Sex Biases in Cancer and Autoimmune Disease Incidence Are Strongly Positively Correlated with Mitochondrial Gene Expression across Human Tissues
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Autoimmune Disease Incidence Data Curation
Estimating Incidence Rates
2.3. Cancer Incidence Data Curation
2.4. Pairing AID and Cancer Incidence Data
2.5. Gene Expression Analysis of Human Tissues
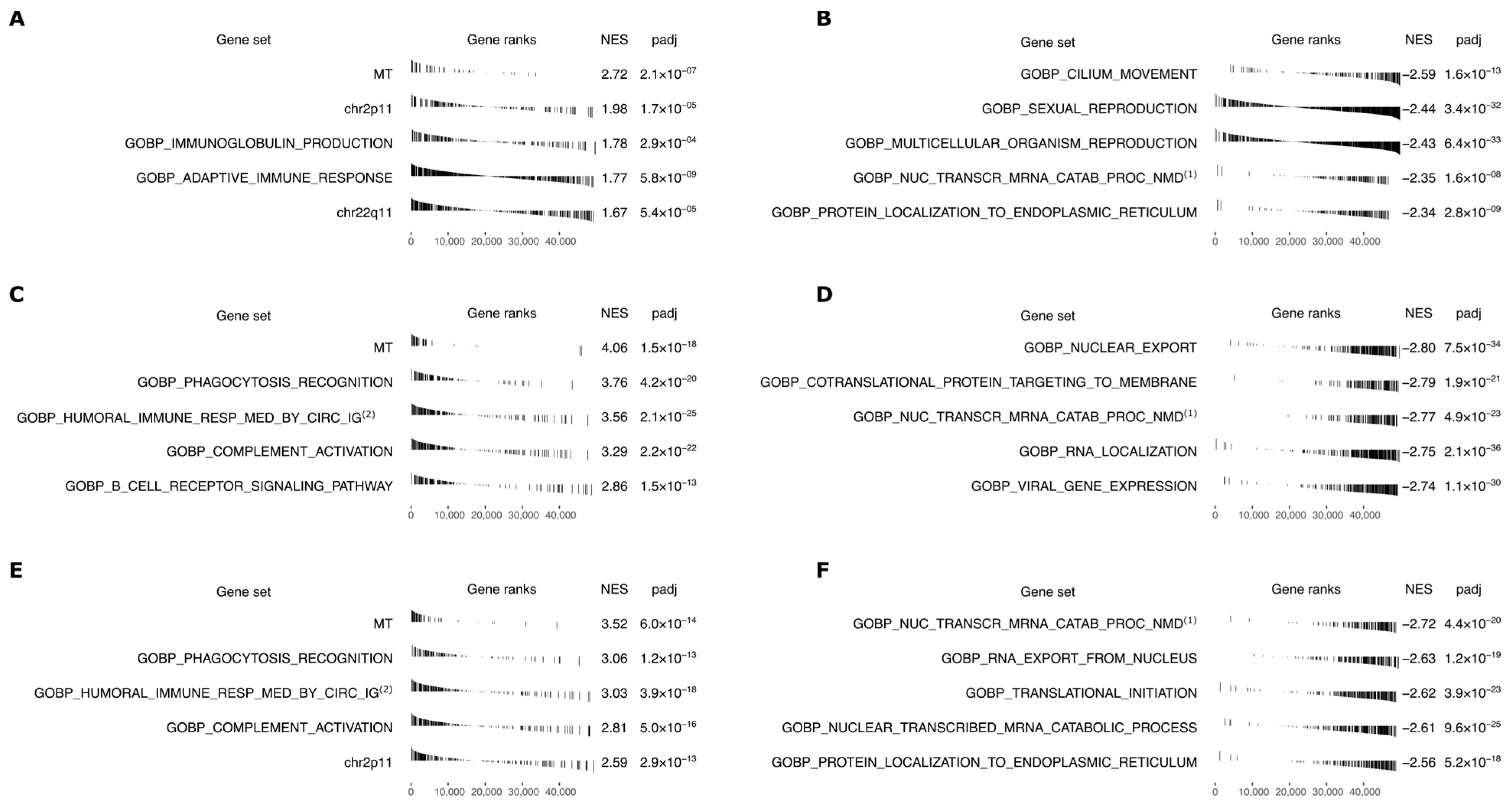
2.6. Gene Set Enrichment Analysis across Human Functional Pathways
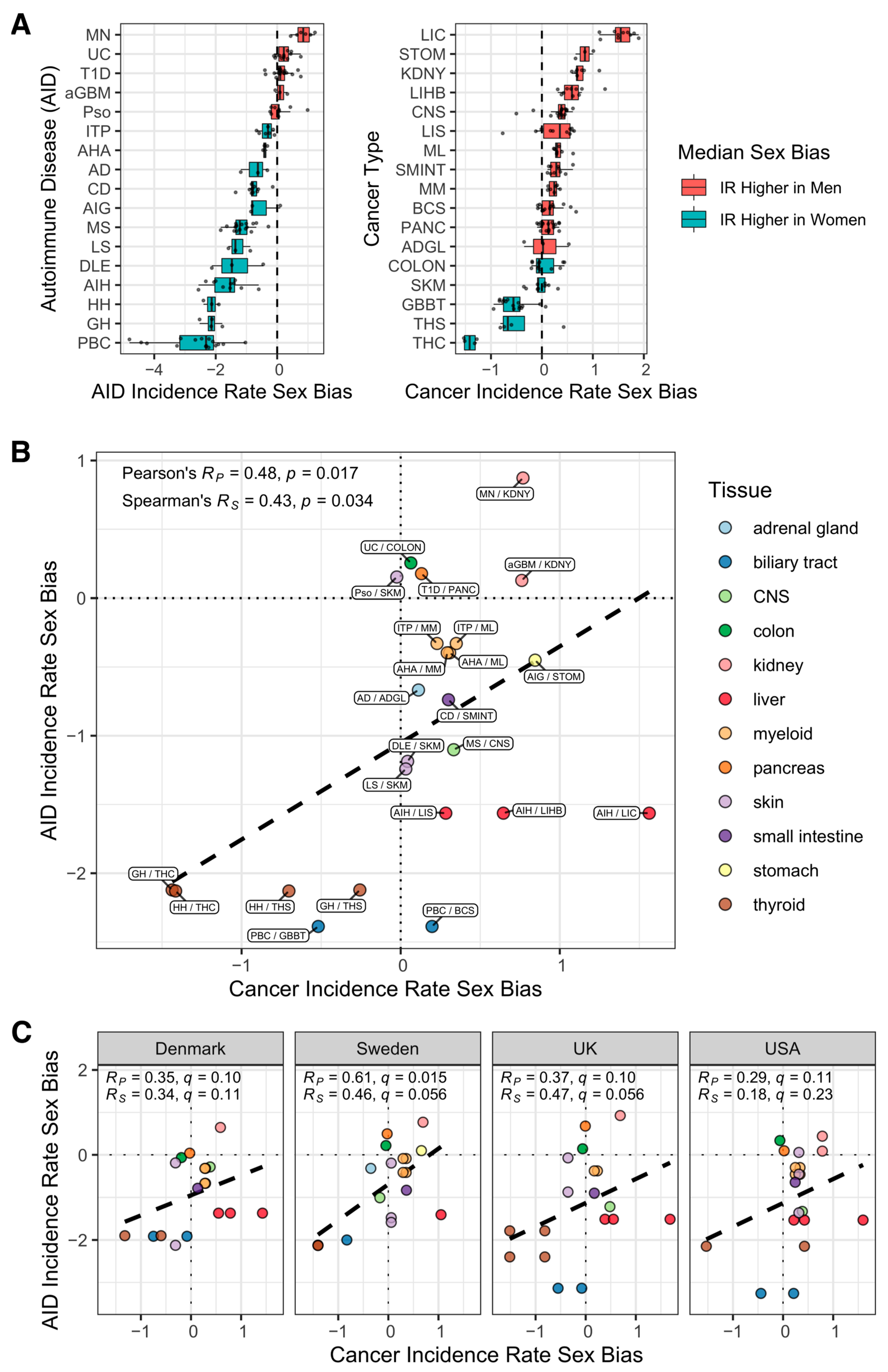
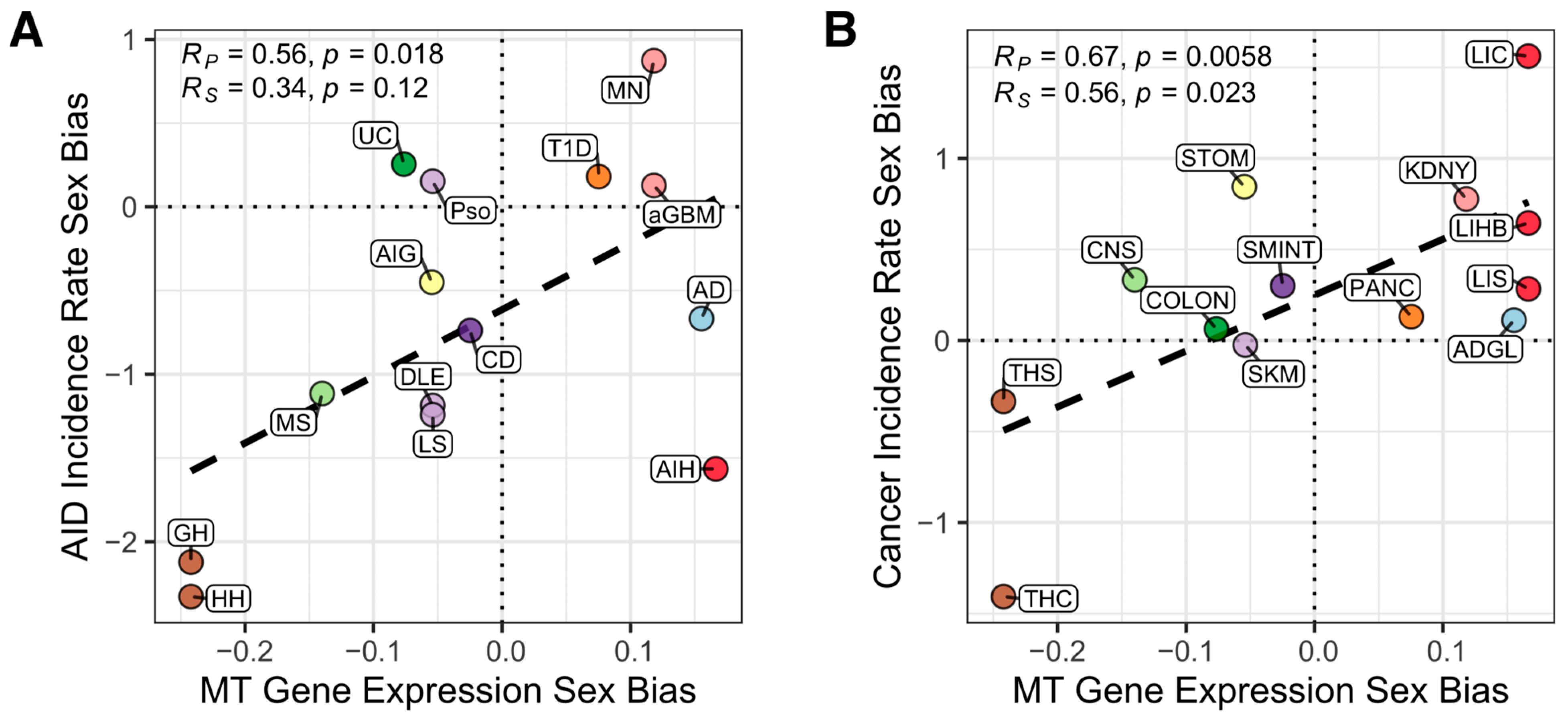
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [PubMed]
- Moroni, L.; Bianchi, I.; Lleo, A. Geoepidemiology, gender and autoimmune disease. Autoimmun. Rev. 2012, 11, A386–A392. [Google Scholar] [CrossRef] [PubMed]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Costa, A.R.; de Oliveira, M.L.; Cruz, I.; Santos, C.R.; Cascalheira, J.F.; Santos, C.R.A. The sex bias of cancer. Trends Endocrinol. Metab. 2020, 31, 785–799. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef]
- Credendino, S.C.; Neumayer, C.; Cantone, I. Genetics and Epigenetics of Sex Bias: Insights from Human Cancer and Autoimmunity. Trends Genet. 2020, 36, 650–663. [Google Scholar] [CrossRef]
- Edgren, G.; Liang, L.; Adami, H.-O.; Chang, E.T. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 2012, 27, 187–196. [Google Scholar] [CrossRef]
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects. 2022. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 23 June 2022).
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. Cancer Incidence in Five Continents. Lyon, IARC. 2017. Available online: http://ci5.iarc.fr (accessed on 18 July 2021).
