Simple Summary
Given the low prevalence and the heterogeneity of childhood cancers, information about the safety of anti-angiogenic drugs in pediatric patients is only partially assessed. We aimed to evaluate the safety of these drugs in children with solid tumors. This systematic review and meta-analysis reported that one out of two pediatric patients using anti-angiogenic drugs in monotherapy experienced a serious adverse event despite proportions varying per single drug.
Abstract
Cancer is a clinical condition that can benefit from anti-angiogenic drugs (AADs). Given the low prevalence and the heterogeneity of childhood cancers, information about the safety of these drugs in pediatric patients is partially assessed. The aim of this study was to evaluate the safety of AADs in pediatric patients with solid tumors. Clinical trials and observational studies were searched in PubMed, ISI Web of Science, and ClinicalTrials database For each included study, adverse events (AEs) were extracted. A meta-analysis was conducted by pooling proportions of AEs using a random intercept logistic regression model. Seventy studies were retrieved. Most part were clinical trials (55 out of 70), and only fifteen observational studies were found. Overall, proportion of serious and non-serious AEs of AADs used as monotherapy was 46% and 89%, respectively. Proportions of serious AEs varied among drugs: sunitinib, 79%; lenvatinib, 64%; sorafenib, 48%; ramucirumab, 41%; pazopanib, 30%; and vandetanib, 27%. A higher proportion of non-serious hematological AEs was found in the patients receiving pazopanib with respect to sunitinib and lenvatinib. The safety profile of AADs has been extensively investigated for mostly drugs in phase I and II trials and is limited to acute toxicities. Overall, one out of two patients using AAD drugs in monotherapy experienced a serious AE despite proportions varied per single drugs. When AADs were combined with standard chemotherapy, the proportion of AEs varied in relation to the single combinations.
Keywords:
anti-VEGF; vegf inhibitors; antiangiogenic drugs; pediatric; children; childhood; cancer; tumor; meta-analysis; systematic review 1. Introduction
Aberrant tumor vessels in solid tumors contribute to maintaining the pro-tumorigenic niche and profoundly influence the success of anticancer therapies [1]. Tumor angiogenesis is mainly driven by an imbalance between pro-angiogenic and anti-angiogenic signaling in the tumor microenvironment. A key pro-angiogenic mediator is vascular endothelial growth factor (VEGF), but other factors can stimulate angiogenesis including fibroblast growth factor-2 (FGF-2); platelet-derived growth factor (PDGF); hepatocyte growth factor (HGF); angiopoietins, and inflammatory mediators, such as interleukins and prostaglandins [2]. VEGF is crucial for tumor angiogenesis, and most antiangiogenic drugs are directed against this factor or its receptors, such as bevacizumab and ramucirumab [3]. However, to avoid resistance to anti-VEGF drugs, over recent decades, alternative strategies that simultaneously target VEGF signaling pathway and other pro-angiogenic signals, including tyrosine kinase inhibitors (TKIs) such as sunitinib, sorafenib, pazopanib, cabozantinib, and others were developed (Table 1) [4,5].

Table 1.
Approved drugs with anti-angiogenic properties (known/potential) in patients with solid tumors.
Despite advances in anticancer therapies, pediatric malignancies continue to be a leading cause of death by disease in people younger than 20 years of age, and while in recent decades, the survival has improved for leukemia and lymphomas, it reached a plateau for many solid tumors [6]. Solid tumors account for 30% of all pediatric cancers. In children, the most common solid tumors are neuroblastoma, central nervous system tumors, sarcomas and Wilms’ tumor [7]. Recent advancements in understanding pediatric tumors have arisen from evaluation of the complex genetic landscape within each tumor subtype. Although for anti-angiogenic drugs there are no clear predictive biomarkers of response, these drugs are considered promising chemosensitizers of anticancer strategies such as chemotherapy, targeted therapies, and immune therapies in several advanced tumors, and while not approved, they are frequently used in pediatric population [5,8,9]. For example, benefits of anti-angiogenic therapy in brain tumors are not clear but it has successfully introduced to treat radiation-induced necrosis in several solid tumors [7,8].
To date, there is limited clinical evidence focusing on safety of anti-angiogenic drugs in pediatric patients. This is mainly due to the low prevalence and the heterogeneity of pediatric cancers. The purpose of this systematic review was to estimate the proportion of adverse events of anti-angiogenic drugs used to treat solid tumors in patients aged 0–18 years and to assess the potential knowledge gaps on safety of these drugs.
2. Materials and Methods
2.1. Study Design
This systematic review and meta-analysis was registered on PROSPERO website (CRD42022325182). This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10].
2.2. Literature Search
We searched PubMed, ISI Web of knowledge and clinicaltrials.gov databases for retrieving the studies of interest. Articles that were published before 31 March 2022 were considered suitable for inclusion. The search strategy was composed by three sets of keywords related to the concepts “anti-VEGF/anti-angiogenic drugs”, “pediatric patients”, and “cancer” (the full strings are available in Table S1). Snowballing search was also conducted to retrieve additional papers of interest by examining the references cited in the included articles and in the excluded reviews that were retrieved from search strategy.
2.3. Eligibility Criteria
Studies including pediatric patients (≤18 years old) with solid cancers were selected for inclusion in the systematic review. If the study included both adults and pediatric patients, the article was considered suitable for inclusion only if reported a stratification of adverse events per pediatric patients or if the median age of included subjects was less than 18 years old. Studies reporting the safety of anti-angiogenic drugs in Table 1 were considered suitable for inclusion. Given the low prevalence of solid tumors in pediatric patients and the difficulty in conducting large comparative studies in this population, both comparative and non-comparative clinical trials and observational studies were considered suitable for inclusion. Case report and case series were not included. Given the possible misclassification between case series and cohort design, we adopted the definition proposed by Mathes et al., where cohort studies were defined as studies where (1) there is a comparison group, (2) a relative risk can be calculated among different exposures, or (3) patients are sampled on the basis of exposure and not on the basis of disease or disease-related outcomes [11]. If one or more of these conditions were true, the study was considered a cohort study. To be included in the meta-analysis, studies had to report at least the severity (grade) or seriousness (serious or non-serious) of adverse events (AEs) occurred. Moreover, to allow for a more formal analysis and to calculate the proportion of AEs [12], only those studies reporting the number of patients experiencing AEs were considered. Finally, as for those studies referring to the same clinicaltrials.gov (NCT) identifier, if the included study reported only a sub-cohort analysis such study was excluded from the meta-analysis to avoid that the same patient was counted twice.
2.4. Study Selection
As for published articles retrieved from PubMed and ISI web of Science, two authors (A.S. and V.C.) screened all titles and abstracts of the references retrieved. Potentially relevant studies were further assessed through examination of full texts. To search for unpublished clinical trials, all records from clinicaltrials.gov with published results were also screened and assessed for inclusion. The reviewers worked independently, in parallel, and blinded to each other. Disagreement between the two reviewers was solved through discussion with a third author (S.D.). As for published studies, eligible studies had to be written in English and studies with no full-text available were excluded.
2.5. Data Extraction
The following information was extracted from both published studies and clinicaltrials.gov records:
- Study characteristics: study type (e.g., phase I, cohort study); as for observational studies, the nature of data collection (i.e., retrospective or prospective) and study design were also extracted (e.g., cohort and case–control studies). The selected studies were associated with an NCT identifier when available.
- Disease and patients’ characteristics: solid tumor type (e.g., glioma) with its respective stage, number of patients included in the safety analysis, number of females, median age (range), and median follow-up time (range) were extracted.
- Exposure: dose, treatment schedule, formulation, and combined regimens to anti-angiogenic drug.
- Adverse events (AEs): type (e.g., nausea, hypertension), severity (i.e., grades 1–2, ≥3), seriousness (serious, non-serious), and number of patients experiencing AEs retrieved from selected studies; AEs reported in the retrieved studies were also assigned to a system organ class (SOC) according to the common terminology criteria for adverse events (CTCAE) version 5.0. Dose limiting toxicities were excluded from extraction. If a study reported separately the number of patients experiencing grades 3 and 4 AEs (or grades 1 and 2), the higher number between those graded 3–4 (as well as 1–2) was extracted to avoid a patient having experienced more than one event. An adverse event (AE) was defined as an unfavorable outcome that occurs during or after the use of a drug but is not necessarily caused by it [13]. As for severity, we considered the CTCAE version 5.0 to identify AE grade [14]. On the other hand, as for seriousness, we followed the ICH E2A guidelines from European Medicine Agency [15].
Information was collected in a specific data sheet and was validated by a second author (V.C.). Disagreement between the two reviewers was solved through discussion with a third author (S.D.).
2.6. Quality Assessment
Quality of the studies eligible for meta-analysis was assessed on the basis of the its study design [16,17]: quality of comparative randomized clinical trials was assessed through the Cochrane risk of bias tool [18], and quality of non-randomized or non-comparative clinical trials was assessed through the methodological index for non-randomized studies (MINORS) tool [19]. As in previous systematic reviews [20,21], a quality assessment of non-comparative observational studies was not conducted because these studies were assumed to be associated with high risk of bias. As for the Cochrane risk of bias tool, seven items were considered, namely random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases, and items were judged as “high risk”, “low risk”, and “unclear risk”. The overall risk of bias will correspond to the worst risk of bias in any of the domains. Moreover, if a study has “some concerns” in multiple items, it will be judged also as high risk of bias.
2.7. Statistical Analysis
Characteristics of the studies were described by year of publication, study design, tumor type, number of patients included in the safety population, number of females, median follow-up, and type of therapy (as well as dose and concomitant drugs).
As for the meta-analysis, the proportion of AEs was defined as the number of patients experiencing AEs divided by the total number of patients receiving a single drug/combination of drugs in the included studies. Clinical trials and observational studies data were also analyzed separately.
Study proportions of AEs were pooled with the “metaprop” command in R software (R Foundation) as follows [22]: we fitted random intercept logistic regression model and used maximum-likelihood estimator for tau2, logit transformation of proportions, and Clopper–Pearson CI for individual studies. Pre-planned heterogeneity investigation was based on different combination of anti-angiogenic drugs (with chemotherapy/as monotherapy): as for chemotherapy, single combinations were also investigated, where a high heterogeneity was found. Heterogeneity was assessed by inspecting I2 (>75% high, 40–74% moderate, <40 low).
