Cost-Effectiveness of Risk-Reducing Surgery for Breast and Ovarian Cancer Prevention: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
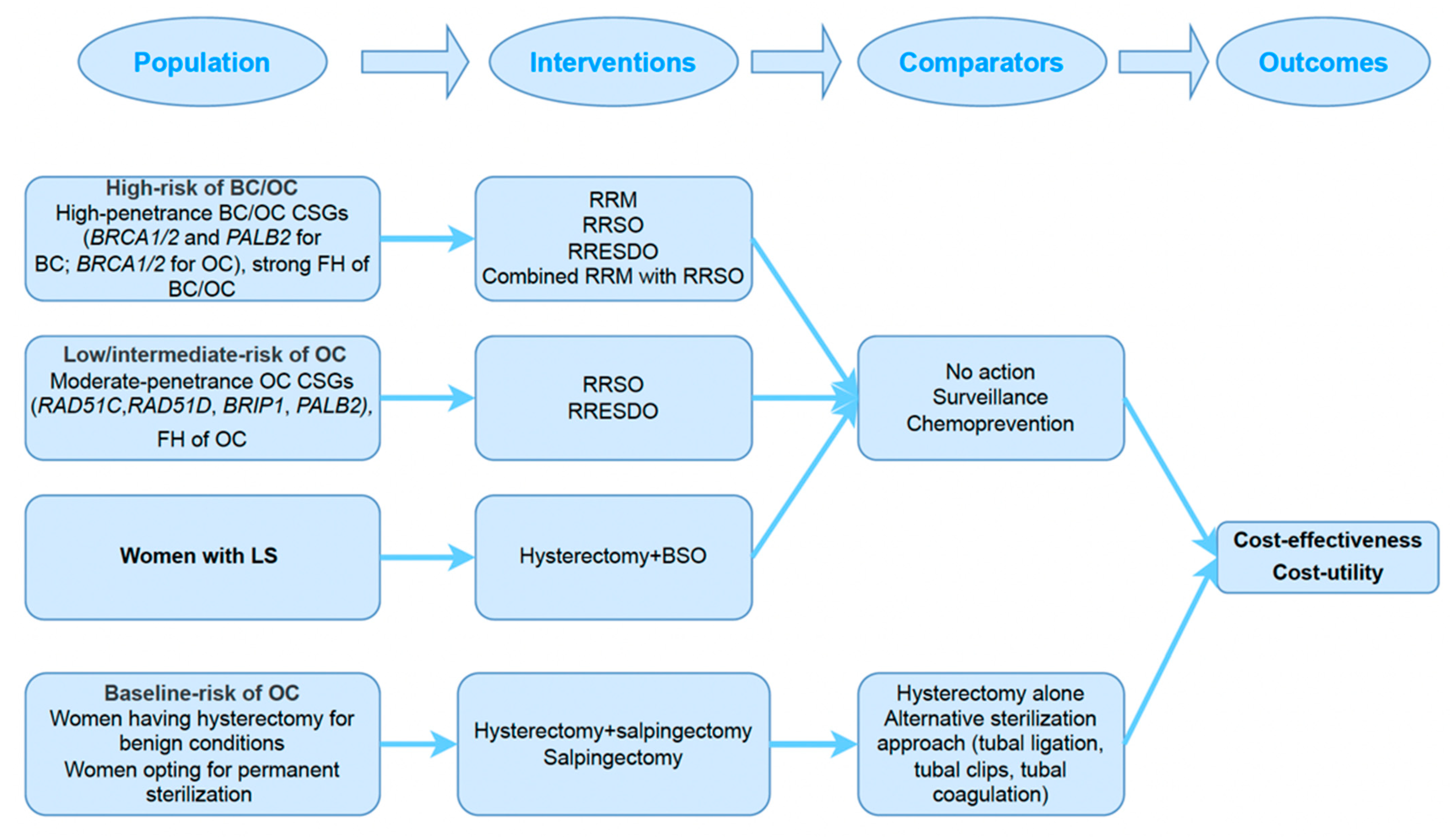
2.2. Study Selection (Inclusion Criteria)
2.3. Screening of the Literature
2.4. Exclusion Criteria
2.5. Data Extraction
2.6. Quality of Reporting
2.7. Evidence Synthesis
2.8. Accuracy of Data
3. Results
3.1. Study Characteristics
3.2. Reported Methodology of Included Studies
3.3. Main Findings: Cost-Effectiveness of RRS
3.3.1. Cost-Effectiveness of RRM/RRSO in Unaffected BRCA1/2 PV Carriers
3.3.2. Cost-Effectiveness of RRM in Affected BRCA1/2 PV Carriers with OC
3.3.3. Cost-Effectiveness of RRESDO in Unaffected BRCA1/2 PV Carriers
3.3.4. Cost-Effectiveness of Hysterectomy and BSO in Women with LS
3.3.5. Cost-Effectiveness of RRSO in Women at Low/Intermediate OC Risk
3.3.6. Cost-Effectiveness of OBS in Women at Baseline Population OC Risk
4. Discussion
Recommendations
- High-quality prospective data is required for utility scores for patients undergoing RRS for BC and OC prevention as well as for preventive hysterectomy.
