Current Insights and Progress in the Clinical Management of Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology and Etiology
3. Clinical Presentation and Diagnosis
3.1. Diagnosis
3.2. An Overview of the Pathophysiology and Histology of HNC
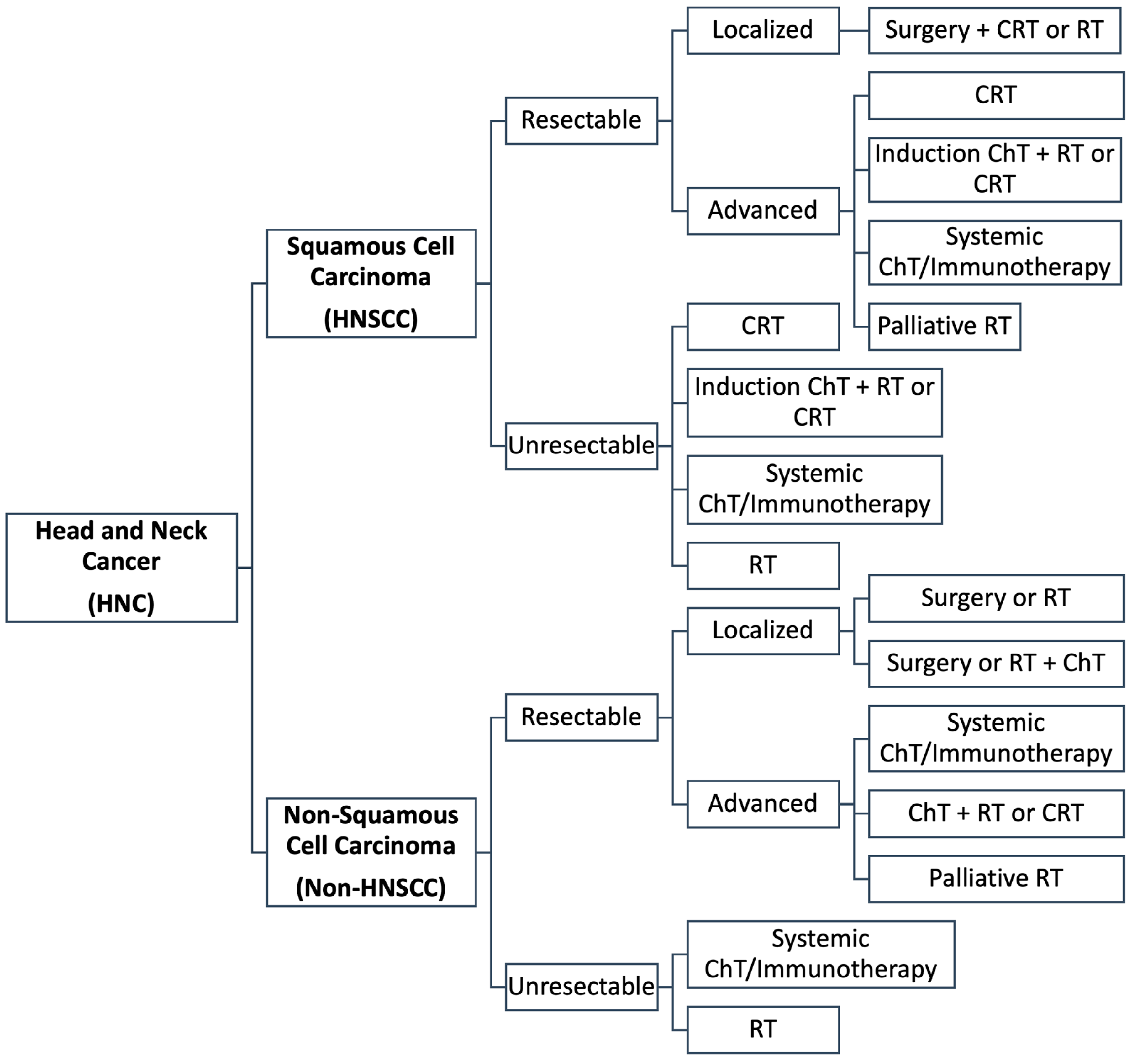
3.3. Current Treatment Approaches
4. Therapeutic Advances in HNC
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leonel, A.; Bonan, R.; Pinto, M.; Kowalski, L.; Perez, D. The Pesticides Use and the Risk for Head and Neck Cancer: A Review of Case-Control Studies. Med. Oral. Patol. Oral Cir. Bucal 2021, 26, e56–e63. [Google Scholar] [CrossRef] [PubMed]
- Heroiu Cataloiu, A.-D.; Danciu, C.E.; Popescu, C.R. Multiple Cancers of the Head and Neck. Maedica 2013, 8, 80–85. [Google Scholar] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Halicek, M.; Little, J.V.; Wang, X.; Chen, A.Y.; Fei, B. Optical Biopsy of Head and Neck Cancer Using Hyperspectral Imaging and Convolutional Neural Networks. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef]
- Poddar, A.; Aranha, R.R.; Muthukaliannan, G.K.; Nachimuthu, R.; Jayaraj, R. Head and Neck Cancer Risk Factors in India: Protocol for Systematic Review and Meta-Analysis. BMJ Open 2018, 8, e020014. [Google Scholar] [CrossRef]
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Jou, A.; Hess, J. Epidemiology and Molecular Biology of Head and Neck Cancer. Oncol. Res. Treat. 2017, 40, 328–332. [Google Scholar] [CrossRef]
- Kase, S.; Baburin, A.; Kuddu, M.; Innos, K. Incidence and Survival for Head and Neck Cancers in Estonia, 1996–2016: A Population-Based Study. Clin. Epidemiol. 2021, 13, 149–159. [Google Scholar] [CrossRef]
- Kawakita, D.; Oze, I.; Iwasaki, S.; Matsuda, T.; Matsuo, K.; Ito, H. Trends in the Incidence of Head and Neck Cancer by Subsite between 1993 and 2015 in Japan. Cancer Med. 2022, 11, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Harvey, A.; Yetukuri, M.; Hansen, A.R.; Simpson, M.C.; Adjei Boakye, E.; Varvares, M.A.; Osazuwa-Peters, N. Rising Incidence of Late-stage Head and Neck Cancer in the United States. Cancer 2020, 126, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.C.; Morrison, S.D.; Dillon, J.K. The Burden of Head and Neck Cancer in the United States, 1990–2017. J. Oral Maxillofac. Surg. 2021, 79, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, K.K.; Grønhøj, C.; Jensen, D.H.; Karnov, K.K.S.; Agander, T.K.; Specht, L.; von Buchwald, C. Increasing Incidence and Survival of Head and Neck Cancers in Denmark: A Nation-Wide Study from 1980 to 2014. Acta Oncol. 2018, 57, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Cabeçadas, J.; Martinez, D.; Andreasen, S.; Mikkelsen, L.H.; Molina-Urra, R.; Hall, D.; Strojan, P.; Hellquist, H.; Bandello, F.; Rinaldo, A.; et al. Lymphomas of the Head and Neck Region: An Update. Virchows Arch. 2019, 474, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Alamgir, W.; Ashraf, A.; Haider, A.; Rashid, S.; Abrar, F. Clinicopathological and Immunohistochemical Profile of Lymphoma of Head and Neck Region. Pak. J. Pathol. 2021, 32, 142–146. [Google Scholar]
- Singh, R.; Shaik, S.; Negi, B.; Rajguru, J.; Patil, P.; Parihar, A.; Sharma, U. Non-Hodgkin’s Lymphoma: A Review. J. Fam. Med. Prim. Care 2020, 9, 1834. [Google Scholar]
- Kuo, C.-Y.; Shih, C.-P.; Cheng, L.-H.; Liu, S.-C.; Chiu, F.-H.; Lin, Y.-Y.; Hu, J.-M.; Chu, Y.-H. Head and Neck Lymphomas: Review of 151 Cases. J. Med. Sci. 2020, 40, 215. [Google Scholar]
- Kamiński, B. Lymphomas of the Head-and-Neck Region. J. Cancer Res. Ther. 2021, 17, 1347. [Google Scholar] [CrossRef]
- Scelsi, C.L.; Wang, A.; Garvin, C.M.; Bajaj, M.; Forseen, S.E.; Gilbert, B.C. Head and Neck Sarcomas: A Review of Clinical and Imaging Findings Based on the 2013 World Health Organization Classification. Am. J. Roentgenol. 2019, 212, 644–654. [Google Scholar] [CrossRef]
- Fabiano, S.; Contiero, P.; Barigelletti, G.; D’Agostino, A.; Tittarelli, A.; Mangone, L.; Bisceglia, I.; Bongiorno, S.; De Lorenzis, L.E.; Mazzoleni, G.; et al. Epidemiology of Soft Tissue Sarcoma and Bone Sarcoma InItaly: Analysis of Data from 15 Population-Based Cancer Registries. Sarcoma 2020, 2020, 6142613. [Google Scholar] [CrossRef]
- Kalavrezos, N.; Sinha, D. Head and Neck Sarcomas in Adulthood: Current Trends and Evolving Management Concepts. Br. J. Oral Maxillofac. Surg. 2020, 58, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Dahlem, K.K.K.; Pfeiffer, J. Prognostic Value of Comorbidities in Patients with Carcinoma of the Major Salivary Glands. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Pfeiffer, J.; Lange, K.; Dahlem, K.K.K. Health-Related Quality of Life in Patients with Major Salivary Gland Carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Thawani, R.; Kim, M.S.; Arastu, A.; Feng, Z.; West, M.T.; Taflin, N.F.; Thein, K.Z.; Li, R.; Geltzeiler, M.; Lee, N.; et al. The Contemporary Management of Cancers of the Sinonasal Tract in Adults. CA Cancer J. Clin. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, S.; Jensen, D.H.; Jakobsen, K.K.; Grønhøj, C.; Geneser, C.; Karnov, K.; Specht, L.; Agander, T.K.; von Buchwald, C. Incidence and Survival in Sinonasal Carcinoma: A Danish Population-Based, Nationwide Study from 1980 to 2014. Acta Oncol. 2018, 57, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; D’Souza, G. Epidemiology of Head and Neck Cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 379–396. [Google Scholar] [CrossRef]
- Park, J.-O.; Nam, I.-C.; Kim, C.-S.; Park, S.-J.; Lee, D.-H.; Kim, H.-B.; Han, K.-D.; Joo, Y.-H. Sex Differences in the Prevalence of Head and Neck Cancers: A 10-Year Follow-Up Study of 10 Million Healthy People. Cancers 2022, 14, 2521. [Google Scholar] [CrossRef]
- Mazul, A.L.; Naik, A.N.; Zhan, K.Y.; Stepan, K.O.; Old, M.O.; Kang, S.Y.; Nakken, E.R.; Puram, S.V. Gender and Race Interact to Influence Survival Disparities in Head and Neck Cancer. Oral Oncol. 2021, 112, 105093. [Google Scholar] [CrossRef]
- Tataru, D.; Mak, V.; Simo, R.; Davies, E.A.; Gallagher, J.E. Trends in the Epidemiology of Head and Neck Cancer in London. Clin. Otolaryngol. 2017, 42, 104–114. [Google Scholar] [CrossRef]
