Diagnostics Using Non-Invasive Technologies in Dermatological Oncology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Techniques
2.1. Digital Photography and Total Body Photography (TBP)
2.2. Dermoscopy
2.3. Confocal Microscopy
2.4. Optical Coherence Tomography
2.5. High Frequency Ultrasound
2.6. Raman Spectroscopy
2.7. Electrical Impedance Spectroscopy
2.8. Multispectral Imaging
2.9. Multiphoton Microscopy
2.10. Multispectral Optoacoustic Tomography
2.11. Artificial Intelligence (AI)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of skin cancer. Adv. Exp. Med. Biol. 2014, 810, 120–140. [Google Scholar] [PubMed]
- Schneider, S.L.; Kholi, I.; Iltefat, H.; Hamzavi, I.H.; Council, M.L.; Rossi, M.R.; Ozog, D.M. Emerging imaging technologies in dermatology: Part II: Basic prinicples. J. Am. Acad. Dermatol. 2019, 80, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Kholi, I.; Iltefat, H.; Hamzavi, I.H.; Council, M.L.; Rossi, M.R.; Ozog, D.M. Emerging imaging technologies in dermatology: Part I: Applications and limitations. J. Am. Acad. Dermatol. 2019, 80, 1121–1131. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization for Research and Treatment of Cancer (EORTC). European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar]
- Adler, N.R.; Kelly, J.W.; Guitera, P.; Menzies, S.W.; Chamberlain, A.J.; Fishburn, P.; Button-Sloan, A.E.; Heal, C.; Soyer, H.P.; Thompson, J.F. Methods of melanoma detection and of skin monitoring for individuals at high risk of melanoma: New Australian clinical practice. Med. J. Aust. 2019, 210, 41–47. [Google Scholar] [CrossRef]
- Quigley, E.A.; Tokay, B.A.; Jewell, S.T.; Marchetti, M.A.; Halpern, A.C. Technology and Technique Standards for Camera-Acquired Digital Dermatologic Images: A Systematic Review. JAMA Dermatol. 2015, 151, 883–890. [Google Scholar] [CrossRef]
- Malvehy, J.; Puig, S. Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: A twostep method. Clin. Dermatol. 2002, 20, 297–304. [Google Scholar] [CrossRef]
- Puig, S.; Malvehy, J. Monitoring Patients with Multiple Nevi. Dermatol. Clin. 2013, 31, 565–577. [Google Scholar] [CrossRef]
- Hornung, A.; Steeb, T.; Wessely, A.; Brinker, T.J.; Breakell, T.; Erdmann, M.; Berking, C.; Heppt, M.V. The Value of Total Body Photography for the Early Detection of Melanoma: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1726. [Google Scholar] [CrossRef]
- Ji-Xu, A.; Dinnes, J.; Matin, R.N. Total body photography for the diagnosis of cutaneous melanoma in adults: A systematic review and meta-analysis. Br. J. Dermatol. 2021, 185, 302–312. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Magdy, M.; Khalaf, E.M.; Mostafa, A.; Arafa, A. Teledermatology in the time of COVID-19. Int. J. Clin. Pract. 2021, 75, e15000. [Google Scholar] [CrossRef]
- Menzies, S.W. Evidence-Based Dermoscopy. Dermatol. Clin. 2013, 31, 521–524. [Google Scholar] [CrossRef]
- Babino, G.; Lallas, A.; Longo, C.; Moscarella, E.; Alfano, R.; Argenziano, G. Dermoscopy of melanoma and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2015, 150, 507–519. [Google Scholar]
- Wolner, Z.J.; Yélamos, O.; Liopyris, K.; Rogers, T.; Marchetti, M.A.; Marghoob, A.A. Enhancing Skin Cancer Diagnosis with Dermoscopy. Dermatol. Clin. 2017, 35, 417–437. [Google Scholar] [CrossRef]
- Vestergaard, M.E.; Macaskill, P.; Holt, P.E.; Menzies, S.W. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008, 159, 669–676. [Google Scholar] [CrossRef]
- Salerni, G.; Terán, T.; Puig, S.; Malvehy, J.; Zalaudek, I.; Argenziano, G.; Kittler, H. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: A study on behalf of the International Dermoscopy Society. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 805–814. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Young, A.; Wong, A.; Stalling, S.; Wei, M.; Hadley, D. Precision Diagnosis Of Melanoma And Other Skin Lesions From Digital Images. AMIA Jt Summits Transl. Sci. Proc. 2017, 26, 220–226. [Google Scholar]
