Simple Summary
Patients with cancer may face bone metastases and osteoporosis due to cancer or treatments, leading to a high risk of developing skeletal-related events. Skeletal-related events may negatively affect patients’ quality and length of life. Although physical exercise has been recognized as a potential adjunctive strategy in the cancer setting, it is often not recommended to patients with bone health impairments due to safety concerns. In the present review, we explore the effects of exercise on safety profile, bone health, and the impact on functional outcomes in patients with cancer affected by bone metastasis, osteoporosis/osteopenia, or at high risk of losing bone. Moreover, the underlying mechanisms of the beneficial effect of exercise on bone are explored, and considerations about exercise prescription are discussed.
Abstract
Bone health is often threatened in cancer patients. Bone metastasis and osteoporosis frequently occur in patients with cancer and may lead to different skeletal-related events, which may negatively affect patients’ quality of life and are associated with high mortality risk. Physical exercise has been recognized as a potential adjunctive strategy in the cancer setting to improve physical function as well as treatment-related side effects. Nevertheless, exercise is often not recommended to patients with bone health impairments due to safety concerns. In the current review, we aimed, through a comprehensive review of the evidence, to explore the impact of exercise in terms of safety profile, bone outcomes, and the effects on other outcomes in patients with cancer affected by bone metastasis or at high risk of losing bone. Additionally, we explored the potential mechanisms by which exercise may act on bone, particularly the impact of mechanical load on bone remodeling. Finally, considerations about exercise prescription and programming in these populations are also discussed.
1. Introduction
Bone health is often threatened in patients with cancer. Cancer and its treatments may damage the skeleton, exposing patients to an increased risk of skeletal-related events (SREs).
Bone metastasis is, firstly, a frequent complication in solid tumors, occurring most frequently in advanced prostate (85%), breast (70%), lung (40%), and kidney (40%) malignancies [1]. The spine, pelvis, skull, ribs, proximal humeri, and femora are the areas most affected by the metastatic spread, probably reflecting the distribution of the red bone marrow [1]. Bone metastases may be classified as osteoblastic, osteolytic, or mixed. Osteoblastic bone metastases, developing through the stimulation of osteoblast proliferation and differentiation, are associated with the deposition of new pathological bone and are typically observed in prostate cancer [2]. On the other hand, osteolytic metastases, often occurring in breast, lung, and renal cancers, are characterized by osteocyte activation, thus resulting in the destruction of normal bone [2]. In some cases, bone metastases may be mixed, i.e., with both components of osteoblastic and osteolytic lesions [2]. Bone metastases expose patients to a high risk of SREs, such as pathological fracture, the need for radiotherapy and/or surgery to bone, spinal cord compression, and hypercalcemia. Skeletal morbidities are reported in lung (53.4%), prostate (45.9%), and breast (43.6%) cancers with bone involvement [3] and occur more frequently in patients with osteolytic lesions [4]. Different treatment options are available, including radiotherapy, surgery, bone-targeted agents (i.e., bisphosphonates and denosumab), as well as systemic treatment for the underlying oncological disease. Nevertheless, these approaches have a major role only in preventing disease progression and palliating symptoms [5].
On the other hand, patients affected by early stage cancer may be subjected to an increased loss of bone mineral density, leading to developing osteoporosis, i.e., a systemic disorder characterized by low bone mass leading to bone fragility. A prospective study revealed a prevalence of 16% for osteoporosis and 44% for osteopenia (a loss of bone density not so severe as osteoporosis) among 1041 patients affected by different malignancies [6]. The peak bone mass, usually reached around 30 years, is the major determinant of an individual bone density [7], which begins to decline due to age and changes in sex-steroid hormones [7]. Beyond genetic predisposition, several lifestyle factors may accelerate bone loss, such as tobacco smoking, high alcohol consumption, impaired mobility, low body weight, nutritional deficiencies (e.g., calcium intake), and low physical activity [8]. Additionally, some drugs, e.g., corticosteroids, and different anticancer treatments, including gonadotropin-releasing hormone (GnRH) agonists, chemotherapy-induced ovarian failure (CIOF), aromatase inhibitors (AIs), and androgen-deprivation therapy (ADT), may decrease bone mineral density [8]. For instance, patients with non-metastatic prostate cancer treated with ADT experience a reduction in bone mineral density ranging from 2.29% to 5.5% during the first year of treatment, which continues throughout the second year at a slower rate [9]. Similarly, patients in postmenopausal status undergoing AIs may report a decrease in bone mass of around 2–3% per year, whereas premenopausal women lose bone by approximately 7 and 7.7% due to CIOF and GnRH agonists, respectively [10]. Despite bone-targeted agents, as well as positive changes in lifestyle factors associated with the risk of osteoporosis may help slow down bone loss [8], patients undergoing the aforementioned anticancer treatments have an elevated risk of fracture [10,11].
SREs related to bone metastases and fractures related to osteoporosis may impair patients’ quality of life and are associated with an increased risk of mortality [12,13] and a high economic burden [14]. In this scenario, strategies addressed to preserve physical function, improve patients’ quality of life, decrease the risk of falls, as well as improve bone health and, thus, diminish the risk of SREs and fractures, are crucial.
Physical activity and exercise have been proven to be beneficial in the oncological setting. Observational evidence suggested a positive association between physical activity and survival, especially in breast, colorectal, and prostate cancers [15]. Additionally, randomized controlled trials have demonstrated the beneficial effects of exercise in improving physical function through an increase in cardiorespiratory fitness [16], muscle strength [17], and optimization of body composition [17]. Exercise intervention may help to enhance the quality of life and ameliorate some side effects of cancer and its treatments, such as fatigue, peripheral neuropathy, and lymphoedema; moderate evidence is available for bone health [18]. Regarding bone health, exercise may be an important tool for improving bone remodeling, matrix mineralization, and marrow health, thus leading to the preservation of bone mineral density [19,20]. Nevertheless, despite the well-known benefits of exercise, some concerns about safety issues in patients suffering from bone fragility may arise. In this sense, a survey found that about 40% of oncology providers working in lung cancer settings report having no opinion or agree that exercise should be avoided in patients with bone metastases [21]. Similarly, another study involving oncologists and palliative care physicians shows that 65% are worried about a potential increase in fracture risk due to physical activity in patients with metastatic bone disease, osteoporosis, or undergoing ADT [22]. In contrast, the large majority of patients with bone metastases consider it important to be physically active, they feel able to exercise, and are interested in participating in exercise programs [23]. Although prior reviews have investigated the effect of exercise in patients with bone metastases or those at risk of bone loss in separate studies [24,25], a comprehensive review including both aspects is currently missing. With these premises, the present review aims to elucidate the impact of exercise in patients with bone metastases and those with or at risk of developing osteoporosis regarding safety, efficacy on bone health, psychological well-being, and health-related fitness components, such as cardiorespiratory fitness, strength, and body composition. Additionally, the potential mechanisms by which exercise could remodel bone mass and considerations about exercise prescription in these populations are also summarized.