- There is a need for large-scale prospective studies to generate high-quality evidence regarding the level of OC risk reduction and menopausal impact with respect to OBS and early-salpingectomy.
- Further prospective evidence is required on the surgical morbidity and OC risk reduction in terms of OBS at caesarean section.
- The lifetime BC risk threshold at which RRM is cost-effective needs to be established.
- The costs and health effects of novel effective therapies such as PARPi should be incorporated into future cost-effectiveness modelling of surgical prevention strategies.
- Economic evaluations of BC, OC and EC prevention should be undertaken in low- and middle-income countries and a broad range of health systems and contexts.
- Given the recent update to the CHEERS checklist, the reporting quality of economic evaluations should meet the newly revised expectations. This will enable researchers to provide more uniformly reported data to facilitate further evidence synthesis and provide robust estimates from which to draw inferences.
- There needs to be a move towards active and greater patient and public involvement in economic evaluation studies, including the dissemination of findings directly to patients as stakeholders and involving them actively in policy implementation.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Ovarian Cancer Mortality Statistics. 2022. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/mortality (accessed on 10 May 2022).
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Sfakianos, G.P.; Havrilesky, L.J. A review of cost-effectiveness studies in ovarian cancer. Cancer Control 2011, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Association Consortium. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, D.; Sobocan, M.; Blyuss, O.; Miller, R.E.; Evans, O.; Crusz, S.M.; Mills-Baldock, T.; Sun, L.; Hammond, R.F.L.; Gaba, F.; et al. Implementation of Multigene Germline and Parallel Somatic Genetic Testing in Epithelial Ovarian Cancer: Signpost Study. Cancers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Domchek, S.M.; Robson, M.E. Update on Genetic Testing in Gynecologic Cancer. J. Clin. Oncol. 2019, 37, 2501–2509. [Google Scholar] [CrossRef]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. 2019, 21, 2167–2180. [Google Scholar] [CrossRef]
- Li, X.; You, R.; Wang, X.; Liu, C.; Xu, Z.; Zhou, J.; Yu, B.; Xu, T.; Cai, H.; Zou, Q. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin. Cancer Res. 2016, 22, 3971–3981. [Google Scholar] [CrossRef]
- Marcinkute, R.; Woodward, E.R.; Gandhi, A.; Howell, S.; Crosbie, E.J.; Wissely, J.; Harvey, J.; Highton, L.; Murphy, J.; Holland, C.; et al. Uptake and efficacy of bilateral risk reducing surgery in unaffected female BRCA1 and BRCA2 carriers. J. Med. Genet. 2022, 59, 133. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Friebel, T.; Lynch, H.T.; Neuhausen, S.L.; van ‘t Veer, L.; Garber, J.E.; Evans, G.R.; Narod, S.A.; Isaacs, C.; Matloff, E.; et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J. Clin. Oncol. 2004, 22, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Graham, J.; O’Connell, S.; Arnold, S.; Fitzsimmons, D. Familial breast cancer: Summary of updated NICE guidance. BMJ 2013, 346, f3829. [Google Scholar] [CrossRef]
- NICE. Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer. NICE Clinical Guideline CG164. 2017. Available online: https://www.nice.org.uk/guidance/cg164 (accessed on 16 July 2022).