- Cinotti, E.; Tognetti, L.; Campoli, M.; Liso, F.; Cicigoi, A.; Cartocci, A.; Rossi, R.; Rubegni, P.; Perrot, J.L. Super-high magnification dermoscopy can aid the differential diagnosis between melanoma and atypical naevi. Clin. Exp. Dermatol. 2021, 46, 1216–1222. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, A.; Mickan, S.; Mallett, S.; Ayya, M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol. Pract. Concept. 2013, 3, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadouch, D.J.; Schram, M.E.; Leeflang, M.M.; Limpens, J.; Spuls, P.I.; de Rie, M.A. In vivo confocal microscopy of basal cell carcinoma: A systematic review of diagnostic accuracy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.P.; Peppelman, M.; Hoogedoorn, L.; van Erp, P.E.J.; Gerritsen, M.J.P. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: A systematic review. Eur. J. Dermatol. 2016, 26, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Caruntu, A.; Boda, D.; Caruntu, C. In Vivo Reflectance Confocal Microscopy-Diagnostic Criteria for Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. J. Clin. Med. 2020, 9, 1987. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Aleissa, S.; Liopyris, K.; Dusza, S.W.; Phillips, W.; Rossi, A.M.; Lee, E.H.; Marghoob, A.A.; Nehal, K.S. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J. Am. Acad. Dermatol. 2019, 81, 417–426. [Google Scholar] [CrossRef]
- Guitera, P.; Moloney, F.J.; Menzies, S.W.; Stretch, J.R.; Quinn, M.J.; Hong, A.; Fogarty, G.; Scolyer, R.A. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013, 149, 692–698. [Google Scholar] [CrossRef] [Green Version]
- Guilera, J.M.; Barreiro Capurro, A.; Carrera Alvárez, C.; Puig Sardá, S. The role of reflectance confocal microscopy in clinical trials for tumor monitoring. Dermatol. Clin. 2016, 34, 519–526. [Google Scholar] [CrossRef]
- Peters, N.; Schubert, M.; Metzler, G.; Geppert, J.P.; Moehrle, M. Diagnostic accuracy of a new ex vivo confocal laser scanning microscope compared to H&E-stained paraffin slides for micrographic surgery of basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol 2019, 33, 298–304. [Google Scholar]
- Horn, M.; Gerger, A.; Koller, S.; Weger, W.; Langsenlehner, U.; Krippl, P.; Kerl, H.; Samonigg, H.; Smolle, J. The use of confocal laser-scanning microscopy in microsurgery for invasive squamous cell carcinoma. Br. J. Dermatol. 2007, 156, 81–84. [Google Scholar] [CrossRef]
- Venturini, M.; Gualdi, G.; Zanca, A.; Lorenzi, L.; Pellacani, G.; Calzavara-Pinton, P.G. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br. J. Dermatol. 2016, 174, 380–385. [Google Scholar] [CrossRef]
- Edwards, S.J.; Osei-Assibey, G.; Patalay, R.; Wakefield, V.; Karner, C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: A systematic review. Clin. Exp. Dermatol. 2017, 42, 266–275. [Google Scholar] [CrossRef]
- Olsen, J.; Themstrup, L.; de Carvalho, N.; Mogensen, M.; Pellacani, G.; Jemec, G.B. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn. Ther. 2016, 16, 44–49. [Google Scholar] [CrossRef]
- Themstrup, L.; Welzel, J.; Ciardo, S.; Kaestle, R.; Ulrich, M.; Holmes, J.; Whitehead, R.; Sattler, E.C.; Kindermann, N.; Pellacani, G.; et al. Validation of Dynamic optical coherence tomography for non-invasive, in vivo microcirculation imaging of the skin. Microvasc. Res. 2016, 107, 97–105. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography-Practical applications in dermatology and comparison with established imaging methods. Skin. Res. Technol. 2021, 27, 340–352. [Google Scholar] [CrossRef]
- Fischman, S.; Pérez-Anker, J.; Tognetti, L.; Di Naro, A.; Suppa, M.; Cinotti, E.; Viel, T.; Monnier, J.; Rubegni, P.; Del Marmol, V.; et al. Non-invasive scoring of cellular atypia in keratinocyte cancers in 3D LC-OCT images using Deep Learning. Sci. Rep. 2022, 12, 481. [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Orte Cano, C.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: A descriptive study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Orte Cano, C.; Perez-Anker, J.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1099–1110. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography May Enhance Monitoring of Superficial Basal Cell Carcinoma Treated with Imiquimod 5% Cream: A Pilot Study. Cancers 2021, 13, 4913. [Google Scholar] [CrossRef]