3. Results
3.1. Study Selection
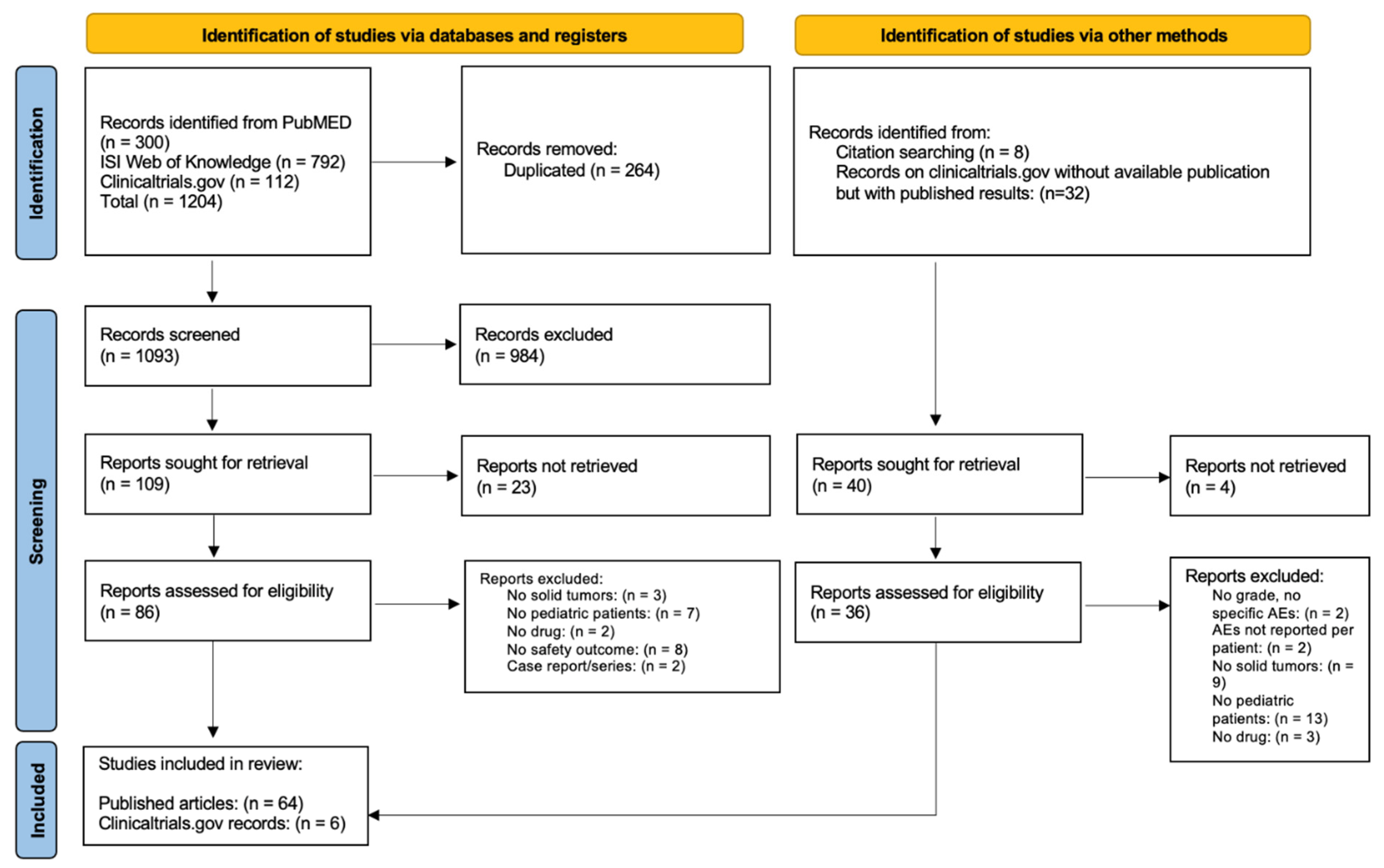
In total 300, 792, and 112 records were identified from PubMed, ISI Web of Knowledge, and clinical trials.gov, respectively (Figure 1). After removing duplicated records, 1093 articles were available for the screening of title and abstract: 109 studies were selected for full-text assessment. Twenty-three were excluded due to missing full texts, eight did not reported any safety outcomes, seven did not included pediatric patients, three did not include patients with solid tumors, two were case series/reports, and two did not analyze anti-angiogenic drugs. Eight records were found through the snowballing procedure, and thirty-two records on clincialtrials.gov without available publication but with study results were found. After screening, six additional clinical studies with results on clinicaltrials.gov were included in the analysis. In total, 70 records (64 published articles and 6 clinical trials with published results retrieved from clinicaltrials.gov) reporting AEs of anti-angiogenic drugs in pediatric patients with solid tumors were included in the systematic review [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93].
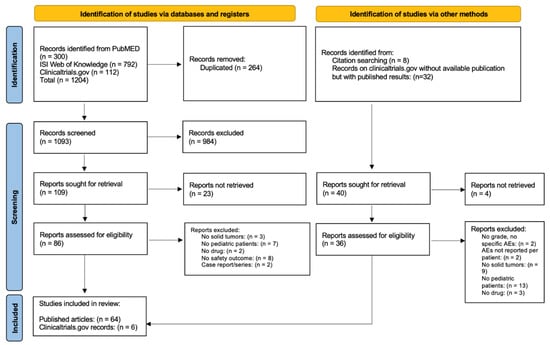
Figure 1.
Prisma flow diagram.
3.2. Study Characteristics
Characteristics of the included records were reported in Table 2. Twenty-four were phase I studies, twenty-two were phase II studies, fifteen were retrospective observational studies, five studies were phase I/II, four studies were clinical trials not otherwise specified, and one was a phase II/III trial for a total of 1837 subjects: the median of patients enrolled per study was 25 (range 2–92). Some publications included in the systematic review referred to the same trial but analyze different indications, outcomes or different drug combinations (i.e., NCT00381797 [33,75,79,80], NCT00665990 [34,85,86], and NCT02432274 [35,36]).

Table 2.
Characteristics of included studies assessing safety of anti-angiogenic drugs in pediatric patients with solid tumors.
Most of the studies evaluated AEs of anti-angiogenic drugs in patients with central nervous system tumors (sixteen various glioma [24,25,29,32,43,44,45,46,65,75,76,80,81,82,83,84], eight various brain tumors [28,33,54,59,61,64,72,88], three ependymoma [30,70,79], two medulloblastoma [23,51], one astrocytoma [47], and two neuroblastoma [56,78]). Twenty-three studies referred to a cohort of patients with various solid tumors [27,31,34,37,38,39,40,41,50,53,55,58,60,63,66,68,71,73,74,85,86,87,89], and ten referred to patients with sarcoma [26,35,36,52,57,62,69,77,78,90]. Additionally, two studies on gastrointestinal tumor [42,67], two on hepatic carcinoma [48,92], one study on thyroid cancer [49], and one on bone tumors [91] were found.
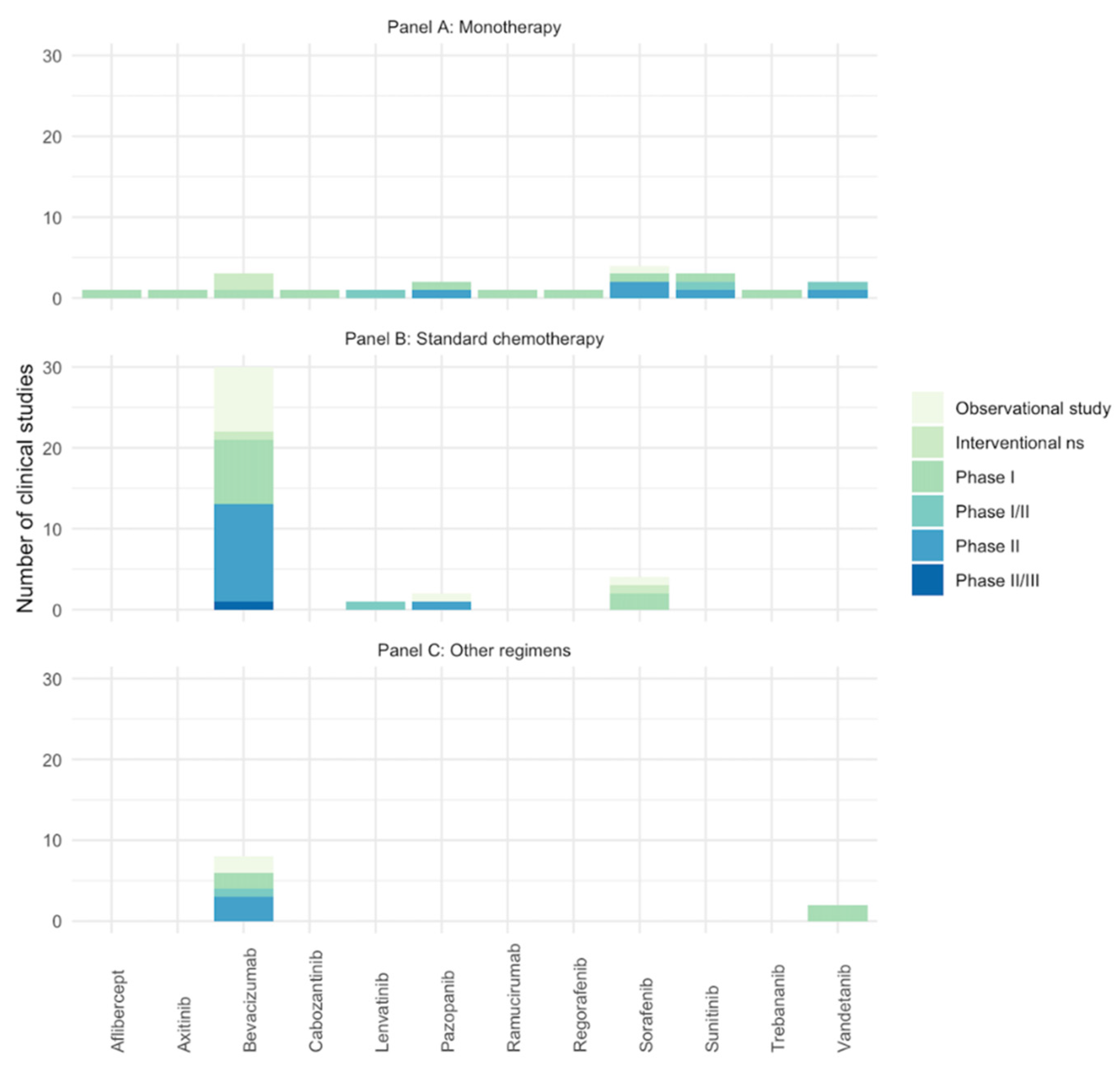
Twenty studies reported the number of AEs of anti-angiogenic drugs used as monotherapy (three for bevacizumab [40,43,72], four for sorafenib [47,71,90,91], three for sunitinib [31,67,70], two for pazopanib [41,73], two for vandetanib [42,49], one for aflibercept [39], one for axitinib [37], one for cabozatinib [27], one for trebananib [50], one for lenvatinib [35], one for regorafenib [38], and one for ramucirumab [74]), thirty-six reported anti-angiogenic drugs used in combination with standard chemotherapy (twenty-nine for bevacizumab [23,26,28,29,33,44,45,46,51,52,54,55,56,57,58,64,66,68,75,76,77,78,79,80,81,82,83,84], four for sorafenib [48,53,60,92], two for pazopanib [62,69], and one for lenvatinib [36]), and ten reported a combination with different regimens (eight for bevacizumab [30,32,59,61,63,65,88,89] and two for vandetanib [24,25]). Finally, four records reported the combination between bevacizumab and sorafenib with cyclophosphamide [34,85,86,87]. See Figure 2 for the number studies retrieved per anti-angiogenic drug.
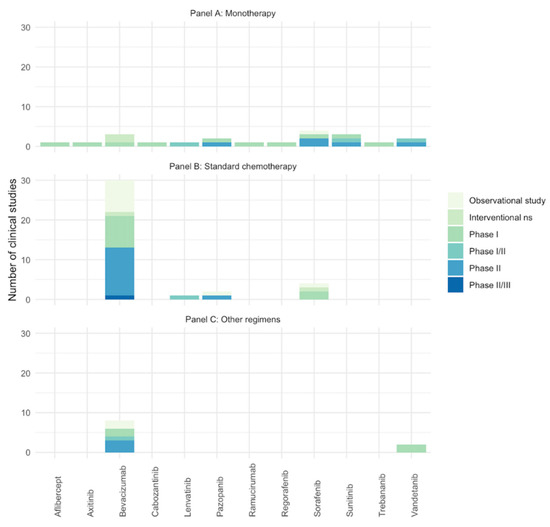
Figure 2.
Number of included studies (clinical trials and observational studies) assessing the AEs of anti-angiogenic drugs in pediatric patients with solid tumors. Studies with same NCT identifier were considered as one. (A) Anti-angiogenic drugs in monotherapy; (B) anti-angiogenic drugs in combination with standard chemotherapy; (C) anti-angiogenic drugs in combination with target therapy/other. Ns: phase non-specified.
Only few studies reported information on median follow-up of patients (12 out of 70), and the maximum follow-up reported was 96 months in a phase I/II study of patients receiving vandetanib as monotherapy [49]. The minimum follow-up reported was 7 months [69].
3.3. Safety
Forty-five studies were included in the meta-analysis for the evaluation of severity, while twenty for the evaluation of seriousness of AEs. Characteristics of the safety population of the selected studies were reported in Supplementary Table S2.
