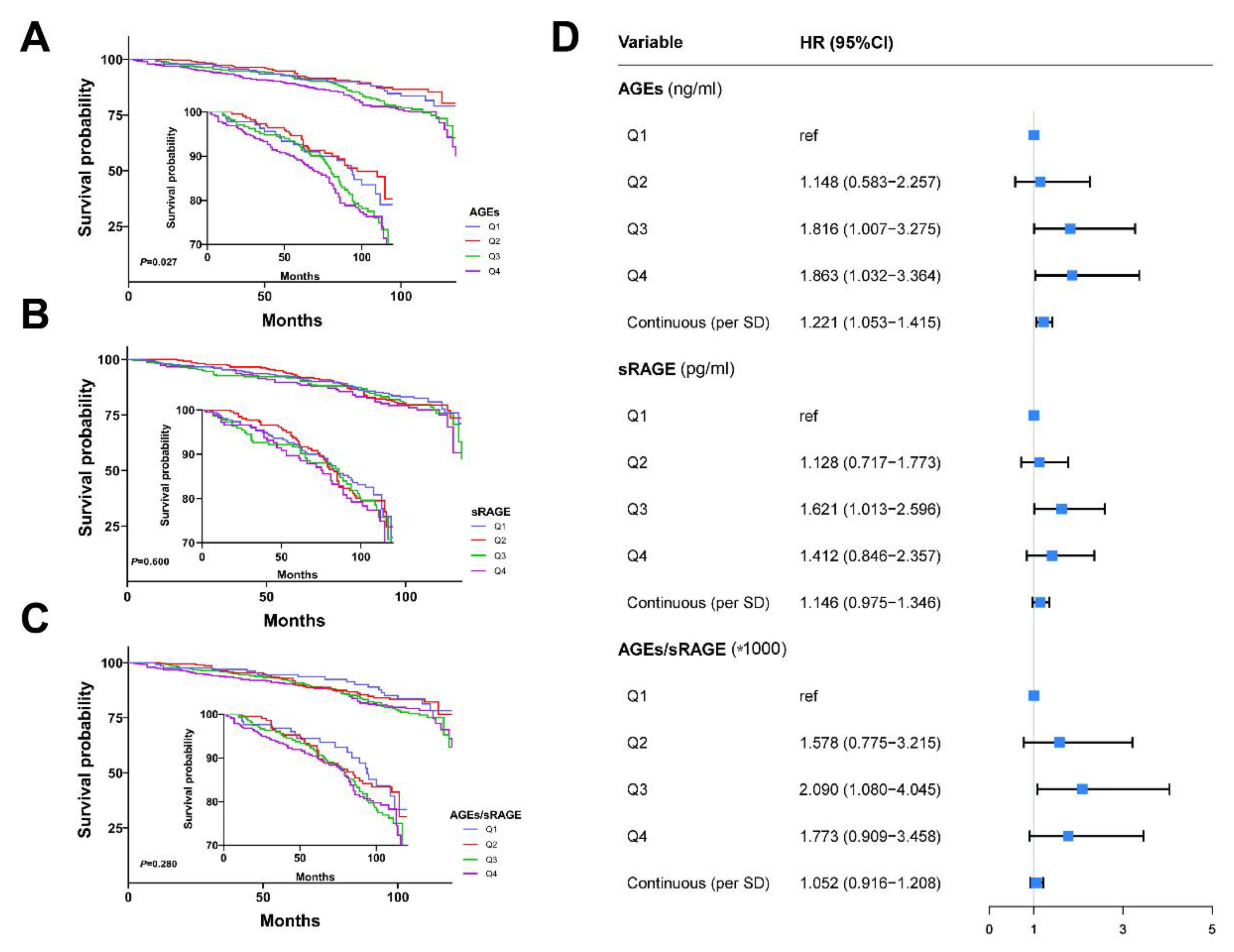
3.4. Association of AGEs and sRAGE Levels with Clinicopathological Features and Prognosis of Breast Cancer Patients
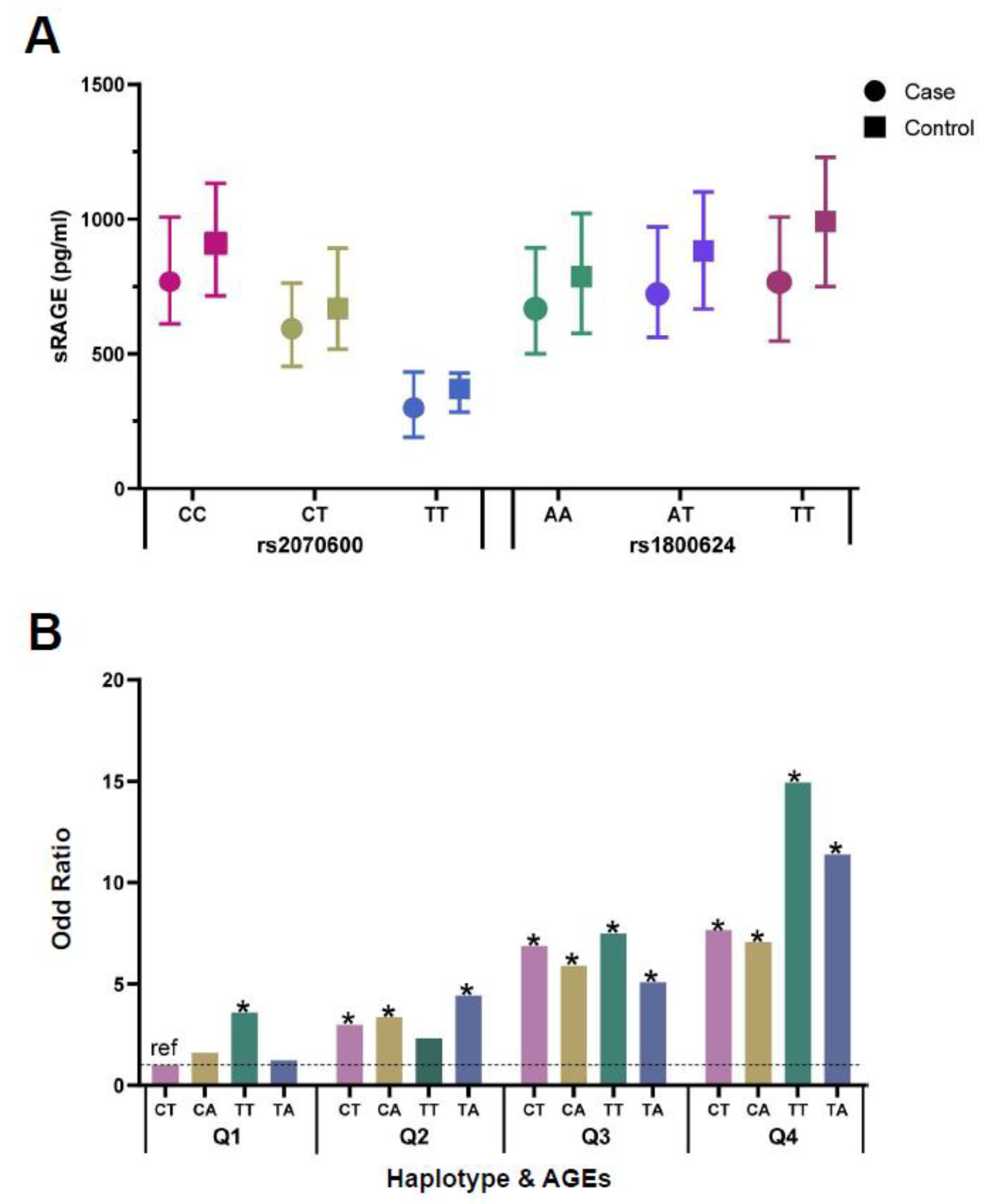
3.5. Analyses of Genetic Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end-products |
| AUC | area under the curve |
| BMI | body mass index |
| CEL | carboxyethyl lysine |
| CI | confidence interval |
| CML | N (6)-carboxymethyl-lysine |
| CV | coefficients of variation |
| DNA | deoxyribonucleic acid |
| E2 | estradiol |
| ELISA | enzyme-linked immunosorbent assay |
| FFQ | food frequency questionnaire |
| FSH | follicle-stimulating hormone |
| HER2 | human epidermal growth factor receptor 2 |
| HR | hazard ratio |
| IDI | integrated discrimination improvement |
| IQR | interquartile range |
| LH | luteinizing hormone |
| MAF | minimum allele frequency |
| NRI | net reclassification improvements |
| OR | odds ratio |
| PCR | polymerase chain reaction |
| RAGE | receptor for advanced glycation end-products |
| RCS | restricted cubic spline |
| ROC | receiver operating curve |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SNPs | Single-nucleotide polymorphisms |
| sRAGE | soluble receptor for advanced glycation end-products |
| TNM | tumor node metastasis |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 2014, 446. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Bohlke, K. Priorities for the primary prevention of breast cancer. CA Cancer J. Clin. 2014, 64, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.R.; Ford, M.E.; Gregoski, M.J.; Kramer, R.M.; Knight, K.D.; Spruill, L.; Nogueira, L.M.; Krisanits, B.A.; Phan, V.; La Rue, A.C.; et al. Advanced glycation end products are elevated in estrogen receptor-positive breast cancer patients, alter response to therapy, and can be targeted by lifestyle intervention. Breast Cancer Res. Treat. 2019, 173, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.; Spruill, L.; Walter, K.R.; Nogueira, L.M.; Fedarovich, H.; Turner, R.Y.; Ahmed, M.; Salley, J.D.; Ford, M.E.; Findlay, V.J.; et al. AGE metabolites: A biomarker linked to cancer disparity? Cancer Epidemiol. Biomark. Prev. 2014, 23, 2186–2191. [Google Scholar] [CrossRef]