- Gambichler, T.; Regeniter, P.; Bechara, F.G.; Orlikov, A.; Vasa, R.; Moussa, G.; Stücker, M.; Altmeyer, P.; Hoffmann, K. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J. Am. Acad. Dermatol. 2007, 57, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.; Whang, T.B.; Bard, R.L.; Marmur, E.S. Ultrasound in dermatology: Principles and applications. J. Am. Acad. Dermatol. 2012, 67, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Roldán, F.A.; Varelli, C.; Bard, R.; Corvino, A.; Wortsman, X. Skin cancer: Findings and role of high-resolution ultrasound. J. Ultrasound. 2019, 22, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, F.; Wortsman, X.; Muñoz, C.; Segovia, L.; Espinoza, M.; Jemec, G.B. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: Correlation with histology. Cancer Imaging 2008, 8, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Jemec, G.B.E. Dermatologic Ultrasound with Clinical and Histologic Correlations, 1st ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- MacFarlane, D.; Shah, K.; Wysong, A.; Wortsman, X.; Humphreys, T.R. The role of imaging in the management of patients with nonmelanoma skin cancer: Diagnostic modalities and applications. J. Am. Acad. Dermatol. 2017, 76, 579–588. [Google Scholar] [CrossRef]
- Catalano, O.; Setola, S.V.; Vallone, P.; Mattace Raso, M.; Gallipoli D’Errico, A. Sonography for locoregional staging and follow-up of cutaneous melanoma: How we do it. J. Ultrasound Med. 2010, 29, 791–802. [Google Scholar] [CrossRef]
- Catalano, O.; Caracò, C.; Mozzillo, N.; Siani, A. Locoregional spread of cutaneous melanoma: Sonography findings. AJR Am. J. Roentgenol. 2010, 194, 735–745. [Google Scholar] [CrossRef]
- Bhatt, K.D.; Tambe, S.A.; Jerajani, H.R.; Dhurat, R.S. Utility of highfrequency ultrasonography in the diagnosis of benign and malignant skin tumors. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 162–182. [Google Scholar]
- Marmur, E.S.; Berkowitz, E.Z.; Fuchs, B.S.; Singer, G.K.; Yoo, J.Y. Use of high-frequency, high-resolution ultrasound before Mohs surgery. Dermatol. Surg. 2010, 36, 841–847. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, H.; Kalia, S.; Lui, H. Using Raman Spectroscopy to Detect and Diagnose Skin Cancer In Vivo. Dermatol. Clin. 2017, 35, 495–504. [Google Scholar] [CrossRef]
- Gniadecka, M.; Philipsen, P.A.; Sigurdsson, S.; Wessel, S.; Nielsen, O.F.; Christensen, D.H.; Hercogova, J.; Rossen, K.; Thomsen, H.K.; Gniadecki, R.; et al. Melanoma diagnosis by Raman spectroscopy and neural networks: Structure alterations in proteins and lipids in intact cancer tissue. J. Investig. Dermatol. 2004, 12, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Sigurdsson, S.; Philipsen, P.A.; Hansen, L.K.; Larsen, J.; Gniadecka, M.; Wulf, H.C. Detection of skin cancer by classification of Raman spectra. IEEE Trans. Biomed. Eng. 2004, 51, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Lui, H.; McLean, D.I.; Zeng, H. Integrated real-time Raman system for clinical in vivo skin analysis. Skin Res. Technol. 2008, 14, 484–492. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, H.; Hamzavi, I.; McLean, D.I.; Lui, H. Rapid near-infrared Raman spectroscopy system for real-time in vivo skin measurements. Opt. Lett. 2001, 26, 1782–1784. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.L.; Xie, X.S. Coherent anti-Stokes Raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef]
- Saar, B.G.; Freudiger, C.W.; Reichman, J.; Stanley, C.M.; Holtom, G.R.; Xie, X.S. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 2010, 330, 1368–1370. [Google Scholar] [CrossRef] [Green Version]
- Dorrell, D.N.; Strowd, L.C. Skin Cancer Detection Technology. Dermatol. Clin. 2019, 37, 527–536. [Google Scholar] [CrossRef]
- Malvehy, J.; Hauschild, A.; Curiel-Lewandrowski, C.; Mohr, P.; Hofmann-Wellenhof, R.; Motley, R.; Berking, C.; Grossman, D.; Paoli, J.; Loquai, C.; et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: An international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br. J. Dermatol. 2014, 171, 1099–1107. [Google Scholar] [CrossRef]
- Liebich, C.; Von Bruehl, M.L.; Schubert, I.; Oberhoffer, R.; Sander, C. Retrospective evaluation of the performance of the electrical impedance spectroscopy system Nevisense in detecting keratinocyte cancers. Skin Res. Technol. 2021, 27, 723–729. [Google Scholar] [CrossRef]