3.3.1. Anti-Angiogenic Drugs as Monotherapy
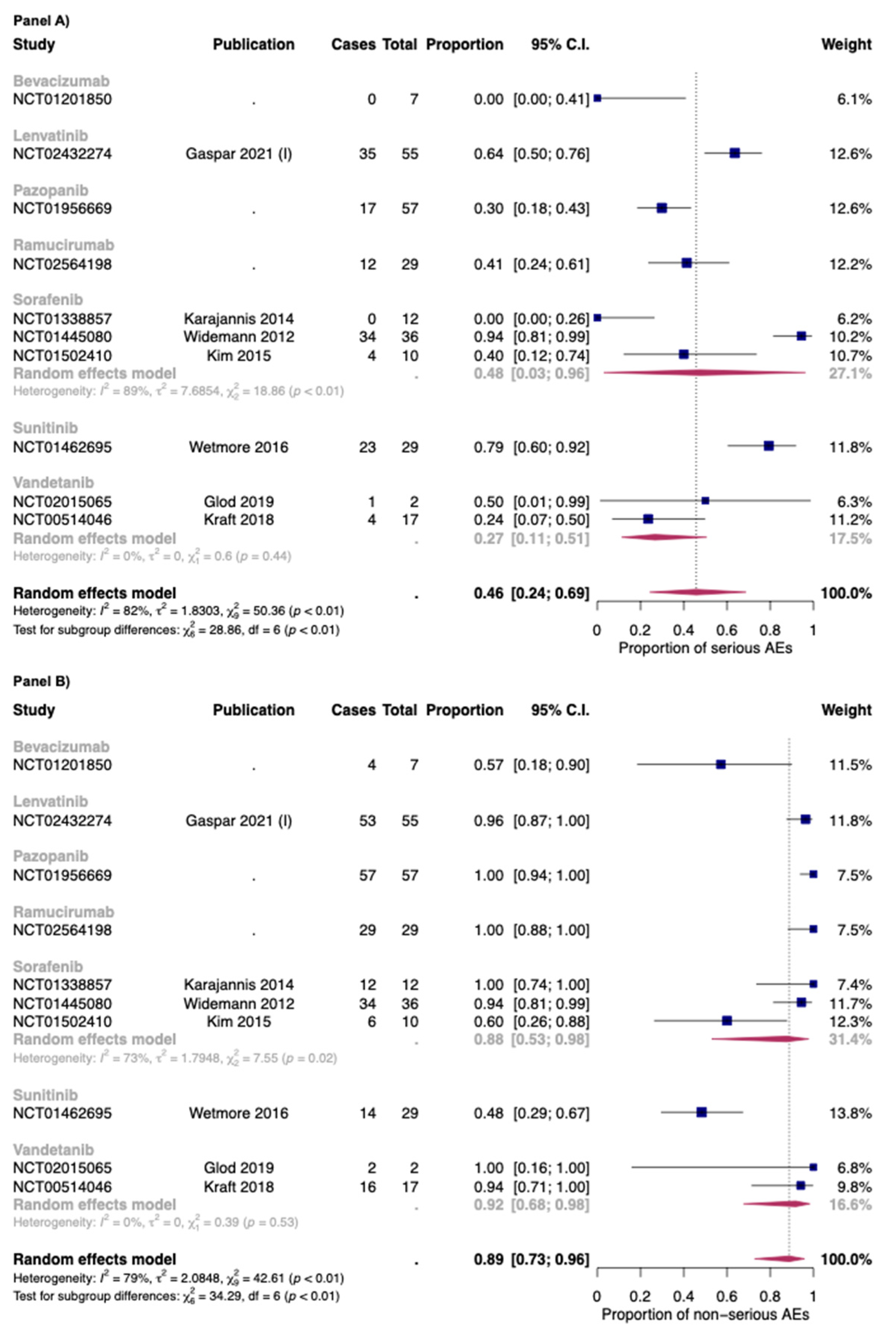
In Figure 3, the overall proportion of serious (Panel A) and non-serious AEs (Panel B) for anti-angiogenic drugs used as monotherapies was reported. Overall, the proportion of serious and non-serious AEs of anti-angiogenic drugs used as monotherapy was 0.46 [95% CI: 0.24–0.69] and 0.89 [95% CI: 0.73–0.96], respectively. The two drugs with the higher proportion of serious AEs were sunitinib (0.79: one study) and lenvatinib (0.64; one study).
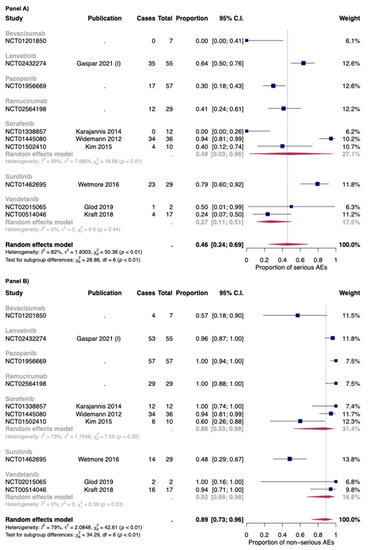
Figure 3.
Overall proportion of serious (Panel A) and non-serious (Panel B) AEs for anti-angiogenic drugs used as monotherapy. Cases: Patients with at least an event. Total: Patients included in the study [35,42,47,49,70,71,90].
As for bevacizumab, only one study with seven patients in the safety analysis was included [72]. No serious AEs were found in this study. As for vandetanib, the two studies included in this review reported a proportion of serious AEs of 0.27 [95% CI: 0.11–0.51], with low heterogeneity (I2: 0%; p > 0.05) [42,49]. As for sorafenib, the proportion was 0.48 but with high heterogeneity between studies (I2: 89%; p < 0.01). Indeed, the dosages of sorafenib were different between the three studies included in this review (Widemann et al.: 150–325 mg/m2/dose; Kim et al.: 200 mg/m2/dose; Karajannis et al.: 200–400 mg/m2/dose) [47,71,90]. Moreover, as for the safety populations, while the rate between male: female was 1:1 for the study of Widemann et al. and Karajannis et al., the study ok Kim et al. is composed almost of female patients (nine out of ten) [47,71,90].
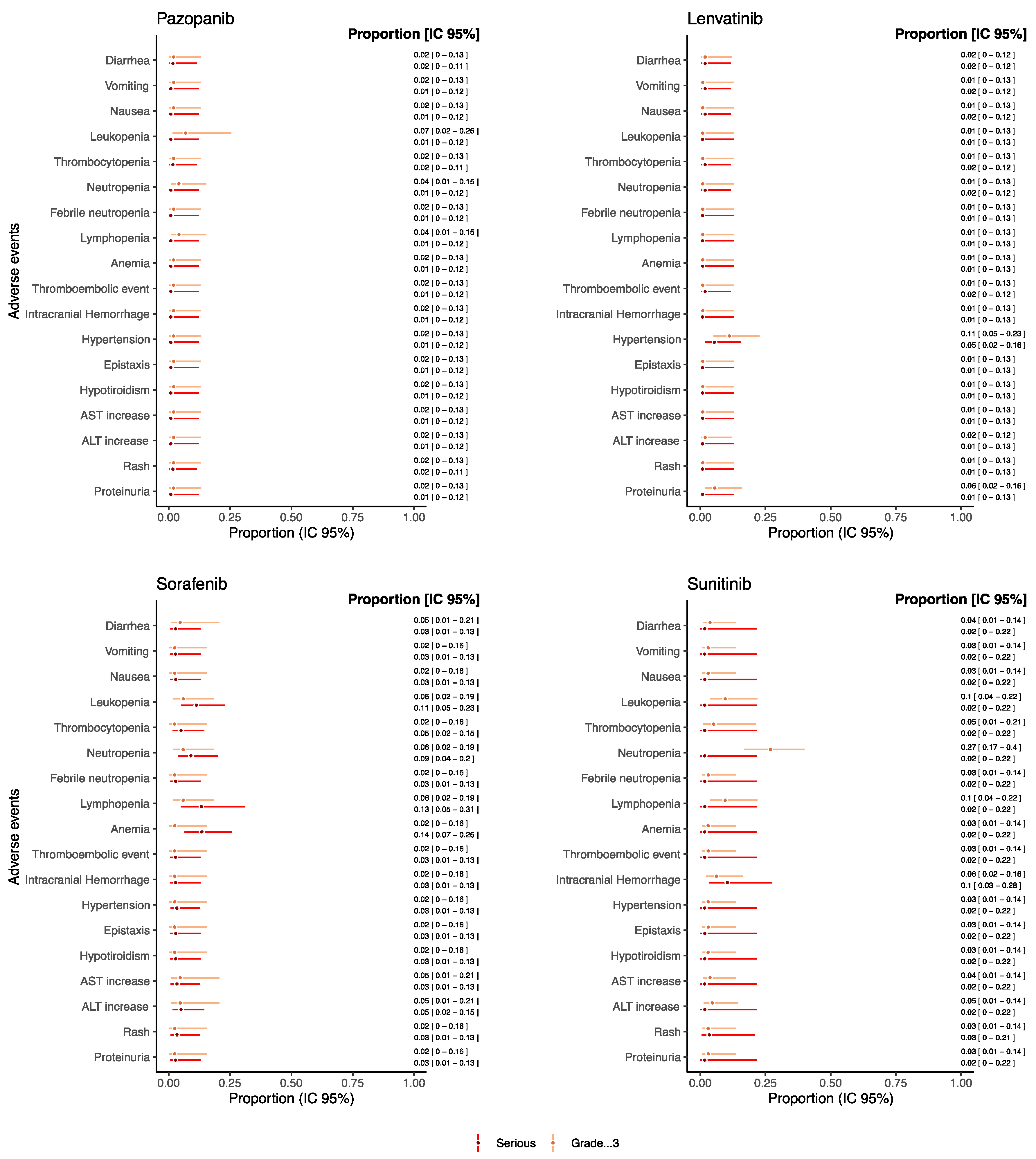
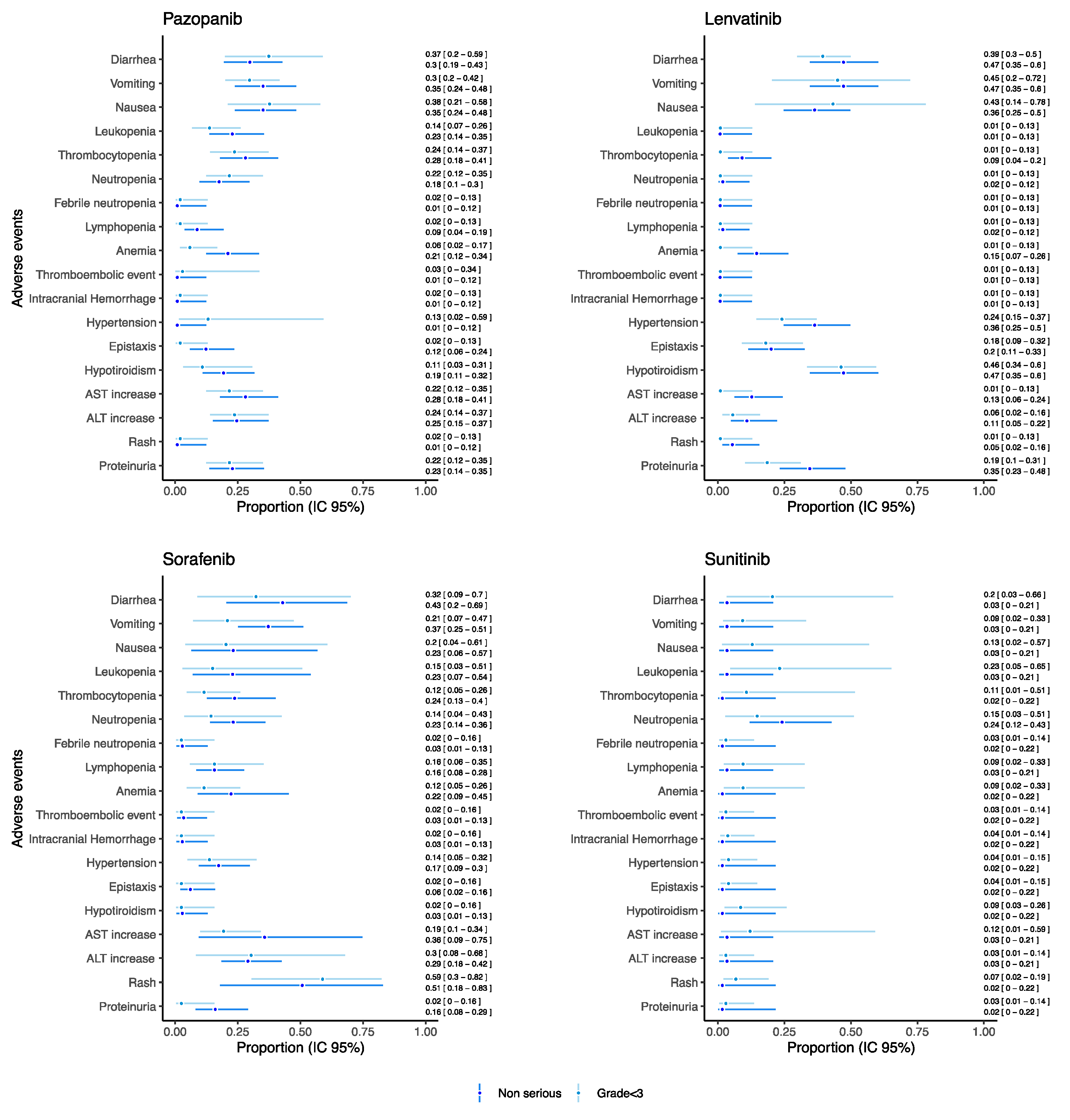
As for drugs used as monotherapy reporting both serious and grade ≥ 3 AEs and which were evaluated in at least 20 patients, the proportion of such AEs was reported in Figure 4 (see Figure S1 in the Supplementary Materials for the other drugs).