- Turner, D.P. Advanced glycation end-products: A biological consequence of lifestyle contributing to cancer disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef]
- Chen, J.H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Omofuma, O.O.; Turner, D.P.; Peterson, L.L.; Merchant, A.T.; Zhang, J.; Steck, S.E. Dietary Advanced Glycation End-products (AGE) and Risk of Breast Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO). Cancer Prev. Res. 2020, 13, 601–610. [Google Scholar] [CrossRef]
- Peterson, L.L.; Park, S.; Park, Y.; Colditz, G.A.; Anbardar, N.; Turner, D.P. Dietary advanced glycation end products and the risk of postmenopausal breast cancer in the National Institutes of Health-AARP Diet and Health Study. Cancer 2020, 126, 2648–2657. [Google Scholar] [CrossRef]
- Omofuma, O.O.; Peterson, L.L.; Turner, D.P.; Merchant, A.T.; Zhang, J.; Thomson, C.A.; Neuhouser, M.L.; Snetselaar, L.G.; Caan, B.J.; Shadyab, A.H.; et al. Dietary Advanced Glycation End-Products and Mortality after Breast Cancer in the Women’s Health Initiative. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, H.; Siddiqui, Z.; Khan, M.Y.; Rehman, S.; Shahab, U.; Godovikova, T.; Silnikov, V.; Moinuddin. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin. Cancer Biol. 2018, 49, 44–55. [Google Scholar] [CrossRef]
- Prasad, K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: Myth or reality. Int. J. Angiol. 2014, 23, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021, 42, 101958. [Google Scholar] [CrossRef] [PubMed]
- Gryszczynska, B.; Budzyn, M.; Begier-Krasinska, B.; Osinska, A.; Boruczkowski, M.; Kaczmarek, M.; Bukowska, A.; Iskra, M.; Kasprzak, M.P. Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension. Int. J. Mol. Sci. 2019, 20, 3942. [Google Scholar] [CrossRef] [PubMed]
- Muthyalaiah, Y.S.; Jonnalagadda, B.; John, C.M.; Arockiasamy, S. Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression. Glycoconj. J. 2022, 38, 717–734. [Google Scholar] [CrossRef]
- Serveaux-Dancer, M.; Jabaudon, M.; Creveaux, I.; Belville, C.; Blondonnet, R.; Gross, C.; Constantin, J.M.; Blanchon, L.; Sapin, V. Pathological Implications of Receptor for Advanced Glycation End-Product (AGER) Gene Polymorphism. Dis. Markers 2019, 2019, 2067353. [Google Scholar] [CrossRef]