- Mehanna, H.; Paleri, V.; West, C.M.L.; Nutting, C. Head and Neck Cancer--Part 1: Epidemiology, Presentation, and Prevention. BMJ 2010, 341, c4684. [Google Scholar] [CrossRef] [PubMed]
- Wyss, A.; Hashibe, M.; Chuang, S.-C.; Lee, Y.-C.A.; Zhang, Z.-F.; Yu, G.-P.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; et al. Cigarette, Cigar, and Pipe Smoking and the Risk of Head and Neck Cancers: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013, 178, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Galbiatti, A.L.S.; Padovani-Junior, J.A.; Maníglia, J.V.; Rodrigues, C.D.S.; Pavarino, É.C.; Goloni-Bertollo, E.M. Head and Neck Cancer: Causes, Prevention and Treatment. Braz. J. Otorhinolaryngol. 2013, 79, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Fedewa, S.; Chen, A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. North Am. 2018, 30, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Del Signore, A.G.; Megwalu, U.C. The Rising Incidence of Major Salivary Gland Cancer in the United States. Ear, Nose Throat J. 2017, 96, E13–E16. [Google Scholar] [CrossRef]
- Pan, S.Y.; de Groh, M.; Morrison, H. A Case-Control Study of Risk Factors for Salivary Gland Cancer in Canada. J. Cancer Epidemiol. 2017, 2017, 4909214. [Google Scholar] [CrossRef]
- Hashim, D.; Sartori, S.; Brennan, P.; Curado, M.P.; Wünsch-Filho, V.; Divaris, K.; Olshan, A.F.; Zevallos, J.P.; Winn, D.M.; Franceschi, S.; et al. The Role of Oral Hygiene in Head and Neck Cancer: Results from International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann. Oncol. 2016, 27, 1619–1625. [Google Scholar] [CrossRef]
- Porceddu, S.V.; Veness, M.J.; Guminski, A. Nonmelanoma Cutaneous Head and Neck Cancer and Merkel Cell Carcinoma: Current Concepts, Advances, and Controversies. J. Clin. Oncol. 2015, 33, 3338–3345. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Chien, C.-Y.; Luo, S.-D.; Huang, T.-L.; Lin, W.-C.; Fang, F.-M.; Chiu, T.-J.; Chen, Y.-H.; Lai, C.-C.; Hsu, C.-M.; et al. Betel Nut Chewing History Is an Independent Prognosticator for Smoking Patients with Locally Advanced Stage IV Head and Neck Squamous Cell Carcinoma Receiving Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil. World J. Surg. Oncol. 2016, 14, 86. [Google Scholar] [CrossRef]
- Rahman, Q.B.; Iocca, O.; Kufta, K.; Shanti, R.M. Global Burden of Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2020, 32, 367–375. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-Associated Head and Neck Cancer: A Virus-Related Cancer Epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Castellsagué, X.; Chaturvedi, A.; Goodman, M.T.; Snijders, P.; Tommasino, M.; Arbyn, M.; Franceschi, S. Eurogin Roadmap: Comparative Epidemiology of HPV Infection and Associated Cancers of the Head and Neck and Cervix. Int. J. Cancer 2014, 134, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Abogunrin, S.; Di Tanna, G.L.; Keeping, S.; Carroll, S.; Iheanacho, I. Prevalence of Human Papillomavirus in Head and Neck Cancers in European Populations: A Meta-Analysis. BMC Cancer 2014, 14, 968. [Google Scholar] [CrossRef] [PubMed]
- Gooi, Z.; Chan, J.Y.K.; Fakhry, C. The Epidemiology of the Human Papillomavirus Related to Oropharyngeal Head and Neck Cancer. Laryngoscope 2016, 126, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Betiol, J.; Villa, L.L.; Sichero, L. Impact of HPV Infection on the Development of Head and Neck Cancer. Braz. J. Med. Biol. Res. 2013, 46, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, C.; Wagner, S.; Mayer, C.S.; Klussmann, J.P. Basics of Tumor Development and Importance of Human Papilloma Virus (HPV) for Head and Neck Cancer. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2012, 11, Doc09. [Google Scholar]
- Syrjänen, S. The Role of Human Papillomavirus Infection in Head and Neck Cancers. Ann. Oncol. 2010, 21, vii243–vii245. [Google Scholar] [CrossRef]
- Sinha, P.; Logan, H.L.; Mendenhall, W.M. Human Papillomavirus, Smoking, and Head and Neck Cancer. Am. J. Otolaryngol. 2012, 33, 130–136. [Google Scholar] [CrossRef]
- Tsang, C.M.; Lui, V.W.Y.; Bruce, J.P.; Pugh, T.J.; Lo, K.W. Translational Genomics of Nasopharyngeal Cancer. Semin. Cancer Biol. 2020, 61, 84–100. [Google Scholar] [CrossRef]
- Shah, K.M.; Young, L.S. Epstein–Barr Virus and Carcinogenesis: Beyond Burkitt’s Lymphoma. Clin. Microbiol. Infect. 2009, 15, 982–988. [Google Scholar] [CrossRef]
- Raghupathy, R.; Hui, E.P.; Chan, A.T.C. Epstein-Barr Virus as a Paradigm in Nasopharyngeal Cancer: From Lab to Clinic. Am. Soc. Clin. Oncol. Educ. B. 2014, 34, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Merhi, M.; Raza, A.; Inchakalody, V.P.; Abdelouahab, N.; Zar Gul, A.R.; Uddin, S.; Dermime, S. Role of Epstein–Barr Virus in the Pathogenesis of Head and Neck Cancers and Its Potential as an Immunotherapeutic Target. Front. Oncol. 2018, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Polverini, P.J.; Lingen, M.W. A History of Innovations in the Diagnosis and Treatment of Oral and Head and Neck Cancer. J. Dent. Res. 2019, 98, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pulido, G.; Medina, D.I.; Barani, M.; Rahdar, A.; Sargazi, G.; Baino, F.; Pandey, S. Nanomaterials for the Diagnosis and Treatment of Head and Neck Cancers: A Review. Materials 2021, 14, 3706. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of Head-and-Neck Cancer from Exhaled Breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, A.A.; Almangush, A.; Youssef, O.; Metsälä, M.; Silén, S.; Nixon, I.J.; Haigentz, M.; Rodrigo, J.P.; Saba, N.F.; Vander Poorten, V.; et al. Exhaled Breath Analysis in the Diagnosis of Head and Neck Cancer. Head Neck 2020, 42, 787–793. [Google Scholar] [CrossRef]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, Plasma and Saliva Biomarkers for Head and Neck Cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef]