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, R.T.; Kauff, N.D.; Domchek, S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009, 101, 80–87. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Flaum, N.; Harkness, E.F.; Clayton, R.D.; Holland, C.; Martin-Hirsch, P.; Wood, N.; Keating, P.; Woodward, E.R.; Lalloo, F.; et al. Specialist oncological surgery for removal of the ovaries and fallopian tubes in BRCA1 and BRCA2 pathogenic variant carriers may reduce primary peritoneal cancer risk to very low levels. Int. J. Cancer 2021, 148, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Gaba, F.; Talaulikar, V.; Pundir, J.; Gessler, S.; Davies, M.; Menon, U. Risk-Reducing Salpingo-Oophorectomy and the Use of Hormone Replacement Therapy Below the Age of Natural Menopause: Scientific Impact Paper No. 66 October 2021: Scientific Impact Paper No. 66. BJOG 2022, 129, e16–e34. [Google Scholar]
- Manchanda, R.; Legood, R.; Antoniou, A.C.; Gordeev, V.S.; Menon, U. Specifying the ovarian cancer risk threshold of ‘premenopausal risk-reducing salpingo-oophorectomy’ for ovarian cancer prevention: A cost-effectiveness analysis. J. Med. Genet. 2016, 53, 591–599. [Google Scholar] [CrossRef]
- Manchanda, R.; Legood, R.; Pearce, L.; Menon, U. Defining the risk threshold for risk reducing salpingo-oophorectomy for ovarian cancer prevention in low risk postmenopausal women. Gynecol. Oncol. 2015, 139, 487–494. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Ryan, N.A.J.; Arends, M.J.; Bosse, T.; Burn, J.; Cornes, J.M.; Crawford, R.; Eccles, D.; Frayling, I.M.; Ghaem-Maghami, S.; et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 2019, 21, 2390–2400. [Google Scholar] [CrossRef]
- Harmsen, M.G.; Arts-de Jong, M.; Hoogerbrugge, N.; Maas, A.H.; Prins, J.B.; Bulten, J.; Teerenstra, S.; Adang, E.M.; Piek, J.M.; van Doorn, H.C.; et al. Early salpingectomy (TUbectomy) with delayed oophorectomy to improve quality of life as alternative for risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers (TUBA study): A prospective non-randomised multicentre study. BMC Cancer 2015, 15, 593. [Google Scholar] [CrossRef]
- Gaba, F.; Robbani, S.; Singh, N.; McCluggage, W.G.; Wilkinson, N.; Ganesan, R.; Bryson, G.; Rowlands, G.; Tyson, C.; Arora, R.; et al. Preventing Ovarian Cancer through early Excision of Tubes and late Ovarian Removal (PROTECTOR): Protocol for a prospective non-randomised multi-center trial. Int. J. Gynecol. Cancer 2021, 31, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Walker, J. A Study to Compare Two Surgical Procedures in Women with BRCA1 Mutations to Assess Reduced Risk of Ovarian Cancer. 2020; ClinicalTrials.gov Identifier: [NCT04251052]. Available online: https://clinicaltrials.gov/ct2/show/NCT04251052 (accessed on 18 June 2022).