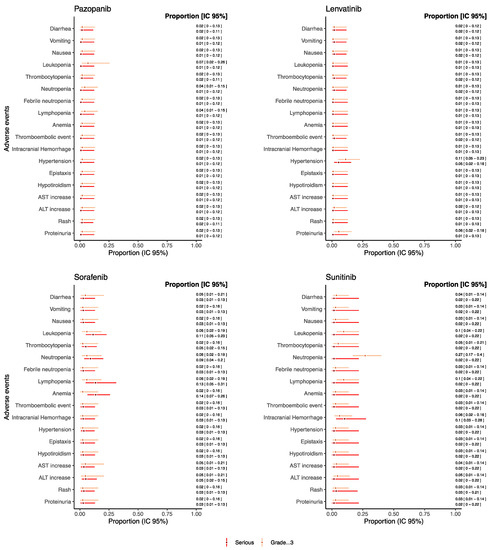
Figure 4.
Proportions of serious/grade ≥ 3 AEs for pediatric patients receiving anti-angiogenic drugs as monotherapies.
Overall, the proportion of grade ≥ 3 and serious AEs, such as gastrointestinal AEs, hematological AEs, thromboembolic event, intracranial hemorrhage, hypertension, hypothyroidism, AST and ALT increase, rush, and proteinuria remained under the threshold of 0.15 for each drug. Sunitinib reported a proportion of neutropenia grade ≥ 3 of 0.26 [95% CI: 0.17–0.39] despite the proportion of serious neutropenia being 0.01 [95% CI: 0.01–0.21]. Additionally, trebananib reported a proportion of neutropenia grade ≥ 3 of 0.21 [95% CI: 0.08–0.44], but no information on seriousness was available from retrieved studies (Figure S1). Sorafenib reported a proportion of serious anemia and lymphopenia of 0.13 [95% CI: 0.06–0.26] and 0.13 [95% CI: 0.05–0.31], respectively. Moreover, for serious intracranial hemorrhage, a proportion of 0.10 [95% CI: 0.03–0.28] was found for patients treated with sunitinib.
As for drugs used as monotherapy reporting both non-serious and grade < 3 AEs and which were evaluated in at least 20 patients, the proportion of such AEs was reported in Figure 5 (see Figure S2 in the Supplementary Materials for the other drugs). For each drug reported in Figure 5, we found a substantial correspondence between proportion of non-serious and grade < 3 AE. A higher proportion of hematological AEs was found in the patients receiving pazopanib (proportion for leukopenia: 0.22 [95% CI: 0.14–0.35], thrombocytopenia 0.28 [95% CI: 0.18–0.41]) and sorafenib (proportion for leukopenia: 0.23 [95% CI: 0.07–0.54], thrombocytopenia 0.24 [95% CI: 0.13–0.40]) with respect to sunitinib (proportion for leukopenia: 0.03 [95% CI: 0.01–0.21], thrombocytopenia 0.02 [95% CI: 0.01–0.22]) and lenvatinib (proportion for leukopenia: 0.01 [95% CI: 0.01–0.12], thrombocytopenia 0.09 [95% CI: 0.04–0.20]). Notably, sunitinib showed the proportion of non-serious neutropenia of 0.24 [95% CI: 0.12–0.43]. On the other hand, lenvatinib reported the higher proportion of non-serious hypertension (0.36 [95% CI: 0.25–0.50]) and of hypothyroidism (0.47 [95% CI: 0.34–0.60]).
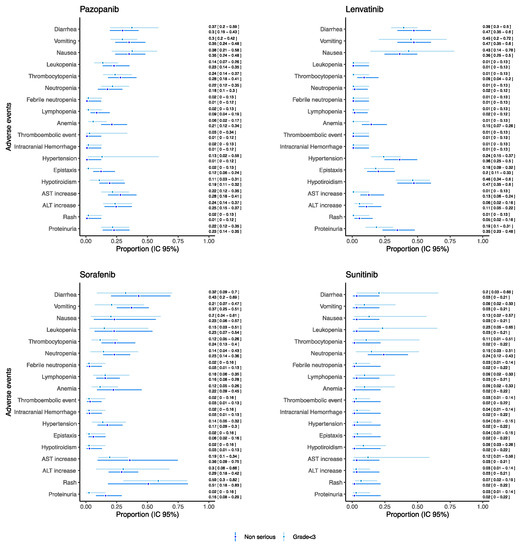
Figure 5.
Proportions of non-serious/grade < 3 AEs for pediatric patients receiving anti-angiogenic drugs as monotherapies.
3.3.2. Anti-Angiogenic Drugs in Combination with Standard Chemotherapy
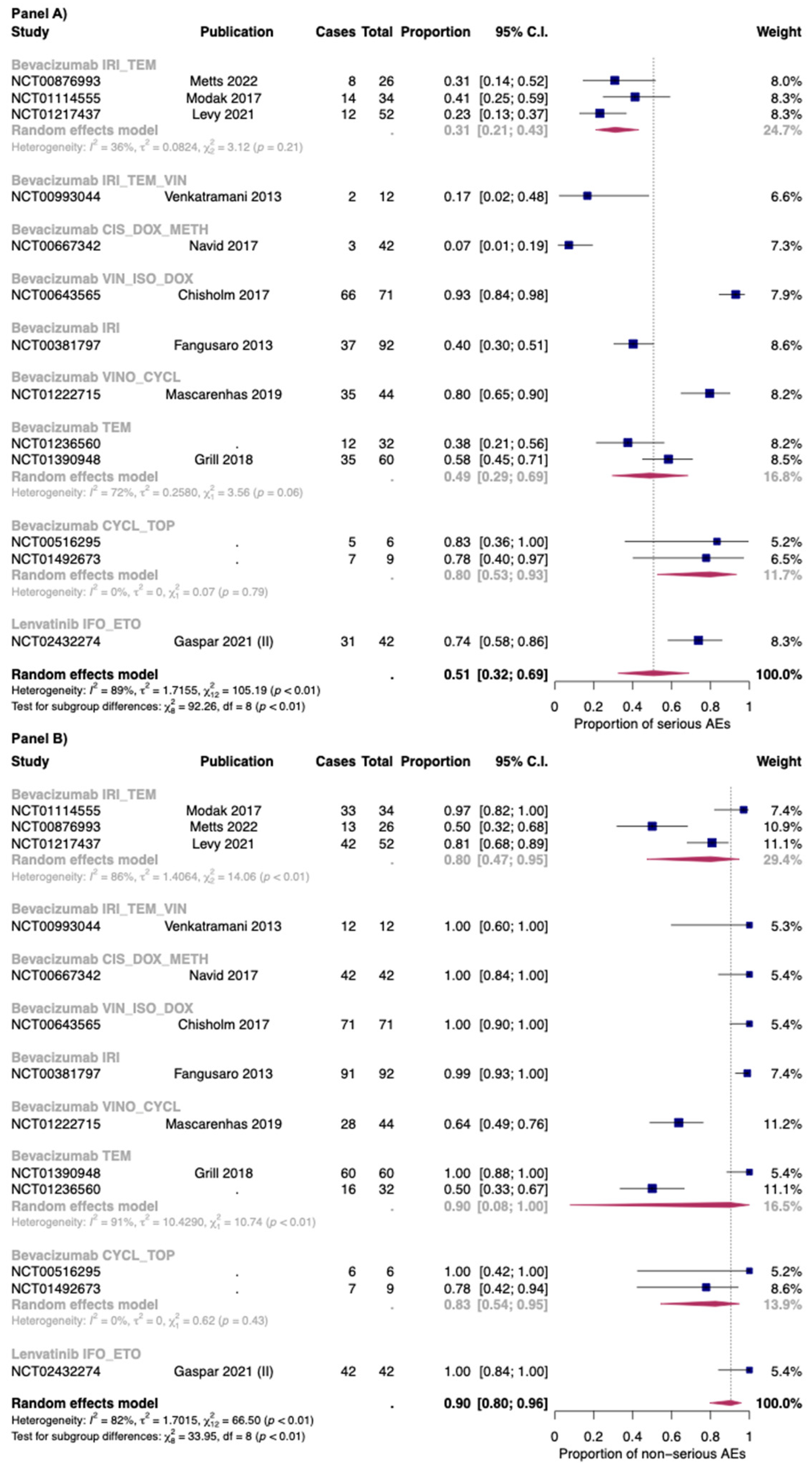
Twenty-five out of twenty-six studies included in the seriousness analysis reported both the overall proportion of serious and non-serious AEs. In Figure S3 of the Supplementary Materials, the overall proportion of serious (Panel A) and non-serious AEs (Panel B) for anti-angiogenic drugs in combination with chemotherapy was reported.
The proportion of serious and non-serious AEs of anti-angiogenic drugs in combination with chemotherapy was 0.51 [95% CI: 0.32–0.69] and 0.90 [95% CI: 0.80–0.96], respectively. In combination with chemotherapy the proportion of serious AEs was found to be 0.74 [95% CI: 0.58–0.86] with lenvatinib (only one study), and 0.48 [95% CI: 0.29–0.68] with bevacizumab (thirteen studies). For non-serious AEs, bevacizumab in combination with chemotherapy reported a proportion of 0.89 [95% CI: 0.77–0.95], while in the only study included for lenvatinib, the authors reported that all the patients have at least one non-serious AEs. However, a high heterogeneity was found between studies reporting serious and non-serious AEs for bevacizumab with chemotherapy: I2 88% and 82%, respectively (Figure 6).
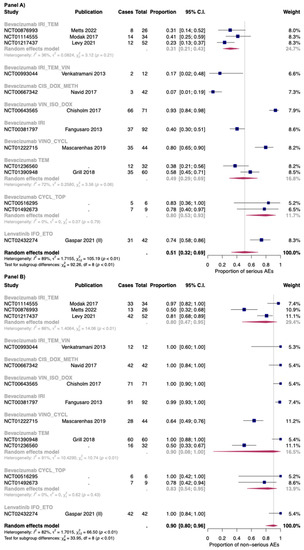
Figure 6.
Proportion of serious (Panel A) and non-serious AEs (Panel B) for bevacizumab and lenvatinib plus chemotherapy by single regimens; IRI_TEM: Irinotecan + temozolomide; IRI_TEM_VIN: Irinotecan + temozolomide + vincristine; CIS_DOX_METH: Cisplatin + doxorubicin + methotrexate; VIN_ISO_DOX: Vincristine + ifosfamide +doxorubicin; IRI: irinotecan; VINO_CYCL: Vinorelbine + cyclophosphamide; TEM: Temozolomide; CYCL_TOP: Cyclophosphamide + topotecan; IFO_ETO: Ifosfamide + etoposide. Cases: Patients with at least an event. Total: Patients included in the study [26,33,36,44,51,52,54,56,57,66].
The combination with a higher proportion of serious AEs was bevacizumab + vincristine + ifosfamide (0.93; 95% CI: 0.84–0.98), while that with less proportion was bevacizumab + doxycycline + methotrexate: (0.07; 95% CI: 0.01–0.19). For non-serious AEs, a high heterogeneity was found for combination of bevacizumab + irinotecan + temozolomide (three studies: I2 86%) and for bevacizumab + temozolomide (two studies: I2 91%). As for the first combination, in the study of Modak et al. [56] (which reported the higher proportion of serious AEs), bevacizumab was administered at 15 mg/kg, while in the other two studies (Levy et al.; Metts et al. [51,54]), bevacizumab was administered at 10 mg/kg. Moreover, the in the study of Metts et al. [54], the irinotecan was administered only at day 1 and 15 of a 28-day cycle, while in the other two studies [51,56], it was administered for the first five days of each cycle. Finally, as for the combination bevacizumab + temozolomide, temozolomide was given as 150–200 mg/m2/dose. These patients also received daily radiotherapy treatment, but its dosage was not reported in one study [76].