- Cabezas-Camarero, S.; Pérez-Segura, P. Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers 2022, 14, 2858. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Dharmawardana, N.; Woods, C.; Watson, D.I.; Yazbeck, R.; Ooi, E.H. A Review of Breath Analysis Techniques in Head and Neck Cancer. Oral Oncol. 2020, 104, 104654. [Google Scholar] [CrossRef] [PubMed]
- Konings, H.; Stappers, S.; Geens, M.; De Winter, B.Y.; Lamote, K.; van Meerbeeck, J.P.; Specenier, P.; Vanderveken, O.M.; Ledeganck, K.J. A Literature Review of the Potential Diagnostic Biomarkers of Head and Neck Neoplasms. Front. Oncol. 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kulasinghe, A.; Perry, C.; Nelson, C.; Punyadeera, C. A Liquid Biopsy for Head and Neck Cancers. Expert Rev. Mol. Diagn. 2016, 16, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Bian, L.; Zhang, M.; Bo, F.; Lu, X.; Li, D. Liquid Biopsy and Their Application Progress in Head and Neck Cancer: Focus on Biomarkers CTCs, CfDNA, CtDNA and EVs. Biomark. Med. 2020, 14, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Chantre-Justino, M.; Alves, G.; Delmonico, L. Clinical Applications of Liquid Biopsy in HPV-negative and HPV-positive Head and Neck Squamous Cell Carcinoma: Advances and Challenges. Explor. Target. Anti-Tumor Ther. 2022, 3, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K. Circulating Tumor Cells in Head and Neck Carcinomas. Clin. Chem. 2019, 65, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, M.; Xie, F.; Lou, J.; Zhou, X.; Zhang, L.; Fang, M.; Zhou, F. Exosomes in Head and Neck Cancer: Roles, Mechanisms and Applications. Cancer Lett. 2020, 494, 7–16. [Google Scholar] [CrossRef]
- Cramer, J.D.; Reddy, A.; Ferris, R.L.; Duvvuri, U.; Samant, S. Comparison of the Seventh and Eighth Edition American Joint Committee on Cancer Oral Cavity Staging Systems. Laryngoscope 2018, 128, 2351–2360. [Google Scholar] [CrossRef]
- Shah, J.P.; Montero, P.H. New AJCC/UICC Staging System for Head and Neck, and Thyroid Cancer. Rev. Médica Clínica Las Condes 2018, 29, 397–404. [Google Scholar] [CrossRef]
- Edge, S.B.; Amin, M.B. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Mishra, M.; Upadhyaya, N.; Dive, A.; Bodhade, A. Histological Patterns of Head and Neck Tumors: An Insight to Tumor Histology. J. Oral Maxillofac. Pathol. 2014, 18, 58. [Google Scholar] [CrossRef]
- Helliwell, T.R.; Giles, T.E. Pathological Aspects of the Assessment of Head and Neck Cancers: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S59–S65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendelsohn, A.H.; Lai, C.K.; Shintaku, I.P.; Elashoff, D.A.; Dubinett, S.M.; Abemayor, E.; St. John, M.A. Histopathologic Findings of HPV and P16 Positive HNSCC. Laryngoscope 2010, 120, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kiess, A.; Chung, C.H. Emerging Biomarkers in Head and Neck Cancer in the Era of Genomics. Nat. Rev. Clin. Oncol. 2015, 12, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Wunschel, M.; Neumeier, M.; Utpatel, K.; Reichert, T.E.; Ettl, T.; Spanier, G. Staging More Important than Grading? Evaluation of Malignancy Grading, Depth of Invasion, and Resection Margins in Oral Squamous Cell Carcinoma. Clin. Oral Investig. 2021, 25, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, U. Malignancy Grading of Invasive Fronts of Oral Squamous Cell Carcinomas. Transl. Res. Oral Oncol. 2017, 2, 2057178X1770887. [Google Scholar] [CrossRef]
- Perez-Ordoñez, B. Neuroendocrine Carcinomas of the Larynx and Head and Neck: Challenges in Classification and Grading. Head Neck Pathol. 2018, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aljabab, A.S.; Nason, R.W.; Kazi, R.; Pathak, K.A. Head and Neck Soft Tissue Sarcoma. Indian J. Surg. Oncol. 2011, 2, 286–290. [Google Scholar] [CrossRef]
- Haines, G.K. Pathology of Head and Neck Cancer I: Epithelial and Related Tumors. In Head & Neck Cancer: Current Perspectives, Advances, and Challenges; Springer: Dordrecht, The Netherlands, 2013; pp. 257–287. [Google Scholar]
- Haines, G.K. Pathology of Head and Neck Cancer II: Mesenchymal and Lymphoid Tumors. In Head & Neck Cancer: Current Perspectives, Advances, and Challenges; Springer: Dordrecht, The Netherlands, 2013; pp. 289–311. [Google Scholar]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical Update on Head and Neck Cancer: Molecular Biology and Ongoing Challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.A.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 203–239. [Google Scholar] [CrossRef] [PubMed]
- de Bree, R.; van der Waal, I.; de Bree, E.; René Leemans, C. Management of Adult Soft Tissue Sarcomas of the Head and Neck. Oral Oncol. 2010, 46, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.J. Lymphoma of the Thyroid and Head and Neck. Clin. Oncol. 2012, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Zapater, E.; Bagán, J.; Carbonell, F.; Basterra, J. Malignant Lymphoma of the Head and Neck. Oral Dis. 2010, 16, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.K.; Nestor, M. Targeted Therapy in Head and Neck Cancer. Tumor Biol. 2012, 33, 707–721. [Google Scholar] [CrossRef]
- Casey Fazer-Posorske, P.A.-C. A Multidisciplinary Approach to Head and Neck Cancer. J. Adv. Pract. Oncol. 2021, 12. [Google Scholar] [CrossRef]
- Barrera-Franco, J.L.; Gallegos-Hernández, J.F.; Granados-García, M.; Gurrola-Machuca, H. Multidisciplinary Approach in Head and Neck Cancer. Gac. Mex. Oncol. 2019, 16. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Specenier, P. Optimal Treatment for Recurrent/Metastatic Head and Neck Cancer. Ann. Oncol. 2010, 21, vii252–vii261. [Google Scholar] [CrossRef]
- Price, K.A.R.; Cohen, E.E. Current Treatment Options for Metastatic Head and Neck Cancer. Curr. Treat. Options Oncol. 2012, 13, 35–46. [Google Scholar] [CrossRef]
- Dok, R.; Nuyts, S. HPV Positive Head and Neck Cancers: Molecular Pathogenesis and Evolving Treatment Strategies. Cancers 2016, 8, 41. [Google Scholar] [CrossRef]