- Falconer, H.; Yin, L.; Grönberg, H.; Altman, D. Ovarian cancer risk after salpingectomy: A nationwide population-based study. J. Natl. Cancer Inst. 2015, 107, dju410. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Yin, L.; Salehi, S.; Altman, D. Association between pelvic inflammatory disease and subsequent salpingectomy on the risk for ovarian cancer. Eur. J. Cancer 2021, 145, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.; Baandrup, L.; Dehlendorff, C.; Kjaer, S.K. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: A nationwide case-control study. Acta Obstet. Gynecol. Scand. 2015, 94, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Jacobson, J.S.; Heitjan, D.F.; Zivin, J.G.; Hershman, D.; Neugut, A.I.; Grann, V.R. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann. Intern. Med. 2006, 144, 397–406. [Google Scholar] [CrossRef]
- Grann, V.R.; Patel, P.R.; Jacobson, J.S.; Warner, E.; Heitjan, D.F.; Ashby-Thompson, M.; Hershman, D.L.; Neugut, A.I. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res. Treat. 2011, 125, 837–847. [Google Scholar] [CrossRef]
- Petelin, L.; Trainer, A.H.; Mitchell, G.; Liew, D.; James, P.A. Cost-effectiveness and comparative effectiveness of cancer risk management strategies in BRCA1/2 mutation carriers: A systematic review. Genet. Med. 2018, 20, 1145–1156. [Google Scholar] [CrossRef]
- Sroczynski, G.; Gogollari, A.; Kuehne, F.; Hallsson, L.R.; Widschwendter, M.; Pashayan, N.; Siebert, U. A Systematic Review on Cost-effectiveness Studies Evaluating Ovarian Cancer Early Detection and Prevention Strategies. Cancer Prev. Res. 2020, 13, 429–442. [Google Scholar] [CrossRef]
- Kwon, J.S.; Sun, C.C.; Peterson, S.K.; White, K.G.; Daniels, M.S.; Boyd-Rogers, S.G.; Lu, K.H. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008, 113, 326–335. [Google Scholar] [CrossRef]
- Yang, K.Y.; Yang, K.Y.; Caughey, A.B.; Little, S.E.; Cheung, M.K.; Chen, L.M. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Fam. Cancer 2011, 10, 535–543. [Google Scholar] [CrossRef]
- Dilley, S.E.; Havrilesky, L.J.; Bakkum-Gamez, J.; Cohn, D.E.; Michael Straughn, J., Jr.; Caughey, A.B.; Rodriguez, M.I. Cost-effectiveness of opportunistic salpingectomy for ovarian cancer prevention. Gynecol. Oncol. 2017, 146, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; McAlpine, J.N.; Hanley, G.E.; Finlayson, S.J.; Cohen, T.; Miller, D.M.; Gilks, C.B.; Huntsman, D.G. Costs and benefits of opportunistic salpingectomy as an ovarian cancer prevention strategy. Obstet. Gynecol. 2015, 125, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Tinker, A.; Pansegrau, G.; McAlpine, J.; Housty, M.; McCullum, M.; Gilks, C.B. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet. Gynecol. 2013, 121, 14–24. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BMJ 2022, 376, e067975. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development. Inflation (CPI). 2022. Available online: https://data.oecd.org/price/inflation-cpi.htm (accessed on 10 May 2022).
- Organisation for Economic Co-operation and Development. Purchasing Power Parities (PPP). 2022. Available online: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm (accessed on 10 May 2022).