2. Materials and Methods
To explore the role of exercise in patients with cancer affected by bone metastases or osteoporosis at risk of bone loss, a comprehensive search on PubMed (MEDLINE), Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and SPORTDiscus was performed. The following keywords drove the research: “metastatic cancer”, “bone metastases”, “osteoporosis”, “osteopenia”, “bone health”, “exercise”, “physical activity”, “physical exercise”. Trials were included if they had a randomized controlled design; included patients with cancer affected by bone metastasis or those with osteoporosis/osteopenia or at high risk of developing it; and investigated the effect of exercise as a form of planned, structured, and repetitive body movement to improve physical fitness components. Published abstracts, non-full text, non-English articles, and interventions involving general physical activity recommendations were excluded. Two independent reviewers screened the literature (G.B. and L.O.). Disagreements were discussed and resolved by a third reviewer (A.A.).
3. Exercise and Bone Metastasis
A series of investigations have explored the impact of exercise on different outcomes, including safety profile, effect on bone mass, and other parameters, in patients with bone metastases (Table 1).

Table 1.
Overview of the randomized controlled studies conducted on patients with bone metastases.
3.1. Safety of Exercise
The National Cancer Institute defines an adverse event (AE) as an “unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medical treatment or procedure that may or may not be considered related to the medical treatment or procedure” [49]. Based on this definition, AEs in studies testing exercise can be categorized as non-exercise-related AEs, i.e., unrelated to exercise intervention, and exercise-related AEs, i.e., occurred during the exercise sessions. One investigation did not measure safety [42]. Whereas eight trials did not record any AEs during the exercise period [26,27,28,29,30,31,36,39,43,45,48,50], two investigations reported exercise-related AEs [33,34,35,44], two described non-exercise-related AEs [38,41], and four recorded both exercise and non-exercise-related AEs [37,40,46,47]. Among them, only two studies reported serious AEs related to exercise training [33,34,40]. Concerning non-exercise-related AEs, the number of reported side effects appears similar among patients who engage in an exercise intervention compared to the controls [37,38,40,41]. On the other hand, most AEs occurring during the exercise sessions were classified as non-serious, such as fatigue, back pain, dizziness, and muscle strain, whereas only two studies have associated exercise with serious SREs [33,34,35,40,41,44]. For instance, Uth and colleagues, in their trial which tested soccer in a sample of 57 patients with advanced prostate cancer (19.3% of them with bone metastasis), reported two fibula fractures and one partial rupture of the Achilles tendon during the training [33,34,35]. Nevertheless, whether these AEs occurred in patients with or without bone metastases is unclear. A second investigation on 214 men with prostate cancer (19% with bone metastases) described two ruptures of the Achilles tendon associated with exercise [40]. In this case, however, a sub-analysis revealed that those side effects occurred in patients without skeletal metastasis, thus excluding the possible association with bone disease [46]. Of note, focusing on the type of exercise, no serious SREs were observed in trials investigating resistance training as part of the exercise sessions.
Overall, the available data support the safety profile of exercise in patients with cancer affected by bone metastases, even in those interventions which included resistance training, an activity traditionally considered at high risk for fracture. However, some considerations are mandatory. Firstly, patients included in the current investigations might be highly selected and, thus, not fully representative of the entire cancer population with bone metastases. In this sense, most studies are addressed to patients with prostate and breast cancers, whereas limited or no information for other cancer types, such as lung or kidney, is available. Additionally, inclusion criteria for selecting patients with bone metastases rarely report detailed information and often exclude the frailest patients, such as those with bone pain or unstable metastases [26,27,33,39,44,45]. Secondly, the adopted criteria for monitoring and reporting AEs are sometimes not specified or heterogeneous across the studies. The introduction of a standardized classification may help to improve the accuracy of AE monitoring, which is fundamental to adequately assess safety, while preserving patient’s safety within a clinical trial. Future investigations should address these gaps in order to definitely consolidate the safety profile of exercise in patients with cancer affected by metastatic bone disease.
3.2. Effect of Exercise on Bone Health
In healthy subjects, physical exercise is a recognized lifestyle component able to maximize bone development, and improve and preserve bone health across the lifespan [51,52]. On the other hand, whether or not exercise may harbor the same benefits in patients with bone metastasis is still a significant subject of debate.
The available studies in this setting show mixed results [29,34,35,41,46,47]. Bjerre and colleagues found no significant differences in total hip and spine bone mineral density after 6 months of soccer training in 41 patients affected by prostate cancers with skeletal metastases [46]. Another similar investigation has explored bone adaptation to soccer training in 57 patients with advanced or metastatic prostate cancer (19.3% of them with bone metastases). Whereas post-intervention evaluations did not detect improvements in total body and leg bone mineral density, the bone mineral content of the leg (mean difference 13.8 g, 95% CI: 7.0 to 20.5 g) and total (mean difference 26.4 g, 95% CI: 5.8 to 46.9 g) statistically increased in the experimental group compared to the controls, thus suggesting a possible response in bone tissue after an exercise intervention [34]. Beyond the systemic impact on bone quality, exercise may directly affect the bone lesion, potentially contributing to its remineralization. In this sense, a randomized controlled trial testing isometric resistance exercise did not show significant differences in the density of the metastatic bone or pathological fracture rate in 60 patients affected by unstable spinal metastases and undergoing palliative radiotherapy. However, this study is characterized by a short survival in both groups (mean 4.4 months), leading to a high dropout rate (73% in the experimental group and 63% in the controls), which makes it difficult to know if the lack of results in bone outcomes are attributable to the small sample size or to exercise ineffectiveness [43]. Another similar investigation has compared the effect of exercise on metastatic bone density during radiotherapy in patients with stable spinal metastases [29]. Sixty patients were randomized to receive passive muscle therapy (controls), or isometric resistance training performed five days per week over two weeks and then three times per week until six months. Compared to controls that remained stable, the experimental group reported an improvement in bone density in all spine metastases, which significantly increased by 28.3% and 80.3% after three and six months, respectively. A sub-analysis by metastasis types revealed that, while no differences emerged from osteoblastic lesions, osteolytic metastases seemed to benefit more from exercise, increasing their density by about 88.8% and 179.3% after three and six months [29]. Moreover, biochemical evaluations found significant enhancements in bone turnover markers, especially pyridinoline and C-terminal cross-linking telopeptide of type I collagen, in the experimental group [32], further strengthening the hypothesis that exercise might be an adjunctive strategy able to produce a synergistic effect on radiotherapy to improve the recalcification of metastases.
3.3. The Overall Effect of Exercise
Across the studies including patients with bone metastases, other outcomes, such as physical function, treatment-related side effects, and quality of life, have been investigated. Most of the investigations reported improvements in cardiorespiratory fitness and muscle strength [26,36,37,38,39,44,45,47], whereas the results on body composition appear more debated [33,34,36,38,39,44,46,47]. For instance, Cormie et al., in a randomized controlled trial in patients with bone metastatic prostate cancer, observed that 12 weeks of resistance training at moderate intensity twice a week was able to improve muscle strength, aerobic capacity, and lean body mass, whereas no effect in fat mass was detected [26]. On the contrary, a similar study combining aerobic and resistance training for three months in patients with metastatic bone disease confirmed a positive increase in strength and cardiorespiratory function but did not find any significant changes in lean and fat mass [36].