- Kitamura, N.; Sento, S.; Yoshizawa, Y.; Sasabe, E.; Kudo, Y.; Yamamoto, T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Kaidar-Person, O.; Gil, Z.; Billan, S. Precision Medicine in Head and Neck Cancer. Drug Resist. Updat. 2018, 40, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Dong, H.; Yang, C.; Liu, Y.; Wu, Y.; Zhu, L.; Tong, X.; Wang, S. A Review on the Advances and Challenges of Immunotherapy for Head and Neck Cancer. Cancer Cell Int. 2021, 21, 406. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for Head and Neck Cancer: Recent Advances and Future Directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.E.H.; Haring, C.T.; Mann, J.E.; Brenner, J.C.; Spector, M.E.; Swiecicki, P.L. Novel Immunotherapeutic Approaches in Head and Neck Cancer. J. Cancer Metastasis Treat. 2019, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Cohen, R.B.; Aggarwal, C. Immunotherapy for Head and Neck Cancer: Latest Developments and Clinical Potential. Ther. Adv. Med. Oncol. 2016, 8, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Vermorken, J.B. Immunotherapy in Head and Neck Cancer: Aiming at EXTREME Precision. BMC Med. 2017, 15, 110. [Google Scholar] [CrossRef]
- Bauml, J.M.; Aggarwal, C.; Cohen, R.B. Immunotherapy for Head and Neck Cancer: Where Are We Now and Where Are We Going? Ann. Transl. Med. 2019, 7, S75. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Di Liello, R.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for Head and Neck Cancer: Present and Future. Crit. Rev. Oncol. Hematol. 2022, 174, 103679. [Google Scholar] [CrossRef]
- Rischin, D.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Braña, I.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Health-Related Quality-of-Life Results from KEYNOTE-048. Oral Oncol. 2022, 128, 105815. [Google Scholar] [CrossRef]
- Marron, T.U.; Ryan, A.E.; Reddy, S.M.; Kaczanowska, S.; Younis, R.H.; Thakkar, D.; Zhang, J.; Bartkowiak, T.; Howard, R.; Anderson, K.G.; et al. Considerations for Treatment Duration in Responders to Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e001901. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, I.; Ascui, R.; Capizzano, A.A. Checkpoint Inhibitors in Head and Neck Cancer. Memo Mag. Eur. Med. Oncol. 2019, 12, 249–252. [Google Scholar] [CrossRef]
- Addeo, R.; Ghiani, M.; Merlino, F.; Ricciardiello, F.; Caraglia, M. CheckMate 141 Trial: All That Glitters Is Not Gold. Expert Opin. Biol. Ther. 2019, 19, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Bahig, H.; Tonneau, M.; Karivedu, V.; Burtness, B. Current Therapy for Metastatic Head and Neck Cancer: Evidence, Opportunities, and Challenges. Am. Soc. Clin. Oncol. Educ. book. Am. Soc. Clin. Oncol. Annu. Meet. 2022, 42, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dickie, J.; Sutavani, R.V.; Pointer, C.; Thomas, G.J.; Savelyeva, N. Targeting Head and Neck Cancer by Vaccination. Front. Immunol. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D. Immunity in Head and Neck Cancer. Cancer Immunol. Res. 2015, 3, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, H.A.; Lee, S.M. Immunotherapy Approaches Beyond PD-1 Inhibition: The Future of Cellular Therapy for Head and Neck Squamous Cell Carcinoma. Curr. Treat. Options Oncol. 2019, 20, 31. [Google Scholar] [CrossRef]
- Simpson, D.R.; Mell, L.K.; Cohen, E.E.W. Targeting the PI3K/AKT/MTOR Pathway in Squamous Cell Carcinoma of the Head and Neck. Oral Oncol. 2015, 51, 291–298. [Google Scholar] [CrossRef]
- Bowles, D.W.; Keysar, S.B.; Eagles, J.R.; Wang, G.; Glogowska, M.J.; McDermott, J.D.; Le, P.N.; Gao, D.; Ray, C.E.; Rochon, P.J.; et al. A Pilot Study of Cetuximab and the Hedgehog Inhibitor IPI-926 in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2016, 53, 74–79. [Google Scholar] [CrossRef]
- Musumeci, F.; Greco, C.; Grossi, G.; Molinari, A.; Schenone, S. Recent Studies on Ponatinib in Cancers Other Than Chronic Myeloid Leukemia. Cancers 2018, 10, 430. [Google Scholar] [CrossRef]
- Forster, M.D.; Mendes, R.; Harrington, K.J.; Guerrero Urbano, T.; Baines, H.; Spanswick, V.J.; Ensell, L.; Hartley, J.A.; Adeleke, S.; Gougis, P.; et al. ORCA-2: A Phase I Study of Olaparib in Addition to Cisplatin-Based Concurrent Chemoradiotherapy for Patients with High Risk Locally Advanced Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2016, 34, TPS6108. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rosen, F.; Stadler, W.M.; Recant, W.; Stenson, K.; Huo, D.; Vokes, E.E. Phase II Trial of ZD1839 in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2003, 21, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK 4/6 Inhibitors as Single Agent in Advanced Solid Tumors. Front. Oncol. 2018, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Wang, H.; Quon, H.; Chung, C.H.; Gillison, M.L.; Agrawal, N.; Richmon, J.; Subramaniam, R.M.; Koch, W.; Gourin, C.G.; et al. A Phase I (Ph1) Study of Dasatinib (D) with Cetuximab (Cet) /Radiation (IMRT) +/− Cisplatin (P) in Stage II, III/IV Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2015, 33, e17036. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.J.; Park, K.; Lee, S.; Sun, J.M.; Keam, B.; An, H.J.; Cho, J.Y.; Kim, J.-S.; Lee, H.; et al. Phase II Trial of Nintedanib in Patients with Recurrent or Metastatic Salivary Gland Cancer: A Multicenter Phase II Study. J. Clin. Oncol. 2016, 34, 6090. [Google Scholar] [CrossRef]
- Karivedu, V.; Riaz, M.K.; Mathews, M.; Kurtzweil, N.; Monroe, I.; Romano, A.; Wise-Draper, T.M. A Phase II Study Evaluating the Efficacy of Niraparib and Dostarlimab (TSR-042) in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2022, 40, TPS6105. [Google Scholar] [CrossRef]
- Sola, A.M.; Johnson, D.E.; Grandis, J.R. Investigational Multitargeted Kinase Inhibitors in Development for Head and Neck Neoplasms. Expert Opin. Investig. Drugs 2019, 28, 351–363. [Google Scholar] [CrossRef]
- Adkins, D.R.; Lin, J.-C.; Sacco, A.; Ley, J.; Oppelt, P.; Vanchenko, V.; Komashko, N.; Yen, C.-J.; Wise-Draper, T.; Lopez-Picazo Gonzalez, J.; et al. Palbociclib and Cetuximab Compared with Placebo and Cetuximab in Platinum-Resistant, Cetuximab-Naïve, Human Papillomavirus-Unrelated Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Double-Blind, Randomized, Phase 2 Trial. Oral Oncol. 2021, 115, 105192. [Google Scholar] [CrossRef]