- Huang, Q.; Mi, J.; Wang, X.; Liu, F.; Wang, D.; Yan, D.; Wang, B.; Zhang, S.; Tian, G. Genetically lowered concentrations of circulating sRAGE might cause an increased risk of cancer: Meta-analysis using Mendelian randomization. J. Int. Med. Res. 2016, 44, 179–191. [Google Scholar] [CrossRef]
- Turner, D.P. The Role of Advanced Glycation End-Products in Cancer Disparity. Adv. Cancer Res. 2017, 133, 1–22. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e912. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2015, 101, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Nass, N.; Ignatov, A.; Andreas, L.; Weissenborn, C.; Kalinski, T.; Sel, S. Accumulation of the advanced glycation end product carboxymethyl lysine in breast cancer is positively associated with estrogen receptor expression and unfavorable prognosis in estrogen receptor-negative cases. Histochem. Cell Biol. 2017, 147, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Waghela, B.N.; Vaidya, F.U.; Ranjan, K.; Chhipa, A.S.; Tiwari, B.S.; Pathak, C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol. Cell. Biochem. 2021, 476, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.A.; Chatzigeorgiou, A.; Papageorgiou, E.; Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G.; Koutsilieris, M.; Diamanti-Kandarakis, E. Advanced glycation end products interfere in luteinizing hormone and follicle stimulating hormone signaling in human granulosa KGN cells. Exp. Biol. Med. 2018, 243, 29–33. [Google Scholar] [CrossRef]
- Sessa, L.; Gatti, E.; Zeni, F.; Antonelli, A.; Catucci, A.; Koch, M.; Pompilio, G.; Fritz, G.; Raucci, A.; Bianchi, M.E. The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs). PLoS ONE 2014, 9, e86903. [Google Scholar] [CrossRef]
- Moy, K.A.; Jiao, L.; Freedman, N.D.; Weinstein, S.J.; Sinha, R.; Virtamo, J.; Albanes, D.; Stolzenberg-Solomon, R.Z. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology 2013, 57, 2338–2345. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Z.; Tinker, L.; Sangi-Haghpeykar, H.; Strickler, H.; Ho, G.Y.; Gunter, M.J.; Rohan, T.; Logsdon, C.; White, D.L.; et al. A prospective study of soluble receptor for advanced glycation end-products and colorectal cancer risk in postmenopausal women. Cancer Epidemiol. 2016, 42, 115–123. [Google Scholar] [CrossRef]