Regarding patient-reported outcomes, more than half of the studies did not report improvement in quality of life, distress, and fatigue levels, nor did they report negative effects [26,36,37,38,40,41,43,44], while other investigations suggest a possible positive impact on these outcomes [27,39,42,45,46]. Intriguingly, pain level has also been monitored. Rief and colleagues, in their trial assessing resistance training in patients with spinal bone metastasis, observed that exercise was able to relieve pain levels and reduce the oral morphine dose, as well as the concomitant non-opioid analgesics over six months [53]. Another three-arm randomized controlled trial including 516 patients with mixed cancer types (51.3% with bone metastases) has compared controls (arm 1) versus telerehabilitation (arm 2) (composed of walking-based program and resistance activities) and telerehabilitation plus pharmacological pain management (arm 3). After six months, compared to controls, both interventions exhibited equal effectiveness in improving pain interference (arm 2, −0.4: 95% CI: −0.78 to −0.09; arm 3, −0.4: 95% CI: −0.79 to −0.10) and intensity (arm 2, −0.4: 95% CI: −0.78 to −0.07; arm 3, −0.5: 95% CI: −0.84 to −0.11). Additionally, the total hospital days (335 days for arm 1 vs. 213 days for arm 2 vs. 284 days for arm 3) and the length of stay (7.4 days for arm 1 vs. 3.5 days for arm 2 vs. 5.0 days for arm 3) were lower in experimental groups than the control arm [42]. Since pain is one of the most impactful consequences of bone metastases, seriously affecting patients’ independence and quality of life, exercise may be considered a non-pharmacological adjunctive therapy with a potential analgesic effect in this setting.
4. Exercise and Bone Loss
Different studies have investigated the role of exercise in both patients with non-metastatic disease at high risk of losing bone and in those with a recognized bone fragility condition, i.e., affected by osteopenia or osteoporosis (Table 2).

Table 2.
Overview of the randomized controlled studies conducted on patients with cancer affected by osteopenia/osteoporosis or at risk of bone loss.
4.1. Safety of Exercise
Although most investigations have not assessed the presence or absence of AEs [54,55,57,58,59,62,63,66,68,70,72], the reported findings support the safety profile of exercise [56,61,62,64,65,67,69,71,73,74,75]. In trials including patients with cancer at high risk of accelerated bone loss, e.g., those undergoing chemotherapy, endocrine therapy, or in postmenopausal status, the majority did not find any serious AEs [56,62,64,65,69,71,74], while three registered mild side effects [61,74,75]. For instance, Nikander and colleagues, in their randomized controlled trial, which consisted of a 12-month exercise intervention involving patients with breast cancer undergoing endocrine therapy, recorded 4 moderate AEs [61]. The reported injuries were related to overuse, such as joint/muscle pain and muscle stiffness. However, these side effects were transient, and patients fully recovered in a few days [61]. Considering the studies including patients with bone health impairments (e.g., osteoporosis or osteopenia), no AEs were registered [55,67,73]. Notably, no skeletal fractures have occurred neither in interventions involving high-impact training, such as that of Taaffe et al., which proposed for patients with prostate cancer (50% with osteopenia, 4% with osteoporosis) initiating ADT a six-month supervised aerobic nor during resistance training at high impact [73]. Considered comprehensively, exercise appears safe in this population; however, given the inconsistency in the collection and reporting of the AEs across the investigations, the abovementioned considerations made for the metastatic bone disease are relevant here as well.
4.2. Effect of Exercise on Bone Health
Exercise has been hypothesized as a strategy able to counteract the acceleration of bone loss due to cancer and its treatments. In this sense, a meta-analysis, including 26 randomized controlled trials, has demonstrated that exercise may produce significant improvements in bone-related outcomes, such as whole body, hip, trochanter, and femoral neck bone density among patients with cancer [25]. Analyzing the trials, which included patients at high risk of losing bone, some reported the inability of exercise to preserve bone in patients with cancer [56,65,66,68,70,71]. On the other hand, different investigations found improvements in bone mineral density among patients at high risk of losing bone tissue, even if considerable heterogeneity regarding the skeletal sites has been observed [59,60,62,64,69,74,75]. For instance, a 12-month randomized controlled trial, including 498 patients with breast cancer treated with chemotherapy and/or radiotherapy and/or undergoing endocrine treatments, has explored the impact of a supervised weekly aerobic or circuit training plus home-based, vigorous-intensity aerobic activity 2–3 times per week on bone tissue. Post-intervention evaluations revealed that compared to usual care, women in premenopausal status who performed the experimental intervention reported preservation in femoral neck bone mineral density (−0.2%, 95% CI: −0.9 to 0.6 vs. −1.4%, 95% CI: −2.1 to 0–07; p = 0.01), but not in the lumbar spine [59]. On the contrary, Winters-Stone and colleagues reported that 12 months of combined aerobic and resistance exercise intervention was able to improve lumbar spine body mass density (0.41 vs. −2.27; p = < 0.01), but not that of the femoral neck (−1.37 vs. −2.06; p = 0.27) in postmenopausal patients with breast cancer [60]. Focusing on studies that included patients with cancer and a diagnosed osteopenia or osteoporosis condition, only one investigation did not report improvements in terms of bone outcomes [67]. A 6-month exercise intervention, composed of a supervised and home-based aerobic training program performed 5 days per week, was shown to maintain bone mineral density in 75 postmenopausal women with breast cancer, 11% affected by osteopenia [55]. Another trial investigating 12 months of supervised and unsupervised strength training twice a week did not produce significant effects in terms of the bone mineral density of the spine and hip. However, the subtle changes in bone tissue were sufficient to produce a shift in the distribution of bone categories favoring the experimental group over the controls: a major number of women allocated in the usual care group became osteopenic at the spine compared to patients who performed the exercise program [63]. However, two main factors seem to influence the effectiveness of exercise in bone enhancement: adherence to exercise training and the timing of starting the exercise program with respect to endocrine therapy. For instance, a randomized controlled trial has investigated the effect of 24-month strength training on bone mineral density, in addition to calcium, vitamin D, and risedronate, in 249 patients with breast cancer affected by osteoporosis or osteopenia. The intention-to-treat analysis did not find significant differences in bone health improvement compared to controls that received medication alone. Per-protocol analysis revealed that those patients who attended at least ≥50% of the exercise sessions were less likely to lose bone than controls. In particular, in this subgroup of subjects, only 1.2% and 12.3% lost total hip and femoral neck bone mineral density, respectively, in contrast to controls, in which 8.6% and 26.7% reported a decrease in bone in the same skeletal sites [58]. Regarding the optimal timing for exercise initiation according to endocrine therapy, a study involving 104 patients with prostate cancer has explored if it is more efficacious to prevent bone loss using exercise from the start of ADT rather than trying to recover bone health initiating training after 6 months of endocrine therapy [73]. In this sense, a group was allocated to an immediate six-month supervised aerobic and resistance training, while the other was assigned to usual care followed by six months of the same training. Although total hip and whole-body bone mineral density declined similarly between the 2 groups, the spine bone mineral density was largely preserved in patients who engaged early in exercise (−0.4% vs. −1.6%), thus suggesting that exercising since the time of treatment may be more efficacious to prevent or attenuate the development of treatment-related side effects [73].