- Grann, V.R.; Jacobson, J.S.; Whang, W.; Hershman, D.; Heitjan, D.F.; Antman, K.H.; Neugut, A.I. Prevention with tamoxifen or other hormones versus prophylactic surgery in BRCA1/2-positive women: A decision analysis. Cancer J. Sci. Am. 2000, 6, 13–20. [Google Scholar] [PubMed]
- Bommer, C.; Lupatsch, J.; Bürki, N.; Schwenkglenks, M. Cost-utility analysis of risk-reducing strategies to prevent breast and ovarian cancer in BRCA-mutation carriers in Switzerland. Eur. J. Health Econ. 2022, 23, 807–821. [Google Scholar] [CrossRef]
- Cadish, L.A.; Shepherd, J.P.; Barber, E.L.; Ridgeway, B. Risks and benefits of opportunistic salpingectomy during vaginal hysterectomy: A decision analysis. Am. J. Obstet. Gynecol. 2017, 217, 603.e1–603.e6. [Google Scholar] [CrossRef]
- Gamble, C.; Havrilesky, L.J.; Myers, E.R.; Chino, J.P.; Hollenbeck, S.; Plichta, J.K.; Kelly Marcom, P.; Shelley Hwang, E.; Kauff, N.D.; Greenup, R.A. Cost Effectiveness of Risk-Reducing Mastectomy versus Surveillance in BRCA Mutation Carriers with a History of Ovarian Cancer. Ann. Surg. Oncol. 2017, 24, 3116–3123. [Google Scholar] [CrossRef]
- Grann, V.R.; Panageas, K.S.; Whang, W.; Antman, K.H.; Neugut, A.I. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J. Clin. Oncol. 1998, 16, 979–985. [Google Scholar] [CrossRef]
- Muller, D.; Danner, M.; Rhiem, K.; Stollenwerk, B.; Engel, C.; Rasche, L.; Borsi, L.; Schmutzler, R.; Stock, S. Cost-effectiveness of different strategies to prevent breast and ovarian cancer in German women with a BRCA 1 or 2 mutation. Eur. J. Health Econ. 2018, 19, 341–353. [Google Scholar] [CrossRef]
- Naumann, R.W.; Hughes, B.N.; Brown, J.; Drury, L.K.; Herzog, T.J. The impact of opportunistic salpingectomy on ovarian cancer mortality and healthcare costs: A call for universal insurance coverage. Am. J. Obstet. Gynecol. 2021, 225, 397.e1–397.e6. [Google Scholar] [CrossRef] [PubMed]
- Norum, J.; Hagen, A.I.; Maehle, L.; Apold, J.; Burn, J.; Møller, P. Prophylactic bilateral salpingo-oophorectomy (PBSO) with or without prophylactic bilateral mastectomy (PBM) or no intervention in BRCA1 mutation carriers: A cost-effectiveness analysis. Eur. J. Cancer 2008, 44, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Petelin, L.; Petelin, L.; Hossack, L.; Shanahan, M.; Mitchell, G.; Liew, D.; James, P.A.; Trainer, A.H. Cost-effectiveness of long-term clinical management of BRCA pathogenic variant carriers. Genet. Med. 2020, 22, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Einerson, B.D.; Blanchard, C.T.; Erickson, B.K.; Szychowski, J.; Leath, C.A., 3rd; Biggio, J.R.; Huh, W.K. The cost-effectiveness of opportunistic salpingectomy versus standard tubal ligation at the time of cesarean delivery for ovarian cancer risk reduction. Gynecol. Oncol. 2019, 152, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Tai, R.M.; Choi, S.K.; Coyte, P.C. The Cost-Effectiveness of Salpingectomies for Family Planning in the Prevention of Ovarian Cancer. J. Obstet. Gynaecol. Can. 2018, 40, 317–327. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Clark, L.H.; Stamilio, D.M. Cost-effectiveness of opportunistic salpingectomy vs tubal ligation at the time of cesarean delivery. Am. J. Obstet. Gynecol. 2019, 220, 106.e1–106.e10. [Google Scholar] [CrossRef]
- Wright, J.D.; Silver, E.R.; Tan, S.X.; Hur, C.; Kastrinos, F. Cost-effectiveness Analysis of Genotype-Specific Surveillance and Preventive Strategies for Gynecologic Cancers among Women with Lynch Syndrome. JAMA Netw. Open 2021, 4, e2123616. [Google Scholar] [CrossRef]