3.3.3. Anti-Angiogenic Drugs in Combination with Different Regimens
Eight studies reported AEs of anti-angiogenic drugs in combination with regimens other than standard chemotherapy (six for bevacizumab [30,32,59,61,63,65], and two for vandetanib [24,25]).
The study of Federico et al. reported AEs for the combination bevacizumab + sorafenib + cyclophosphamide [34]. The study did not reported information about the seriousness but provided results about severity of AEs. The authors reported a proportion of 0.46 [95% CI: 0.27–0.65], 0.71 [95% CI: 0.51–0.85], 0.29 [95% CI: 0.15–0.50], 0.13 [95% CI: 0.04–0.31], and 0 [95% CI: 0–0.14] for grade ≥ 3 AEs leukopenia, lymphopenia, neutropenia, thrombocytopenia, and anemia, respectively. Moreover, the authors reported proportions of grade ≥ 3 for proteinuria of 0.08 [95% CI: 0.02–0.26] and for hypertension of 0.17 [95% CI: 0.07–0.36].
One study reported the combination of bevacizumab with temsirolimus [59] and one with everolimus [63]. As for the combination with everolimus, proportions of grade ≥ 3 of 0.27 [95% CI: 0.10–0.52] for lymphopenia and of 0.13 [95% CI: 0.03–0.37] for neutropenia and thrombocytopenia were reported. No vomiting or diarrhea grade ≥ 3 AEs were observed. In the study of bevacizumab + temsirolimus (six patients), no hematological and gastrointestinal AEs grade ≥ 3 were observed, except for one event of thrombocytopenia. The safety of bevacizumab was studies also in combination with lapatinib (anti-HER2) [30]. In this study, only the seriousness of AEs was reported. Overall, a proportion of 0.45 [95% CI: 0.28–0.65] was found for serious AEs. The authors reported proportions of 0.04 [95% CI: 0.01–0.20] for serious febrile neutropenia and increase AST and of 0.12 [95% CI: 0.04–0.31] for increase ALT. All patients had at least one non-serious AEs (proportion for diarrhea: 0.75 [95% CI: 0.55–0.88]; vomiting 0.33 [95% CI: 0.18–0.53]; hypertension: 0.13 [95% CI: 0.04–0.31]).
One study by Su et al. reported information for safety of bevacizumab + radiotherapy + valproic acid in pediatric patients with glioma [65]. Serious AEs occurred in the 10% of the safety population. No hematological and gastrointestinal serious AEs were found. Non-serious AEs occurred in the 87% of the safety population and the proportion of non-serious hypertension was 0.32 [95% CI: 0.19–0.47].
Finally, two studies evaluated severity of AEs for vandetanib: one study in combination with radiotherapy + dasatinib [25], and the other study with the only radiotherapy [24]. The study of Broniscer et al., 2013 (vandetanib + dasatinib + radiotherapy) reported proportions of grade ≥ 3 for neutropenia of 0.16 [95% CI: 0.06–0.34] and for anemia of 0.08 [95% CI: 0.02–0.24]. The proportion of grade ≥ 3 for diarrhea was 0.08 [95% CI: 0.02–0.24] [25]. The study of Broniscer et al., 2010 (vandetanib + radiotherapy) found proportions of grade ≥ 3 for lymphopenia of 0.69 [95% CI: 0.52–0.81] and for neutropenia of 0.09 [95% CI: 0.03–0.22] [24].
3.4. Quality of Included Studies
We found seven randomized studies out of 52 clinical trials (13%) included in the meta-analysis that were evaluated with the Cochrane Risk of Bias tool. All randomized studies were open label and were considered as a high risk of bias for both blinding of participants/personnel and outcome. The other included clinical trials were not comparative and were evaluated with the MINORS tool. Twenty-eight studies reported a moderate quality, seven reported a good quality, and seven reported a poor quality. Table S3 (risk of bias tool) and Table S4 (MINORS tool) reported the quality of the included studies.
4. Discussion
This is the most comprehensive systematic review and meta-analysis aimed to assess the safety of anti-angiogenic drugs in pediatric population with solid cancer. Anti-angiogenic drugs are widely used in childhood cancers, although none of these are approved in pediatric oncology. However, this study did not take in consideration those drugs with anti-angiogenic proprieties that are proposed to be repurposed for solid malignancies (i.e., propranolol and sirolimus) in adults [94,95]. Overall, our review found 70 articles and our meta-analysis is based on 56 studies, which included about 1500 patients, since 2008 to 2022.
Most anti-angiogenic treatments targeting VEGF in pediatric population have been investigated up to a phase II study. We found only one phase II/III trial in which bevacizumab safety in combination with temozolomide and radiotherapy was assessed (NCT01236560) [76]. Bevacizumab was the most investigated drug in the pediatric oncology population, followed by sorafenib.
In our analysis, lenvatinib and sunitinib showed the higher proportion of serious AEs in monotherapy. However, both lenvatinib and sunitinib evidence was drawn by two single studies (55 patients for lenvatinib; 29 patients for sunitinib).
The results from our review showed that gastrointestinal as well as hematological events were the most common AEs in patients receiving anti-angiogenic drugs in monotherapy, despite the proportion of serious/severe remained under 0.15 for each drug (except for sunitinib, which reported a severe neutropenia proportion of 0.27 [CI 95%: 0.17–0.40]). This higher proportion of severe neutropenia reported for sunitinib might be explained by the direct toxicity of multi-TKIs on hematopoietic progenitor cells [96].
Overall, the frequency and severity of myelosuppression vary among anti-angiogenic drugs, based on their different anti-kinase selectivity (lenvatinib reported the lowest rate of proportion of hematological AEs—see Figure 4 and Figure 5). Their activity against fms-related tyrosine kinase 3 (FLT3 or CD135) and c-kit, which are essential for survival and differentiation of hemopoietic progenitor cells, is critical to determine their hematologic toxicity profiles [97]. A possible mechanism might also involve ROS generation, related to both their efficacy and toxicity [98].
In agreement with what observed in adult cancer population, the most common cardiovascular event in pediatric cancer patients exposed to antiangiogenic drugs was hypertension [41,69,70]. The proportion of serious thromboembolic event and intracranial hemorrhage was found less than 5% for each drug except for sunitinib (0.10 CI 95%: 0.17–0.40) However, one of two studies assessing sunitinib for serious AEs was conducted on patients with ependymoma (brain tumor), which need to be considered as a confounding factor for the occurrence of this event. Several mechanisms were suggested for the association between VEGF signaling inhibition and the development of cardiovascular events. VEGF induces the production of two vasodilators, nitric oxide, and prostacyclin, as well as inhibits the production of the vasoconstrictor endothelin-1 [99]. In addition, VEGF promotes proliferation and inhibits apoptosis of endothelial cells, thus contributing to maintenance of vascular homeostasis and tumor angiogenesis [100].
Interestingly, the results from our study showed that pediatric patients treated with lenvatinib reported a high proportion of non-serious hypothyroidism (0.47 CI 95%: 0.34–0.60). This AE associated with lenvatinib is also reported in adult patients affected by hepatocarcinoma, with a proportion varying from 0.16–0.21 [101,102]. However, the mechanism by which lenvatinib induce thyroiditis is not clear. Two studies reported that TKIs induce hypothyroidism through tissue ischemia (inhibition of thyroid blood flow) or apoptosis of the thyroid follicular cells [103,104]. Unfortunately, this systematic review retrieved only seven observational studies (six for bevacizumab, one for pazopanib) that meet the inclusion criteria for meta-analysis [23,28,29,46,55,61,62], and given the different regimens analyzed, indications and doses these studies were not pooled together and no conclusive evidence on the long-term safety of anti-angiogenic drugs in pediatric patients could possibly be drawn. This aspect is important in light of preclinical and clinical evidence on adults patients: in particular, pazopanib is reported to increase risk for bone shortening and fragility, and tooth remodeling (the effects are reported in young rats at ≥10 mg/kg/day) as well as evidence coming from some TKIs, which are associated with cardiovascular events [105,106]. Thus, while the use of pazopanib is not recommended in patients <2 years of age due to safety concerns related to growth and organ maturation [107], that of anti-angiogenic TKIs is currently not regulated due to lack of clear data on safety. Data from observational studies, as well as pharmacovigilance studies, could help to define mechanism of action-depending toxicities of newer multi-TKIs and to evaluate the long-term safety of anti-angiogenic drugs in pediatric patients.
Finally, as for serious and non-serious AEs of anti-angiogenic drugs used in monotherapy, this study highlighted a high heterogeneity among the studies reporting on sorafenib. In the three studies on sorafenib, the drug was used with different posology. Unfortunately, exclusion and inclusion criteria presented by single protocols for each study assessing sorafenib in monotherapy were not retrieved, and we are not able to provide any conclusive assumptions on the heterogeneity observed.
As expected, the combination of anti-angiogenic drugs with chemotherapy leads to an increase in AEs compared with monotherapy. Regarding serious Aes, the combination of bevacizumab with vincristine, ifosfamide, and doxorubicin (only one study) showed the worst safety profile, reaching a proportion of 0.93 [CI 95%: 0.84–0.98]. On the other hand, bevacizumab in combination with doxycycline and methotrexate exhibited the better safety profile (serious AEs 0.07; CI 95%: 0.01–0.19).
The various proportion of serious AEs seemed to be due by the different standard chemotherapy regimen and its different posology. To confirm this observation, among different groups of standard chemotherapy regimens, we did not observe a high heterogeneity regarding serious AEs. As for non-serious AEs, a high heterogeneity was found for the group of bevacizumab + temozolomide and in the group of bevacizumab + irinotecan + temozolomide. A possible explanation of this heterogeneity could be related to different reporting/monitoring of non-serious AEs between the studies.
Although in the adult population, there are no indications on the efficacy of the bevacizumab and sorafenib combination and a clear evidence of excessive toxicity [108,109], in pediatric patients, one study investigated this combination (phase II study with 44 patients with solid tumors) [34]. We found a high proportion of grade ≥ 3 hematological AEs for this combination (proportion of leukopenia 0.46, lymphopenia 0.71, and neutropenia 0.29). In the literature, two other studies, not included in the systematic review due to eligibility criteria, reported information on the safety of bevacizumab in combination with sorafenib in pediatric patients [85,87]. Two safety notes could be derived from such studies: patients with lung lesions and dermatological lesions receiving bevacizumab and sorafenib should be carefully monitored for signs and symptoms of pneumothorax and hand-foot syndrome, respectively. Both toxicities have been described in trials evaluating anti-angiogenic agents [86,110].To our knowledge, this is the first systematic review and meta-analysis assessing the safety of anti-angiogenic drugs in pediatric patients with solid tumors. We conducted a systematic review and meta-analysis according to PRISMA guidelines, including more than 50 clinical studies. To allow a more formal analysis of proportion of AEs we included only studies reporting number of patients with AEs and we minimized retrieved available literature by also including studies reporting results on clinicaltrials.gov.
However, this study also has some limitations. First, only seven studies were randomized clinical trials and most of the included studies were non-comparative clinical trials (42 studies). Given the low prevalence of solid tumors in pediatric patients and the difficulties in conducting large comparative trials in such population, the non-comparative nature of the most of the studies was expected. Second, as for non-comparative trials, most of them were found to have a moderate quality (28/42) and seven were found to have low quality, while clinical trials were open label and so each of them was considered as a high risk of blinding of participants, personnel, outcome assessment. Third, in this work, we decided not to report pharmacokinetic parameters to reveal the safety profile of anti-angiogenic drugs in relation to plasma concentration because it was outside the scope of this review. This systematic review could represent a starting point for the evaluation of pharmacokinetics parameters on safety of anti-angiogenic drug use in pediatric patients. Additionally, gender may affect the metabolic activity of enzymes involved in pharmacokinetics of pro-angiogenic drugs. Nevertheless, despite gender seemingly playing a role in the survival of patients exposed to anticancer drugs [111,112], the role of sex and gender on safety for anticancer drug use, in particular, for anti-angiogenic drugs, is still controversial (especially in the pediatric population). Notably, none of the study included in this review discussed the tolerability results of anti-angiogenic drugs with respect to sex and gender. Finally, it should be considered that selective reporting is also very frequent when dealing with reviews of adverse events. The observation period of the included studies in the meta-analysis was very heterogeneous (most of them did not report it), and the probability of observing events for longer studies is higher than that for short studies.
According to the results of this systematic review and meta-analysis, we can also provide some recommendations. First, we did not obtain any information on the long-term toxicities, and observational studies with long-term follow-up using routinely collected electronical healthcare data are required. Second, this study did not aim to collect evidence from studies using spontaneous reporting system; however, the use of these platforms could be fundamental to filling the gap in the safety of anti-angiogenic drugs in special populations.
