Impact of Bevacizumab on Visual Function, Tumor Size, and Toxicity in Pediatric Progressive Optic Pathway Glioma: A Retrospective Nationwide Multicentre Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Clinical Data Collection
2.3. Response Evaluation
2.3.1. Outcomes
2.3.2. Visual Function
2.3.3. Radiologic Response
2.3.4. Toxicity
2.3.5. Progression
2.4. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Visual Function
3.3. Radiologic Evaluation
3.4. Toxicity Profile
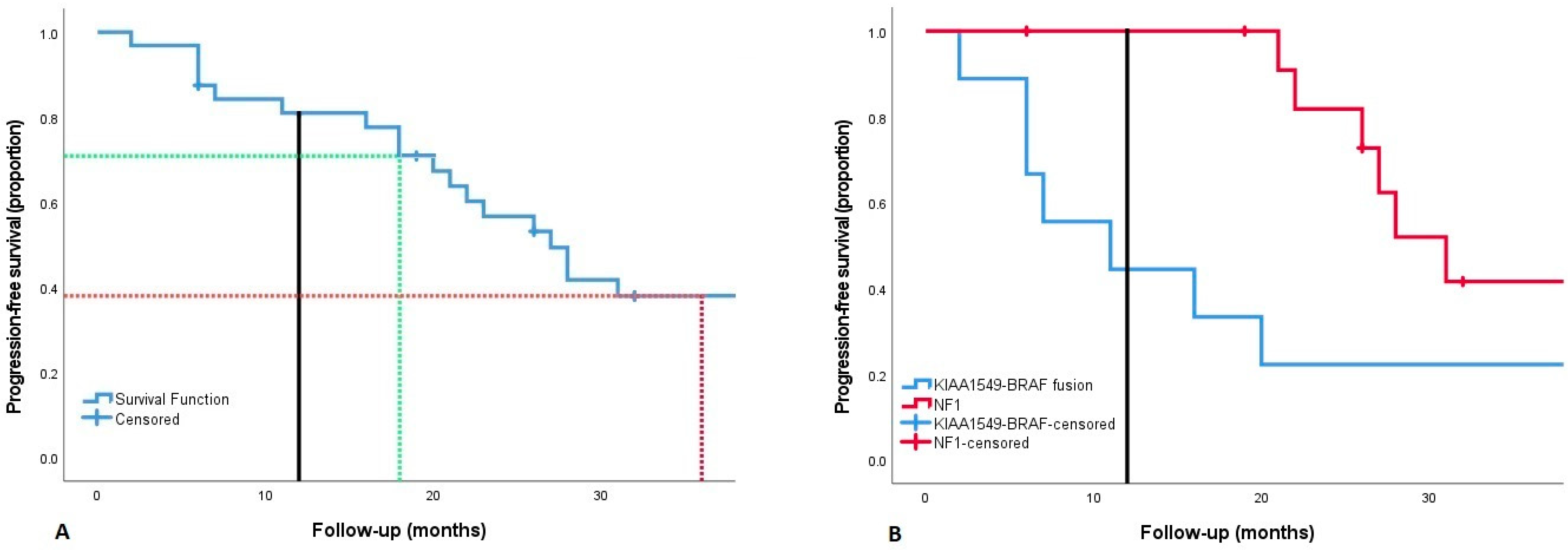
3.5. Progression
4. Discussion
5. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCVA | Best corrected visual acuity |
| BEFIE | Behavioral Visual Field Screening test |
| BVZ | Bevacizumab |
| CI | Confidence interval |
| CR | Complete response |
| CTCAE | Common Terminology Criteria for Adverse Events |
| HFA | Humphrey Visual Field Analyzer |
| IRI | Irinotecan |
| LGG | Low grade glioma |
| MDC | Modified Dodge classification |
| MR | Minor response |
| NF1 | Neurofibromatosis type 1 |
| nNF1 | No Neurofibromatosis type 1 |
| OPG | Optic pathway glioma |
| PA | Pilocytic astrocytoma |
| PD | Progressive disease |
| PFS | Progression-free survival |
| RAPNO | Response Assessment in Pediatric Neuro-Oncology |
| SAT | Systemic anticancer therapy |
| SD | Stable disease |
| UMCU | University Medical Center Utrecht |
| VEGF | Vascular endothelial growth factor |
| VBL | Vinblastine |
| VF | Visual field |
References
- Rasool, N.; Odel, J.G.; Kazim, M. Optic pathway glioma of childhood. Curr. Opin. Ophthalmol. 2017, 28, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.A.; Schouten-van Meeteren, A.Y.N. Current and emerging treatment strategies for children with progressive chiasmatic-hypothalamic glioma diagnosed as infants: A web-based survey. J. Neuro-Oncol. 2018, 136, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Jakacki, R.; Horn, M.; Rood, B.; Vezina, G.; MacDonald, T.; Fisher, M.J.; Cohen, B. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr. Blood Cancer 2009, 52, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Sikkema, A.H.; de Bont, E.S.; Molema, G.; Dimberg, A.; Zwiers, P.J.; Diks, S.H.; Hoving, E.W.; Kamps, W.A.; Peppelenbosch, M.P.; den Dunnen, W.F. Vascular endothelial growth factor receptor 2 (VEGFR-2) signalling activity in paediatric pilocytic astrocytoma is restricted to tumour endothelial cells. Neuropathol. Appl. Neurobiol. 2011, 37, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Couec, M.L.; André, N.; Thebaud, E.; Minckes, O.; Rialland, X.; Corradini, N.; Aerts, I.; Marec Bérard, P.; Bourdeaut, F.; Leblond, P. Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: Toxicity and efficacy trends. Pediatr. Blood Cancer 2012, 59, 34–38. [Google Scholar] [CrossRef]