- Jolivot, R.; Benezeth, Y.; Marzani, F. Skin Parameter Map Retrieval from a dedicated Multispectral Imaging System Applied to Dermatology/Cosmetology. J. Biomed. Imaging 2013, 2013, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Delpueyo, X.; Vilaseca, M.; Royo, S.; Ares, M.; Rey-Barroso, L.; Sanabria, F.; Puig, S.; Malvehy, J.; Pellacani, J.; Noguero, F.; et al. Multispectral imaging system based on light-emitting diodes for the detection of melanomas and basal cell carcinomas: A pilot study. J. Biomed. Opt. 2017, 22, 065006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Cho, D.; Kim, J.; Kim, M.; Youn, S.; Jang, J.E.; Je, M.; Lee, D.H.; Lee, B.; Farkas, D.L.; et al. Smartphone-based multiespectral imaging: System development and potential for mobile skin diagnosis. Biomed. Opt. Express 2016, 7, 005294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, E.; Girotti, M.R.; Viros, A.; Hooper, S.; Spencer-Dene, B.; Matsuda, M.; Larkin, J.; Marais, R.; Sahai, E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell 2015, 27, 574–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Downes, A.; Chau, Y.Y.; Serrels, B.; Hastie, N.; Elfick, A.; Brunton, V.; Frame, M.; Serrels, A. In vivo imaging of the tumor and its associated microenvironment using combined CARS / 2-photon microscopy. Intravital 2015, 4, e1055430. [Google Scholar] [CrossRef] [PubMed]
- Welzel, J.; Schuh, S. Noninvasive diagnosis in dermatology. J. Dtsch. Dermatol. Ges. 2017, 15, 999–1016. [Google Scholar] [CrossRef] [Green Version]
- Ratner, D.; Thomas, C.O.; Bickers, D. The uses of digital photography in dermatology. J. Am. Acad. Dermatol. 1999, 41, 749–756. [Google Scholar] [CrossRef]
- Price, M.A.; Goldstein, G.D. The use of a digital imaging system in a dermatologic surgery practice. Dermatol. Surg. 1997, 23, 31–32. [Google Scholar] [CrossRef]
- Schiff, T.; Stiller, M.J. Photography without film: Low-cost digital cameras come of age in dermatology. Int. J. Dermatol. 1994, 33, 113–115. [Google Scholar]
- Stone, J.L.; Peterson, R.L.; Wolf, J.E. Digital imaging techniques in dermatology. J. Am. Acad. Dermatol. 1990, 5, 913–917. [Google Scholar] [CrossRef]
- Kvedar, J.C.; Edwards, R.A.; Menn, E.R.; Mofid, M.; Gonzalez, E.; Dover, J.; Parrish, J.A. The substitution of digital images for dermatologic physical examination. Arch. Dermatol. 1997, 133, 161–170. [Google Scholar] [CrossRef]
- Perednia, D.A. What dermatologists should know about digital imaging. J. Am. Acad. Dermatol. 1991, 25, 89–108. [Google Scholar] [CrossRef]
- Stoecker, W.V.; Moss, R.H. Digital imaging in dermatology. Comput. Med. Imaging Graph. 1992, 16, 145–150. [Google Scholar] [CrossRef]
- Braun, R.P.; Rabinovitz, H.S.; Oliviero, M.; Kopf, A.W.; Saurat, J.H. Dermoscopy of pigmented skin lesions. J. Am. Acad. Dermatol. 2005, 52, 109–121. [Google Scholar] [CrossRef]
- Carli, P.; de Giorgi, V.; Crocetti, E.; Mannone, F.; Massi, D.; Chiarugi, A.; Giannotti, B. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: A retrospective study 1997–2001. Br. J. Dermatol. 2004, 150, 687–692. [Google Scholar] [CrossRef]
- Argenziano, G.; Fabbrocini, G.; Carli, P.; de Giorgi, V.; Sammarco, E.; Delfino, M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch. Dermatol. 1998, 134, 1563–1570. [Google Scholar] [CrossRef]
- Henning, J.S.; Dusza, S.W.; Wang, S.Q.; Marghoob, A.A.; Rabinovitz, H.S.; Polsky, D.; Kopf, A.W. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J. Am. Acad. Dermatol. 2007, 56, 45–52. [Google Scholar] [CrossRef]
- Argenziano, G.; Puig, S.; Zalaudek, I.; Sera, F.; Corona, R.; Alsina, M.; Barbato, F.; Carrera, C.; Ferrara, G.; Guilabert, A.; et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J. Clin. Oncol. 2006, 24, 1877–1882. [Google Scholar] [CrossRef] [Green Version]
- Dusi, D.; Rossi, R.; Simonacci, M.; Ferrara, G. Image gallery: The new age of dermoscopy: Optical super-high magnification. Br. J. Dermatol. 2018, 178, e330. [Google Scholar] [CrossRef] [Green Version]
- Cinotti, E.; Ekinde, S.; Labeille, B.; Raberin, H.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image gallery: Pigmented hyphae can be identified in vivo by high and super-high magnification dermoscopy. Br. J. Dermatol. 2019, 181, e4. [Google Scholar] [CrossRef]
- Cinotti, E.; Rossi, R.; Ferrara, G.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image Gallery: Super-high magnification dermoscopy can identify pigmented cells: Correlation with reflectance confocal microscopy. Br. J. Dermatol. 2019, 181, e1. [Google Scholar] [CrossRef] [PubMed]
