Association between Proton Pump Inhibitor Use and the Risk of Female Cancers: A Nested Case-Control Study of 23 Million Individuals
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
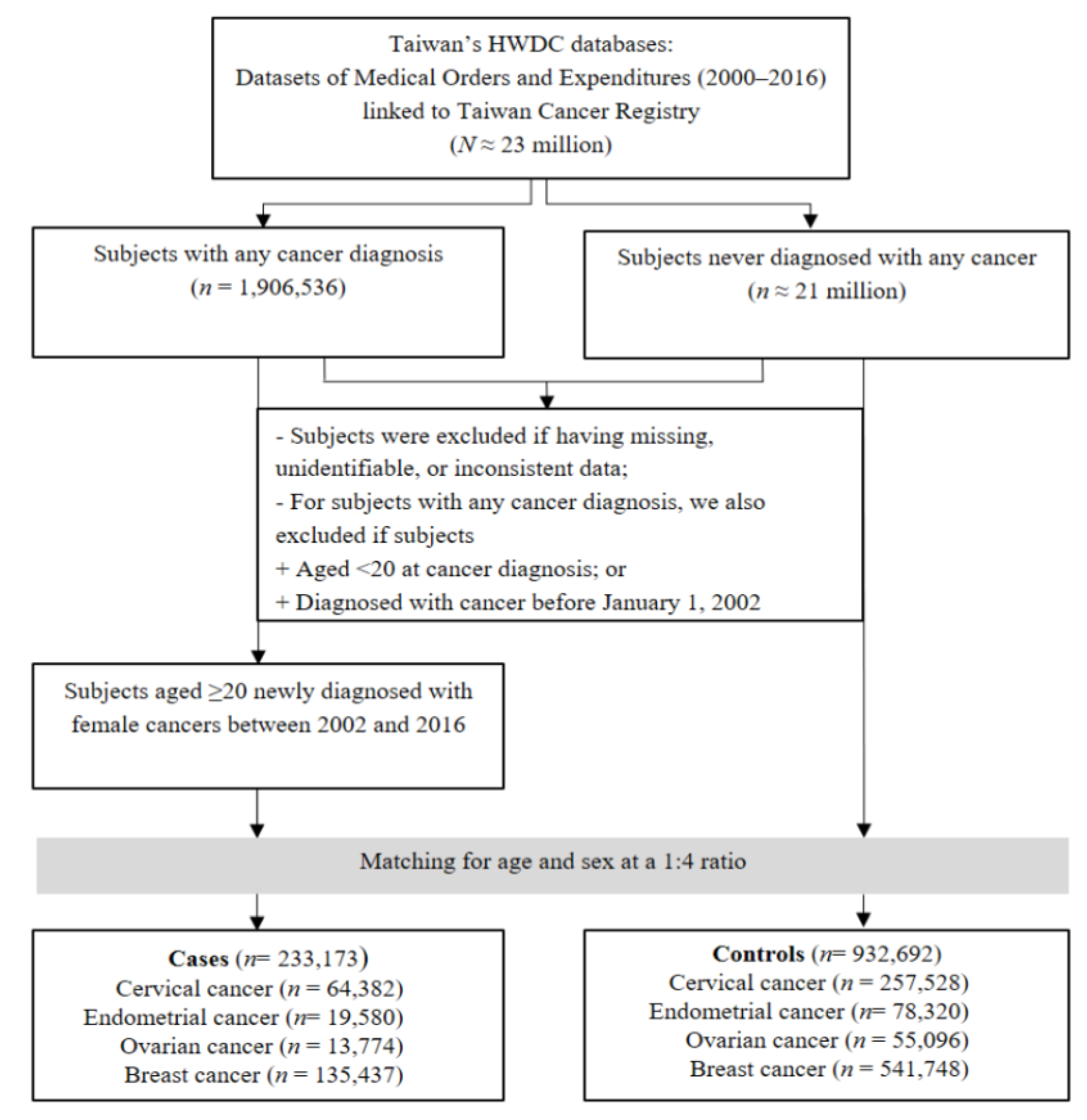
2.2. Study Population
2.3. PPI Exposure
2.4. Confounding Factors
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Associations of PPI Use with Overall Female Cancers
3.3. Associations of PPI Use with Breast, Cervical, Endometrial, and Ovarian Cancers
4. Discussion
4.1. Main Findings
4.2. Biological Plausibility
4.2.1. Breast Cancer
4.2.2. Cervical Cancer
4.2.3. Endometrial Cancer
4.2.4. Ovarian Cancer
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aOR | Adjusted odds ratio |
| ATC Classification | Anatomical Therapeutic Chemical classification |
| CCI | Charlson comorbidity index |
| CI | Confidence interval |
| FASN | Fatty acid synthase |
| HWDC | Health and welfare data science |
| ICD-9-CM | International Classification of Diseases, 9th revision, Clinical Modification |
| MOHW | Ministry of Health and Welfare |
| NHI | National health insurance |
| PPI | Proton pump inhibitor |
| TMU-JIRB | Taipei Medical University—Joint Institutional Review Board |
| TCR | Taiwan Cancer Registry |
| V-ATPase | Vacuolar H+-ATPase |
Appendix A
| ATC Code | Name | Available in Taiwan |
|---|---|---|
| A02BC01 | omeprazole | 1995~ |
| A02BC02 | pantoprazole | 1998~ |
| A02BC03 | lansoprazole | 2004~ |
| A02BC04 | rabeprazole | 2000~ |
| A02BC05 | esomeprazole | 2002~ |
| A02BC06 | dexlansoprazole | 2004~ |
| A02BC07 | dexrabeprazole | Not Available |
| A02BC08 | vonoprazan | Not Available |
| A02BC09 | tegoprazan | Not Available |
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 25 September 2022).
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Facts and Statistics. Available online: https://www.breastcancer.org/facts-statistics (accessed on 25 September 2022).
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A. Ovarian Cancer, Version 2 2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Endometrial Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/ (accessed on 25 September 2022).