- Yamauchi, H.; Nakagawa, C.; Kobayashi, M.; Kobayashi, Y.; Mano, T.; Nakamura, S.; Arai, M. Cost-effectiveness of surveillance and prevention strategies in BRCA1/2 mutation carriers. Breast Cancer 2018, 25, 141–150. [Google Scholar] [CrossRef]
- Grann, V.R.; Patel, P.; Bharthuar, A.; Jacobson, J.S.; Warner, E.; Anderson, K.; Warner, E.; Tsai, W.Y.; Hill, K.A.; Neugut, A.I.; et al. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res. Treat. 2010, 119, 177–184. [Google Scholar] [CrossRef]
- Gaba, F.; Blyuss, O.; Chandrasekaran, D.; Osman, M.; Goyal, S.; Gan, C.; Izatt, L.; Tripathi, V.; Esteban, I.; McNicol, L.; et al. Attitudes towards risk-reducing early salpingectomy with delayed oophorectomy for ovarian cancer prevention: A cohort study. BJOG 2021, 128, 714–726. [Google Scholar] [CrossRef]
- Gaba, F.; Manchanda, R. Systematic review of acceptability, cardiovascular, neurological, bone health and HRT outcomes following risk reducing surgery in BRCA carriers. Best Pract. Res. Clin. Obs. Gynaecol. 2020, 65, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Blanchard, C.T.; Erickson, B.K.; Szychowski, J.; Leath, C.A.; Biggio, J.R.; Huh, W.K. Feasibility of Complete Salpingectomy Compared with Standard Postpartum Tubal Ligation at Cesarean Delivery: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 132, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Boolbol, S.; Degnim, A.; Kuerer, H.; Leitch, A.M.; Morrow, M. Society of Surgical Oncology: Position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Ann. Surg. Oncol. 2007, 14, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.K.; Euhus, D.M.; Boughey, J.C.; Chagpar, A.B.; Feldman, S.M.; Hansen, N.M.; Kulkarni, S.A.; McCready, D.R.; Mamounas, E.P.; Wilke, L.G.; et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk-Reducing) Mastectomy. Ann. Surg. Oncol. 2017, 24, 375–397. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27 (Suppl. 5), v103–v110. [Google Scholar] [CrossRef]
- Cancer Australia. Recommendations for the Management of Women at High Risk of Ovarian Cancer. 2011. Available online: https://www.canceraustralia.gov.au/publications-and-resources/clinical-practice-guidelines/recommendations-management-women-high-risk-ovarian-cancer (accessed on 10 June 2022).
- Ntoumanoglou-Schuiki, A.; Tomasch, G.; Laky, R.; Taumberger, N.; Bjelic-Radisic, V.; Tamussino, K. Opportunistic prophylactic salpingectomy for prevention of ovarian cancer: What do national societies advise? Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 110–112. [Google Scholar] [CrossRef]
- Manchanda, R.; Chandrasekaran, D.; Saridogan, E.; Burnell, M.; Crawford, R.; Brockbank, E.; Kalsi, J.; Jurkovic, D.; Menon, U. Should Opportunistic Bilateral Salpingectomy (OBS) for Prevention of Ovarian Cancer Be Incorporated Into Routine Care or Offered in the Context of a Clinical Trial? Int. J. Gynecol. Cancer 2016, 26, 31–33. [Google Scholar] [CrossRef]
- Hanley, G.E.; Pearce, C.L.; Talhouk, A.; Kwon, J.S.; Finlayson, S.J.; McAlpine, J.N.; Huntsman, D.G.; Miller, D. Outcomes From Opportunistic Salpingectomy for Ovarian Cancer Prevention. JAMA Netw. Open 2022, 5, e2147343. [Google Scholar] [CrossRef]
- Lessard-Anderson, C.R.; Handlogten, K.S.; Molitor, R.J.; Dowdy, S.C.; Cliby, W.A.; Weaver, A.L.; Sauver, J.S.; Bakkum-Gamez, J.N. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecol. Oncol. 2014, 135, 423–427. [Google Scholar] [CrossRef]