- Jiao, L.; Weinstein, S.J.; Albanes, D.; Taylor, P.R.; Graubard, B.I.; Virtamo, J.; Stolzenberg-Solomon, R.Z. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: A prospective study. Cancer Res. 2011, 71, 3582–3589. [Google Scholar] [CrossRef]
- Tesarova, P.; Kalousova, M.; Jachymova, M.; Mestek, O.; Petruzelka, L.; Zima, T. Receptor for advanced glycation end products (RAGE)-soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Investig. 2007, 25, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Zahabi, E.; Sharafabad, F.H.; Abdollahzad, H.; Malekahmadi, M.; Rad, N.B. Circulating Advanced Glycation End Products and Their Soluble Receptors in Relation to All-Cause and Cardiovascular Mortality: A Systematic Review and Meta-analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 2157–2171. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Nakashima, A.; Fujimura, N.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 2015, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.; Tahara, A.; Ishibashi, M.; Hayabuchi, N.; Takeuchi, M.; Imaizumi, T. Adiponectin is inversely associated with ratio of serum levels of AGEs to sRAGE and vascular inflammation. Int. J. Cardiol. 2012, 158, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; He, L.; Wang, B.; Niu, W. The relationship between RAGE gene four common polymorphisms and breast cancer risk in northeastern Han Chinese. Sci. Rep. 2014, 4, 4355. [Google Scholar] [CrossRef][Green Version]
- Feng, L.-J.; Liu, H.-L.; Tan, Q.; Jin, P. 374T/A polymorphism of the receptor for advanced glycation end products is associated with decreased risk of breast cancer in a Chinese population. Int. J. Clin. Exp. Med. 2015, 8, 10109–10113. [Google Scholar]
- Yue, L.; Zhang, Q.; He, L.; Zhang, M.; Dong, J.; Zhao, D.; Ma, H.; Pan, H.; Zheng, L. Genetic predisposition of six well-defined polymorphisms in HMGB1/RAGE pathway to breast cancer in a large Han Chinese population. J. Cell. Mol. Med. 2016, 20, 1966–1973. [Google Scholar] [CrossRef]
- Sin, S.; Lim, M.N.; Kim, J.; Bak, S.H.; Kim, W.J. Association between plasma sRAGE and emphysema according to the genotypes of AGER gene. BMC Pulm. Med. 2022, 22, 58. [Google Scholar] [CrossRef]