- Thomson, A.B.; Sauve, M.D.; Kassam, N.; Kamitakahara, H. Safety of the long-term use of proton pump inhibitors. World J. Gastroenterol. 2010, 16, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Rotman, S.R.; Bishop, T.F. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS ONE 2013, 8, 56060. [Google Scholar] [CrossRef]
- Zink, D.A.; Pohlman, M.; Barnes, M.; Cannokn, M.E. Long-term use of acid suppression started inappropriately during hospitalization. Aliment. Pharmacol. Ther. 2005, 21, 1203–1209. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, C.Z.; Lin, Y.C.; Kao, L.T.; Lin, H.C. Negative Association of Proton Pump Inhibitors with Subsequent Development of Breast Cancer: A Nationwide Population-Based Study. J. Clin. Pharmacol. 2019, 59, 350–355. [Google Scholar] [CrossRef]
- Kamal, H.; Sadr-Azodi, O.; Engstrand, L.; Brusselaers, N. Association between Proton Pump Inhibitor Use and Biliary Tract Cancer Risk: A Swedish Population-Based Cohort Study. Hepatology 2021, 74, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Ihraiz, W.G.; Ahram, M.; Bardaweel, S.K. Proton pump inhibitors enhance chemosensitivity, promote apoptosis, and suppress migration of breast cancer cells. Acta Pharm. 2020, 70, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Djuric, Z.; Sardesai, S.; Hovey, K.; Andrews, C.; Braskey, T.M. Proton Pump Inhibitor Use and Obesity-Associated Cancers in the Women’s Health Initiative. Cancer Epidemiol Biomark. Prev. 2022, 31, 1511. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jeon, H.K.; Hong, J.E.; Cho, Y.J.; Ryu, J.Y.; Choi, J.J. Proton pump inhibitors enhance the effects of cytotoxic agents in chemoresistant epithelial ovarian carcinoma. Oncotarget 2015, 6, 35040–35050. [Google Scholar] [CrossRef]
- He, J.; Shi, X.Y.; Li, Z.M.; Pan, X.H.; Li, Z.L.; Che, Y. Proton pump inhibitors can reverse the YAP mediated paclitaxel resistance in epithelial ovarian cancer. BMC Mol. Cell Biol. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Jeon, H.K.; Hong, J.E.; Choi, J.J.; Kim, T.J.; Choi, C.H. Proton Pump Inhibition Enhances the Cytotoxicity of Paclitaxel in Cervical Cancer. Cancer Res. Treat. 2017, 49, 595–606. [Google Scholar] [CrossRef]
- Cancer Registry Annual Report, 2019, Taiwan. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269 (accessed on 25 September 2022).