- Darelius, A.; Lycke, M.; Kindblom, J.M.; Kristjansdottir, B.; Sundfeldt, K.; Strandell, A. Efficacy of salpingectomy at hysterectomy to reduce the risk of epithelial ovarian cancer: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 880–889. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, L.A.; Steenbeek, M.P.; De Hullu, J.A.; Vos, M.C.; Houterman, S.; Wilkinson, J.; Piek, J.M. Hysterectomy with opportunistic salpingectomy versus hysterectomy alone. Cochrane Database Syst. Rev. 2019, 8, CD012858. [Google Scholar] [CrossRef]
- Garcia, C.; Moskowitz, O.M.; Chisholm, C.A.; Duska, L.R.; Warren, A.L.; Lyons, G.R.; Pettit, K.E. Salpingectomy Compared with Tubal Ligation at Cesarean Delivery: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 132, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Danis, B.R.; Della Badia, C.R.; Richard, S.D. Postpartum Permanent Sterilization: Could Bilateral Salpingectomy Replace Bilateral Tubal Ligation? J. Minim. Invasive Gynecol. 2016, 23, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Grann, V.R.; Jacobson, J.S.; Sundararajan, V.; Albert, S.M.; Troxel, A.B.; Neugut, A.I. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J. Sci. Am. 1999, 5, 283–292. [Google Scholar]
- Lugnér, A.K.; Krabbe, P.F.M. An overview of the time trade-off method: Concept, foundation, and the evaluation of distorting factors in putting a value on health. Expert Rev. Pharm. Outcomes Res. 2020, 20, 331–342. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Astley, S.; Stavrinos, P.; Harkness, E.; Donnelly, L.S.; Dawe, S.; Jacob, I.; Harvie, M.; Cuzick, J.; Brentnall, A.; et al. Improvement in risk prediction, early detection and prevention of breast cancer in the NHS Breast Screening Programme and family history clinics: A dual cohort study. Program. Grants Appl. Res. 2016, 4, xxiii–xxvii. [Google Scholar] [CrossRef]
- Giannini, A.; Giannini, A.; Bogani, G.; Vizza, E.; Chiantera, V.; Laganà, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; D’Oria, O. Advances on Prevention and Screening of Gynecologic Tumors: Are We Stepping Forward? Healthcare 2022, 10, 1605. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Olaparib for Maintenance Treatment of BRCA-Mutated Ovarian, Fallopian Tube and Peritoneal Cancer after Response to First-Line Platinum-BASED Chemotherapy. Single Technology Appraisal [ID1124]. 2019. Available online: https://www.nice.org.uk/guidance/ta598/evidence (accessed on 2 December 2022).
- Guy, H.; Walder, L.; Fisher, M. Cost-Effectiveness of Niraparib Versus Routine Surveillance, Olaparib and Rucaparib for the Maintenance Treatment of Patients with Ovarian Cancer in the United States. Pharmacoeconomics 2019, 37, 391–405. [Google Scholar] [CrossRef] [PubMed]

| Study | Economic Evaluation Type | Perspective | Study Design | Time Horizon | Sources for Costs | Sources for Effectiveness | Outcome Measures | Discount | Incremental Analysis | Sensitivity Analysis | CHEERS Checklist Score (28.0) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson, 2006 [28] | CEA/CUA | Payer | Markov | Lifetime | Secondary/Literature | Primary data/Literature | LYG/QALY | Yes | Yes | One-way | 21.0 |
| Bommer, 2022 [41] | CEA/CUA | Payer | Markov | Lifetime | Secondary | Literature | LYG/QALY | Yes | Yes | One-way/PSA | 22.0 |
| Cadish, 2017 [42] | CEA | NR | Decision tree | NR | Secondary/Literature | Literature | Cancer/death prevented | No | No | One-way | 17.0 |
| Dilley, 2017 [34] | CUA | Payer | Decision tree | NR | Secondary/Literature | Literature | QALY | Yes | Yes | One-way/PSA | 20.0 |
| Gamble, 2017 [43] | CEA | Payer | Markov | Lifetime | Literature | Primary data/Literature | LYG | Yes | Yes | One-way/PSA | 21.0 |