5. Conclusions
In conclusion, anti-angiogenic drugs are frequently used in cancer childhood, from bevacizumab to the more recent molecularly targeted agents. Their toxicity profiles have mostly been studied in phase I and II trials and are limited to acute toxicities, while observational studies are limited on few drugs, such as bevacizumab and pazopanib.
Overall, we observed a correlation between seriousness and severity of AEs. Among monotherapy TKIs, the drugs with a higher proportion of serious and non-serious AEs were sunitinib and lenvatinib, while sorafenib reported a high heterogeneity among studies included. As expected, the proportion of AEs varied in relation to the single combination of anti-angiogenic drugs with standard chemotherapy or other targeted therapies/radiotherapy.
Currently, growth and developmental toxicity, such as those related to TKIs, still remain inadequately addressed. Data from observational studies could help to define mechanism of action-depending toxicities of newer multi-TKIs and to evaluate the long term safety of anti-angiogenic drugs in pediatric patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers14215315/s1, Figure S1: Proportion of serious/grade ≥ 3 AEs for pediatric patients receiving anti-angiogenic drugs as monotherapies (drugs assessed in less than 20 patients or not reporting both serious/grade ≥ 3 AEs); Figure S2: Proportion of non-serious/grade < 3 AEs for pediatric patients receiving anti-angiogenic drugs as monotherapies (drugs assessed in less than 20 patients or not reporting both non-serious/grade < 3 AEs); Figure S3: Overall proportion of serious (Panel A) and non-serious AEs (Panel B) for bevacizumab and lenvatinib plus chemotherapy (no strata by chemotherapy combinations), Table S1: Search string for PubMed, ISI Web of Science and Clinicaltrials.gov; Table S2: Characteristic of the safety population of the included studies, Table S3: Cochrane risk of bias tool assessment for randomized controlled studies, Table S4: MINORS tool assessment.
Author Contributions
Conceptualization, S.D., A.S., V.C. and M.Z.; methodology, S.D, F.S. and A.S.; software, A.S.; formal analysis, A.S. and E.L.; data curation, A.S., V.C. and P.R.; writing—original draft preparation, A.S. and V.C.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be shared upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagl, L.; Horvath, L.; Pircher, A.; Wolf, D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment–New Findings and Future Perspectives. Front. Cell Dev. Biol. 2020, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncol. 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, A.; Ciccone, V.; Donnini, S.; Ziche, M.; Morbidelli, L. Molecular Mechanisms of Resistance to Anti-Angiogenic Drugs. Crit. Rev. Oncog. 2021, 26, 39–66. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Filippelli, A.; Ciccone, V.; Spini, A.; Ristori, E.; Ziche, M.; Morbidelli, L. Chapter 2—Antiangiogenic Drugs: Chemosensitizers for Combination Cancer Therapy. In Antiangiogenic Drugs as Chemosensitizers in Cancer Therapy; Morbidelli, L., Ed.; Cancer Sensitizing Agents for Chemotherapy; Academic Press: Cambridge, MA, USA, 2022; Volume 18, pp. 29–66. [Google Scholar]
- Recent Progress in the Treatment of Cancer in Children-Butler-2021-CA: A Cancer Journal for Clinicians-Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21665 (accessed on 4 March 2022).
- Kline, N.E.; Sevier, N. Solid tumors in children. J. Pediatr. Nurs. 2003, 18, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ollauri-Ibáñez, C.; Astigarraga, I. Use of Antiangiogenic Therapies in Pediatric Solid Tumors. Cancers 2021, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Available online: http://www.prisma-statement.org/ (accessed on 22 October 2019).
- Mathes, T.; Pieper, D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: Potential impact on body of evidence and workload. BMC Med. Res. Methodol. 2017, 17, 107. [Google Scholar] [CrossRef]
- Hopewell, S.; Wolfenden, L.; Clarke, M. Reporting of adverse events in systematic reviews can be improved: Survey results. J. Clin. Epidemiol. 2008, 61, 597–602. [Google Scholar] [CrossRef]
- Zorzela, L.; Golder, S.; Liu, Y.; Pilkington, K.; Hartling, L.; Joffe, A.; Loke, Y.; Vohra, S. Quality of reporting in systematic reviews of adverse events: Systematic review. BMJ 2014, 348, f7668. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60 (accessed on 19 April 2022).
- EMA ICH E2A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. Available online: https://www.ema.europa.eu/en/ich-e2a-clinical-safety-data-management-definitions-standards-expedited-reporting (accessed on 23 May 2022).
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evidence-Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Wang, X.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Deluiz, D.; Tinoco, E.M.B. Horizontal Alveolar Ridge Augmentation with Allogeneic Bone Block Graft Compared with Autogenous Bone Block Graft: A Systematic Review. J. Oral Maxillofac. Res. 2020, 11, e1. [Google Scholar] [CrossRef]
- Pundir, J.; Achilli, C.; Bhide, P.; Sabatini, L.; Legro, R.S.; Rombauts, L.; Teede, H.; Coomarasamy, A.; Zamora, J.; Thangaratinam, S. Risk of foetal harm with letrozole use in fertility treatment: A systematic review and meta-analysis. Hum. Reprod. Updat. 2021, 27, 474–485. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R.; Use R! Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-21415-3. [Google Scholar]
- Aguilera, D.; Mazewski, C.; Fangusaro, J.; Macdonald, T.J.; McNall-Knapp, R.Y.; Hayes, L.L.; Kim, S.; Castellino, R.C. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: A multi-institutional experience. Child’s Nerv. Syst. 2013, 29, 589–596. [Google Scholar] [CrossRef]
- Broniscer, A.; Baker, J.N.; Tagen, M.; Onar-Thomas, A.; Gilbertson, R.J.; Davidoff, A.M.; Panandiker, A.P.; Leung, W.; Chin, T.K.; Stewart, C.F.; et al. Phase I Study of Vandetanib During and After Radiotherapy in Children With Diffuse Intrinsic Pontine Glioma. J. Clin. Oncol. 2010, 28, 4762–4768. [Google Scholar]
- Broniscer, A.; Baker, S.D.; Wetmore, C.; Panandiker, A.S.P.; Huang, J.; Davidoff, A.M.; Onar-Thomas, A.; Panetta, J.C.; Chin, T.K.; Merchant, T.E.; et al. Phase I Trial, Pharmacokinetics, and Pharmacodynamics of Vandetanib and Dasatinib in Children with Newly Diagnosed Diffuse Intrinsic Pontine Glioma. Clin. Cancer Res. 2013, 19, 3050–3058. [Google Scholar] [CrossRef]
- Chisholm, J.C.; Merks, J.H.; Casanova, M.; Bisogno, G.; Orbach, D.; Gentet, J.-C.; Defachelles, A.-S.; Chastagner, P.; Lowis, S.; Ronghe, M.; et al. Open-label, multicentre, randomised, phase II study of the EpSSG and the ITCC evaluating the addition of bevacizumab to chemotherapy in childhood and adolescent patients with metastatic soft tissue sarcoma (the BERNIE study). Eur. J. Cancer 2017, 83, 177–184. [Google Scholar] [CrossRef]
- Chuk, M.K.; Widemann, B.C.; Minard, C.G.; Liu, X.; Kim, A.; Bernhardt, M.B.; Kudgus, R.A.; Reid, J.M.; Voss, S.D.; Blaney, S.; et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children’s Oncology Group. Pediatr. Blood Cancer 2018, 65, e27077. [Google Scholar] [CrossRef] [PubMed]
- Couec, M.-L.; André, N.; Thebaud, E.; Minckes, O.; Rialland, X.; Corradini, N.; Aerts, I.; Bérard, P.M.; Bourdeaut, F.; Leblond, P.; et al. Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: Toxicity and efficacy trends. Pediatr. Blood Cancer 2012, 59, 34–38. [Google Scholar] [CrossRef] [PubMed]
- de Marcellus, C.; Tauziède-Espariat, A.; Cuinet, A.; Pasqualini, C.; Robert, M.P.; Beccaria, K.; Puget, S.; Boddaert, N.; Figarella-Branger, D.; De Carli, E.; et al. The role of irinotecan-bevacizumab as rescue regimen in children with low-grade gliomas: A retrospective nationwide study in 72 patients. J. Neuro-Oncol. 2022, 157, 355–364. [Google Scholar] [CrossRef] [PubMed]
- DeWire, M.; Fouladi, M.; Turner, D.C.; Wetmore, C.; Hawkins, C.; Jacobs, C.; Yuan, Y.; Liu, D.; Goldman, S.; Fisher, P.; et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: A collaborative ependymoma research network study (CERN). J. Neuro-Oncol. 2015, 123, 85–91. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.G.; Shusterman, S.; Ingle, A.M.; Ahern, C.H.; Reid, J.M.; Wu, B.; Baruchel, S.; Glade-Bender, J.; Ivy, P.; Grier, H.E.; et al. Phase I and Pharmacokinetic Study of Sunitinib in Pediatric Patients with Refractory Solid Tumors: A Children’s Oncology Group Study. Clin. Cancer Res. 2011, 17, 5113–5122. [Google Scholar] [CrossRef]
- El-Khouly, F.E.; van Zanten, S.E.M.V.; Jansen, M.H.A.; Bakker, D.P.; Aliaga, E.S.; Hendrikse, N.H.; Vandertop, W.P.; van Vuurden, D.G.; Kaspers, G.J.L. A phase I/II study of bevacizumab, irinotecan and erlotinib in children with progressive diffuse intrinsic pontine glioma. J. Neuro-Oncol. 2021, 153, 263–271. [Google Scholar] [CrossRef]
- Fangusaro, J.; Gururangan, S.; Poussaint, T.Y.; McLendon, R.E.; Onar-Thomas, A.; Warren, K.E.; Wu, S.; Packer, R.J.; Banerjee, A.; Gilbertson, R.J.; et al. Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11). Cancer 2013, 119, 4180–4187. [Google Scholar] [CrossRef]
- Federico, S.M.; Caldwell, K.J.; McCarville, M.B.; Daryani, V.M.; Stewart, C.F.; Mao, S.; Wu, J.; Davidoff, A.M.; Santana, V.M.; Furman, W.L.; et al. Phase I expansion cohort to evaluate the combination of bevacizumab, sorafenib and low-dose cyclophosphamide in children and young adults with refractory or recurrent solid tumours. Eur. J. Cancer 2020, 132, 35–42. [Google Scholar] [CrossRef]
- Gaspar, N.; Campbell-Hewson, Q.; Melcon, S.G.; Locatelli, F.; Venkatramani, R.; Hecker-Nolting, S.; Gambart, M.; Bautista, F.; Thebaud, E.; Aerts, I.; et al. Phase I/II study of single-agent lenvatinib in children and adolescents with refractory or relapsed solid malignancies and young adults with osteosarcoma (ITCC-050). ESMO Open 2021, 6, 100250. [Google Scholar] [CrossRef]
- Gaspar, N.; Venkatramani, R.; Hecker-Nolting, S.; Melcon, S.G.; Locatelli, F.; Bautista, F.; Longhi, A.; Lervat, C.; Entz-Werle, N.; Casanova, M.; et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): A multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021, 22, 1312–1321. [Google Scholar] [CrossRef]
- Geller, J.I.; Fox, E.; Turpin, B.K.; Goldstein, S.L.; Liu, X.; Minard, C.G.; Kudgus, R.A.; Reid, J.M.; Berg, S.L.; Weigel, B.J. A study of axitinib, a VEGF receptor tyrosine kinase inhibitor, in children and adolescents with recurrent or refractory solid tumors: A Children’s Oncology Group phase 1 and pilot consortium trial (ADVL1315). Cancer 2018, 124, 4548–4555. [Google Scholar] [CrossRef] [PubMed]