- Gaens, K.H.; Ferreira, I.; van der Kallen, C.J.; van Greevenbroek, M.M.; Blaak, E.E.; Feskens, E.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; ‘t Hart, L.M.; et al. Association of polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating RAGE levels. J. Clin. Endocrinol. Metab. 2009, 94, 5174–5180. [Google Scholar] [CrossRef]
- Qayyum, S.; Afzal, M.; Naveed, A.K. Association analysis of 374T/A (rs1800624) receptor for advanced glycation end-products (RAGE) gene polymorphism with diabetic retinopathy in Pakistani patients. Pak. J. Med. Sci. 2021, 37, 733–739. [Google Scholar] [CrossRef]
- Dabritz, J.; Friedrichs, F.; Weinhage, T.; Hampe, J.; Kucharzik, T.; Lugering, A.; Broeckel, U.; Schreiber, S.; Spieker, T.; Stoll, M.; et al. The functional -374T/A polymorphism of the receptor for advanced glycation end products may modulate Crohn’s disease. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G823–G832. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Control (N = 1021) | Case (N = 1018) | p |
|---|---|---|---|
| Age (Mean ± SD) | 53.6 ± 5.6 | 53.6 ± 6.1 | 0.925 |
| BMI | <0.0001 | ||
| ≤23.9 | 494 (49.1) | 397 (39.2) | |
| 24–27.9 | 393 (39.1) | 420 (41.5) | |
| ≥28 | 119 (11.8) | 195 (19.3) | |
| Education | <0.0001 | ||
| Under primary school | 40 (4.0) | 185 (19.0) | |
| Junior/senior high school | 734 (72.9) | 614 (63.0) | |
| Junior college or above | 233 (23.1) | 176 (18.0) | |
| Smoke | 0.044 | ||
| No | 873 (91.7) | 891 (89.0) | |
| Yes | 79 (8.3) | 110 (11.0) | |
| Drinking | 0.013 | ||
| No | 915 (95.81) | 975 (97.79) | |
| Yes | 40 (4.19) | 22 (2.21) | |
| Negative events | <0.0001 | ||
| No | 869 (89.6) | 656 (68.2) | |
| Yes | 101 (10.4) | 306 (31.8) | |
| Menarche | |||
| ≤13 | 170 (16.9) | 261 (26.2) | <0.0001 |
| 14 | 192 (19.0) | 182 (18.3) | |
| 15 | 261 (25.9) | 148 (14.9) | |
| ≥16 | 385 (38.2) | 404 (40.6) | |
| Breast feeding | 0.488 | ||
| No | 103 (10.3) | 90 (9.00) | |
| Yes | 896 (89.7) | 916 (91.0) | |
| Abortion | <0.0001 | ||
| No | 411 (41.8) | 261 (26.1) | |
| Yes | 573 (58.2) | 738 (73.9) | |
| Menopause | <0.0001 | ||
| No | 254 (26.0) | 348 (34.6) | |
| Yes | 724 (74.0) | 659 (65.4) | |
| Estrogen replacement therapy | <0.001 | ||