| Grann, 1998 [44] | CEA/CUA | NR | Markov | 50 years | Secondary | Primary data/Literature | LYG/QALY | Yes | Yes | One-way | 16.0 |
| Grann, 2011 [29] | CEA/CUA | Societal | Markov | Lifetime | Secondary/Literature | Primary data/Literature | LYG/QALY | Yes | Yes | One-way/PSA | 19.0 |
| Kwon, 2015 [35] | CEA | Societal | Markov | 40 years | Secondary | Literature | LYG | Yes | Yes | One-way | 19.5 |
| Kwon, 2008 [32] | CUA | Societal | Markov | Lifetime | Secondary | Literature | QALY | Yes | Yes | One-way | 18.0 |
| Kwon, 2013 [36] | CEA/CUA | Societal | Markov | Lifetime | Secondary | Literature | LYG/QALY | Yes | Yes | One-way/two-way | 18.5 |
| Manchanda, 2016 [19] | CUA | Payer | Decision tree | Lifetime | Secondary/Literature | Literature | QALY | Yes | Yes | One-way/PSA | 22.0 |
| Manchanda, 2015 [20] | CUA | NR | Decision tree | Lifetime | Secondary | Literature | QALY | Yes | Yes | One-way/PSA | 21.5 |
| Muller, 2018 [45] | CEA/CUA | Payer | Markov | Lifetime | Primary data/Literature | Primary data/Literature | LYG/QALY | Yes | Yes | One-way/PSA | 20.5 |
| Naumann, 2021 [46] | CEA/CUA | Payer | Markov | Lifetime | Secondary/Literature | Literature | LYG/QALY | Yes | Yes | One-way | 15.0 |
| Norum, 2008 [47] | CEA | NR | Markov | Lifetime | Secondary/Literature | Literature | LYG | Yes | Yes | One-way | 17.5 |
| Petelin, 2020 [48] | CEA/CUA | Payer | Microsimulation | Lifetime | Secondary/Literature | Primary data/Literature | LYG/QALY | Yes | Yes | One-way/PSA | 22.5 |
| Subramaniam, 2019 [49] | CUA | Societal | Decision tree | Lifetime | Primary data/Literature | Primary data/Literature | QALY | Yes | Yes | One-way/two-way/PSA | 20.5 |
| Tai, 2018 [50] | CEA/CUA | Societal | Markov | Lifetime | Secondary/Literature | Literature | LYG/QALY | Yes | Yes | One-way/two-way/PSA | 20.5 |
| Venkatesh, 2019 [51] | CUA | Societal | Decision tree | Lifetime | Secondary/Literature | Literature | QALY | Yes | Yes | One-way/two-way/three-way/PSA | 22.0 |
| Wright, 2021 [52] | CEA/CUA | Payer | Markov | Lifetime | Secondary/Literature | Literature | LYG/QALY | Yes | Yes | One-way/PSA | 23.0 |
| Yamauchi, 2018 [53] | CEA/CUA | Societal | Markov | Lifetime | Primary data/Literature | Secondary/Literature | LYG/QALY | Yes | Yes | One-way | 21.5 |
| Yang, 2011 [33] | CUA | Societal | Decision tree | Lifetime | Secondary/Literature | Secondary/Literature | QALY | Yes | No | One-way/PSA | 19.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Oxley, S.; Sideris, M.; Kalra, A.; Sun, L.; Yang, L.; Legood, R.; Manchanda, R. Cost-Effectiveness of Risk-Reducing Surgery for Breast and Ovarian Cancer Prevention: A Systematic Review. Cancers 2022, 14, 6117. https://doi.org/10.3390/cancers14246117
Wei X, Oxley S, Sideris M, Kalra A, Sun L, Yang L, Legood R, Manchanda R. Cost-Effectiveness of Risk-Reducing Surgery for Breast and Ovarian Cancer Prevention: A Systematic Review. Cancers. 2022; 14(24):6117. https://doi.org/10.3390/cancers14246117
Chicago/Turabian StyleWei, Xia, Samuel Oxley, Michail Sideris, Ashwin Kalra, Li Sun, Li Yang, Rosa Legood, and Ranjit Manchanda. 2022. "Cost-Effectiveness of Risk-Reducing Surgery for Breast and Ovarian Cancer Prevention: A Systematic Review" Cancers 14, no. 24: 6117. https://doi.org/10.3390/cancers14246117
APA StyleWei, X., Oxley, S., Sideris, M., Kalra, A., Sun, L., Yang, L., Legood, R., & Manchanda, R. (2022). Cost-Effectiveness of Risk-Reducing Surgery for Breast and Ovarian Cancer Prevention: A Systematic Review. Cancers, 14(24), 6117. https://doi.org/10.3390/cancers14246117