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Universal Health Coverage in Taiwan. National Health Insurance Administration, MOHAW, Taiwan. Available online: https://www.nhi.gov.tw/English/Content_List.aspx?n=4D7051840BF42F52&topn=ED4A30E51A609E49 (accessed on 25 September 2022).
- ICD-9-CM and ICD-10-CM/PCS Mapping Table; National Health Insurance Administration, MOHAW: Taipei, Taiwan, 2020.
- Grimes, D.A.; Schulz, K.F. Compared to what? Finding controls for case-control studies. Lancet 2005, 365, 1429–1433. [Google Scholar] [CrossRef]
- Rosenbaum, P.A.; Rubin, B.D. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biom. J. 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Gadducci, A.; Biglia, N.; Tana, R.; Cosio, S.; Gallo, M. Metformin use and gynecological cancers: A novel treatment option emerging from drug repositioning. Crit. Rev. Oncol. Hematol. 2016, 105, 73–83. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, T.S. Associations between metabolic syndrome and gynecologic cancer. Obstet. Gynecol. Sci. 2020, 63, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, P.H.; Chen, P.N.; Yang, S.F.; Hsiao, Y.H. Molecular and Cellular Mechanisms of Metformin in Cervical Cancer. Cancers 2021, 13, 2545. [Google Scholar]
- Wang, Y.; Zhao, J.; Chen, X.; Zhang, F.; Li, X. Aspirin use and endometrial cancer risk: A meta-analysis and systematic review. Ann. Transl. Med. 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bai, B.; Xi, Y.; Wang, T.; Zhao, Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016, 142, 368–377. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals 2020, 13, 422. [Google Scholar] [CrossRef]
- Yuan-Yu, L.; Sun, L.N.; Zhang, X.H.; Li, Y.Q.; Yu, L.; Yuan, Z.Q.Y. A Review of the Novel Application and Potential Adverse Effects of Proton Pump Inhibitors. Adv. Ther. 2017, 34, 1070–1086. [Google Scholar]
- Wang, B.Y.; Zhang, J.; Wang, J.L.; Sun, S.; Wang, Z.H.; Wang, L.P. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J. Exp. Clin. Cancer Res. 2015, 34, 85. [Google Scholar] [CrossRef]
- Marino, M.L.; Fais, S.; Djavaheri-Mergny, M.; Villa, A.; Meschini, S.; Lozupone, F. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010, 1, 87. [Google Scholar] [CrossRef]
- Goh, W.; Sleptsova-Freidrich, I.; Petrovic, N. Use of proton pump inhibitors as adjunct treatment for triple-negative breast cancers. An introductory study. J. Pharm. Pharm. Sci. 2014, 17, 439–446. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, M.; Huang, S.; Zou, X. Pantoprazole inhibits human gastric adenocarcinoma SGC-7901 cells by downregulating the expression of pyruvate kinase M2. Oncol. Lett. 2016, 11, 717–722. [Google Scholar] [CrossRef]
- De Milito, A.; Lessi, E.; Logozzi, M.; Lozupone, F.; Spada, M.; Marino, M.L. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007, 67, 5408–5417. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; De Milito, A.; You, H.; Qin, W. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 2007, 67, 10627–10630. [Google Scholar] [CrossRef] [PubMed]
- Mizunashi, K.; Furukawa, Y.; Katano, K.; Abe, K. Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcif. Tissue Int. 1993, 53, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Forgac, M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002, 3, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Larsson, H.; Mattson, H.; Sundell, G.; Carlsson, E. Animal pharmacodynamics of omeprazole. A survey of its pharmacological properties in vivo. Scand. J. Gastroenterol. Suppl. 1985, 108, 23–35. [Google Scholar] [PubMed]