4.3. The Overall Effect of Exercise
Beyond the impact on bone health status, exercise may confer several other benefits to patients with cancer in this setting. Although not all the studies reported positive results on other outcomes, and most found no changes or even an increase in fat tissue [56,60,61,66], exercise may improve physical parameters, as well as patients’ psychological status and quality of life [62,65,66,71,72]. Cormie and colleagues proposed a supervised combined exercise program involving aerobic and strength sessions for 63 patients with prostate cancer scheduled to undergo ADT. After three months, compared to men allocated in the controls, those in the experimental arm experienced significant preservation in appendicular lean mass (mean difference 0.4, CI. 0.1 to 0.7, p = 0.01), a decrease in fat mass (mean difference −1.4, CI: −2.3 to −0.6, p = 0.001), and an increase in cardiorespiratory fitness (mean difference 1.1, CI: 0.4 to 1.9, p = 0.004) and strength. Additionally, the exercisers experienced improvements in treatment-related symptoms, fatigue, sexual activity and function, psychological status (distress and depression), and total cholesterol [65]. Similarly, another investigation on 100 patients with breast cancer in postmenopausal status, which tested 16 weeks of aerobic and strength training thrice a week, found similar results, e.g., improvements in cardiorespiratory fitness, muscle strength fatigue, depression, and quality of life [71]. However, most of the data come from studies that excluded patients affected by bone fragility conditions (osteopenia or osteoporosis), thus necessitating an expansion of research on the impact of exercise in these populations in the future.
5. Mechanisms by Which Exercise Improves Bone
Bone is a dynamic tissue that continuously undergoes remodeling throughout life, thanks to the constant activities of renewal and repair [76]. In this sense, bone homeostasis is strictly regulated by the well-balanced actions of osteoclasts, responsible for bone resorption, and osteoblasts involved in the formation of new bone. Whereas these two processes, if stable, guarantee a constant amount of bone, some conditions may impair the regulatory pathways shifting the balance towards an accelerated bone turnover (e.g., osteoporosis, bone metastases) and/or an increase bone production [76]. The main determinant of bone remodeling is represented by the mechanical stress (and, thus, the obtained tissue deformation—strain) induced by the loads carried by the bones. This system, known as “mechanostat theory”, involves bone cells that, if stimulated above a certain threshold of strain, react to strain, shifting the balance toward an increase in bone formation [77]. On the other hand, if the strain produced is lower than the homeostasis threshold, bone loss occurs [77].
In this context, exercise may produce an adequate load stimulus able to enhance bone formation, with deposition predominating over resorption [78]. However, not all the stimuli generated by exercise are similar and produce the same effects on bone turnover. For instance, activities with low/absent mechanical load, such as swimming and cycling, are unable to generate an adequate signal to shift the balance toward bone formation [79]. On the contrary, weight-bearing training, such as walking, stair climbing, and jogging, has been shown to have a great degree of load and, therefore, a greater capacity to induce osteogenesis. Bone modifications are site-specific and not systemic, in other words, a better anabolic response occurs in those skeletal sites subjected to a greater load [79]. Moreover, evidence states that bone mechanical loading is more effective if dynamic rather than static. In addition, the rate of applied strain affects the osteogenic capacity of exercise, i.e., bone responds better if loads are applied at a high rate [79]. In practice, exercises with high impact, e.g., those which include jumping, should be preferred to build bones, even if safety issues regarding these types of activities should always be kept in mind, especially in frail and elderly populations [79]. Finally, bone cells acquire desensitization to the mechanical loading immediately after a few repetitions; thus, inserting rest periods between exercises is the best way to maximize the anabolic response in bone [79].
From a closer perspective (Figure 1), the load produced by exercise is usually perceived by ion channels, cell adhesion/cytoskeletal molecules, and G protein-related molecules, which are classified as mechanoreceptors in bone cells and translate the mechanical stimuli into biological signals [79]. Subsequently, a series of biochemical signaling have been identified as potential pathways to propagate the stimuli within cells and thus activate osteogenesis. In this sense, it has been found that mechanical stimulation activates the prostaglandin G/H synthase (or cyclooxygenase [COX])-prostaglandin E2(PGE2) and nitric oxide (NO) pathways, as well as the OPG/RANKL/RANK signaling pathways which, in turn, have been related to the suppression of bone resorption, and to enhancement in bone formation, thus favoring bone anabolic response [79]. PGE2 has been found to stimulate osteoblasts proliferation and differentiation. The mechanical load may increase osteocyte-derived PGE2 release and the expression of COX-2, the key enzyme involved in PGE2 production [78]. Conversely, NO exhibits dual effects on osteoblasts activity, depending on its concentration. A high dosage of NO induced by cytokine-stimulated cells inhibits bone formation by reducing osteoblasts’ proliferation, enhancing their apoptosis, and increasing the osteoclast-mediated resorption [80]. On the contrary, a low amount of NO, released by mechanically stimulated osteoblasts and osteocytes, has been shown to increase osteoblasts’ proliferation [80]. Exercise may enhance bone by regulating bone morphogenic proteins (BMP). BMP are members of the transforming growth factor beta (TGFβ) superfamily and are directly implied in osteoblastogenesis. The mechanical strain induced by exercise has been shown to upregulate several types of BMP, such as BPM-2, and BMP-7, which enhance the osteoblasts’ differentiation [81]. Moreover, the activity of osteoclasts is highly modulated by the OPG/RANKL/RANK signaling pathways. RANKL is a mediator produced by osteoblasts that can bind RANK, a specific receptor expressed on osteoclast progenitor cells and mature osteoclasts, which in turn enhances the transformation of mononuclear precursors into mature osteoclasts. The OPG, on the other hand, binds RANKL before its interaction with RANK, thus preventing osteoclast differentiation. Exercise acts on this pathway by increasing the level of OPG and reducing the expression of RANKL, finally resulting in an inhibition of osteoclasts’ differentiation and activity [82]. Another pathway triggered by exercise load and suggested as the major contributor to bone cell mechanotransduction is the Wnt signaling pathway [83]. The Wnt pathway modulates the expression of osteoblastic factors which, through the stimulation of the mesenchymal stem cells, promotes the proliferation and differentiation of osteoblast precursors. Moreover, Wnt signaling is also implied in the downregulation of osteoclastic activity and osteoclastogenesis, slowing down bone resorption [83]. The activity of the Wnt signaling is highly modulated by sclerostin, a protein produced by the SOST gene, which inhibits the pathway, thus reducing osteoblastogenesis and bone formation [83]. Mechanical loading can downregulate the sclerostin expression in bone, allowing for the subsequent activation of the Wnt pathway, thereby increasing bone formation and decreasing the resorption through the inhibition of osteoclast activity [84].
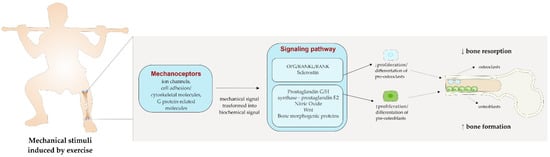
Figure 1.
The effect of mechanical load induced by exercise on bone.