- Provvidenziale, L.; Cinotti, E.; Campoli, M.; Rubegni, P. Superhigh magnification dermoscopy and management of a pediatric Spitz nevus mimicking melanoma. G. Ital. Dermatol. Venereol. 2021, 156, 111–112. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; de Giorgi, V.; Tomasini, C.; Deboli, T.; Quaglino, P.; Fierro, M.T.; Broganelli, P. Fluorescence-advanced videodermatoscopy: A new method for in vivo skin evaluation. Br. J. Dermatol. 2017, 177, e209–e210. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Minagawa, A.; Suzuki, R.; Koga, H.; Okuyama, R. Dermoscopy with near-ultraviolet light highlights the demarcation of melanin distribution in cutaneous melanoma. J. Am. Acad. Dermatol. 2021, 84, e23–e24. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance confocal microscopy for in vivo skin imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef]
- Que, S.K.; Fraga-Braghiroli, N.; Grant-Kels, J.M.; Rabinovitz, H.S.; Oliviero, M.; Scope, A. Through the looking glass: Basics and principles of reflectance confocal microscopy. J. Am. Acad. Dermatol. 2015, 73, 276–284. [Google Scholar] [CrossRef]
- Pellacani, G.; Farnetani, F.; Ciardo, S.; Chester, J.; Kaleci, S.; Mazzoni, L.; Bassoli, S.; Casari, A.; Pampena, R.; Mirra, M.; et al. Effect of Reflectance Confocal Microscopy for Suspect Lesions on Diagnostic Accuracy in Melanoma: A Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 754–761. [Google Scholar] [CrossRef]
- Brand, F.L.; Seyed Jafari, S.M.; Hunger, R.E. Confocal microscopy and lentigo maligna: An in vivo pilot study for the assessment of response to imiquimod therapy. Dermatology 2019, 235, 150–155. [Google Scholar] [CrossRef]
- Alarcon, I.; Carrera, C.; Alos, L.; Palou, J.; Malvehy, J.; Puig, S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J. Am. Acad. Dermatol. 2014, 71, 49–55. [Google Scholar] [CrossRef]
- Ulrich, M.; Krueger-Corcoran, D.; Roewert-Huber, J.; Sterry, W.; Stockfleth, E.; Astner, S. Reflectance confocal microscopy for noninvasive monitoring of therapy and detection of subclinical actinic keratoses. Dermatology 2010, 220, 15–24. [Google Scholar] [CrossRef]
- Segura, S.; Puig, S.; Carrera, C.; Lecha, M.; Borges, V.; Malvehy, J. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: A role for confocal microscopy and photodynamic therapy. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 819–827. [Google Scholar] [CrossRef]
- Malvehy, J.; Pérez-Anker, J.; Toll, A.; Pigem, R.; Garcia, A.; Alos, L.L.; Puig, S. Ex vivo confocal microscopy: Revolution in fast pathology in dermatology. Br. J. Dermatol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef]
- Cinotti, E.; Perrot, J.L.; Labeille, B.; Cambazard, F.; Rubegni, P. Ex vivo confocal microscopy: An emerging technique in dermatology. Dermatol. Pract. Concept. 2018, 8, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Que, S.K.T. Research techniques made simple: Noninvasive imaging technologies for the delineation of basal cell carcinomas. J. Investig. Dermatol. 2016, 136, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Anker, J.; Puig, S.; Malvehy, J. A fast and effective option for tissue flattening: Optimizing time and efficacy in ex vivo confocal microscopy. J. Am. Acad. Dermatol. 2020, 82, 157–158. [Google Scholar] [CrossRef]
- Hartmann, D.; Krammer, S.; Vural, S.; Bachmann, M.R.; Ruini, C.; Sárdy, M.; Ruzicka, T.; Berking, C.; Von Braunmühl, T. Immunofluorescence and confocal microscopy for ex vivo diagnosis of melanocytic and non-melanocytic skin tumours: A pilot study. J. Biophotonics. 2018, 11, e201700211. [Google Scholar] [CrossRef]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.M.; Patalay, R.; Lynch, M.D. Applications and future directions for optical coherence tomography in dermatology. Br. J. Dermatol. 2021, 184, 1014–1022. [Google Scholar] [CrossRef]
- Olsen, J.; Holmes, J.; Jemec, G.B.E. Advances in optical coherence tomography in dermatology—A review. JBO 2018, 23, 040901. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, M.; von Braunmuehl, T.; Kurzen, H.; Dirschka, T.; Kellner, C.; Sattler, E.; Berking, C.; Welzel, J.; Reinhold, U. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: An observational study. Br. J. Dermatol. 2015, 173, 428–435. [Google Scholar] [CrossRef]