- De Milito, A.; Canese, R.; Marino, M.L.; Borghi, M.; Lero, M.; Villa, A. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 2010, 127, 207–219. [Google Scholar] [CrossRef]
- Ferrari, S.; Perut, F.; Fagioli, F.; Brach Del Prever, A.; Meazza, C.; Parafioriti, A. Proton pump inhibitor chemosensitization in human osteosarcoma: From the bench to the patients’ bed. J. Transl. Med. 2013, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Buglioni, S.; Carocci, F.; Francesco, M.; Vincenzi, B.; Fanciulli, M. High dose lansoprazole combined with metronomic chemotherapy: A phase I/II study in companion animals with spontaneously occurring tumors. J. Transl. Med. 2014, 12, 225. [Google Scholar] [CrossRef]
- Hálfdánarson, Ó.; Fall, K.; Ogmundsdottir, M.H.; Lund, S.H.; Steingrímsson, E.; Ogmundsdottir, H.M. Proton pump inhibitor use and risk of breast cancer, prostate cancer, and malignant melanoma: An Icelandic population-based case-control study. Pharmacoepidemiol. Drug Saf. 2019, 28, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lee, C.; Wang, M.; Tannock, I.F. Influence of the proton pump inhibitor lansoprazole on distribution and activity of doxorubicin in solid tumors. Cancer Sci. 2015, 106, 1438–1447. [Google Scholar] [CrossRef]
- Sabolić, I.; Brown, D.; Verbavatz, J.M.; Kleinman, J. H(+)-ATPases of renal cortical and medullary endosomes are differentially sensitive to Sch-28080 and omeprazole. Am. J. Physiol. 1994, 266, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A.; Juneau, P.L.; Heppner, G.H. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int. J. Cancer 1991, 48, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Luciani, F.; Spada, M.; De Milito, A.; Molinari, A.; Rivoltini, L.; Montinaro, A. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl. Cancer Inst. 2004, 96, 1702–1713. [Google Scholar] [CrossRef]
- Ikemura, K.; Hiramatsu, S.; Okuda, M. Drug Repositioning of Proton Pump Inhibitors for Enhanced Efficacy and Safety of Cancer Chemotherapy. Front. Pharmacol. 2017, 8, 911. [Google Scholar] [CrossRef]
- Tozzi, M.; Sørensen, C.E.; Magni, L.; Christensen, N.M.; Bouazzi, R.; Buch, C.M. Proton Pump Inhibitors Reduce Pancreatic Adenocarcinoma Progression by Selectively Targeting H(+), K(+)-ATPases in Pancreatic Cancer and Stellate Cells. Cancers 2020, 12, 640. [Google Scholar] [CrossRef]
- Fako, V.E.; Wu, X.; Pflug, B.; Liu, J.Y.; Zhang, J.T. Repositioning proton pump inhibitors as anticancer drugs by targeting the thioesterase domain of human fatty acid synthase. J. Med. Chem. 2015, 58, 778–784. [Google Scholar] [CrossRef]
- Bauerschlag, D.O.; Maass, N.; Leonhardt, P.; Verburg, F.A.; Pecks, U.; Zeppernick, F. Fatty acid synthase overexpression: Target for therapy and reversal of chemoresistance in ovarian cancer. J. Transl. Med. 2015, 13, 146. [Google Scholar] [CrossRef]
- Uddin, S.; Jehan, Z.; Ahmed, M.; Alyan, A.; Al-Dayel, F.; Hussain, A. Overexpression of fatty acid synthase in Middle Eastern epithelial ovarian carcinoma activates AKT and Its inhibition potentiates cisplatin-induced apoptosis. Mol. Med. 2011, 17, 635–645. [Google Scholar] [CrossRef]
- Tan, M.-M.; Ho, W.K.; Yoon, S.Y.; Mariapun, S.; Hasan, S.N.; Lee, D.S.C. A case-control study of breast cancer risk factors in 7663 women in Malaysia. PLoS ONE 2018, 13, 0203469. [Google Scholar] [CrossRef]