| No | 831 (96.6) | 895 (92.5) | |
| Yes | 29 (3.4) | 73 (7.5) | |
| Birth control pills | 0.156 | ||
| No | 810 (87.3) | 818 (85.0) | |
| Yes | 118 (12.7) | 144 (15.0) | |
| History of benign breast diseases | |||
| No | 699 (72.0) | 640 (64.8) | <0.001 |
| Yes | 272 (28.0) | 348 (35.2) | |
| History of breast cancer in first-degree relatives | |||
| No | 935 (97.1) | 892 (93.7) | <0.001 |
| Yes | 28 (2.9) | 60 (6.3) | |
| TNM | |||
| Early stage (0-IIA) | / | 579 (64.2) | |
| Late stage (IIB-IV) | / | 323 (35.8) | |
| Molecular Subtype | |||
| Luminal A | / | 575 (56.5) | |
| Luminal B | / | 179 (17.6) | |
| Her2+ | / | 180 (17.7) | |
| Basal-like | / | 84 (8.2) | |
| AGEs (ng/mL) | <0.0001 | ||
| Q1 (<=1.714) | 367 (36.0) | 143 (14.0) | |
| Q2 (1.715–4.404) | 267 (26.0) | 243 (23.9) | |
| Q3 (4.405–10.241) | 214 (21.0) | 295 (29.0) | |
| Q4 (≥10.242) | 173 (17.0) | 337 (33.1) | |
| Continuous | 2.6 (1.1, 7.4) | 6.5 (3.0, 12.7) | <0.0001 |
| sRAGE (pg/mL) | <0.0001 | ||
| Q1 (≤557.728) | 197 (19.3) | 313 (30.7) | |
| Q2 (557.729–742.104) | 231 (22.6) | 279 (27.4) | |
| Q3 (742.105–987.953) | 274 (26.8) | 234 (23.0) | |
| Q4 (≥987.954) | 319 (31.2) | 192 (18.9) | |
| Continuous | 826.1 (606.1, 1050.6) | 682.5 (522.3, 914.7) | <0.0001 |
| AGEs/sRAGE (* 1000) | <0.0001 | ||
| Q1 (≤2.100) | 376 (36.8) | 134 (13.2) | |
| Q2 (2.101–5.820) | 281 (27.5) | 229 (22.5) | |
| Q3 (5.821–15.129) | 211 (20.7) | 298 (29.3) | |
| Q4 (≥15.130) | 153 (15.0) | 357 (35.0) | |
| Continuous | 3.4 (1.3, 9.3) | 9.3 (4.0, 21.1) | <0.0001 |
| AGEs(ng/mL) | p | sRAGE(pg/mL) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| BMI | 0.002 | <0.001 | ||||||||
| ≤23.9 | 250 (49.4) | 237 (46.8) | 217 (43.4) | 187 (37.0) | 170 (33.5) | 210 (41.4) | 219 (43.5) | 292 (58.3) | ||
| 24–27.9 | 194 (38.3) | 194 (38.2) | 196 (39.2) | 229 (45.2) | 239 (47.1) | 208 (41.0) | 205 (40.8) | 161 (32.1) | ||
| ≥28 | 62 (12.3) | 75 (14.8) | 87 (17.4) | 90 (17.8) | 98 (19.3) | 89 (17.6) | 79 (15.7) | 48 (9.6) | ||
| Education | 0.001 | 0.022 | ||||||||
| under primary school | 40 (8.0) | 45 (9.0) | 73 (14.8) | 67 (13.8) | 73 (15.0) | 59 (11.9) | 51 (10.3) | 42 (8.4) | ||
| Junior/Senior High School | 340 (67.7) | 361 (72.1) | 315 (63.9) | 332 (68.3) | 319 (65.4) | 349 (70.2) | 337 (67.9) | 343 (68.5) | ||