- Hwang, E.I.; Jakacki, R.I.; Fisher, M.J.; Kilburn, L.B.; Horn, M.; Vezina, G.; Rood, B.R.; Packer, R.J. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr. Blood Cancer 2013, 60, 776–782. [Google Scholar] [CrossRef]
- Gururangan, S.; Fangusaro, J.; Poussaint, T.Y.; McLendon, R.E.; Onar-Thomas, A.; Wu, S.; Packer, R.J.; Banerjee, A.; Gilbertson, R.J.; Fahey, F.; et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—A Pediatric Brain Tumor Consortium study. Neuro-Oncology 2014, 16, 310–317. [Google Scholar] [CrossRef]
- Kalra, M.; Heath, J.A.; Kellie, S.J.; Dalla Pozza, L.; Stevens, M.M.; Swamy, S.; McCowage, G.B. Confirmation of Bevacizumab Activity, and Maintenance of Efficacy in Retreatment After Subsequent Relapse, in Pediatric Low-grade Glioma. J. Pediatr. Hematol./Oncol. 2015, 37, e341–e346. [Google Scholar] [CrossRef]
- Green, K.; Panagopoulou, P.; D’Arco, F.; O’Hare, P.; Bowman, R.; Walters, B.; Dahl, C.; Jorgensen, M.; Patel, P.; Slater, O.; et al. A Nationwide Evaluation of Bevacizumab-based Treatments in Paediatric Low-Grade Glioma in the UK: Safety. Efficacy, Visual Morbidity and Outcomes. Neuro-Oncology, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Zhukova, N.; Rajagopal, R.; Lam, A.; Coleman, L.; Shipman, P.; Walwyn, T.; Williams, M.; Sullivan, M.; Campbell, M.; Bhatia, K.; et al. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019, 8, 40–50. [Google Scholar] [CrossRef]
- Gorsi, H.S.; Khanna, P.C.; Tumblin, M.; Yeh-Nayre, L.; Milburn, M.; Elster, J.D.; Crawford, J.R. Single-agent bevacizumab in the treatment of recurrent or refractory pediatric low-grade glioma: A single institutional experience. Pediatr. Blood Cancer 2018, 65, e27234. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Lober, R.M.; Li, M.D.; Partap, S.; Murphy, P.A.; Barnes, P.D.; Fisher, P.G.; Yeom, K.W. Decreased tumor apparent diffusion coefficient correlates with objective response of pediatric low-grade glioma to bevacizumab. J. Neuro-Oncol. 2015, 122, 491–496. [Google Scholar] [CrossRef]
- de Marcellus, C.; Tauziède-Espariat, A.; Cuinet, A.; Pasqualini, C.; Robert, M.P.; Beccaria, K.; Puget, S.; Boddaert, N.; Figarella-Branger, D.; De Carli, E.; et al. The role of irinotecan-bevacizumab as rescue regimen in children with low-grade gliomas: A retrospective nationwide study in 72 patients. J. Neuro-Oncol. 2022, 157, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J.; Witt, O.; Hernáiz Driever, P.; Bag, A.K.; de Blank, P.; Kadom, N.; Kilburn, L.; Lober, R.M.; Robison, N.J.; Fisher, M.J.; et al. Response assessment in paediatric low-grade glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e305–e316. [Google Scholar] [CrossRef] [PubMed]
- Shofty, B.; Ben-Sira, L.; Freedman, S.; Yalon, M.; Dvir, R.; Weintraub, M.; Toledano, H.; Constantini, S.; Kesler, A. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr. Blood Cancer 2011, 57, 481–485. [Google Scholar] [CrossRef]