- Jingtao Hu, W.H.; Chenyu Sun, C.C.; Yue, L. Association between the Use of Proton Pump Inhibitors with the Risk and the Prognosis of Breast Cancer: A Systematical Review. World J. Surg. 2021, 4, 1327. [Google Scholar]
- Ding, D.C.; Sung, F.C.; Chen, W.; Wang, J.H.; Lin, S.Z. Proton pump inhibitors reduce breast cancer risk in gastric ulcer patients: A population-based cohort study. Breast J. 2020, 26, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Metz, D.C. Safety of proton pump inhibitor exposure. Gastroenterology 2010, 139, 1115–1127. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Cases (with Cancer) (n = 233,173) | Controls (without Cancer) (n = 932,692) | p-Value |
|---|---|---|---|
| Age | |||
| Mean ± SD | 52.05 ± 12.75 | 52.05 ± 12.74 | 1 |
| 20–39 years, n (%) | 36,968 (15.85) | 147,872 (15.85) | 1 |
| 40–64 years, n (%) | 156,120 (66.96) | 624,480 (66.96) | 1 |
| ≥65 years, n (%) | 40,085 (17.19) | 160,340 (17.19) | 1 |
| Comorbid conditions, n (%) | |||
| Myocardial infarction | 421 (0.18) | 1921 (0.21) | 0.015 |
| Congestive heart failure | 3202 (1.37) | 14,451 (1.55) | <0.0001 |
| Peripheral vascular disease | 1551 (0.67) | 7319 (0.78) | <0.0001 |
| Cerebrovascular disease | 8985 (3.85) | 41,794 (4.48) | <0.0001 |
| Dementia | 1583 (0.68) | 7660 (0.82) | <0.0001 |
| Chronic pulmonary disease | 6538 (2.8) | 29,240 (3.14) | <0.0001 |
| Rheumatic disease | 3140 (1.35) | 15,928 (1.71) | <0.0001 |
| Peptic ulcer disease | 25,949 (11.13) | 120,765 (12.95) | <0.0001 |
| Liver disease | 12,912 (5.54) | 59,355 (6.36) | <0.0001 |
| Diabetes | 29,356 (12.59) | 141,444 (15.17) | <0.0001 |
| Hemiplegia or paraplegia | 352 (0.15) | 1742 (0.19) | <0.001 |
| Renal disease | 4876 (2.09) | 22,314 (2.39) | <0.0001 |
| CCI | |||
| Mean ± SD | 0.47 ± 0.92 | 0.53 ±0.94 | |
| Other drugs, n (%) | |||
| Metformin | 21,758 (9.33) | 106,760 (11.46) | <0.0001 |
| Aspirin | 15,508 (6.65) | 73,217 (7.91) | <0.0001 |
| Statin | 17,244 (7.40) | 86,129 (9.26) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.T.H.; Huang, C.-W.; Wang, C.-H.; Lin, M.-C.; Hsu, J.C.; Hsu, M.-H.; Iqbal, U.; Nguyen, P.-A.; Yang, H.-C. Association between Proton Pump Inhibitor Use and the Risk of Female Cancers: A Nested Case-Control Study of 23 Million Individuals. Cancers 2022, 14, 6083. https://doi.org/10.3390/cancers14246083
Nguyen NTH, Huang C-W, Wang C-H, Lin M-C, Hsu JC, Hsu M-H, Iqbal U, Nguyen P-A, Yang H-C. Association between Proton Pump Inhibitor Use and the Risk of Female Cancers: A Nested Case-Control Study of 23 Million Individuals. Cancers. 2022; 14(24):6083. https://doi.org/10.3390/cancers14246083
Chicago/Turabian StyleNguyen, Nhi Thi Hong, Chih-Wei Huang, Ching-Huan Wang, Ming-Chin Lin, Jason C. Hsu, Min-Huei Hsu, Usman Iqbal, Phung-Anh Nguyen, and Hsuan-Chia Yang. 2022. "Association between Proton Pump Inhibitor Use and the Risk of Female Cancers: A Nested Case-Control Study of 23 Million Individuals" Cancers 14, no. 24: 6083. https://doi.org/10.3390/cancers14246083
APA StyleNguyen, N. T. H., Huang, C.-W., Wang, C.-H., Lin, M.-C., Hsu, J. C., Hsu, M.-H., Iqbal, U., Nguyen, P.-A., & Yang, H.-C. (2022). Association between Proton Pump Inhibitor Use and the Risk of Female Cancers: A Nested Case-Control Study of 23 Million Individuals. Cancers, 14(24), 6083. https://doi.org/10.3390/cancers14246083