- Geoerger, B.; Morland, B.; Jiménez, I.; Frappaz, D.; Pearson, A.D.; Vassal, G.; Maeda, P.; Kincaide, J.; Mueller, U.; Schlief, S.; et al. Phase 1 dose-escalation and pharmacokinetic study of regorafenib in paediatric patients with recurrent or refractory solid malignancies. Eur. J. Cancer 2021, 153, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.G.; Blaney, S.M.; Borinstein, S.; Reid, J.M.; Baruchel, S.; Ahern, C.; Ingle, A.M.; Yamashiro, D.J.; Chen, A.; Weigel, B.; et al. A Phase I Trial and Pharmacokinetic Study of Aflibercept (VEGF Trap) in Children with Refractory Solid Tumors: A Children’s Oncology Group Phase I Consortium Report. Clin. Cancer Res. 2012, 18, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.L.G.; Adamson, P.C.; Reid, J.M.; Xu, L.; Baruchel, S.; Shaked, Y.; Kerbel, R.S.; Cooney-Qualter, E.M.; Stempak, D.; Chen, H.X.; et al. Phase I Trial and Pharmacokinetic Study of Bevacizumab in Pediatric Patients With Refractory Solid Tumors: A Children’s Oncology Group Study. J. Clin. Oncol. 2008, 26, 399–405. [Google Scholar] [CrossRef]
- Bender, J.L.G.; Lee, A.; Reid, J.M.; Baruchel, S.; Roberts, T.; Voss, S.D.; Wu, B.; Ahern, C.H.; Ingle, A.M.; Harris, P.; et al. Phase I Pharmacokinetic and Pharmacodynamic Study of Pazopanib in Children With Soft Tissue Sarcoma and Other Refractory Solid Tumors: A Children’s Oncology Group Phase I Consortium Report. J. Clin. Oncol. 2013, 31, 3034–3043. [Google Scholar] [CrossRef]
- Glod, J.; Arnaldez, F.I.; Wiener, L.; Spencer, M.; Killian, J.K.; Meltzer, P.S.; Dombi, E.; Derse-Anthony, C.; Derdak, J.; Srinivasan, R.; et al. A Phase II Trial of Vandetanib in Children and Adults with Succinate Dehydrogenase–Deficient Gastrointestinal Stromal Tumor. Clin. Cancer Res. 2019, 25, 6302–6308. [Google Scholar] [CrossRef]
- Gorsi, H.S.; Khanna, P.C.; Tumblin, M.; Yeh-Nayre, L.; Milburn, M.; Elster, J.D.; Crawford, J.R. Single-agent bevacizumab in the treatment of recurrent or refractory pediatric low-grade glioma: A single institutional experience. Pediatr. Blood Cancer 2018, 65, e27234. [Google Scholar] [CrossRef]
- Grill, J.; Massimino, M.; Bouffet, E.; Azizi, A.; McCowage, G.; Cañete, A.; Saran, F.; Le Deley, M.-C.; Varlet, P.; Morgan, P.; et al. Phase II, Open-Label, Randomized, Multicenter Trial (HERBY) of Bevacizumab in Pediatric Patients With Newly Diagnosed High-Grade Glioma. J. Clin. Oncol. 2018, 36, 951–958. [Google Scholar] [CrossRef]
- Hummel, T.R.; Salloum, R.; Drissi, R.; Kumar, S.; Sobo, M.; Goldman, S.; Pai, A.; Leach, J.; Lane, A.; Pruitt, D.; et al. A pilot study of bevacizumab-based therapy in patients with newly diagnosed high-grade gliomas and diffuse intrinsic pontine gliomas. J. Neuro-Oncology 2015, 127, 53–61. [Google Scholar] [CrossRef]
- Kalra, M.; Heath, J.A.; Kellie, S.J.; Pozza, L.D.; Stevens, M.M.; Swamy, S.; McCowage, G.B. Confirmation of Bevacizumab Activity, and Maintenance of Efficacy in Retreatment After Subsequent Relapse, in Pediatric Low-grade Glioma. J. Pediatr. Hematol. 2016, 37, e341–e346. [Google Scholar] [CrossRef]
- Karajannis, M.A.; Legault, G.; Fisher, M.J.; Milla, S.S.; Cohen, K.J.; Wisoff, J.; Harter, D.H.; Goldberg, J.D.; Hochman, T.; Merkelson, A.; et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro-Oncology 2014, 16, 1408–1416. [Google Scholar] [CrossRef]
- Keino, D.; Yokosuka, T.; Hirose, A.; Sakurai, Y.; Nakamura, W.; Fujita, S.; Hayashi, A.; Miyagawa, N.; Iwasaki, F.; Hamanoue, S.; et al. Pilot Study of the Combination of Sorafenib and Fractionated Irinotecan in Pediatric Re-lapse/Refractory Hepatic Cancer (FINEX Pilot Study). Pediatr. Blood Cancer 2020, 67, e28655. [Google Scholar] [CrossRef]
- Kraft, I.L.; Akshintala, S.; Zhu, Y.; Lei, H.; Derse-Anthony, C.; Dombi, E.; Steinberg, S.M.; Lodish, M.; Waguespack, S.G.; Kapustina, O.; et al. Outcomes of Children and Adolescents with Advanced Hereditary Medullary Thyroid Carcinoma Treated with Vandetanib. Clin. Cancer Res. 2018, 24, 753–765. [Google Scholar] [CrossRef]
- Leary, S.E.; Park, J.R.; Reid, J.M.; Ralya, A.T.; Baruchel, S.; Wu, B.; Roberts, T.P.; Liu, X.; Minard, C.G.; Fox, E.; et al. Pediatric Phase I Trial and Pharmacokinetic Study of Trebananib in Relapsed Solid Tumors, Including Primary Tumors of the Central Nervous System ADVL1115: A Children’s Oncology Group Phase I Consortium Report. Clin. Cancer Res. 2017, 23, 6062–6069. [Google Scholar] [CrossRef]
- Levy, A.S.; Krailo, M.; Chi, S.; Villaluna, D.; Springer, L.; Williams-Hughes, C.; Fouladi, M.; Gajjar, A. Temozolomide with irinotecan versus temozolomide, irinotecan plus bevacizumab for recurrent medulloblastoma of childhood: Report of a COG randomized Phase II screening trial. Pediatr. Blood Cancer 2021, 68, e29031. [Google Scholar] [CrossRef]
- Mascarenhas, L.; Chi, Y.-Y.; Hingorani, P.; Anderson, J.R.; Lyden, E.R.; Rodeberg, D.A.; Indelicato, D.J.; Kao, S.; Dasgupta, R.; Spunt, S.L.; et al. Randomized Phase II Trial of Bevacizumab or Temsirolimus in Combination With Chemotherapy for First Relapse Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2019, 37, 2866–2874. [Google Scholar] [CrossRef]
- Meany, H.J.; Widemann, B.C.; Hinds, P.S.; Bagatell, R.; Shusterman, S.; Stern, E.; Jayaprakash, N.; Peer, C.J.; Figg, W.D.; Hall, O.M.; et al. Phase 1 study of sorafenib and irinotecan in pediatric patients with relapsed or refractory solid tumors. Pediatr. Blood Cancer 2021, 68, e29282. [Google Scholar] [CrossRef]
- Metts, J.; Harrington, B.; Salman, E.; Bradfield, S.M.; Flanary, J.; Mosha, M.; Amankwah, E.; Stapleton, S. A phase I study of irinotecan and temozolomide with bevacizumab in children with recurrent/refractory central nervous system tumors. Child’s Nerv. Syst. 2022, 38, 919–928. [Google Scholar] [CrossRef]
- Millan, N.C.; Poveda, M.J.; Cruz, O.; Mora, J. Safety of bevacizumab in patients younger than 4 years of age. Clin. Transl. Oncol. 2016, 18, 464–468. [Google Scholar] [CrossRef]
- Modak, S.; Kushner, B.H.; Basu, E.; Roberts, S.S.; Cheung, N.-K.V. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: Results of a phase II study. Pediatr. Blood Cancer 2017, 64, e26448. [Google Scholar] [CrossRef]
- Navid, F.; Santana, V.M.; Neel, M.; McCarville, M.B.; Shulkin, B.L.; Wu, J.; Billups, C.A.; Mao, S.; Daryani, V.M.; Stewart, C.F.; et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int. J. Cancer 2017, 141, 1469–1477. [Google Scholar] [CrossRef]
- Okada, K.; Yamasaki, K.; Tanaka, C.; Fujisaki, H.; Osugi, Y.; Hara, J. Phase I Study of Bevacizumab Plus Irinotecan in Pediatric Patients with Recurrent/Refractory Solid Tumors. Jpn. J. Clin. Oncol. 2013, 43, 1073–1079. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Shin, S.J.; Vats, T.; Guha-Thakurta, N.; Aaron, J.; Rytting, M.; Kleinerman, E.; Kurzrock, R. Pediatric Pa-tients with Refractory Central Nervous System Tumors: Experiences of a Clinical Trial Combining Bevacizumab and Temsirolimus. Anticancer Research 2014, 34, 1939–1945. [Google Scholar]
- Reed, D.R.; Mascarenhas, L.; Manning, K.; Hale, G.A.; Goldberg, J.; Gill, J.; Sandler, E.; Isakoff, M.S.; Smith, T.; Caracciolo, J.; et al. Pediatric phase I trial of oral sorafenib and topotecan in refractory or recurrent pediatric solid malignancies. Cancer Med. 2016, 5, 294–303. [Google Scholar] [CrossRef]
- Reismüller, B.; Azizi, A.A.; Peyrl, A.; Heinrich, M.; Gruber-Olipitz, M.; Luckner, D.; Rothschild, K.V.; Slavc, I. Feasibility and tolerability of bevacizumab in children with primary CNS tumors. Pediatr. Blood Cancer 2010, 54, 681–686. [Google Scholar] [CrossRef]
- Russo, I.; Di Paolo, V.; Crocoli, A.; Mastronuzzi, A.; Serra, A.; Di Paolo, P.L.; Di Giannatale, A.; Miele, E.; Milano, G.M. A Chart Review on the Feasibility and Safety of the Vincristine Irinotecan Pazopanib (VIPaz) Association in Children and Adolescents With Resistant or Relapsed Sarcomas. Front. Oncol. 2020, 10, 1228. [Google Scholar] [CrossRef]
- Santana, V.M.; Sahr, N.; Tatevossian, R.G.; Jia, S.; Campagne, O.; Sykes, A.; Stewart, C.F.; Furman, W.L.; McGregor, L.M. A phase 1 trial of everolimus and bevacizumab in children with recurrent solid tumors. Cancer 2020, 126, 1749–1757. [Google Scholar] [CrossRef]
- Schiavetti, A.; Varrasso, G.; Mollace, M.G.; Dominici, C.; Ferrara, E.; Papoff, P.; Di Biasi, C. Bevacizumab-containing regimen in relapsed/progressed brain tumors: A single-institution experience. Child’s Nerv. Syst. 2019, 35, 1007–1012. [Google Scholar] [CrossRef]
- Su, J.M.; Murray, J.C.; McNall-Knapp, R.Y.; Bowers, D.C.; Shah, S.; Adesina, A.M.; Paulino, A.C.; Jo, E.; Mo, Q.; Baxter, P.A.; et al. A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr. Blood Cancer 2020, 67, e28283. [Google Scholar] [CrossRef]
- Venkatramani, R.; Malogolowkin, M.; Davidson, T.B.; May, W.; Sposto, R.; Mascarenhas, L. A Phase I Study of Vincristine, Irinotecan, Temozolomide and Bevacizumab (Vitb) in Pediatric Patients with Relapsed Solid Tumors. PLoS ONE 2013, 8, e68416. [Google Scholar] [CrossRef]
- Verschuur, A.C.; Bajčiová, V.; Mascarenhas, L.; Khosravan, R.; Lin, X.; Ingrosso, A.; Janeway, K.A. Sunitinib in pediatric patients with advanced gastrointestinal stromal tumor: Results from a phase I/II trial. Cancer Chemother. Pharmacol. 2019, 84, 41–50. [Google Scholar] [CrossRef]
- Wagner, L.; Turpin, B.; Nagarajan, R.; Weiss, B.; Cripe, T.; Geller, J. Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr. Blood Cancer 2013, 60, 1447–1451. [Google Scholar] [CrossRef]
- Weiss, A.R.; Chen, Y.-L.; Scharschmidt, T.J.; Chi, Y.-Y.; Tian, J.; Black, J.O.; Davis, J.L.; Fanburg-Smith, J.C.; Zambrano, E.; Anderson, J.; et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 1110–1122. [Google Scholar] [CrossRef]
- Wetmore, C.; Daryani, V.M.; Billups, C.A.; Boyett, J.M.; Leary, S.; Tanos, R.; Goldsmith, K.C.; Stewart, C.F.; Blaney, S.M.; Gajjar, A. Phase II evaluation of sunitinib in the treatment of recurrent or refractory high-grade glioma or ependymoma in children: A children’s Oncology Group Study ACNS1021. Cancer Med. 2016, 5, 1416–1424. [Google Scholar] [CrossRef]
- Widemann, B.C.; Kim, A.; Fox, E.; Baruchel, S.; Adamson, P.C.; Ingle, A.M.; Bender, J.G.; Burke, M.; Weigel, B.; Stempak, D.; et al. A Phase I Trial and Pharmacokinetic Study of Sorafenib in Children with Refractory Solid Tumors or Leukemias: A Children’s Oncology Group Phase I Consortium Report. Clin. Cancer Res. 2012, 18, 6011–6022. [Google Scholar] [CrossRef]
- University of Colorado, Denver The Role of Bevacizumab in the Treatment of Radiation Necrosis in Children With Central Nervous System Tumors; clinicaltrials.gov: Bethesda, MD, USA, 2021.