In a more indirect manner, exercise may favor bone anabolic response through the modulation of the inflammatory status. Indeed, emerging evidence suggests that inflammation may elicit a direct impact on bone turnover. The effect of inflammatory processes on bone has been described in several chronic inflammatory diseases, such as periodontitis, rheumatoid arthritis, aseptic prosthesis loosening, and chronic obstructive pulmonary disease [85]. Tumor-promoting inflammation is a hallmark of cancer, making cancer a full-fledged inflammatory disease [86]. Although the exact mechanisms by which inflammation may regulate bone remodeling remain to be elucidated, several cytokines and growth factors have been shown to regulate the osteoblasts’ and osteoclasts’ activity [87]. Some inflammatory mediators, including IL-1, IL-6, and IL-11, may act through the OPG/RANKL/RANK pathway, upregulating the RANKL expression and thus stimulating osteoclastogenesis [88], while others, such as TNF-α, may impair bone remodeling through the disruption of osteoblasts’ differentiation [85]. On the other hand, other cytokines, such as IL-4 and IFN-gamma, have demonstrated an inhibitory effect on osteoclasts’ differentiation, even if it is often overshadowed by those which promote osteoclasts’ activation [85].
6. Consideration about Exercise Prescription in Patients with Bone Metastases or with Osteoporosis
Exercise is a crucial intervention in the oncological setting, able to improve physical parameters and counteract treatment-related side effects [18]. In patients with cancer affected by bone impairments, exercise is able to increase physical function, enhance quality of life and potentially improve bone health. However, to prescribe and deliver a safe and feasible exercise program in a frail population and prevent/reduce the risk of SREs and fractures related to osteoporosis, some considerations should be applied.
To date, specific screening tools to determine the risk and benefit ratio of an exercise program in this setting are currently absent [89]. In this situation, thus, patient assessment is a crucial step for obtaining relevant information to program a safe and personalized exercise program. Beyond the patient’s medical history and anticancer treatment plan, physical and psychological evaluation, including cardiorespiratory fitness, strength, body composition, as well as the barriers and preferences experienced during exercising, allow consideration of the expected heterogeneity among patients [90]. In accordance with the current exercise recommendations for people with bone metastases, physical testing should be adapted (e.g., avoiding tests that apply a high load on metastatic bone sites) [89]. In this sense, the assessment of bone health status is fundamental. Acquiring information regarding the severity of bone impairments, e.g., the status of osteopenia or osteoporosis in non-metastatic patients, as well as the number, type, size, and location of skeletal lesions in people with bone metastases, is essential to target exercise testing and programming [89]. Additionally, whereas osteoporosis is often painless, pain at rest or during movements at the skeletal lesion is one of the most common symptoms in patients with bone metastases [91]. Evaluation of pain, e.g., using the brief pain inventory or visual analogue scale, may be useful to establish its severity [91]. Since pain during functional activity is associated with increased fracture risk, it should also be strictly monitored during exercise [91].
In 2019, the American College of Sports Medicine updated the guidelines for exercise in people with cancer [18]. These recommendations suggest that patients with cancer should engage in moderate-intensity aerobic activity, at least 30 min per session, and resistance training, i.e., contracting the muscles against a resistance to overload and bring about a training effect in the muscular system, 2 times per week, utilizing 2 sets of 8–15 repetitions at moderate intensity [18]. In addition to these cancer-specific exercise guidelines, recommendations about exercise in osteoporotic people may offer additional guidance. Particularly, balance training, i.e., exercises aiming to improve controls of rapid balance reaction, can be included in the exercise sessions in order to prevent falls and, thus, the fracture risk [52,92]. Clearly, these recommendations are general, and exercise should be personalized, taking into account the effect on bone remodeling on the one hand, and safety issues on the other. For instance, concerning aerobic activity, training on a treadmill produces a greater bone anabolic effect compared to cycling; nevertheless, walking on a treadmill may expose patients to higher risk of falls than cycling. A similar comparison could be applied to resistance training. Free-weight resistance training or high-impact training (e.g., jumping) may be more effective in increasing mechanical load and, thus, bone formation with respect to other forms of strength training, e.g., those with isotonic machines or with elastic bands. Even in this case, free-weight resistance and high-impact training may have a greater risk of injury than other activities. To cope with these complex situations, as advised by the International Bone Metastases Exercise Working Group, the prescription and delivery of exercise should be performed by university-qualified exercise professionals who have additional cancer exercise education and appropriate experience in working with patients with bone metastases [89]. These experts possess the professional expertise to adequately weigh the risk–benefit ratio of testing and exercise programs/activities based on the patient’s condition, and may offer appropriate monitoring of the patient’s exercise response while paying attention to the correct exercise technique on postural alignment [89]. A final consideration to keep in mind is related to the fact that most patients might be highly deconditioned. In this sense, it might be necessary to start with a low dose of exercise and progressively increase it over the weeks, according to the patient’s response.
7. Conclusions
According to the available evidence, exercise may offer a safe approach to improving physical function and self-reported outcomes, and to potentially enhance bone health in patients with cancer affected by bone impairments. Although some trials are currently ongoing to enrich the currently available and evidence-based data (Table 3.), additional studies are needed in order to consolidate the impact of exercise on safety and bone outcomes, as well as to develop adequate tools to screen patients’ eligibility for exercise intervention. Based on the currently available evidence, exercise has been shown to be safe and feasible in patients with cancer suffering from bone metastases, affected by osteoporosis/osteopenia, or at risk of bone loss. Moreover, exercise may help improve bone health, physical function, and quality of life, and help manage cancer and treatment-related side effects. Therefore, patients should be supported to engage in sufficient physical activity and encouraged to include in their exercise routine those activities that may favor a bone anabolic response. Nevertheless, given the recognized peculiarity of this population, the prescription and delivery of exercise should be performed by suitable experts who have specific training in these settings.

Table 3.
Randomized controlled trials currently ongoing in patients with bone metastases or in patients with cancer affected by osteoporosis/osteopenia or at risk of bone loss.
Author Contributions
Conceptualization, A.A., S.P., G.B. and L.O.; methodology, A.A. and S.P.; software, A.A., S.P., G.B. and L.O.; validation, A.A., S.P., G.B. and L.O.; formal analysis, A.A., S.P., G.B., L.O. and A.B.; investigation, A.A., S.P., G.B., L.O. and A.B.; resources, A.A., S.P., G.B. and L.O.; data curation, A.A.; writing—original draft preparation, A.A., G.B. and L.O; writing—review and editing, A.A. and S.P.; visualization, A.A., S.P., G.B., L.O., A.B., I.T., D.T., L.B., J.I., M.S., F.S., M.M., F.Z. and E.F.; supervision, A.A. and S.P.; project administration, A.A. and S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. M.M. reports personal fees from Pfizer, EUSA Pharma, and Astra Zeneca, outside the submitted manuscript. S.P. received honoraria or speakers’ fees from Astra-Zeneca, Eli-Lilly, BMS, Boehringer Ingelheim, MSD, and Roche, outside the submitted manuscript. All remaining authors declare that they have no competing interests.