- Boone, M.A.; Suppa, M.; Marneffe, A.; Miyamoto, M.; Jemec, G.B.; Del Marmol, V. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1714–1725. [Google Scholar] [CrossRef]
- Ferrante di Ruffano, L.; Dinnes, J.; Deeks, J.J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013189. [Google Scholar] [CrossRef] [PubMed]
- Marneffe, A.; Suppa, M.; Miyamoto, M.; Del Marmol, V.; Boone, M. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp. Dermatol. 2016, 25, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, V.; Themstrup, L.; Theut Riis, P.; Olsen, J.; Jemec, G.B. In vivo measurements of blood vessels’ distribution in non-melanoma skin cancer by dynamic optical coherence tomography—A new quantitative measure? Skin Res. Technol. 2018, 24, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Themstrup, L.; Pellacani, G.; Welzel, J.; Holmes, J.; Jemec, G.B.E.; Ulrich, M. In vivo microvascular imaging of cutaneous actinic keratosis, Bowe’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1655–1662. [Google Scholar] [CrossRef]
- Welzel, J.; Schuh, S.; de Carvalho, N.; Themstrup, L.; Ulrich, M.; Jemec, G.B.E.; Holmes, J.; Pellacani, G. Dynamic optical coherence tomography shows characteristic alterations of blood vessels in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1087–1093. [Google Scholar] [CrossRef]
- Dinnes, J.; Bamber, J.; Chuchu, N.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; Deeks, J.J.; et al. Cochrane Skin Cancer Diagnostic Test Accuracy Group. High-frequency ultrasound for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013188. [Google Scholar]
- Harland, C.C.; Bamber, J.C.; Gusterson, B.A.; Mortimer, P.S. High frequency, high resolution B-scan ultrasound in the assessment of skin tumours. Br. J. Dermatol. 1993, 128, 525–532. [Google Scholar] [CrossRef]
- Lindberg, C.A.; Grace, A. The Oxford American College Dictionary; G.P. Putnam’s Sons: New York, NY, USA, 2002. [Google Scholar]
- Svoboda, R.M.; Prado, G.; Mirsky, R.S.; Rigel, D.S. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J. Am. Acad. Dermatol. 2019, 80, 285–287. [Google Scholar] [CrossRef] [Green Version]
- Rocha, L.; Menzies, S.W.; Lo, S.; Avramidis, M.; Khoury, R.; Jackett, L.; Guitera, P. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br. J. Dermatol. 2017, 177, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Bourgeois, M.; Ribero, S.; Barreiro, A.; Espinoza, N.; Carrera, C.; Garcia, A.; Alos, L.; Puig, S.; Malvehy, J. Reflectance Confocal Microscopy and Electrical Impedance Spectroscopy in the Early Detection of Melanoma in Changing Lesions during Long-term Follow-up of Very High-risk Patients. Acta Derm. Venereol. 2022, 102, adv00751. [Google Scholar] [CrossRef]
- Rey-Barroso, L.; Burgos-Fernández, F.J.; Delpueyo, X.; Ares, M.; Royo, S.; Malvehy, J.; Puig, S.; Vilaseca, M. Visible and Extended Near-Infrared Multispectral Imaging for Skin Cancer Diagnosis. Sensors 2018, 18, 1441. [Google Scholar] [CrossRef] [Green Version]
- Tomatis, S.; Carrara, M.; Bono, A.; Bartoli, C.; Lualdi, M.; Tragni, G.; Colombo, A.; Marchesini, R. Automated melanoma detection with a novel multispectral imaging system: Results of a prospective study. Phys. Med. Biol. 2005, 50, 1675–1687. [Google Scholar] [CrossRef]
- Bekina, A.; Diebele, I.; Rubins, U.; Zaharans, J.; Dejarbo, A.; Sigulis, J. Multispectral assessment of skin malformations using a modified video-microscope. Latv. J. Phys. Tech. Sci. 2012, 49, 4–8. [Google Scholar]
- Jakovels, D.; Lihacova, I.; Kuzmina, I.; Sigulis, J. Evaluation of Skin Melanoma in Spectral Range 450–950 nm Using Principal Component Analysis. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 12–16 May 2013; Volume 8803. [Google Scholar]
- Hoover, E.E.; Squier, J.A. Advances in multiphoton microscopy technology. Nat. Photonics 2013, 7, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.S.; Dancik, Y.; Prow, T.W.; Thorling, C.A.; Lin, L.L.; Grice, J.E.; Robertson, T.A.; König, K.; Becker, W. Non-invasive imaging of skin physiology and percutaneous penetration using fluorescence spectral and lifetime imaging with multiphoton and confocal microscopy. Eur. J. Pharm. Biopharm. 2011, 77, 469–488. [Google Scholar] [CrossRef]