- Fisher, M.J.; Loguidice, M.; Gutmann, D.H.; Listernick, R.; Ferner, R.E.; Ullrich, N.J.; Packer, R.J.; Tabori, U.; Hoffman, R.O.; Ardern-Holmes, S.L.; et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: A multicenter retrospective analysis. Neuro-Oncology 2012, 14, 790–797. [Google Scholar] [CrossRef]
- Banerjee, A.; Jakacki, R.I.; Onar-Thomas, A.; Wu, S.; Nicolaides, T.; Young Poussaint, T.; Fangusaro, J.; Phillips, J.; Perry, A.; Turner, D.; et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncology 2017, 19, 1135–1144. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Young Poussaint, T.; Lensing, S.; Wu, S.; Ligon, A.H.; Lindeman, N.; Stewart, C.F.; Jones, D.T.W.; Pfister, S.M.; et al. Selumetinib in pediatric patients with non-neurofibromatosis type 1-associated, nonoptic pathway (opg) and non-pilocytic recurrent/progressive low-grade glioma harboring BRAF V600E mutation or BRAF-kiaa1549 fusion: A multicenter prospective pediatric brain tumor consortium (PBTC) phase 2 trial. Neuro-Oncology 2022, 24, i88. [Google Scholar] [CrossRef]
- Hawkins, C.; Walker, E.; Mohamed, N.; Zhang, C.; Jacob, K.; Shirinian, M.; Alon, N.; Kahn, D.; Fried, I.; Scheinemann, K.; et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin. Cancer Res. 2011, 17, 4790–4798. [Google Scholar] [CrossRef]
- Jacob, K.; Albrecht, S.; Sollier, C.; Faury, D.; Sader, E.; Montpetit, A.; Serre, D.; Hauser, P.; Garami, M.; Bognar, L.; et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br. J. Cancer 2009, 101, 722–733. [Google Scholar] [CrossRef]
- Taylor, T.; Jaspan, T.; Milano, G.; Gregson, R.; Parker, T.; Ritzmann, T.; Benson, C.; Walker, D. Radiological classification of optic pathway gliomas: Experience of a modified functional classification system. Br. J. Radiol. 2008, 81, 761–766. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems: 10th Revision. ICD-10 Version 2019. Available online: https://icd.who.int/browse10/2019/en#/H54.9 (accessed on 3 November 2022).
- Moussa, G.; Bassilious, K.; Mathews, N. A novel excel sheet conversion tool from Snellen fraction to LogMAR including ‘counting fingers’, ‘hand movement’, ‘light perception’ and ‘no light perception’ and focused review of literature of low visual acuity reference values. Acta Ophthalmol. 2021, 99, e963–e965. [Google Scholar] [CrossRef] [PubMed]
- Koenraads, Y.; Braun, K.P.; van der Linden, D.C.; Imhof, S.M.; Porro, G.L. Perimetry in young and neurologically impaired children: The Behavioral Visual Field (BEFIE) Screening Test revisited. JAMA Ophthalmol. 2015, 133, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Greve, E.L.; Dannheim, F.; Bakker, D. The Peritest, a new automatic and semi-automatic perimeter. Int. Ophthalmol. 1982, 5, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events: Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 3 November 2022).