| Junior college or above | 122 (24.3) | 95 (18.9) | 105 (21.3) | 87 (17.9) | 96 (19.6) | 89 (17.9) | 108 (21.8) | 116 (23.2) | ||
| Smoking | 0.010 | 0.027 | ||||||||
| No | 460 (93.3) | 449 (90.9) | 422 (87.0) | 433 (90.0) | 441 (89.8) | 455 (93.0) | 444 (91.0) | 424 (87.4) | ||
| Yes | 33 (6.7) | 45 (9.1) | 63 (13.0) | 48 (10.0) | 50 (10.2) | 34 (7.0) | 44 (9.0) | 61 (12.6) | ||
| Drinking | 0.559 | 0.490 | ||||||||
| No | 478 (96.0) | 475 (97.1) | 467 (96.7) | 470 (97.5) | 472 (96.7) | 477 (97.2) | 465 (95.9) | 476 (97.5) | ||
| Yes | 20 (4.0) | 14 (2.9) | 16 (3.3) | 12 (2.5) | 16 (3.3) | 14 (2.8) | 20 (4.1) | 12 (2.5) | ||
| Negative events | 0.050 | 0.091 | ||||||||
| No | 408 (82.9) | 382 (78.9) | 378 (77.9) | 357 (75.8) | 373 (77.2) | 369 (76.7) | 381 (79.0) | 402 (82.7) | ||
| Yes | 84 (17.1) | 102 (21.1) | 107 (22.1) | 114 (24.2) | 110 (22.8) | 112 (23.3) | 101 (21.0) | 84 (17.3) | ||
| Menopause | 0.017 | 0.018 | ||||||||
| No | 130 (21.6) | 150 (24.9) | 148 (24.6) | 174 (28.9) | 170 (28.2) | 165 (27.4) | 130 (21.6) | 137 (22.8) | ||
| Yes | 371 (26.8) | 350 (25.3) | 342 (24.7) | 320 (23.1) | 332 (24.0) | 332 (24.0) | 364 (26.3) | 355 (25.7) | ||
| BMI 1 | p for Interaction | Menopause 2 | p for Interaction | Age 3 | p for Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤23.9 | 24–27.9 | ≥28 | No | Yes | <60 | ≥60 | ||||
| AGEs (ng/mL) | 0.898 | 0.070 | 0.390 | |||||||
| Q1 | ref | ref | ref | |||||||
| Q2 | 2.827 (1.776–4.502) | 2.512 (1.505–4.193) | 1.337 (0.572–3.124) | 3.723 (1.899–7.299) | 2.159 (1.478–3.154) | 2.830 (1.992–4.021) | 1.339 (0.621–2.887) | |||
| Q3 | 4.371 (2.694–7.094) | 4.158 (2.469–7.001) | 2.857 (1.175–6.952) | 8.718 (4.325–17.575) | 3.002 (2.044–4.408) | 4.586 (3.192–6.590) | 2.179 (0.985–4.822) | |||
| Q4 | 5.744 (3.446–9.574) | 5.049 (3.018–8.446) | 5.738 (2.320–14.195) | 8.879 (4.566–17.267) | 4.898 (3.267–7.342) | 6.155 (4.234–8.947) | 3.203 (1.482–6.924) | |||
| sRAGE (pg/mL) | 0.533 | 0.947 | 0.019 | |||||||
| Q1 | ref | ref | ref | |||||||
| Q2 | 0.562 (0.338–0.936) | 0.931 (0.584–1.484) | 0.502 (0.212–1.189) | 0.705 (0.401–1.240) | 0.694 (0.469–1.028) | 0.631 (0.445–0.895) | 1.337 (0.638–2.803) | |||