- Novartis Pharmaceuticals A Phase II Study of Pazopanib GW786034, NSC# 737754 in Children, Adolescents and Young Adults With Refractory Solid Tumors; clinicaltrials.gov: Bethesda, MD, USA, 2020.
- Eli Lilly and Company A Phase 1 Study Of Ramucirumab, a Human Monoclonal Antibody Against the Vascular Endothelial Growth Factor-2 (VEGFR-2) Receptor in Children With Refractory Solid Tumors, Including CNS Tumors; clinicaltrials.gov: Bethesda, MD, USA, 2021.
- Gururangan, S.; Chi, S.N.; Poussaint, T.Y.; Onar-Thomas, A.; Gilbertson, R.J.; Vajapeyam, S.; Friedman, H.S.; Packer, R.J.; Rood, B.N.; Boyett, J.M.; et al. Lack of Efficacy of Bevacizumab Plus Irinotecan in Children With Recurrent Malignant Glioma and Diffuse Brainstem Glioma: A Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2010, 28, 3069–3075. [Google Scholar] [CrossRef]
- Vorinostat, Temozolomide, or Bevacizumab in Combination With Radiation Therapy Followed by Bevacizumab and Te-mozolomide in Young Patients With Newly Diagnosed High-Grade Glioma-Tabular View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01236560 (accessed on 28 April 2022).
- Vincristine Sulfate, Topotecan Hydrochloride, and Cyclophosphamide With or Without Bevacizumab in Treating Young Patients With Refractory or First Recurrent Extracranial Ewing Sarcoma-Study Results-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00516295 (accessed on 28 April 2022).
- Cyclophosphamide, Topotecan, and Bevacizumab (CTB) in Patients With Relapsed/Refractory Ewing’s Sarcoma and Neuroblastoma-Study Results-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01492673 (accessed on 28 April 2022).
- Gururangan, S.; Fangusaro, J.; Poussaint, T.Y.; Onar-Thomas, A.; Gilbertson, R.J.; Vajapeyam, S.; Gajjar, A.; Goldman, S.; Friedman, H.S.; Packer, R.J.; et al. Lack of efficacy of bevacizumab + irinotecan in cases of pediatric recurrent ependymoma--a Pediatric Brain Tumor Consortium study. Neuro-Oncology 2012, 14, 1404–1412. [Google Scholar] [CrossRef]
- Gururangan, S.; Fangusaro, J.; Poussaint, T.Y.; McLendon, R.E.; Onar-Thomas, A.; Wu, S.; Packer, R.J.; Banerjee, A.; Gilbertson, R.J.; Fahey, F.; et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—A Pediatric Brain Tumor Consortium study. Neuro-Oncology 2014, 16, 310–317. [Google Scholar] [CrossRef]
- Packer, R.J.; Jakacki, R.; Horn, M.; Rood, B.; Vezina, G.; MacDonald, T.; Fisher, M.J.; Cohen, B. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr. Blood Cancer 2009, 52, 791–795. [Google Scholar] [CrossRef]
- Crotty, E.E.; Leary, S.E.S.; Geyer, J.R.; Olson, J.M.; Millard, N.E.; Sato, A.A.; Ermoian, R.P.; Cole, B.L.; Lockwood, C.M.; Paulson, V.A.; et al. Children with DIPG and high-grade glioma treated with temozolomide, irinotecan, and bevacizumab: The Seattle Children’s Hospital experience. J. Neuro-Oncol. 2020, 148, 607–617. [Google Scholar] [CrossRef]
- Zhukova, N.; Rajagopal, R.; Lam, A.; Coleman, L.; Shipman, P.; Walwyn, T.; Williams, M.; Sullivan, M.; Campbell, M.; Bhatia, K.; et al. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019, 8, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Parekh, C.; Jubran, R.; Erdreich-Epstein, A.; Panigrahy, A.; Blüml, S.; Finlay, J.; Dhall, G. Treatment of children with recurrent high grade gliomas with a bevacizumab containing regimen. J. Neuro-Oncol. 2011, 103, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Navid, F. Phase I and Clinical Pharmacology Study of Bevacizumab, Sorafenib, and Low-Dose Cyclophosphamide in Children and Young Adults with Refractory/Recurrent Solid Tumors (Vol 19, Pg 236, 2013). Clin. Cancer Res. 2013, 19, 1914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inaba, H.; Panetta, J.C.; Pounds, S.B.; Wang, L.; Li, L.; Navid, F.; Federico, S.M.; Eisenmann, E.D.; Vasilyeva, A.; Wang, Y.-D.; et al. Sorafenib Population Pharmacokinetics and Skin Toxicities in Children and Adolescents with Refractory/Relapsed Leukemia or Solid Tumor Malignancies. Clin. Cancer Res. 2019, 25, 7320–7330. [Google Scholar] [CrossRef]
- Interiano, R.B.; McCarville, M.B.; Wu, J.; Davidoff, A.M.; Sandoval, J.; Navid, F. Pneumothorax as a complication of combination antiangiogenic therapy in children and young adults with refractory/recurrent solid tumors. J. Pediatr. Surg. 2015, 50, 1484–1489. [Google Scholar] [CrossRef][Green Version]
- Peyrl, A.; Chocholous, M.; Kieran, M.W.; Azizi, A.A.; Prucker, C.; Czech, T.; Dieckmann, K.; Schmook, M.-T.; Haberler, C.; Leiss, U.; et al. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors: Antiangio-genic Therapy for Embryonal Tumors. Pediatr. Blood Cancer 2012, 59, 511–517. [Google Scholar] [CrossRef]
- Benesch, M.; Windelberg, M.; Sauseng, W.; Witt, V.; Fleischhack, G.; Lackner, H.; Gadner, H.; Bode, U.; Urban, C. Compassionate use of bevacizumab (Avastin®) in children and young adults with refractory or recurrent solid tumors. Ann. Oncol. 2008, 19, 807–813. [Google Scholar] [CrossRef]
- Kim, A.; Widemann, B.C.; Krailo, M.; Ms, N.J.; Fox, E.; Weigel, B.; Blaney, S.M. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 1562–1566. [Google Scholar] [CrossRef]
- Raciborska, A.; Bilska, K. Sorafenib in patients with progressed and refractory bone tumors. Med. Oncol. 2018, 35, 126. [Google Scholar] [CrossRef]
- Schmid, I.; Häberle, B.; Albert, M.H.; Corbacioglu, S.; Fröhlich, B.; Graf, N.; Kammer, B.; Kontny, U.; Leuschner, I.; Scheel-Walter, H.-G.; et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr. Blood Cancer 2012, 58, 539–544. [Google Scholar] [CrossRef]
- Fox, E.; Widemann, B.C.; Chuk, M.K.; Marcus, L.; Aikin, A.; Whitcomb, P.O.; Merino, M.J.; Lodish, M.; Dombi, E.; Steinberg, S.M.; et al. Vandetanib in Children and Adolescents with Multiple Endocrine Neoplasia Type 2B Associated Medullary Thyroid Carcinoma. Clin. Cancer Res. 2013, 19, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Spini, A.; Roberto, G.; Gini, R.; Bartolini, C.; Bazzani, L.; Donnini, S.; Crispino, S.; Ziche, M. Evidence of β-blockers drug repurposing for the treatment of triple negative breast cancer: A systematic review. Neoplasma 2019, 66, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Spini, A.; Donnini, S.; Pantziarka, P.; Crispino, S.; Ziche, M. Repurposing of drugs for triple negative breast cancer: An overview. ecancermedicalscience 2020, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Barber, N.A.; Afzal, W.; Akhtari, M. Hematologic toxicities of small molecule tyrosine kinase inhibitors. Target. Oncol. 2011, 6, 203–215. [Google Scholar] [CrossRef]
- Santoni, M.; Rizzo, M.; Burattini, L.; Farfariello, V.; Berardi, R.; Santoni, G.; Carteni, G.; Cascinu, S. Present and future of tyrosine kinase inhibitors in renal cell carcinoma: Analysis of hematologic toxicity. Recent Patents Anti-Infective Drug Discov. 2012, 7, 104–110. [Google Scholar] [CrossRef]
- Bekeschus, S. Acquired cancer tyrosine kinase inhibitor resistance: ROS as critical determinants. Signal Transduct. Target. Ther. 2021, 6, 437. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef]
- Ciccone, V.; Morbidelli, L.; Ziche, M.; Donnini, S. How to conjugate the stemness marker ALDH1A1 with tumor angiogenesis, progression, and drug resistance. Cancer Drug Resist 2020, 3, 26–37. [Google Scholar] [CrossRef]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Illouz, F.; Braun, D.; Briet, C.; Schweizer, U.; Rodien, P. ENDOCRINE SIDE-EFFECTS OF ANTI-CANCER DRUGS: Thyroid effects of tyrosine kinase inhibitors. Eur. J. Endocrinol. 2014, 171, R91–R99. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Salti, I. Tyrosine Kinase Inhibitors Induced Thyroid Dysfunction: A Review of Its Incidence, Pathophysiology, Clinical Relevance, and Treatment. BioMed Res. Int. 2013, 2013, e725410. [Google Scholar] [CrossRef] [PubMed]
- Vashty, M. A Phase II Study of Pazopanib (GW786034, NSC# 737754) in Children, Adolescents and Young Adults with Refractory Solid Tumors—The Study Protocol. 183. Available online: https://clinicaltrials.gov/ProvidedDocs/69/NCT01956669/Prot_000.pdf (accessed on 1 September 2022).
- Cirmi, S.; El Abd, A.; Letinier, L.; Navarra, M.; Salvo, F. Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS). Cancers 2020, 12, 826. [Google Scholar] [CrossRef] [PubMed]
- Votri-ent-Epar-Product-Information_en.Pdf. Available online: https://www.ema.europa.eu/en/documents/product-information/votrient-epar-product-information_en.pdf (accessed on 1 September 2022).
- Lee, J.-M.; Annunziata, C.M.; Hays, J.L.; Cao, L.; Choyke, P.; Yu, M.; An, D.; Turkbey, I.B.; Minasian, L.M.; Steinberg, S.M.; et al. Phase II trial of bevacizumab and sorafenib in recurrent ovarian cancer patients with or without prior-bevacizumab treatment. Gynecol. Oncol. 2020, 159, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.M.; Mahoney, M.R.; Loui, W.S.; Roberts, L.R.; Smyrk, T.C.; Gatalica, Z.; Borad, M.; Kumar, S.; Alberts, S.R. Phase I/II Randomized Trial of Sorafenib and Bevacizumab as First-Line Therapy in Patients with Locally Advanced or Metastatic Hepatocellular Carcinoma: North Central Cancer Treatment Group Trial N0745 (Alliance). Target. Oncol. 2017, 12, 201–209. [Google Scholar] [CrossRef]
- Tyrosine Kinase Inhibitors of Vascular Endothelial Growth Factor Receptors in Clinical Trials: Current Status and Future Directions | The Oncologist | Oxford Academic. Available online: https://academic.oup.com/oncolo/article/11/7/753/6397106 (accessed on 8 March 2022).
- Spini, A.; Gini, R.; Rosellini, P.; Singier, A.; Bellan, C.; Pascucci, A.; Leoncini, L.; Mathieu, C.; Martellucci, I.; Furiesi, F.; et al. First-Line Pharmacotherapies and Survival among Patients Diagnosed with Non-Resectable NSCLC: A Real-Life Setting Study with Gender Prospective. Cancers 2021, 13, 6129. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; He, J.; Ren, S.; Wu, F.; Zhang, J.; Wang, F. Gender differences in colorectal cancer survival: A meta-analysis. Int. J. Cancer 2017, 141, 1942–1949. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).