- de Haas, V.; Grill, J.; Raquin, M.A.; Couanet, D.; Habrand, J.L.; Sainte-Rose, C.; Laithier, V.; Kieffer, V.; Kalifa, C. Relapses of optic pathway tumors after first-line chemotherapy. Pediatr. Blood Cancer 2009, 52, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, C.; Ellis, H.P.; Shaw, A.; Penman, C.; Palmer, A.; Wragg, C.; Greenslade, M.; Haynes, H.R.; Williams, H.; Lowis, S.; et al. BRAF Fusion Analysis in Pilocytic Astrocytomas: KIAA1549-BRAF 15-9 Fusions Are More Frequent in the Midline Than Within the Cerebellum. J. Neuropathol. Exp. Neurol. 2015, 74, 867–872. [Google Scholar] [CrossRef]
- Dodgshun, A.J.; Elder, J.E.; Hansford, J.R.; Sullivan, M.J. Long-term visual outcome after chemotherapy for optic pathway glioma in children: Site and age are strongly predictive. Cancer 2015, 121, 4190–4196. [Google Scholar] [CrossRef]
- Heidary, G.; Fisher, M.J.; Liu, G.T.; Ferner, R.E.; Gutmann, D.H.; Listernick, R.H.; Kapur, K.; Loguidice, M.; Ardern-Holmes, S.L.; Avery, R.A.; et al. Visual field outcomes in children treated for neurofibromatosis type 1-associated optic pathway gliomas: A multicenter retrospective study. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 349.e341–349.e345. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Poussaint, T.Y.; Wu, S.; Ligon, A.H.; Lindeman, N.; Campagne, O.; Banerjee, A.; Gururangan, S.; Kilburn, L.B.; et al. A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: A Pediatric Brain Tumor Consortium study. Neuro-Oncology 2021, 23, 1777–1788. [Google Scholar] [CrossRef]
| Characteristics | Nr of Patients (%) |
|---|---|
| Total population | 33 |
| Sex | |
| Male | 20 (60.6) |
| Female | 13 (39.4) |
| NF1 | 13 (39.4) |
| Diagnosis based on clinical signs | 6 |
| DNA pathogenic mutation | 7 |
| Anatomic location: stage MDC | |
| MDC 2 | 4 (12.1) |
| MDC combined 1&2 | 4 (12.1) |
| MDC combined 2&3 | 154 (45.5) |
| MDC combined ≥ 3 stages | 10 (30.3) |
| Hypothalamic involvement | 28 (84.8) |
| Lepto-meningeal metastases | 6 (18.2) |
| Biopsy performed (NF1/nNF1) | 17 (3/14) |
| Obtained during surgery | 9 (1/8) |
| Biopsy only | 8 (2/6) |
| Pathology | 17 (51.2) |
| Pilocytic astrocytoma | 13 |
| Fibrillary astrocytoma | 2 |
| Pilomyxoid astrocytoma | 2 |
| Tumor biology (NF1/nNF1) | 13 (3/10) |
| BRAF V600E mutation (analysed in 12 samples) | 0 |
| KIAA1549-BRAF fusion (analysed in 15 samples) | 9 (0/9) (8 of 9: PA) |
| Age at diagnosis of OPG | |
| Median (yr) (NF1/nNF1) | 2.4 (5.2/1.1) |
| Range (yr) | 0.3–10.2 |
| NF1 | 2.4–10.2 |
| nNF1 | 0.3–5.1 |
| IQR (yr) | 0.8–5.0 |
| Age at start of all therapy | |
| Median (yr) (NF1/nNF1) | 2.4 (5.6–1.2) |
| Range (yr) | 0.3–16.0 |
| NF1 | 2.4–16.0 |
| nNF1 | 0.3–5.4 |
| IQR (yr) | 1.1–5.3 |
| Interval diagnosis OPG-start 1th therapy | 0.1 (0.0–9.3) |
| Age at start of BVZ | |
| Median (yr) (NF1/nNF1) | 7.2 (11.0/4.2) |
| Range (yr) | 0.7–17.7 |
| NF1 | 4.7–17.7 |
| nNF1 | 0.7–15.1 |
| IQR (yr) | 3.4–11.0 |
| Indication start BVZ | |
| Radiologic progression | 19 (57.6) |
| Radiologic progression and visual deterioration | 1 (3.0) |
| Visual deterioration | 12 (36.4) |
| New metastases | 1 (3.0) |
| BVZ initiation in SAT episode | |
| 1st | 1 (3.0) |