| Q3 | 0.486 (0.295–0.802) | 0.732 (0.453–1.184) | 0.293 (0.127–0.676) | 0.516 (0.281–0.947) | 0.548 (0.374–0.804) | 0.445 (0.313–0.632) | 1.492 (0.699–3.182) | |||
| Q4 | 0.321 (0.198–0.523) | 0.554 (0.332–0.924) | 0.307 (0.120–0.786) | 0.456 (0.251–0.827) | 0.393 (0.265–0.582) | 0.406 (0.285–0.578) | 0.464 (0.210–1.023) | |||
| AGEs\ sRAGE (* 1000) | 0.400 | 0.524 | 0.141 | |||||||
| Q1 | ref | ref | ref | |||||||
| Q2 | 2.474 (1.561–3.921) | 2.410 (1.417–4.100) | 1.031 (0.437–2.432) | 2.864 (1.462–5.610) | 1.921 (1.311–2.815) | 2.578 (1.808–3.676) | 1.001 (0.460–2.179) | |||
| Q3 | 4.679 (2.855–7.667) | 3.087 (1.861–5.119) | 4.228 (1.728–10.344) | 5.783 (2.904–11.517) | 3.203 (2.179–4.708) | 4.125 (2.879–5.909) | 2.665 (1.184–5.997) | |||
| Q4 | 7.412 (4.442–12.369) | 7.276 (4.196–12.619) | 6.055 (2.456–14.926) | 11.302 (5.775–22.116) | 6.396 (4.210–9.718) | 8.538 (5.809–12.550) | 3.280 (1.484–7.249) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Liu, F.; Qiao, Y.; Wang, P.; Du, H.; Si, C.; Wang, X.; Chen, K.; Song, F. Genetically Modified Circulating Levels of Advanced Glycation End-Products and Their Soluble Receptor (AGEs-RAGE Axis) with Risk and Mortality of Breast Cancer. Cancers 2022, 14, 6124. https://doi.org/10.3390/cancers14246124
Peng Y, Liu F, Qiao Y, Wang P, Du H, Si C, Wang X, Chen K, Song F. Genetically Modified Circulating Levels of Advanced Glycation End-Products and Their Soluble Receptor (AGEs-RAGE Axis) with Risk and Mortality of Breast Cancer. Cancers. 2022; 14(24):6124. https://doi.org/10.3390/cancers14246124
Chicago/Turabian StylePeng, Yu, Fubin Liu, Yating Qiao, Peng Wang, Han Du, Changyu Si, Xixuan Wang, Kexin Chen, and Fangfang Song. 2022. "Genetically Modified Circulating Levels of Advanced Glycation End-Products and Their Soluble Receptor (AGEs-RAGE Axis) with Risk and Mortality of Breast Cancer" Cancers 14, no. 24: 6124. https://doi.org/10.3390/cancers14246124
APA StylePeng, Y., Liu, F., Qiao, Y., Wang, P., Du, H., Si, C., Wang, X., Chen, K., & Song, F. (2022). Genetically Modified Circulating Levels of Advanced Glycation End-Products and Their Soluble Receptor (AGEs-RAGE Axis) with Risk and Mortality of Breast Cancer. Cancers, 14(24), 6124. https://doi.org/10.3390/cancers14246124