- Breunig, H.G.; Weinigel, M.; König, K. Multiphoton spectroscopy of human skin in vivo. SPIE Proc. 2012, 137, 82251D. [Google Scholar]
- Wyckoff, J.B.; Wang, Y.; Lin, E.Y.; Li, J.F.; Goswami, S.; Stanley, E.R.; Segall, J.E.; Pollard, J.W.; Condeelis, J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007, 67, 2649–2656. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.Y.; Haberman, A.M.; Greco, V. Preclinical Advances with Multiphoton Microscopy in Live Imaging of Skin Cancers. J. Investig. Dermatol. 2017, 137, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G. Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef]
- Murphy, R. Introduction to AI Robotics; MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Mitra, B.; Craswell, N. An Introduction to Neural Information Retrieval; Now Publishers Inc.: Boston, MA, USA, 2018. [Google Scholar]
- Takiddin, A.; Schneider, J.; Yang, Y.; Abd-Alrazaq, A.; Househ, M. Artificial Intelligence for Skin Cancer Detection: Scoping Review. J. Med. Internet Res. 2021, 23, e22934. [Google Scholar] [CrossRef] [PubMed]
- Haggenmüller, S.; Maron, R.C.; Hekler, A.; Utikal, J.S.; Barata, C.; Barnhill, R.L.; Beltraminelli, H.; Berking, C.; Betz-Stablein, B.; Blum, A.; et al. Skin cancer classification via convolutional neural networks: Systematic review of studies involving human experts. Eur. J. Cancer. 2021, 156, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Tschandl, P.; Rinner, C.; Apalla, Z.; Argenziano, G.; Codella, N.; Halpern, A.; Janda, M.; Lallas, A.; Longo, C.; Malvehy, J.; et al. Human-computer collaboration for skin cancer recognition. Nat. Med. 2020, 26, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Daneshjou, R.; Barata, C.; Betz-Stablein, B.; Celebi, M.E.; Codella, N.; Combalia, M.; Guitera, P.; Gutman, D.; Halpern, A.; Helba, B.; et al. Checklist for Evaluation of Image-Based Artificial Intelligence Reports in Dermatology: CLEAR Derm Consensus Guidelines From the International Skin Imaging Collaboration Artificial Intelligence Working Group. JAMA Dermatol. 2022, 158, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Artificial Intelligence-Based Skin Cancer Phone Apps Unreliable. JAMA 2020, 323, 1336. [Google Scholar] [CrossRef]
- Sun, M.D.; Kentley, J.; Mehta, P.; Dusza, S.; Halpern, A.C.; Rotemberg, V. Accuracy of commercially available smartphone applications for the detection of melanoma. Br. J. Dermatol. 2022, 186, 744–746. [Google Scholar] [CrossRef]
- Sangers, T.; Reeder, S.; van der Vet, S.; Jhingoer, S.; Mooyaart, A.; Siegel, D.M.; Nijsten, T.; Wakkee, M. Validation of a Market-Approved Artificial Intelligence Mobile Health App for Skin Cancer Screening: A Prospective Multicenter Diagnostic Accuracy Study. Dermatology 2022, 238, 649–656. [Google Scholar] [CrossRef]
- Jahn, A.S.; Navarini, A.A.; Cerminara, S.E.; Kostner, L.; Huber, S.M.; Kunz, M.; Maul, J.T.; Dummer, R.; Sommer, S.; Neuner, A.D.; et al. Over-Detection of Melanoma-Suspect Lesions by a CE-Certified Smartphone App: Performance in Comparison to Dermatologists, 2D and 3D Convolutional Neural Networks in a Prospective Data Set of 1204 Pigmented Skin Lesions Involving Patients’ Perception. Cancers 2022, 14, 3829. [Google Scholar] [CrossRef]

| Technique | Applications | Limitations | Perspectives |
|---|---|---|---|
| Clinical photography | Accurate health records, providing medico-legal documentation, evaluation of the patient’s lesions over time, pre-surgical planning, educating patients [3]. | Lack of the image acquisition standardization [7] | Wider use in the documentation of skin tumors, Image standards, Integration in the EHR (electronic health record), teledermatology |
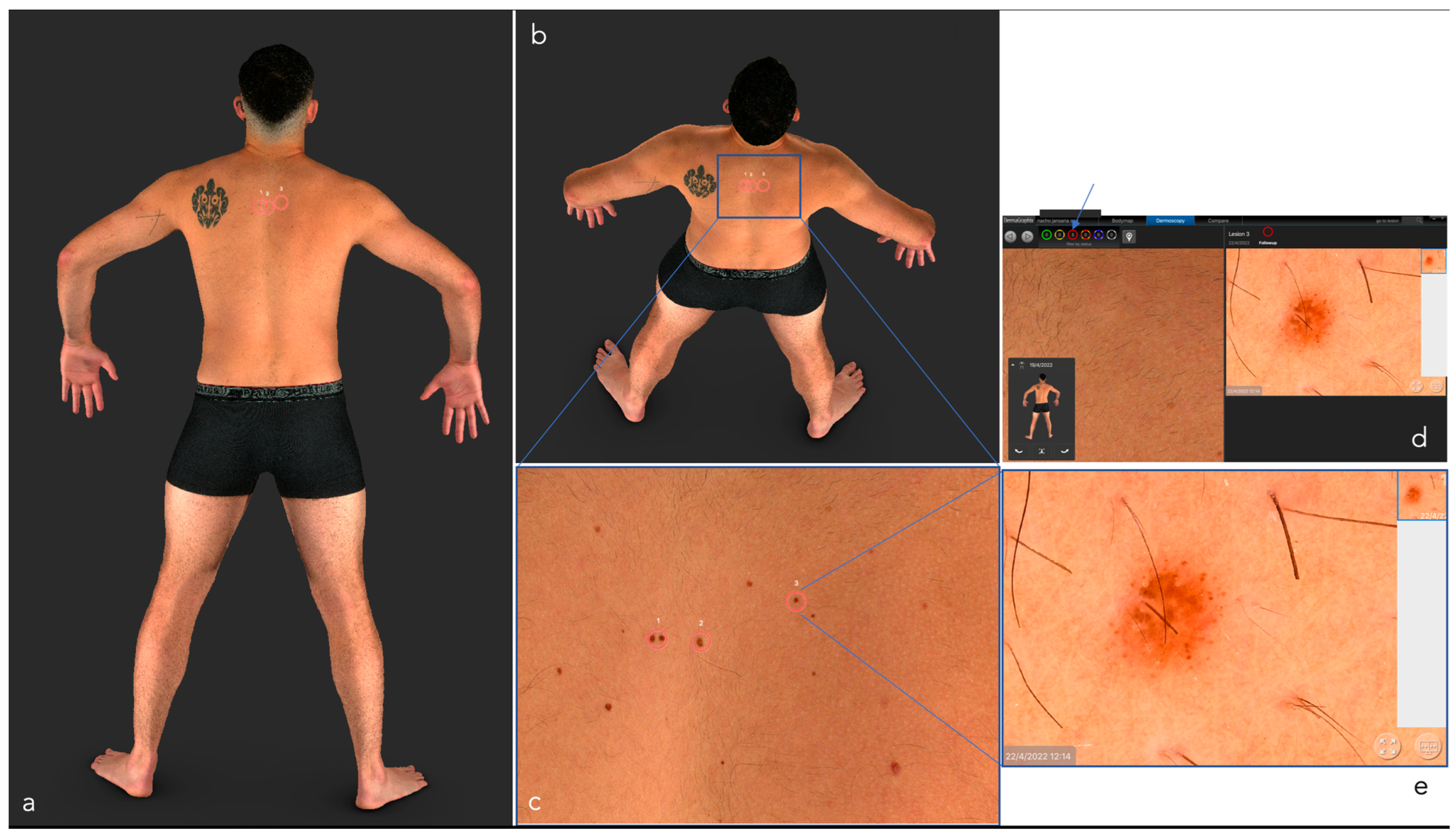
| Total Body Photography (TBP) 1 | TBP provides a 2-D or 3-D reconstruction of the entire skin surface, making it possible to follow up patients with a high number of atypical melanocytic lesions in a relatively short time and increasing the accuracy of the early diagnosis of melanoma [8,9,10,11] | Lack of image acquisition standardization, high prices of some TBP systems, large amount of storage space required [7] | Better resolution and faster examinations, integration, new standards (DICOM), incorporation of AI |
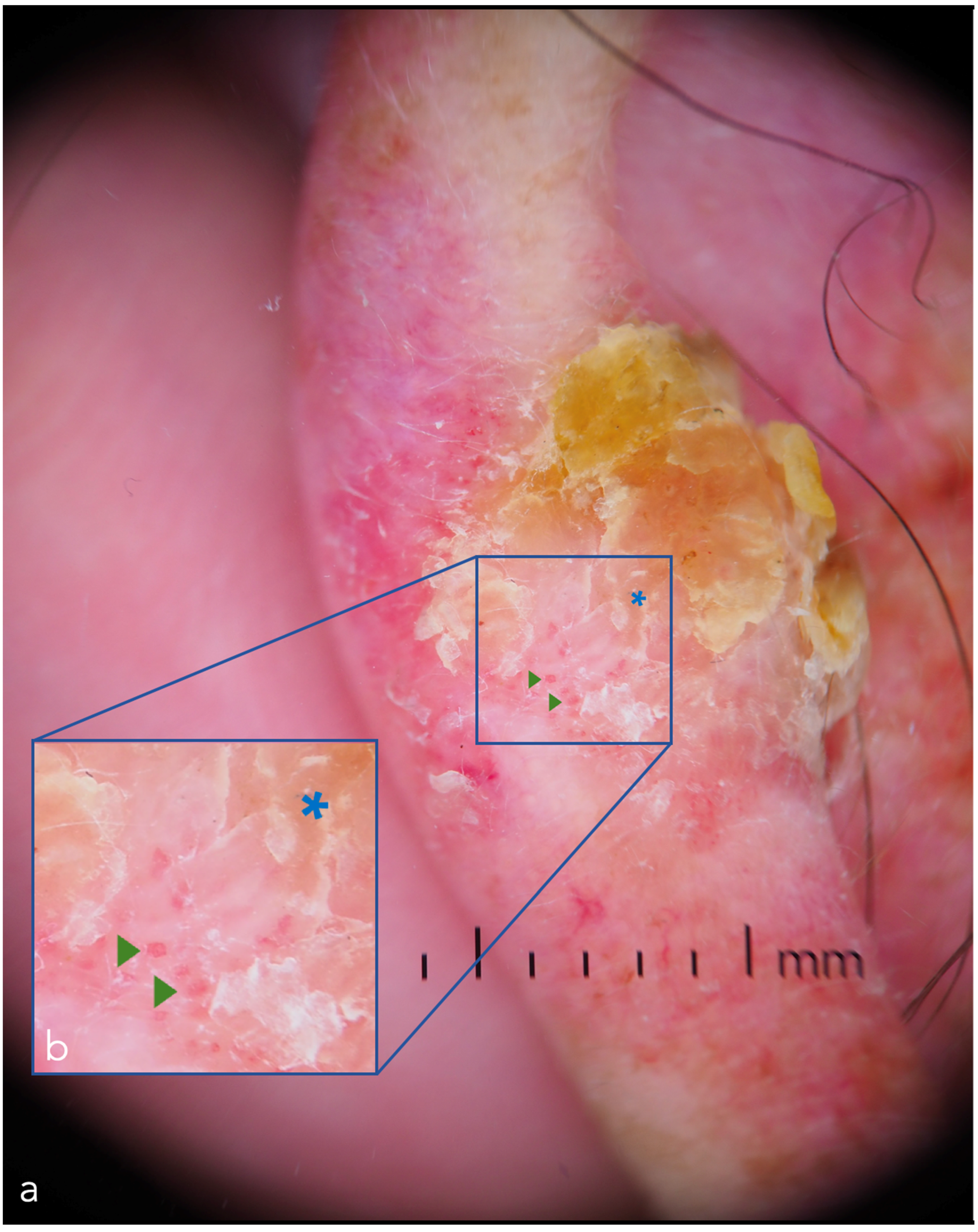