| 2nd | 13 (39.4) |
| 3rd | 16 (48.5) |
| 4th | 3 (9.1) |
| Visual Function & Change | |
|---|---|
| BCVA at Start of BVZ (n = eyes) | 52 |
| Bilateral blindness (n = patients) | 3 |
| Blind eyes | 13 |
| No data (n = per eye) | 14 |
| BCVA per eye 1 (n = eyes) | 39 |
| Median (LogMAR) | 0.4 |
| Range | −0.1–2.7 |
| IQR | 0.1–1.3 |
| Binocular BCVA 1 at start BVZ (n = patients) | 23 |
| Median (LogMAR) | 0.2 |
| Range | −0.1–1.8 |
| IQR | 0.0–1.0 |
| BCVA after end BVZ (n = eyes) | 52 |
| Bilateral blindness (n = patients) | 3 |
| Monocular blindness (n = eyes) | 13 |
| No data (n= eyes) | 10 |
| BCVA per eye 1 (n = eyes) | 39 |
| Median (LogMAR) | 0.3 |
| Range | −0.1–3.0 |
| IQR | 0.0–1.3 |
| Change in BCVA 1 (n = eyes) | 39 |
| Median (LogMAR) | 0 |
| Range | −0.7–1.2 |
| IQR | −0.1–0.02 |
| Improvement (≤0.2 LogMAR) | 8 (20.5%) |
| Stable (change within 0.2 LogMAR) | 29 (74.4%) |
| Decrease (≥0.2 LogMAR) | 2 (5.1%) |
| Change in VF (n = eyes) | 26 |
| Improvement | 19 (73.1%) |
| Stable | 4 (15.4%) |
| Decrease | 2 (7.7%) |
| Shift 2 | 1 (3.8%) |
| Side Effect | Grade CTCAE | N (%) |
|---|---|---|
| Nausea | Grade 2 | 4 (12.1) |
| Hypertension | Grade 2 | 1 (3.0) |
| Grade 3 | 3 (9.1) | |
| Proteinuria | Grade 2 | 2 (6.1) |
| Grade 3 | 1 (3.0) | |
| Fatigue | Grade 2 | 2 (6.1) |
| Abdominal pain | Grade 3 | 1 (3.0) |
| Colitis | Grade 2 | 1 (3.0) |
| Gastric hemorrhage | Grade 3 | 1 (3.0) |
| Pneumonia | Grade 3 | 1 (3.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennebroek, C.A.M.; van Zwol, J.; Porro, G.L.; Oostenbrink, R.; Dittrich, A.T.M.; Groot, A.L.W.; Pott, J.W.; Janssen, E.J.M.; Bauer, N.J.; van Genderen, M.M.; et al. Impact of Bevacizumab on Visual Function, Tumor Size, and Toxicity in Pediatric Progressive Optic Pathway Glioma: A Retrospective Nationwide Multicentre Study. Cancers 2022, 14, 6087. https://doi.org/10.3390/cancers14246087
Bennebroek CAM, van Zwol J, Porro GL, Oostenbrink R, Dittrich ATM, Groot ALW, Pott JW, Janssen EJM, Bauer NJ, van Genderen MM, et al. Impact of Bevacizumab on Visual Function, Tumor Size, and Toxicity in Pediatric Progressive Optic Pathway Glioma: A Retrospective Nationwide Multicentre Study. Cancers. 2022; 14(24):6087. https://doi.org/10.3390/cancers14246087
Chicago/Turabian StyleBennebroek, Carlien A. M., Judith van Zwol, Giorgio L. Porro, Rianne Oostenbrink, Anne T. M. Dittrich, Annabel L. W. Groot, Jan W. Pott, Etienne J. M. Janssen, Noël J. Bauer, Maria M. van Genderen, and et al. 2022. "Impact of Bevacizumab on Visual Function, Tumor Size, and Toxicity in Pediatric Progressive Optic Pathway Glioma: A Retrospective Nationwide Multicentre Study" Cancers 14, no. 24: 6087. https://doi.org/10.3390/cancers14246087
APA StyleBennebroek, C. A. M., van Zwol, J., Porro, G. L., Oostenbrink, R., Dittrich, A. T. M., Groot, A. L. W., Pott, J. W., Janssen, E. J. M., Bauer, N. J., van Genderen, M. M., Saeed, P., Lequin, M. H., de Graaf, P., & Schouten-van Meeteren, A. Y. N. (2022). Impact of Bevacizumab on Visual Function, Tumor Size, and Toxicity in Pediatric Progressive Optic Pathway Glioma: A Retrospective Nationwide Multicentre Study. Cancers, 14(24), 6087. https://doi.org/10.3390/cancers14246087