References
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Prim. 2020, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Virk, M.S.; Lieberman, J.R. Tumor metastasis to bone. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S5. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS ONE 2020, 15, e0234927. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Cruz, C.; Smith, C. Risk factors of skeletal-related events in patients with bone metastasis from non-small cell lung cancer undergoing treatment with zoledronate—A post hoc analysis of a randomized clinical trial. Support. Care Cancer 2021, 29, 1629–1633. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Reuss-Borst, M.; Hartmann, U.; Scheede, C.; Weiß, J. Prevalence of osteoporosis among cancer patients in Germany: Prospective data from an oncological rehabilitation clinic. Osteoporos. Int. 2012, 23, 1437–1444. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Van Poznak, C.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers with Nonmetastatic Disease: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2916–2946. [Google Scholar] [CrossRef]
- Morote, J.; Orsola, A.; Abascal, J.M.; Planas, J.; Trilla, E.; Raventos, C.X.; Cecchini, L.; Encabo, G.; Reventos, J. Bone Mineral Density Changes in Patients with Prostate Cancer During the First 2 Years of Androgen Suppression. J. Urol. 2006, 175, 1679–1683; discussion 1683. [Google Scholar] [CrossRef]
- Shapiro, C.L. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers 2020, 12, 3094. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, H.S.; Park, J.-Y.; Kim, J.W.; Ahn, H.K.; Ha, J.S.; Cho, K.S. Androgen-deprivation therapy and the risk of newly developed fractures in patients with prostate cancer: A nationwide cohort study in Korea. Sci. Rep. 2021, 11, 10057. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.E.; De Hoedt, A.M.; Aronson, W.J.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Terris, M.K.; Divers, C.H.; Valderrama, A.; Freedland, S.J. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016, 19, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Lix, L.M.; Azimaee, M.; Metge, C.; Caetano, P.; Leslie, W.D. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos. Int. 2011, 22, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.A.; Bansal, A.; Goulart, B.H.; McCune, J.S.; Karnopp, A.; Fedorenko, C.; Greenlee, S.; Valderrama, A.; Sullivan, S.D.; Ramsey, S.D. The Clinical and Economic Impacts of Skeletal-Related Events Among Medicare Enrollees with Prostate Cancer Metastatic to Bone. Oncologist 2016, 21, 320–326. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sport. Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef]
- Scott, J.M.; Zabor, E.C.; Schwitzer, E.; Koelwyn, G.J.; Adams, S.C.; Nilsen, T.S.; Moskowitz, C.S.; Matsoukas, K.; Iyengar, N.M.; Dang, C.T.; et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2018, 36, 2297–2305. [Google Scholar] [CrossRef]
- Köppel, M.; Mathis, K.; Schmitz, K.H.; Wiskemann, J. Muscle hypertrophy in cancer patients and survivors via strength training. A meta-analysis and meta-regression. Crit. Rev. Oncol. Hematol. 2021, 163, 103371. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sport. Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Schwab, P.; Scalapino, K. Exercise for bone health: Rationale and prescription. Curr. Opin. Rheumatol. 2011, 23, 137–141. [Google Scholar] [CrossRef]
- Pilotto, S.; Avancini, A.; Menis, J.; Sperduti, I.; Levra, M.G.; Berghmans, T.; Bironzo, P.; Brandão, M.; De Ruysscher, D.; Edwards, J.; et al. Exercise in lung Cancer, the healthcare providers opinion (E.C.H.O.): Results of the EORTC lung cancer Group (LCG) survey. Lung Cancer 2022, 169, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sheill, G.; Guinan, E.; Neill, L.O.; Hevey, D.; Hussey, J. Physical activity and advanced cancer: The views of oncology and palliative care physicians in Ireland. Ir. J. Med. Sci. 2018, 187, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.S.; Watanabe, S.M.; Baracos, V.E.; Courneya, K.S. Physical activity interests and preferences in palliative cancer patients. Support. Care Cancer 2009, 18, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.; Hart, N.H.; Bolam, K.A.; Mansfield, S.; Mina, D.S.; Winters-Stone, K.M.; Campbell, A.; Rosenberger, F.; Wiskemann, J.; Quist, M.; et al. Exercise for individuals with bone metastases: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 166, 103433. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Toohey, K. The effect of exercise for improving bone health in cancer survivors—A systematic review and meta-analysis. J. Sci. Med. Sport 2022, 25, 31–40. [Google Scholar] [CrossRef]
- Cormie, P.; Newton, R.U.; Spry, N.; Joseph, D.; Taaffe, D.R.; Galvão, D.A. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013, 16, 328–335. [Google Scholar] [CrossRef]
- Litterini, A.J.; Fieler, V.K.; Cavanaugh, J.T.; Lee, J.Q. Differential Effects of Cardiovascular and Resistance Exercise on Functional Mobility in Individuals with Advanced Cancer: A Randomized Trial. Arch. Phys. Med. Rehabil. 2013, 94, 2329–2335. [Google Scholar] [CrossRef]
- Rief, H.; Omlor, G.; Akbar, M.; Welzel, T.; Bruckner, T.; Rieken, S.; Haefner, M.F.; Schlampp, I.; Gioules, A.; Habermehl, D.; et al. Feasibility of isometric spinal muscle training in patients with bone metastases under radiation therapy—First results of a randomized pilot trial. BMC Cancer 2014, 14, 67. [Google Scholar] [CrossRef]
- Rief, H.; Petersen, L.C.; Omlor, G.; Akbar, M.; Bruckner, T.; Rieken, S.; Haefner, M.F.; Schlampp, I.; Förster, R.; Debus, J.; et al. The effect of resistance training during radiotherapy on spinal bone metastases in cancer patients—A randomized trial. Radiother. Oncol. 2014, 112, 133–139. [Google Scholar] [CrossRef]