- Seebacher, N.A.; Stacy, A.E.; Porter, G.M.; Merlot, A.M. Clinical Development of Targeted and Immune Based Anti-Cancer Therapies. J. Exp. Clin. Cancer Res. 2019, 38, 156. [Google Scholar] [CrossRef]
- Guo, C.-X.; Huang, X.; Xu, J.; Zhang, X.-Z.; Shen, Y.-N.; Liang, T.-B.; Bai, X.-L. Combined Targeted Therapy and Immunotherapy for Cancer Treatment. World J. Clin. Cases 2021, 9, 7643–7652. [Google Scholar] [CrossRef]
- Harrington, K.; Cohen, E.; Siu, L.; Rischin, D.; Licitra, L.; Vermorken, J.; Le, Q.-T.; Tahara, M.; Machiels, J.-P.; Hawk, N.; et al. 351 Pembrolizumab plus Lenvatinib vs Chemotherapy and Lenvatinib Monotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma That Progressed on Platinum Therapy and Immunotherapy: LEAP-009. J. Immunother. Cancer 2020, 8, A214. [Google Scholar]
- Kordbacheh, F.; Farah, C.S. Current and Emerging Molecular Therapies for Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5471. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.S.; Morris, T.A.; Gramza, A.; Liu, S.; Khan, S.A.; Colevas, A.D.; Pearce, T.; Rischin, D. A Phase II Study of Tarloxotinib (a Hypoxia Activated Prodrug of a Pan-Erb Tyrosine Kinase Inhibitor) in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck or Skin. Investig. New Drugs 2022, 40, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Filippelli, A.; Ciccone, V.; Spini, A.; Ristori, E.; Ziche, M.; Morbidelli, L. Antiangiogenic Drugs: Chemosensitizers for Combination Cancer Therapy. In Antiangiogenic Drugs as Chemosensitizers in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–66. [Google Scholar]
- Suh, Y.; Amelio, I.; Guerrero Urbano, T.; Tavassoli, M. Clinical Update on Cancer: Molecular Oncology of Head and Neck Cancer. Cell Death Dis. 2014, 5, e1018. [Google Scholar] [CrossRef]
- Vander Broek, R.; Mohan, S.; Eytan, D.; Chen, Z.; Van Waes, C. The PI3K/Akt/MTOR Axis in Head and Neck Cancer: Functions, Aberrations, Cross-Talk, and Therapies. Oral Dis. 2015, 21, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Santuray, R.T.; Johnson, D.E.; Grandis, J.R. New Therapies in Head and Neck Cancer. Trends Cancer 2018, 4, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/MTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef] [PubMed]
- Marquard, F.E.; Jücker, M. PI3K/AKT/MTOR Signaling as a Molecular Target in Head and Neck Cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef]
- Anna Szymonowicz, K.; Chen, J. Biological and Clinical Aspects of HPV-Related Cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef]
- Kroonen, J.S.; Vertegaal, A.C.O. Targeting SUMO Signaling to Wrestle Cancer. Trends Cancer 2021, 7, 496–510. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic Therapy in Head and Neck Cancer: Indications, Outcomes, and Future Prospects. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.F.; Takes, R.P.; Vander Poorten, V.; Ferlito, A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv. Ther. 2018, 35, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Marchal, S.; Dolivet, G.; Lassalle, H.-P.; Guillemin, F.; Bezdetnaya, L. Targeted Photodynamic Therapy in Head and Neck Squamous Cell Carcinoma: Heading into the Future. Lasers Med. Sci. 2015, 30, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.; Stevenson, H.; Jerjes, W. From Basic Mechanisms to Clinical Research: Photodynamic Therapy Applications in Head and Neck Malignancies and Vascular Anomalies. J. Clin. Med. 2021, 10, 4404. [Google Scholar] [CrossRef]
- Saini, R.; Lee, N.; Liu, K.; Poh, C. Prospects in the Application of Photodynamic Therapy in Oral Cancer and Premalignant Lesions. Cancers 2016, 8, 83. [Google Scholar] [CrossRef]
- Silva, C.O.; Rijo, P.; Molpeceres, J.; Ascensão, L.; Roberto, A.; Fernandes, A.S.; Gomes, R.; Pinto Coelho, J.M.; Gabriel, A.; Vieira, P.; et al. Bioproduction of Gold Nanoparticles for Photothermal Therapy. Ther. Deliv. 2016, 7, 287–304. [Google Scholar] [CrossRef]
- Costa, E.; Ferreira-Gonçalves, T.; Cardoso, M.; Coelho, J.M.P.; Gaspar, M.M.; Faísca, P.; Ascensão, L.; Cabrita, A.S.; Reis, C.P.; Figueiredo, I.V. A Step Forward in Breast Cancer Research: From a Natural-Like Experimental Model to a Preliminary Photothermal Approach. Int. J. Mol. Sci. 2020, 21, 9681. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Gaspar, M.M.; Coelho, J.M.P.; Marques, V.; Viana, A.S.; Ascensão, L.; Carvalho, L.; Rodrigues, C.M.P.; Ferreira, H.A.; Ferreira, D.; et al. The Role of Rosmarinic Acid on the Bioproduction of Gold Nanoparticles as Part of a Photothermal Approach for Breast Cancer Treatment. Biomolecules 2022, 12, 71. [Google Scholar] [CrossRef]
- Amaral, M.; Charmier, A.J.; Afonso, R.A.; Catarino, J.; Faísca, P.; Carvalho, L.; Ascensão, L.; Coelho, J.M.P.; Gaspar, M.M.; Reis, C.P. Gold-Based Nanoplataform for the Treatment of Anaplastic Thyroid Carcinoma: A Step Forward. Cancers 2021, 13, 1242. [Google Scholar] [CrossRef]
- Lopes, J.; Ferreira-Gonçalves, T.; Figueiredo, I.V.; Rodrigues, C.M.P.; Ferreira, H.; Ferreira, D.; Viana, A.S.; Faísca, P.; Gaspar, M.M.; Coelho, J.M.P.; et al. Proof-of-Concept Study of Multifunctional Hybrid Nanoparticle System Combined with NIR Laser Irradiation for the Treatment of Melanoma. Biomolecules 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Coelho, J.M.P.; Vieira, P.M.C.; Viana, A.S.; Gaspar, M.M.; Reis, C. Preliminary Assays towards Melanoma Cells Using Phototherapy with Gold-Based Nanomaterials. Nanomaterials 2020, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. How to Treat Melanoma? The Current Status of Innovative Nanotechnological Strategies and the Role of Minimally Invasive Approaches like PTT and PDT. Pharmaceutics 2022, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cai, W. Nanomedicine for Targeted Photothermal Cancer Therapy: Where Are We Now? Nanomedicine 2015, 10, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.; Wang, F.; Yang, M.; Xie, L.; Zeng, X. Biosafety, Nontoxic Nanoparticles for VL–NIR Photothermal Therapy Against Oral Squamous Cell Carcinoma. ACS Omega 2021, 6, 11240–11247. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the Application of Inorganic Nanomaterials in Cancer Photothermal Therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, Long-Term Toxicity, and Mechanistic Studies of Gold Nanorods Photothermal Therapy of Cancer in Xenograft Mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold Nanorod Assisted Near-Infrared Plasmonic Photothermal Therapy (PPTT) of Squamous Cell Carcinoma in Mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Betzer, O.; Ankri, R.; Motiei, M.; Popovtzer, R. Theranostic Approach for Cancer Treatment: Multifunctional Gold Nanorods for Optical Imaging and Photothermal Therapy. J. Nanomater. 2015, 16, 381. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold Nanoparticle-mediated Photothermal Therapy: Applications and Opportunities for Multimodal Cancer Treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]