- Finnish Cancer Registry. 27 April 2021. Available online: https://cancerregistry.fi/statistics/cancer-statistics/ (accessed on 21 July 2021).
- The Swedish Cancer Register. 6 December 2020. Available online: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/swedish-cancer-register/ (accessed on 21 July 2021).
- Taiwan Cancer Registry. 4 May 2012. Available online: http://tcr.cph.ntu.edu.tw/main.php?Page=N2 (accessed on 21 July 2021).
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, H.X.; Yao, Y.; Bian, Z.H.; Zheng, Y.J.; Li, L.; Moutsopoulos, H.M.; Gershwin, M.E.; Lian, Z.X. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J. Autoimmun. 2019, 104, 102333. [Google Scholar] [CrossRef]
- Wagner, M.; Jasek, M.; Karabon, L. Immune Checkpoint Molecules—Inherited Variations as Markers for Cancer Risk. Front. Immunol. 2021, 11, 606721. [Google Scholar] [CrossRef]
- Dunford, A.; Weinstock, D.M.; Savova, V.; Schumacher, S.E.; Cleary, J.P.; Yoda, A.; Sullivan, T.J.; Hess, J.M.; Gimelbrant, A.A.; Beroukhim, R.; et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet. 2016, 49, 10–16. [Google Scholar] [CrossRef]
- Di Dalmazi, G.; Hirshberg, J.; Lyle, D.; Freij, J.B.; Caturegli, P. Reactive oxygen species in organ-specific autoimmunity. Autoimmun. Highlights 2016, 7, 11. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers, or Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Hu, L.; Yao, X.; Shen, Y. Altered mitochondrial DNA copy number contributes to human cancer risk: Evidence from an updated meta-analysis. Sci. Rep. 2016, 6, 35859. [Google Scholar] [CrossRef]
- Chen, L.; Duvvuri, B.; Grigull, J.; Jamnik, R.; Wither, J.E.; Wu, G.E. Experimental evidence that mutated-self peptides derived from mitochondrial DNA somatic mutations have the potential to trigger autoimmunity. Hum. Immunol. 2014, 75, 873–879. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Pontier, D.B.; Gribnau, J. Xist regulation and function eXplored. Hum. Genet. 2011, 130, 223–236. [Google Scholar] [CrossRef]
- di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, I.; Starskaia, I.; Nagy, T.; Guo, J.; Yatkin, E.; Väänänen, K.; Watford, W.T.; Chen, Z. Estrogen receptor α contributes to T cell–mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci. Signal. 2018, 11, eaap9415. [Google Scholar] [CrossRef]
- Papa, L.; Germain, D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J. Cell Sci. 2011, 124, 1396–1402. [Google Scholar] [CrossRef]
- Kenny, T.C.; Craig, A.J.; Villanueva, A.; Germain, D. Mitohormesis Primes Tumor Invasion and Metastasis. Cell Rep. 2019, 27, 2292–2303. [Google Scholar] [CrossRef]
- Liao, T.-L.; Lee, Y.-C.; Tzeng, C.-R.; Wang, Y.-P.; Chang, H.-Y.; Lin, Y.-F.; Kao, S.-H. Mitochondrial translocation of estrogen receptor beta affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free. Radic. Biol. Med. 2019, 134, 359–373. [Google Scholar] [CrossRef]
- Vento, S.; Cainelli, F. Autoimmune Diseases in Low and Middle Income Countries: A Neglected Issue in Global Health. Isr. Med. Assoc. J. 2016, 18, 54–55. [Google Scholar] [PubMed]
- Lleo, A. Geoepidemiology and the Impact of Sex on Autoimmune Diseases. In Principles of Gender-Specific Medicine; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Defining and analyzing geoepidemiology and human autoimmunity. J. Autoimmun. 2010, 34, J168–J177. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.W.; Alfredsson, L.; Costenbader, K.H.; Kamen, D.L.; Nelson, L.M.; Norris, J.M.; De Roos, A.J. Epidemiology of environmental exposures and human autoimmune diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J. Autoimmun. 2012, 39, 259–271. [Google Scholar] [CrossRef]