- Rief, H.; Akbar, M.; Keller, M.; Omlor, G.; Welzel, T.; Bruckner, T.; Rieken, S.; Häfner, M.F.; Schlampp, I.; Gioules, A.; et al. Quality of life and fatigue of patients with spinal bone metastases under combined treatment with resistance training and radiation therapy- a randomized pilot trial. Radiat. Oncol. 2014, 9, 151. [Google Scholar] [CrossRef]
- Rief, H.; Bruckner, T.; Schlampp, I.; Bostel, T.; Welzel, T.; Debus, J.; Förster, R. Resistance training concomitant to radiotherapy of spinal bone metastases—Survival and prognostic factors of a randomized trial. Radiat. Oncol. 2016, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Rief, H.; Omlor, G.; Akbar, M.; Bruckner, T.; Rieken, S.; Foerster, R.; Schlampp, I.; Welzel, T.; Bostel, T.; Roth, H.J.; et al. Biochemical markers of bone turnover in patients with spinal metastases after resistance training under radiotherapy—A randomized trial. BMC Cancer 2016, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Uth, J.; Hornstrup, T.; Schmidt, J.F.; Christensen, J.F.; Frandsen, C.; Christensen, K.B.; Helge, E.W.; Brasso, K.; Rørth, M.; Midtgaard, J.; et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand. J. Med. Sci. Sport. 2014, 24 (Suppl. S1), 105–112. [Google Scholar] [CrossRef] [PubMed]
- Uth, J.; Hornstrup, T.; Christensen, J.F.; Christensen, K.B.; Jørgensen, N.R.; Helge, E.W.; Schmidt, J.F.; Brasso, K.; Helge, J.W.; Jakobsen, M.D.; et al. Football training in men with prostate cancer undergoing androgen deprivation therapy: Activity profile and short-term skeletal and postural balance adaptations. Eur. J. Appl. Physiol. 2016, 116, 471–480. [Google Scholar] [CrossRef]
- Uth, J.; Hornstrup, T.; Christensen, J.F.; Christensen, K.B.; Jørgensen, N.R.; Schmidt, J.F.; Brasso, K.; Jakobsen, M.D.; Sundstrup, E.; Andersen, L.L.; et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos. Int. 2016, 27, 1507–1518. [Google Scholar] [CrossRef]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Cormie, P.; Joseph, D.; Chambers, S.K.; Chee, R.; Peddle-McIntyre, C.J.; Hart, N.H.; Baumann, F.T.; et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med. Sci. Sport. Exerc. 2018, 50, 393–399. [Google Scholar] [CrossRef]
- Rosenberger, F.; Wiskemann, J.; Vallet, S.; Haag, G.M.; Schembri, E.; Jäger, D.; Grüllich, C. Resistance training as supportive measure in advanced cancer patients undergoing TKI therapy—A controlled feasibility trial. Support. Care Cancer 2017, 25, 3655–3664. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachex-Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Dawson, J.K.; Dorff, T.B.; Schroeder, E.T.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef]
- Bjerre, E.D.; Brasso, K.; Jørgensen, A.B.; Petersen, T.H.; Eriksen, A.R.; Tolver, A.; Christensen, J.F.; Poulsen, M.H.; Madsen, S.S.; Østergren, P.B.; et al. Football Compared with Usual Care in Men with Prostate Cancer (FC Prostate Community Trial): A Pragmatic Multicentre Randomized Controlled Trial. Sport. Med. 2019, 49, 145–158. [Google Scholar] [CrossRef]
- Bjerre, E.D.; Petersen, T.H.; Jørgensen, A.B.; Johansen, C.; Krustrup, P.; Langdahl, B.; Poulsen, M.H.; Madsen, S.S.; Østergren, P.B.; Borre, M.; et al. Community-based football in men with prostate cancer: 1-year follow-up on a pragmatic, multicentre randomised controlled trial. PLoS Med. 2019, 16, e1002936. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A.L.; Moynihan, T.; Herrin, J.; Loprinzi, C.; Kroenke, K. Effect of Collaborative Telerehabilitation on Functional Impairment and Pain Among Patients with Advanced-Stage Cancer: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Sprave, T.; Rosenberger, F.; Verma, V.; Förster, R.; Bruckner, T.; Schlampp, I.; Bostel, T.; Welzel, T.; Akbaba, S.; Rackwitz, T.; et al. Paravertebral Muscle Training in Patients with Unstable Spinal Metastases Receiving Palliative Radiotherapy: An Exploratory Randomized Feasibility Trial. Cancers 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, B.R.; Jorgensen, M.G.; Frystyk, J.; Hordam, B.; Borre, M. Home-based ‘exergaming’ was safe and significantly improved 6-min walking distance in patients with prostate cancer: A single-blinded randomised controlled trial. Br. J. Urol. Int. 2019, 124, 600–608. [Google Scholar] [CrossRef]
- Yee, J.; Davis, G.M.; Hackett, D.; Beith, J.M.; Wilcken, N.; Currow, D.; Emery, J.; Phillips, J.; Martin, A.; Hui, R.; et al. Physical Activity for Symptom Management in Women with Metastatic Breast Cancer: A Randomized Feasibility Trial on Physical Activity and Breast Metastases. J. Pain Symptom Manag. 2019, 58, 929–939. [Google Scholar] [CrossRef]
- Bjerre, E.D.; Weller, S.; Poulsen, M.H.; Madsen, S.S.; Bjerre, R.D.; Østergren, P.B.; Borre, M.; Brasso, K.; Midtgaard, J. Safety and Effects of Football in Skeletal Metastatic Prostate Cancer: A Subgroup Analysis of the FC Prostate Community Randomised Controlled Trial. Sport. Med. Open 2021, 7, 27. [Google Scholar] [CrossRef]
- Via, J.D.; Owen, P.J.; Daly, R.M.; Mundell, N.L.; Livingston, P.M.; Rantalainen, T.; Foulkes, S.J.; Millar, J.L.; Murphy, D.G.; Fraser, S.F. Musculoskeletal Responses to Exercise Plus Nutrition in Men with Prostate Cancer on Androgen Deprivation: A 12-Month RCT. Med. Sci. Sport. Exerc. 2021, 53, 2054–2065. [Google Scholar] [CrossRef]
- Galvão, D.A.; Taaffe, D.R.; Chambers, S.K.; Fairman, C.M.; Spry, N.; Joseph, D.; Newton, R.U. Exercise intervention and sexual function in advanced prostate cancer: A randomised controlled trial. BMJ Support. Palliat. Care 2022, 12, 29–32. [Google Scholar] [CrossRef]
- Institute, N.C. Common Terminology Criteria for Adverse Events (CTCAE). CTEP. Available online: cancer.gov (accessed on 3 September 2022).