| Non-infectious Risk Factors | Substance Use | Tobacco |
| Betel chewing | ||
| Alcohol | ||
| Behavioural | Poor oral and dental hygiene | |
| Solar exposure | ||
| Dietary Deficiencies | Inadequate nutrition | |
| Vitamin A | ||
| Iron (in Plummer–Vinson Syndrome) | ||
| Occupational Hazards | Asbestos | |
| Radium | ||
| Mustard Gas | ||
| Nickel | ||
| Radiation | ||
| Leather tanning by-products | ||
| Woodworking by-products | ||
| Chromium | ||
| Infectious Risk Factors | HPV | |
| EBV | ||
| Anatomical Location | T | N | M | Stage | ||
|---|---|---|---|---|---|---|
| Unknown | T0 (location unknown) | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | M0 | III | ||
| N2 (3–6 cm ipsilateral nodal metastasis and ENE-) | M0 | IVA | ||||
| N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | M0 | IVB | ||||
| Any N | M1 | IVC | ||||
| Lip and Oral Cavity | T1 (tumour ≤ 2 cm, DOI ≤ 5 mm) | N0 (no regional nodal metastasis) | M0 | I | ||
| T2 (tumour ≤ 2 cm, DOI 5–10 mm) | II | |||||
| T3 (tumour > 4 cm or DOI >10 mm) | III | |||||
| T1,2,3 | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | |||||
| T4a (moderate local disease) | N0,1 | IVA | ||||
| T1,2,3,4a | N2 (3–6 cm ipsilateral nodal metastasis and ENE-, or multiple ≤6 cm ipsilateral nodal metastasis and ENE-, or ≤6 cm bilateral or contralateral nodal metastasis) | |||||
| Any T | N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | IVB | ||||
| T4b (very advanced local disease) | Any N | |||||
| Any T | M1 | IVC | ||||
| Major Salivary Glands | Tis (carcinoma in situ) | N0 (no regional nodal metastasis) | M0 | 0 | ||
| T1 (tumour ≤ 2 cm, w/o extraparenchymal extension) | I | |||||
| T2 (tumour 2–4 cm, w/o extraparenchymal extension) | II | |||||
| T3 (tumour ≥ 4 cm, and/or with extraparenchymal extension) | III | |||||
| T0,1,2,3 | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | |||||
| T4a (moderate local disease) | N0,1 | IVA | ||||
| T0, 1, 2, 3, 4a | N2 (3–6 cm ipsilateral nodal metastasis and ENE-, or multiple ≤ 6 cm ipsilateral nodal metastasis and ENE-, or ≤ 6 cm bilateral or contralateral nodal metastasis) | |||||
| Any T | N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | IVB | ||||
| T4b (very advanced local disease) | Any N | |||||
| Any T | M1 | IVC | ||||
| Nasopharynx | Tis | N0 (no regional nodal metastasis) | M0 | 0 | ||
| T1 (tumour limited to the naso-, or with locoregional invasion of the oropharynx and nasal cavity) | I | |||||
| T1,0 (tumour not identified, EBV+ cervical nodes) | N1 (≤6 cm unilateral cervical lymph nodes metastasis, or uni-/bilateral retropharyngeal lymph nodes metastasis, above the cricoidal cartilage caudal border) | II | ||||
| T2 (tumour invading parapharyngeal space and/or adjacent soft tissue) | N0 | |||||
| N1 | ||||||
| T1, 0 | N2 (≤6 cm bilateral retropharyngeal lymph nodes metastasis above the cricoidal cartilage caudal border) | III | ||||
| T2 | ||||||
| T3 (tumour invading surrounding bone structures) | N0 | |||||
| N1 | ||||||
| N2 | ||||||
| T4 (tumour with extensive soft tissue invasion and/or with intracranial extension) | N0 | IVA | ||||
| N1 | ||||||
| N2 | ||||||
| Any T | N3 (>6 cm uni-/bilateral retropharyngeal lymph nodes metastasis and/or below the cricoidal cartilage caudal border) | |||||
| Any N | M1 | IVB | ||||
| HPV+ Oropharynx | T0,1,2 (tumour not identified; tumour ≤ 2 cm or 2–4 cm) | N0,1 (no regional nodal metastasis; one or more ≤6 cm ipsilateral nodal metastasis) | M0 | I | ||
| N2 (≤6 cm contralateral or bilateral nodal metastasis) | II | |||||
| T3 (tumour < 4 cm or with invasion of epiglottis lingual surface) | N0,1,2 | |||||
| T0,1,2,3,4 | N3 (>6 cm nodal metastasis) | III | ||||
| T4 (moderate locoregional invasion) | N0,1,2,3 | |||||
| Any T | Any N | M1 | IV | |||
| HPV- Oropharynx and Hypopharynx | Tis (carcinoma in situ) | N0 (no regional nodal metastasis) | M0 | 0 | ||
| T1 (tumour ≤ 2 cm) | I | |||||
| T2 (tumour 2–4 cm) | II | |||||
| T3 (tumour < 4 cm or with invasion of epiglottis lingual surface) | III | |||||
| T1,2,3 | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | |||||
| T4a (tumour with moderate locoregional invasion) | N0,1 | IVA | ||||
| T1,2,3,4a | N2 (3–6 cm ipsilateral nodal metastasis and ENE-, or multiple ≤ 6 cm ipsilateral nodal metastasis and ENE-) | |||||
| Any T | N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | IVB | ||||
| T4b (very advanced locoregional invasion) | Any N | |||||
| Any T | M1 | IVC | ||||
| Nasal Cavity and Paranasal Sinuses | Tis (carcinoma in situ) | N0 (no regional nodal metastasis) | M0 | 0 | ||
| T1 (limited to one subsite, with or without bone invasion) | I | |||||
| T2 (two subsites or invading adjacent subsites, with or without bone invasion) | II | |||||
| T3 (locoregional invasion of orbital medial wall or floor, maxillary sinus, palate, and cribriform plate) | III | |||||
| T1,2,3 | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | |||||
| T4a (moderate locoregional invasion) | N0,1 | IVA | ||||
| T1,2,3,4a | N2 (3–6 cm ipsilateral nodal metastasis and ENE-, or multiple ≤ 6 cm ipsilateral nodal metastasis and ENE-, or ≤ 6 cm bilateral or contralateral nodal metastasis) | |||||