- Tiniakou, E.; Costenbader, K.H.; Kriegel, M.A. Sex-specific environmental influences on the development of autoimmune diseases. Clin. Immunol. 2013, 149, 182–191. [Google Scholar] [CrossRef]
- Jackson, S.S.; Marks, M.A.; Katki, H.A.; Cook, M.B.; Hyun, N.; Freedman, N.D.; Kahle, L.L.; Castle, P.E.; Graubard, B.I.; Chaturvedi, A.K. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer 2022, 128, 3531–3540. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Michels, N.; van Aart, C.; Morisse, J.; Mullee, A.; Huybrechts, I. Chronic inflammation towards cancer incidence: A systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol. 2021, 157, 103177. [Google Scholar] [CrossRef]
- He, M.-M.; Lo, C.-H.; Wang, K.; Polychronidis, G.; Wang, L.; Zhong, R.; Knudsen, M.D.; Fang, Z.; Song, M. Immune-Mediated Diseases Associated with Cancer Risks. JAMA Oncol. 2022, 8, 209. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
| (a) Measure to Estimate | (b) Measures Needed for Estimator | (1) Datasets with (a) | (2) Datasets with (a) & (b) | (3) Datasets with (b) but Not (a) | (4) Datasets with Neither (a) nor (a) |
|---|---|---|---|---|---|
| IRSB | casesM/casesF | 125 (66%) | 105 (56%) | 63 (34%) | 0 (0%) |
| IRM, IRF | IRTOTAL, casesM/casesF | 123 (65%) | 84 (45%) | 41 (22%) | 24 (13%) |
| IRTOTAL | IRM, IRF | 143 (76%) | 101 (54%) | 22 (12%) | 23 (12%) |
| Measure | Estimator | β | α | r2 | r | p |
|---|---|---|---|---|---|---|
| 0.922 | −0.0254 | 0.966 | 0.983 | 6.04 × 10−78 | ||
| 0.923 | −0.0585 | 0.963 | 0.981 | 5.54 × 10−76 | ||
| 1.020 | −0.303 | 0.997 | 0.998 | 1.15 × 10−107 | ||
| 1.035 | −0.307 | 0.997 | 0.999 | 5.04 ×10−105 | ||
| 0.975 | 0.228 | 0.997 | 0.999 | 6.10 × 10−108 | ||
| 0.959 | 0.236 | 0.997 | 0.999 | 2.26 × 10−105 | ||
| 1.013 | −0.123 | 1.000 | 1.000 | 1.09 × 10−182 | ||
| 1.013 | −0.136 | 1.000 | 1.000 | 1.39 × 10−182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, D.R.; Sinha, S.; Nair, N.U.; Ryan, B.M.; Barnholtz-Sloan, J.S.; Mount, S.M.; Erez, A.; Aldape, K.; Castle, P.E.; Rajagopal, P.S.; et al. Sex Biases in Cancer and Autoimmune Disease Incidence Are Strongly Positively Correlated with Mitochondrial Gene Expression across Human Tissues. Cancers 2022, 14, 5885. https://doi.org/10.3390/cancers14235885
Crawford DR, Sinha S, Nair NU, Ryan BM, Barnholtz-Sloan JS, Mount SM, Erez A, Aldape K, Castle PE, Rajagopal PS, et al. Sex Biases in Cancer and Autoimmune Disease Incidence Are Strongly Positively Correlated with Mitochondrial Gene Expression across Human Tissues. Cancers. 2022; 14(23):5885. https://doi.org/10.3390/cancers14235885
Chicago/Turabian StyleCrawford, David R., Sanju Sinha, Nishanth Ulhas Nair, Bríd M. Ryan, Jill S. Barnholtz-Sloan, Stephen M. Mount, Ayelet Erez, Kenneth Aldape, Philip E. Castle, Padma S. Rajagopal, and et al. 2022. "Sex Biases in Cancer and Autoimmune Disease Incidence Are Strongly Positively Correlated with Mitochondrial Gene Expression across Human Tissues" Cancers 14, no. 23: 5885. https://doi.org/10.3390/cancers14235885
APA StyleCrawford, D. R., Sinha, S., Nair, N. U., Ryan, B. M., Barnholtz-Sloan, J. S., Mount, S. M., Erez, A., Aldape, K., Castle, P. E., Rajagopal, P. S., Day, C.-P., Schäffer, A. A., & Ruppin, E. (2022). Sex Biases in Cancer and Autoimmune Disease Incidence Are Strongly Positively Correlated with Mitochondrial Gene Expression across Human Tissues. Cancers, 14(23), 5885. https://doi.org/10.3390/cancers14235885