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef]
- Beck, B.R.; Daly, R.M.; Singh, M.A.; Taaffe, D.R. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 2017, 20, 438–445. [Google Scholar] [CrossRef]
- Rief, H.; Welzel, T.; Omlor, G.; Akbar, M.; Bruckner, T.; Rieken, S.; Haefner, M.F.; Schlampp, I.; Gioules, A.; Debus, J. Pain response of resistance training of the paravertebral musculature under radiotherapy in patients with spinal bone metastases—A randomized trial. BMC Cancer 2014, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Winters-Stone, K.; Gallucci, B. Exercise Effects on Bone Mineral Density in Women with Breast Cancer Receiving Adjuvant Chemotherapy. Oncol. Nurs. Forum 2007, 34, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Alvarez-Reeves, M.; Cadmus, L.; Mierzejewski, E.; Mayne, S.T.; Yu, H.; Chung, G.G.; Jones, B.; Knobf, M.T.; DiPietro, L. Exercise Improves Body Fat, Lean Mass, and Bone Mass in Breast Cancer Survivors. Obesity 2009, 17, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Hopkins-Price, P.; Vicari, S.; Pamenter, R.; Courneya, K.S.; Markwell, S.; Verhulst, S.; Hoelzer, K.; Naritoku, C.; Jones, L.; et al. A Randomized Trial to Increase Physical Activity in Breast Cancer Survivors. Med. Sci. Sport. Exerc. 2009, 41, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Twiss, J.J.; Waltman, N.L.; Berg, K.; Ott, C.D.; Gross, G.J.; Lindsey, A.M. An Exercise Intervention for Breast Cancer Survivors with Bone Loss. J. Nurs. Sch. 2009, 41, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Waltman, N.L.; Twiss, J.J.; Ott, C.D.; Gross, G.J.; Lindsey, A.M.; Moore, T.E.; Berg, K.; Kupzyk, K. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: A 24-month randomized controlled trial. Osteoporos. Int. 2010, 21, 1361–1369. [Google Scholar] [CrossRef]
- Saarto, T.; Sievänen, H.; Kellokumpu-Lehtinen, P.; Nikander, R.; Vehmanen, L.; Huovinen, R.; Kautiainen, H.; Järvenpää, S.; Penttinen, H.M.; Utriainen, M.; et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos. Int. 2012, 23, 1601–1612. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.; Bennett, J.A.; Leo, M.C.; Naik, A.; Schwartz, A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: A randomized, controlled trial. Breast Cancer Res. Treat. 2011, 127, 447–456. [Google Scholar] [CrossRef]
- Nikander, R.; Sievänen, H.; Ojala, K.; Kellokumpu-Lehtinen, P.L.; Palva, T.; Blomqvist, C.; Luoto, R.; Saarto, T. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients—A 12-month RCT. J. Musculoskelet. Neuronal Interact. 2012, 12, 127–135. [Google Scholar]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.M.; Bennett, J.A.; Leo, M.C.; Torgrimson-Ojerio, B.; Luoh, S.-W.; Schwartz, A. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: A randomized controlled trial. Osteoporos. Int. 2013, 24, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Laudermilk, M.; Woo, K.; Brown, J.C.; Schmitz, K.H. Influence of weight training on skeletal health of breast cancer survivors with or at risk for breast cancer-related lymphedema. J. Cancer Surviv. 2014, 8, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Dobek, J.C.; Bennett, J.A.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Skeletal Response to Resistance and Impact Training in Prostate Cancer Survivors. Med. Sci. Sport. Exerc. 2014, 46, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Galvão, D.A.; Spry, N.; Joseph, D.; Chee, R.; Taaffe, D.R.; Chambers, S.K.; Newton, R.U. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015, 115, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.S.; Raastad, T.; Skovlund, E.; Courneya, K.S.; Langberg, C.W.; Lilleby, W.; Fosså, S.D.; Thorsen, L. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015, 54, 1805–1813. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, Y.U.; Kim, S.J.; Hong, S.; Han, M.S.; Choi, E. The Effect on Bone Outcomes of Adding Exercise to Supplements for Osteopenic Breast Cancer Survivors: A Pilot Randomized Controlled Trial. Cancer Nurs. 2016, 39, 144–152. [Google Scholar] [CrossRef]
- Knobf, M.T.; Jeon, S.; Smith, B.; Harris, L.; Kerstetter, J.; Thompson, A.S.; Insogna, K. Effect of a randomized controlled exercise trial on bone outcomes: Influence of adjuvant endocrine therapy. Breast Cancer Res. Treat. 2016, 155, 491–500. [Google Scholar] [CrossRef]
- Kim, S.H.; Seong, D.H.; Yoon, S.M.; Choi, Y.D.; Choi, E.; Song, Y.; Song, H. The Effect on Bone Outcomes of Home-based Exercise Intervention for Prostate Cancer Survivors Receiving Androgen Deprivation Therapy: A Pilot Randomized Controlled Trial. Cancer Nurs. 2018, 41, 379–388. [Google Scholar] [CrossRef]
- De Paulo, T.R.S.; Winters-Stone, K.M.; Viezel, J.; Rossi, F.E.; Simões, R.R.; Tosello, G.; Freitas, I.F. Effects of resistance plus aerobic training on body composition and metabolic markers in older breast cancer survivors undergoing aromatase inhibitor therapy. Exp. Gerontol. 2018, 111, 210–217. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Thomas, G.A.; Cartmel, B.; Harrigan, M.; Fiellin, M.; Capozza, S.; Zhou, Y.; Ercolano, E.; Gross, C.P.; Hershman, D.; Ligibel, J.; et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity 2017, 25, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Galvão, D.A.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Hayne, D.; Cormie, P.; Shum, D.H.K.; Newton, R.U. Immediate versus delayed exercise in men initiating androgen deprivation: Effects on bone density and soft tissue composition. BJU Int. 2019, 123, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, L.S.; Bloom, J.; Stewart, S.; Sellmeyer, D.E. A Randomized Controlled Trial of Exercise to Prevent Bone Loss in Premenopausal Women with Breast Cancer. J. Women’s Health 2019, 28, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Uth, J.; Fristrup, B.; Sørensen, V.; Helge, E.W.; Christensen, M.K.; Kjærgaard, J.B.; Møller, T.K.; Helge, J.W.; Jørgensen, N.R.; Rørth, M.; et al. One year of Football Fitness improves L1–L4 BMD, postural balance, and muscle strength in women treated for breast cancer. Scand. J. Med. Sci. Sport. 2021, 31, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.; Fernandez-Patron, C. Destroy to Rebuild: The Connection Between Bone Tissue Remodeling and Matrix Metalloproteinases. Front. Physiol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Turner, C.H.; Robling, A.G. Mechanisms by which exercise improves bone strength. J. Bone Miner. Metab. 2005, 23, 16–22. [Google Scholar] [CrossRef]
- Robling, A.G.; Daly, R.; Fuchs, R.K.; Burr, D.B. Mechanical adaptation. In Basic and Applied Bone Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–233. [Google Scholar]
- Klein-Nulend, J.; van Oers, R.F.; Bakker, A.D.; Bacabac, R.G. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos. Int. 2014, 25, 1427–1437. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.; Ge, L.; Pathak, J.L. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications. Curr. Osteoporos. Rep. 2020, 18, 67–80. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.; Zhang, L.; Wu, J.; Guo, J.; Zou, D.; Chen, B.; Sun, Z.; Shen, C.; Zou, J. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog. Biophys. Mol. Biol. 2016, 122, 122–130. [Google Scholar] [CrossRef]
- Choi, R.B.; Robling, A.G. The Wnt pathway: An important control mechanism in bone’s response to mechanical loading. Bone 2021, 153, 116087. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2020, 11, 511799. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Ofotokun, I. Physiological and pathophysiological bone turnover—Role of the immune system. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 215. [Google Scholar] [CrossRef]
- Campbell, K.L.; Cormie, P.; Weller, S.; Alibhai, S.M.H.; Bolam, K.A.; Campbell, A.; Cheville, A.L.; Dalzell, M.-A.; Hart, N.H.; Higano, C.S.; et al. Exercise Recommendation for People with Bone Metastases: Expert Consensus for Health Care Providers and Exercise Professionals. JCO Oncol. Pract. 2022, 18, e697–e709. [Google Scholar] [CrossRef]
- Avancini, A.; Sartori, G.; Gkountakos, A.; Casali, M.; Trestini, I.; Tregnago, D.; Bria, E.; Jones, L.W.; Milella, M.; Lanza, M.; et al. Physical Activity and Exercise in Lung Cancer Care: Will Promises Be Fulfilled? Oncologist 2020, 25, e555–e569. [Google Scholar] [CrossRef]
- Sheill, G.; Guinan, E.M.; Peat, N.; Hussey, J. Considerations for Exercise Prescription in Patients with Bone Metastases: A Comprehensive Narrative Review. PM&R 2018, 10, 843–864. [Google Scholar] [CrossRef]
- Giangregorio, L.M.; Papaioannou, A.; MacIntyre, N.J.; Ashe, M.C.; Heinonen, A.; Shipp, K.; Wark, J.; McGill, S.; Keller, H.; Jain, R.; et al. Too Fit to Fracture: Exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos. Int. 2014, 25, 821–835. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).