| Any T | N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | IVB | ||||
| T4b (very advanced locoregional invasion) | Any N | |||||
| Any T | M1 | IVC | ||||
| Larynx (T classification depends on tumour location: supraglottis, glottis, subglottis) | Tis (carcinoma in situ) | N0 (no regional nodal metastasis) | M0 | 0 | ||
| T1 | I | |||||
| T2 | II | |||||
| T3 | III | |||||
| T1,2,3 | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | |||||
| T4a | N0,1 | IVA | ||||
| T1,2,3,4a | N2 (3–6 cm ipsilateral nodal metastasis and ENE-, or multiple ≤ 6 cm ipsilateral nodal metastasis and ENE-, or ≤ 6 cm bilateral or contralateral nodal metastasis) | |||||
| Any T | N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | IVB | ||||
| T4b | Any N | |||||
| Any T | M1 | IVC | ||||
| Cutaneous HNSCC | Tis (carcinoma in situ) | N0 (no regional nodal metastasis) | M0 | 0 | ||
| T1 (tumour ≤ 2 cm) | I | |||||
| T2 (tumour 2–4 cm) | II | |||||
| T3 (tumour ≥ 4 cm, or minor bone invasion, or perineural invasion, or deep soft tissue invasion) | III | |||||
| T1 | N1 (≤3 cm ipsilateral nodal metastasis and ENE-) | |||||
| T2 | ||||||
| T3 | ||||||
| T1 | N2 (3–6 cm ipsilateral nodal metastasis and ENE-, or multiple ≤ 6 cm ipsilateral nodal metastasis and ENE-, or ≤6 cm bilateral or contralateral nodal metastasis) | IV | ||||
| T2 | ||||||
| T3 | ||||||
| Any T | N3 (≥6 cm ipsilateral nodal metastasis and ENE-, or any ENE+ nodal metastasis) | |||||
| T4 (major cortical bone/marrow invasion, skull base invasion or skull base foramen invasion) | Any N | |||||
| Any T | M1 | |||||
| Thyroid Cancer | Differentiated | <55 years old | Any T | Any N | M0 | I |
| M1 | II | |||||
| ≥55 years old | T1 (tumour ≤ 2 cm, confined to the thyroid) | N0/NX (no regional nodal metastasis; regional lymph nodes cannot be assessed) | M0 | I | ||
| N1 (regional nodal metastasis) | II | |||||
| T2 (tumour 2–4 cm, confined to the thyroid) | N0/NX | I | ||||
| N1 | II | |||||
| T3 (tumour ≥ 4 cm, confined to the thyroid or locoregional invasion of surrounding muscles) | Any N | |||||
| T4a (gross extrathyroidal invasion of subcutaneous soft tissues, larynx, trachea, oesophagus) | III | |||||
| T4b (gross extrathyroidal invasion of prevertebral fascia, or encasing of the carotid artery or mediastinal vessels of any size) | IVA | |||||
| Any T | M1 | IVB | ||||
| Anaplastic (T and N w/ same definition as for differentiated thyroid carcinoma) | T1,2,3a | N0/X | M0 | IVA | ||
| N1 | IVB | |||||
| T3b | Any N | |||||
| T4 | ||||||
| Any T | M1 | IVC | ||||
| Medullary (T and N with same definition as for differentiated thyroid carcinoma) | T1 | N0 | M0 | I | ||
| T2 | II | |||||
| T3 | ||||||
| T1,2,3 | N1a | III | ||||
| T4a | Any N | IVA | ||||
| T1,2,3 | N1b | |||||
| T4b | Any N | IVB | ||||
| Any T | M1 | IVC | ||||
| Histopathological Subtype | Main Characteristics | ||
|---|---|---|---|
| Epithelial | Conventional | Squamous Cell Carcinoma | Characteristics of squamous epithelium that penetrated the basement membrane. Different degrees of differentiation. |
| Verrucous | Resembles common wart with a pushing border and blunt bulbous projections in a chronically inflamed stroma. | ||
| Papillary | Narrow papillae with fibrovascular cores (rare variant). HPV-positive or -negative. | ||
| Basaloid | Cribriform nests of small cells with high nuclear to cytoplasm ratio (aggressive variant). Oropharyngeal forms are likely to be HPV+. | ||
| Spindle cell | Polypoid mass with areas of conventional SCC (rare variant). Bone and cartilage may also be present. | ||
| Lymphoepithelial | EBV+ undifferentiated carcinoma presents as a nest or single-cell population in the presence of lymphocytes. | ||
| Intestinal-type | Sinonasal Adenocarcinoma | Resembles the aggressive form of colon adenocarcinoma. | |
| Non-intestinal-type | Resemble normal sero-mucinous glands, with solid growth pattern, necrosis and marked nuclear atypia. | ||
| Sinonasal Undifferentiated | Carcinoma | Unknown etiology, presenting as sheets of mitotically active large cells with large nucleoli (aggressive variant). Sometimes presents p16 expression when negative for HPV. | |
| Nasopharyngeal | Keratinizing, non-keratinizing or non-keratinizing undifferentiated. The latter two are EBV+. | ||
| Large Cell Neuroendocrine | Large cells with abundant cytoplasm and typical neuroendocrine immunophenotype. | ||
| Small Cell Neuroendocrine | Resembles its lung namesake. | ||
| Mucoepidermoid | Low-grade tumours usually present larger populations of mucous cells, while high-grade forms present epithelioid and intermediate cells. Translocation t(11;19) is a marker of favourable prognostic. | ||
| Adenoid Cystic | Tumour of the major or minor salivary glands, presenting as tubules, cribriform or solid nests, with patterns of luminal or abluminal differentiation. | ||
| Acinic Cell | Resembles the normal parotid gland, with absent ducts. | ||
| Epithelial–Myoepithelial | Tumours with the highest degree of luminal and abluminal differentiation, with focal areas containing bi-layered ducts, with columnar epithelial and myoepithelial cells. | ||
| Salivary Duct | Infiltrating cord, papillae, and large nests with necrosis. Usually expresses androgen receptor (AR), overexpresses HER2, and is negative for oestrogen (ER) and progesterone (PR) receptors. | ||
| Polymorphous Low Grade | Adenocarcinoma | Tumour of the minor salivary glands similar to adenoid cystic carcinoma, but with polymorphous architecture, and no luminal or abluminal differentiation. | |
| Basal Cell | Prominent basement membrane and hyaline globules scattered within a nest architecture. | ||
| Ceruminous Gland | Similar features to salivary gland carcinomas, presenting as an infiltrative cribriform nest, with luminal and/or abluminal cells. | ||
| Olfactory Neuroblastoma | Uniform blue cells arranged in rosettes in neurofibrillary background. Poorly differentiated forms loose lobular architecture and present necrosis. | ||
| Ewing’s Sarcoma | Small blue cells with t(11;22) translocation involving EWS and FLI-1 genes, seen in adolescents and young adults. | ||
| Primitive Neuroectodermal Tumour | Similar to Ewing’s sarcoma but seen in any age group and presents neuroendocrine differentiation. Mutation in p53 signifies a poorer prognosis. | ||
| Melanoma | Same presentation of melanoma at other sites. | ||
| Carcinoma ex Pleomorphic Adenoma | A benign tumour mixed with adenocarcinoma, composed of epithelial and/or mesenchymal entities. | ||
| Ameloblastoma | Odontogenic epithelium, resembling the enamel organ of developing tooth. | ||
| Mesenchymal | Osteosarcoma | Resembles osteosarcoma of other sites. | |
| Laryngeal | Chondrosarcoma | Lobulated nodules of cartilage, with increased cellularity with nuclear pleomorphism. Spindle cells may be present. | |
| Mesenchymal | Rare form with distinctive biphasic appearance, presenting as nodules of hyaline cartilage surrounded by sheets of small or spindle cells. | ||
| Conventional | Chondromas | Pseudoencapsulated tumours with fibrous bands, with the cells growing in cords, sheets, or pseudo-glandular entities, in a mucinous matrix. | |
| Dedifferentiated | Like conventional chondromas, but lack mucinous matrix. | ||
| Chondroid | Conventional chondrosarcoma with the addition of cartilage foci. | ||
| Embryonal | Rhabdo-myosarcoma | A wide range of presentations, from undifferentiated cells to those resembling foetal muscle. Stains for skeletal muscle-specific markers. | |
| Botryoid | Polypoid masses, formed by hypocellular zones separated from the epithelium by a hypocellular layer of connective tissue. | ||
| Alveolar | Small round cells separated by dense fibrous septae into nests, with dicohesive cells in the centre. | ||
| Mesenchymal Extrarenal Rhabdoid Tumour | Rhabdoid cells and loss of nuclear INI1 expression. | ||
| Alveolar Soft Parts | Sarcoma | Highly vascular tumours presenting as nests, formed by dicohesive cells, with haemorrhagic and necrotic areas. | |
| Synovial | t(X;18) (p11;q11) translocation. | ||
| Follicular Dendritic Cell | Spindle cells present in fascicular, storiform or diffuse pattern, in the presence of lymphocytes. | ||
| Lymphoid | Conventional | Lymphoma | Can present as Hodgkin’s or non-Hodgkin’s lymphoma (NHL). |
| Diffuse Large B-cell | Most common lymphoma of the head and neck, presenting as sheets/clusters of intermediate-to-large cells, with large necrotic areas. | ||
| Plasmablastic | Rare variant of diffuse large B-cell lymphoma, presenting as large plasmocytic cells, proliferating diffusely, with abundant apoptotic bodies. They may also present necrotic regions. | ||
| MALT | Small-to-medium lymphocytes in size with pale cytoplasm, or plasmacytic features. Translocation t(11;18) (q21;q21) is frequent. | ||
| Extranodal NK-/-T-Cell of Nasal Type | Mixture of cells with different dimensions, irregular nuclei and amounts of pale cytoplasm, and chronic inflammatory infiltrates. The overlying mucosa usually presents as ulcerated but can range to hyperplastic. Commonly EBV+. | ||
| Burkitt’s | Uniform mitotic lymphoid cells, with interspersed macrophages and phagocytized apoptotic tumour cells. Can be EBV+. | ||
| Plasmacytoma | Sheets and nests of plasma cells, sometimes with plasmablastic features, and scattered multinucleated giant tumour cells. | ||
| Target | Drug | Therapeutic Indication(s) | Phase | ClinicalTrials.Gov ID |
|---|---|---|---|---|
| PI3K | Buparlisib | R/M HNSCC | III | NCT04338399 |
| Alpelisib | II | NCT04997902 | ||
| Copanlisib | I | NCT03735628 | ||
| Eganelisib | I | NCT02637531 | ||
| Duvelisib | R/M and non-R/M HNSCC | II | NCT05057247 | |
| PI3K and mTOR | Gedatolisib | R/M or Advanced HNC | I | NCT03065062 |
| AKT | Ipatasertib | I | NCT05172245 NCT05172258 | |
| II | ||||
| p53 | COTI-2 | HNC | I | NCT02433626 |
| Advexin | R/M HNSCC | II | NCT03544723 | |
| EGFR | Erlotinib | II | NCT00076310 NCT01927744 NCT00954226 | |
| HNSCC | II | |||
| HNC | I | |||
| Afatinib | R/M HNSCC | II | NCT02979977 | |
| Resectable HNSCC | II | NCT05517330 NCT05516589 | ||
| II | ||||
| R/M HNSCC | III | NCT01856478 | ||
| Dacomitinib | II | NCT04946968 | ||
| Lapatinib | Localized HNSCC | II | NCT01612351 | |
| HNC | II | NCT01711658 | ||
| PARP | Olaparib | I | NCT02308072 | |
| I | NCT02229656 | |||
| Niraparib | R/M HNSCC | II | NCT04313504 | |
| HNC | II | NCT05169437 | ||
| II | NCT04779151 | |||
| Smoothened | Sonidegib | R/M HNSCC | I | NCT04007744 |
| VEGFR | Bevacizumab | Localized HNSCC | II | NCT01588431 |
| HNC | III | NCT05063552 NCT00588770 | ||
| R/M HNSCC | III | |||
| Localized HNSCC | I and II | NCT03134846 | ||
| HNC | II | NCT03818061 | ||
| Lenvatinib | R/M HNSCC | II | NCT04977453 | |
| Cabozantinib | HNC | II | NCT05136196 | |
| I | NCT03170960 | |||
| HNSCC | I | NCT03667482 | ||
| R/M HNSCC | II | NCT03468218 | ||
| R/M HNC | I | NCT04514484 | ||
| Sorafenib | R/M HNSCC | II | NCT00494182 | |
| Surufatinib | R/M HNC | II | NCT04910854 | |
| Abemaciclib | R/M HNSCC | II | NCT03356223 | |
| HNSCC | II | NCT04169074 | ||
| Ribociclib | I | NCT04000529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, M.N.; Faísca, P.; Ferreira, H.A.; Gaspar, M.M.; Reis, C.P. Current Insights and Progress in the Clinical Management of Head and Neck Cancer. Cancers 2022, 14, 6079. https://doi.org/10.3390/cancers14246079
Amaral MN, Faísca P, Ferreira HA, Gaspar MM, Reis CP. Current Insights and Progress in the Clinical Management of Head and Neck Cancer. Cancers. 2022; 14(24):6079. https://doi.org/10.3390/cancers14246079
Chicago/Turabian StyleAmaral, Mariana Neves, Pedro Faísca, Hugo Alexandre Ferreira, Maria Manuela Gaspar, and Catarina Pinto Reis. 2022. "Current Insights and Progress in the Clinical Management of Head and Neck Cancer" Cancers 14, no. 24: 6079. https://doi.org/10.3390/cancers14246079
APA StyleAmaral, M. N., Faísca, P., Ferreira, H. A., Gaspar, M. M., & Reis, C. P. (2022). Current Insights and Progress in the Clinical Management of Head and Neck Cancer. Cancers, 14(24), 6079. https://doi.org/10.3390/cancers14246079