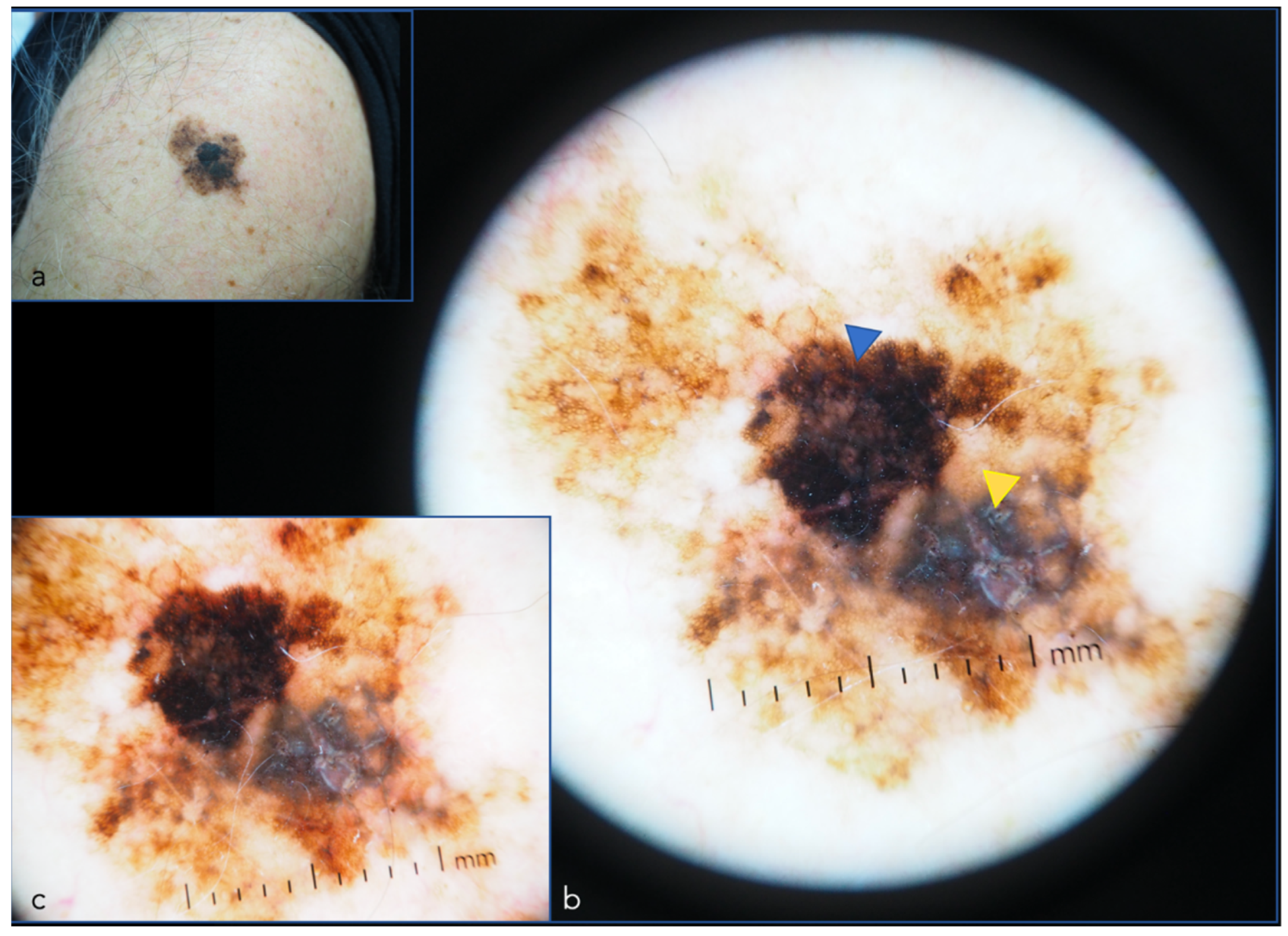
| Dermoscopy | A cornerstone of dermatologic diagnostics. Allows the visualization of skin lesions located in the epidermis and upper dermis [12,13] increasing diagnostic accuracy and reducing the benign-to-malignant biopsy ratio [14,15,16,17] | Diagnostic accuracy varies greatly according to the experience of the clinician and consequently a training period is necessary [18] | Computer image processing and AI, super-high (400×) magnification dermoscopy [19], new devices with multiple light sources, use in teledermatology, use with smartphones |
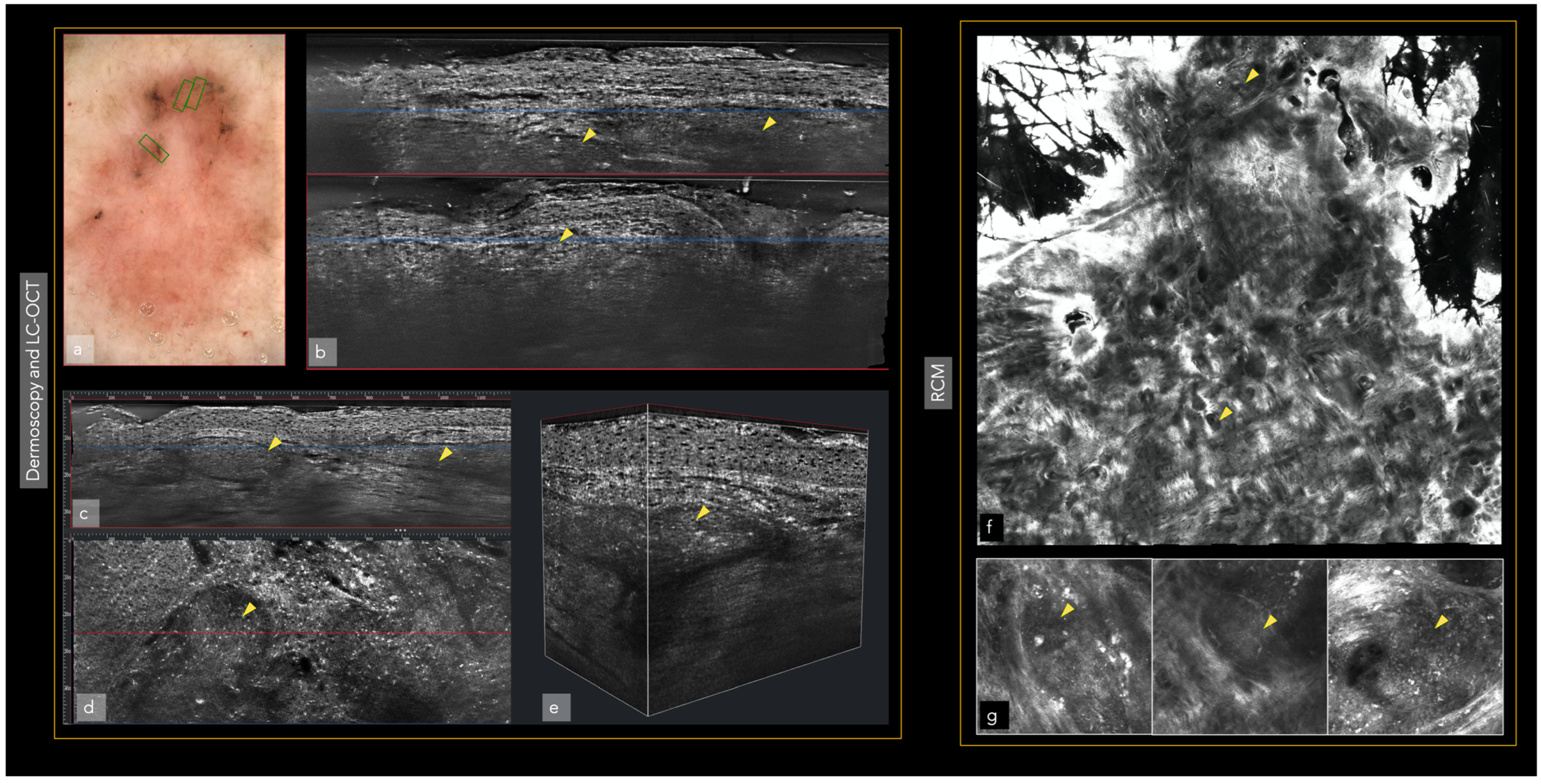
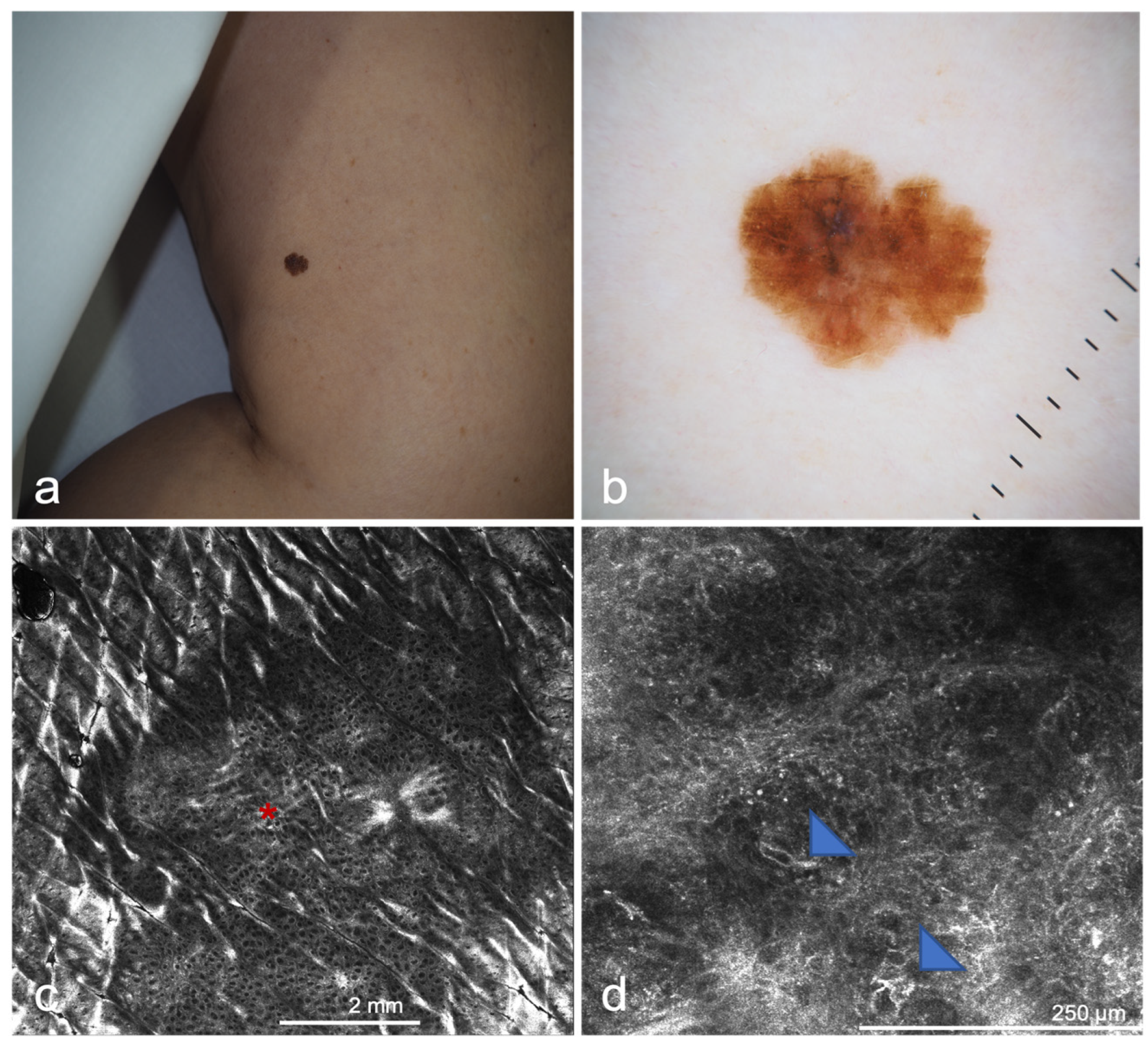
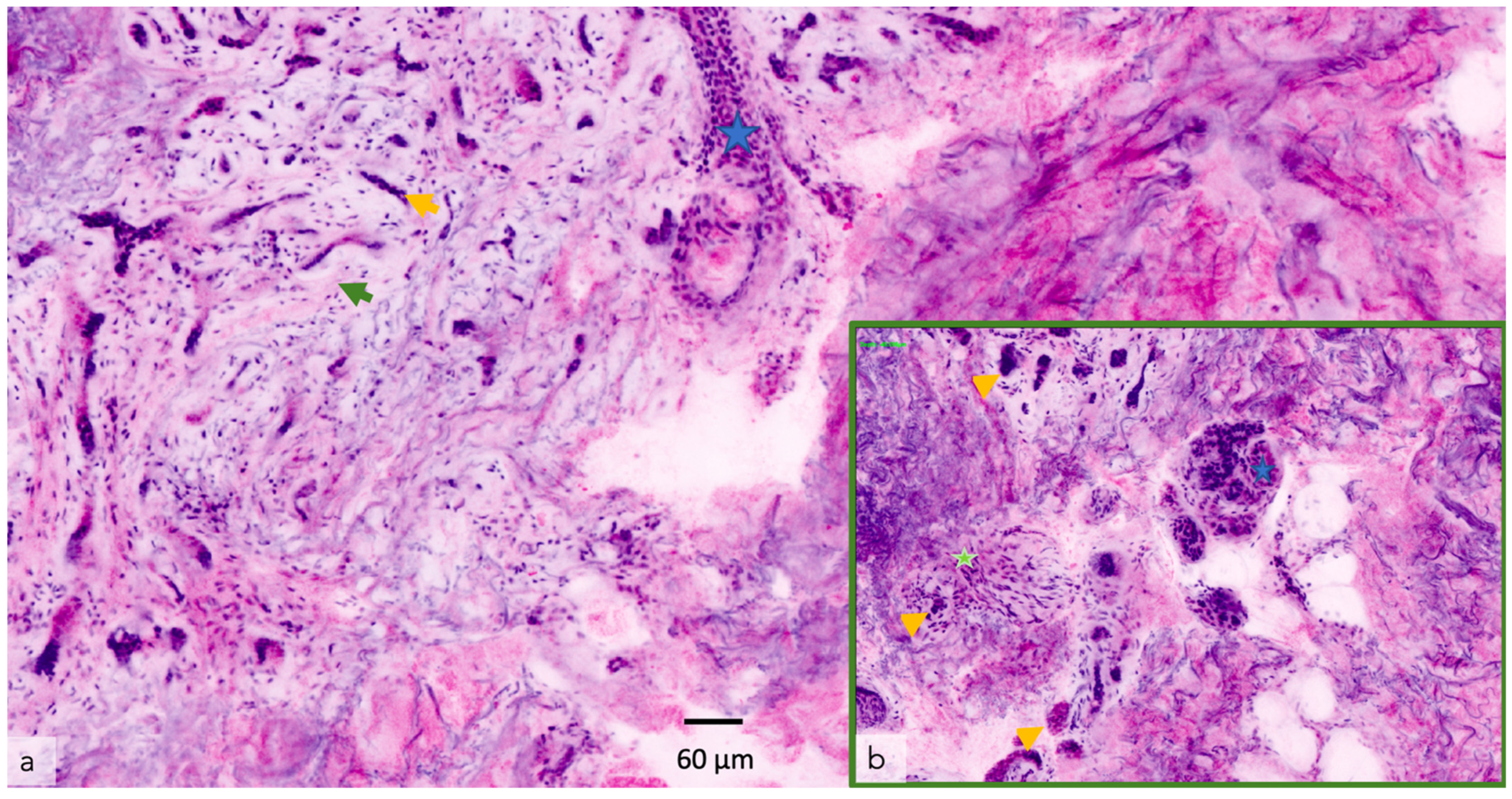
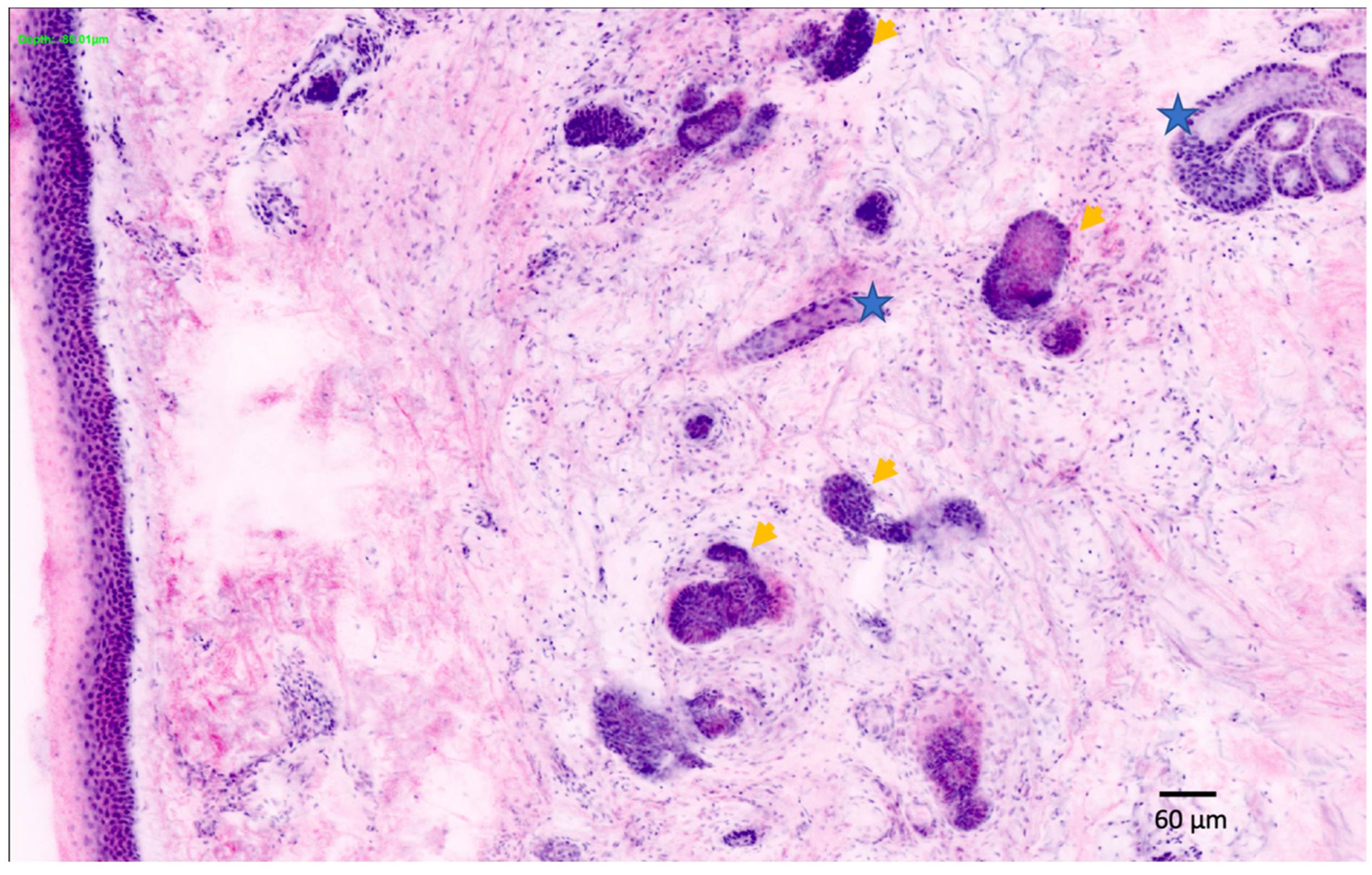
| Confocal microscopy (CM) 2 | Allows the capture of cellular-resolution images of skin lesions, parallel to the skin surface, at different depths from the stratum corneum to the superficial dermis up to a depth of 250–300 µm [20]. Reflectance confocal microscopy utilizes melanin and keratin as the main endogenous chromophores [21], increasing the diagnostic accuracy of melanoma and non-melanoma skin cancers [22,23,24], allowing the evaluation of pre-surgical skin tumor margins [25,26,27] and the non-invasive monitoring of the response of treatments [28]. Fluorescent confocal microscopy, uses fluorochromes ex vivo, to increase the cell-to-stroma contrast, allowing a fast assessment of tumor margins during surgery [29,30] | The devices are expensive [31], an extensive training period is necessary to master the procedure, limited depth of laser penetration [32], large amount of storage space required | CM/OCT/DERMOSCOPIC integrated devices, increase in image capture speed, use of new ex vivo fluorochromes to allow the staining of different types of cells (i.e., melanocytes), incorporation of AI |
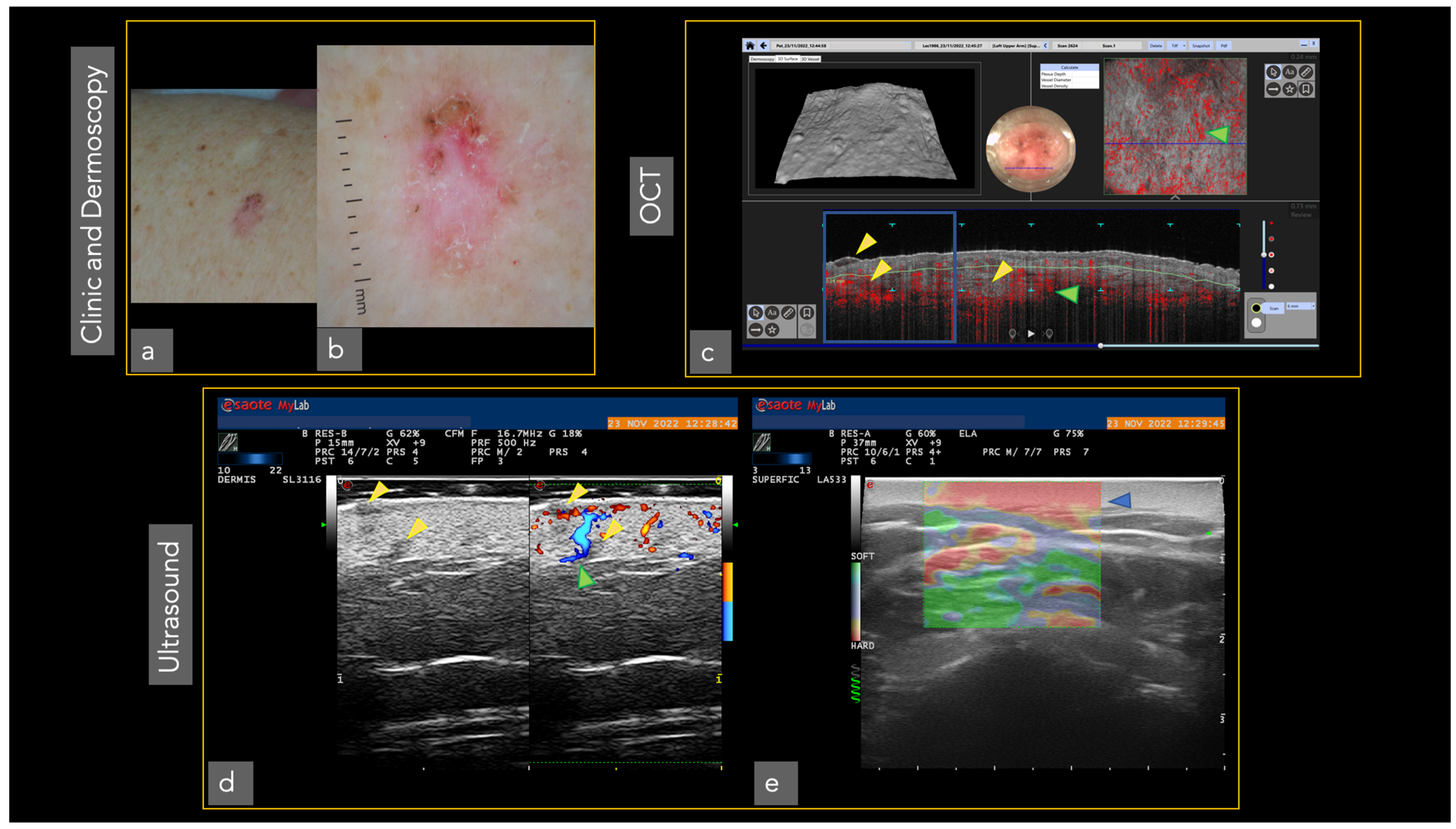
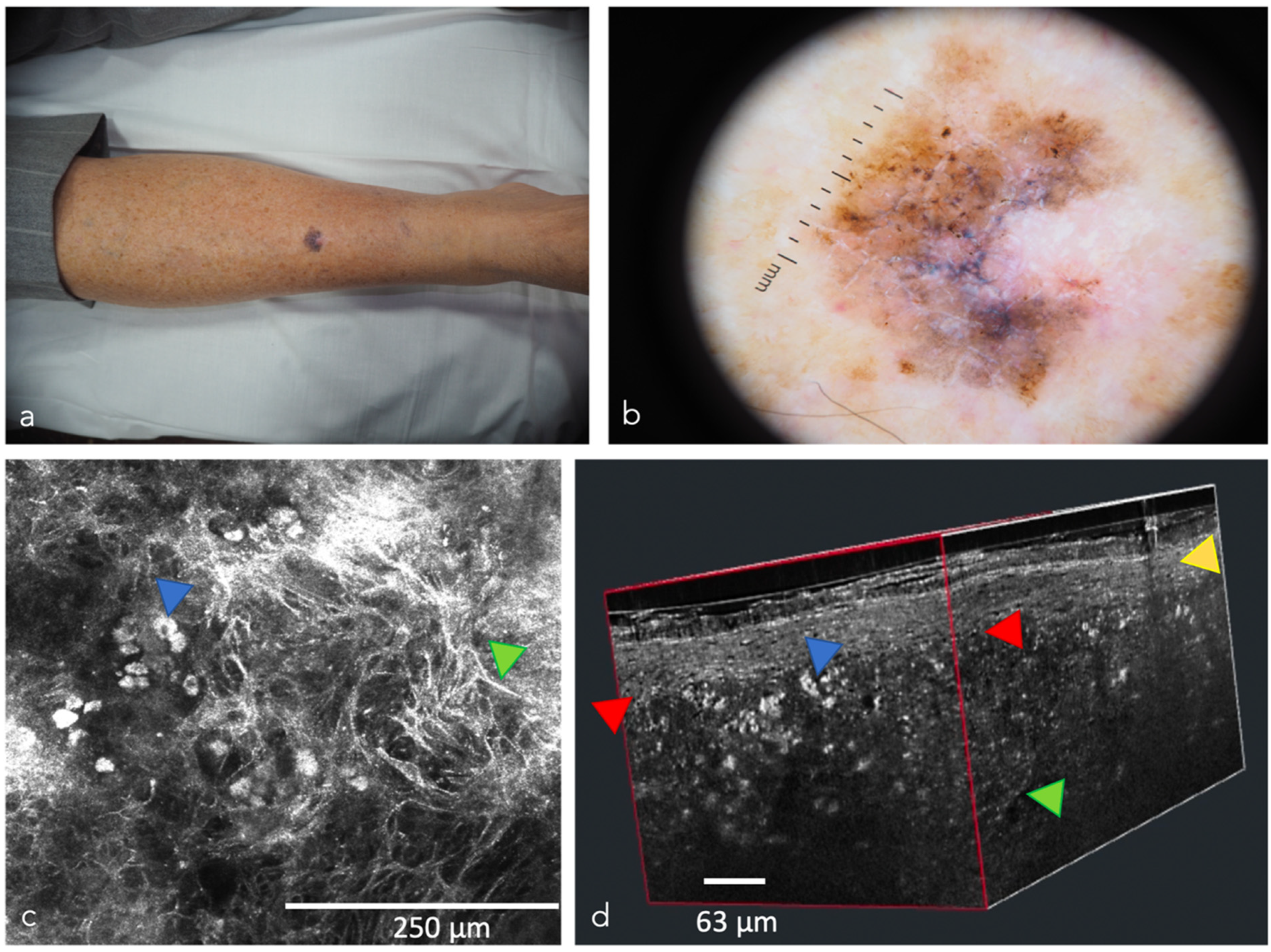
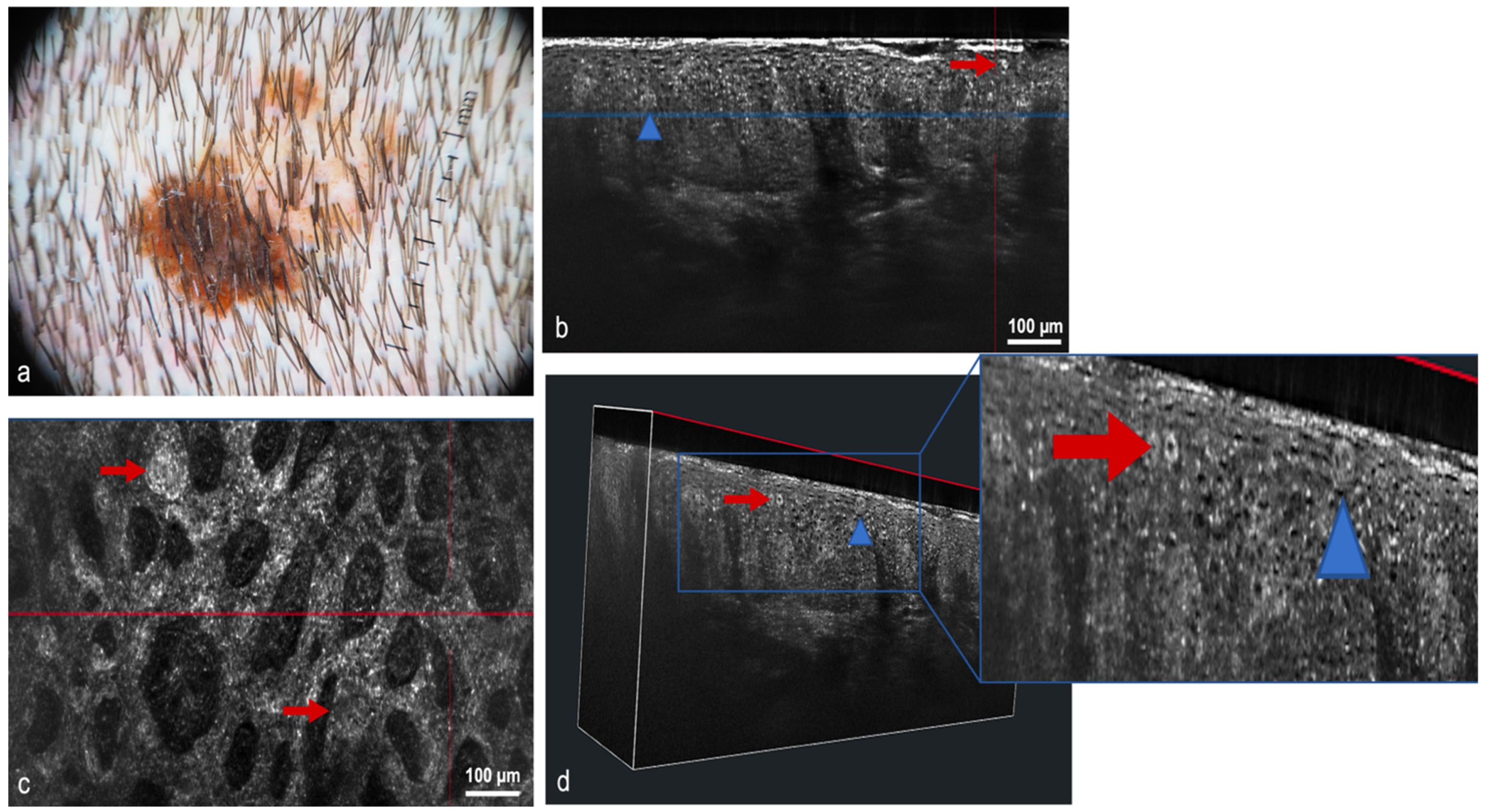
| Optical Coherence Tomography (OCT) 3 | OCT generates high-resolution en face and cross-sectional in vivo images of the skin to a maximum depth of 2 mm. Used mainly for the diagnosis of non-melanoma skin cancer [33]. Angiographic OCT (dynamic OCT), permits the detection of blood flow, achieving high-resolution two-dimensional and three-dimensional images of combined vascular structures within the skin’s structural organization [34]. LC-OCT 4 combines the principle of OCT interferometry with the spatial filtering of CM, providing three in vivo imaging modalities with cellular resolution: histological-like vertical, CM-like horizontal and a new unique 3-D reconstruction [35]. It has already shown excellent performance in the diagnosis of non-melanoma skin cancer [36,37,38,39,40] | The devices are expensive, extensive training is necessary to master the procedure, lack of cellular resolution for OCT and angiographic OCT [41] | Better contrast and optical resolution, definition of dynamic OCT diagnostic criteria, application of LC-OCT to all fields of dermatological oncology, incorporation of AI |
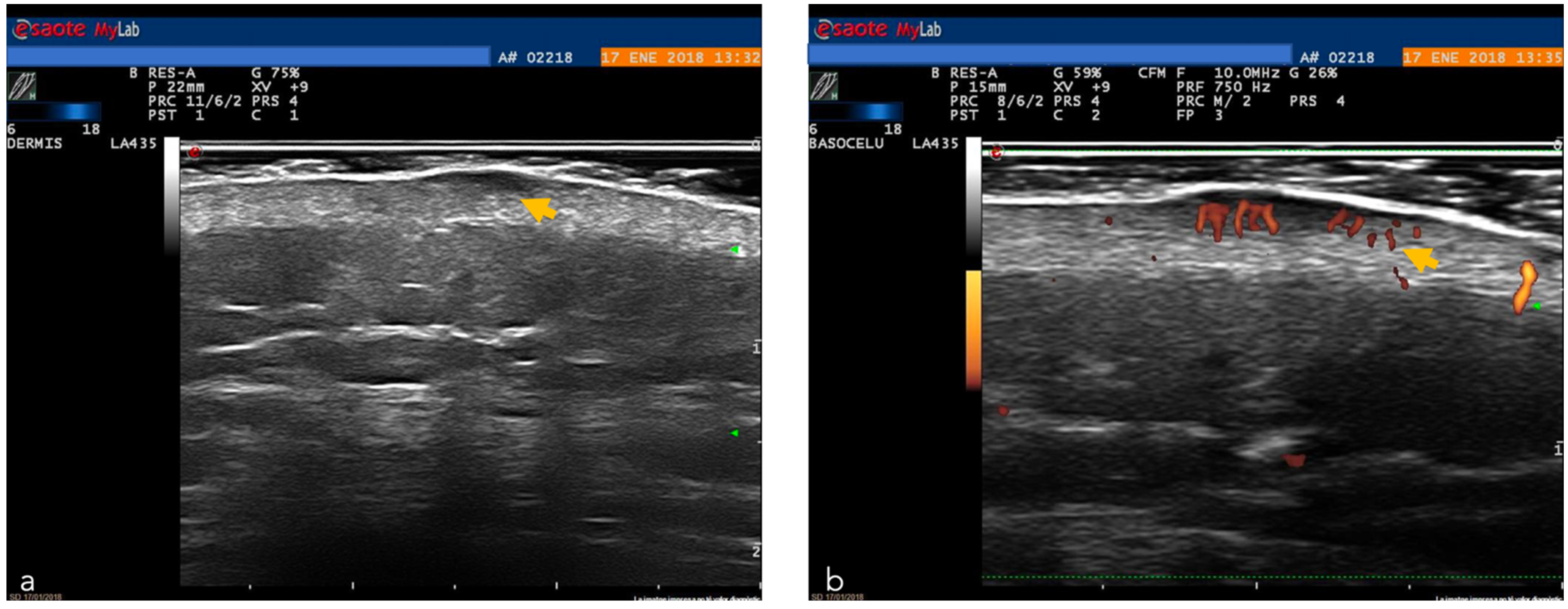
| Ultrasound | Non-invasive imaging technique based on the measurement of sound wave reflections from the tissues of the body [42]. Thanks to multiple frequencies which allow different depths of penetration, this can be a useful tool in the diagnosis of skin tumors, evaluating tumor depth and allowing pre-surgical planning [43,44,45,46,47,48,49] | Low image resolution, operator-dependent examination [50] extensive training is necessary | Higher ultrasound frequencies with better resolution of skin tumors, introduction of new devices with reduced costs for the use in practice in dermatological oncology. |
| RAMAN Spectroscopy | Measures the different Raman spectra, due to different molecular composition, between pathological and healthy tissue [51]. It has been used for the diagnosis of skin tumors, both in an ex vivo and in vivo context, with promising results in terms of sensitivity [52,53,54,55] | Elevated cost and limited clinical use | Development of less expensive and faster devices. New studies evaluating the diagnostic performance of coherent anti-Stokes Raman scattering and stimulated Raman scattering [56,57] are needed |
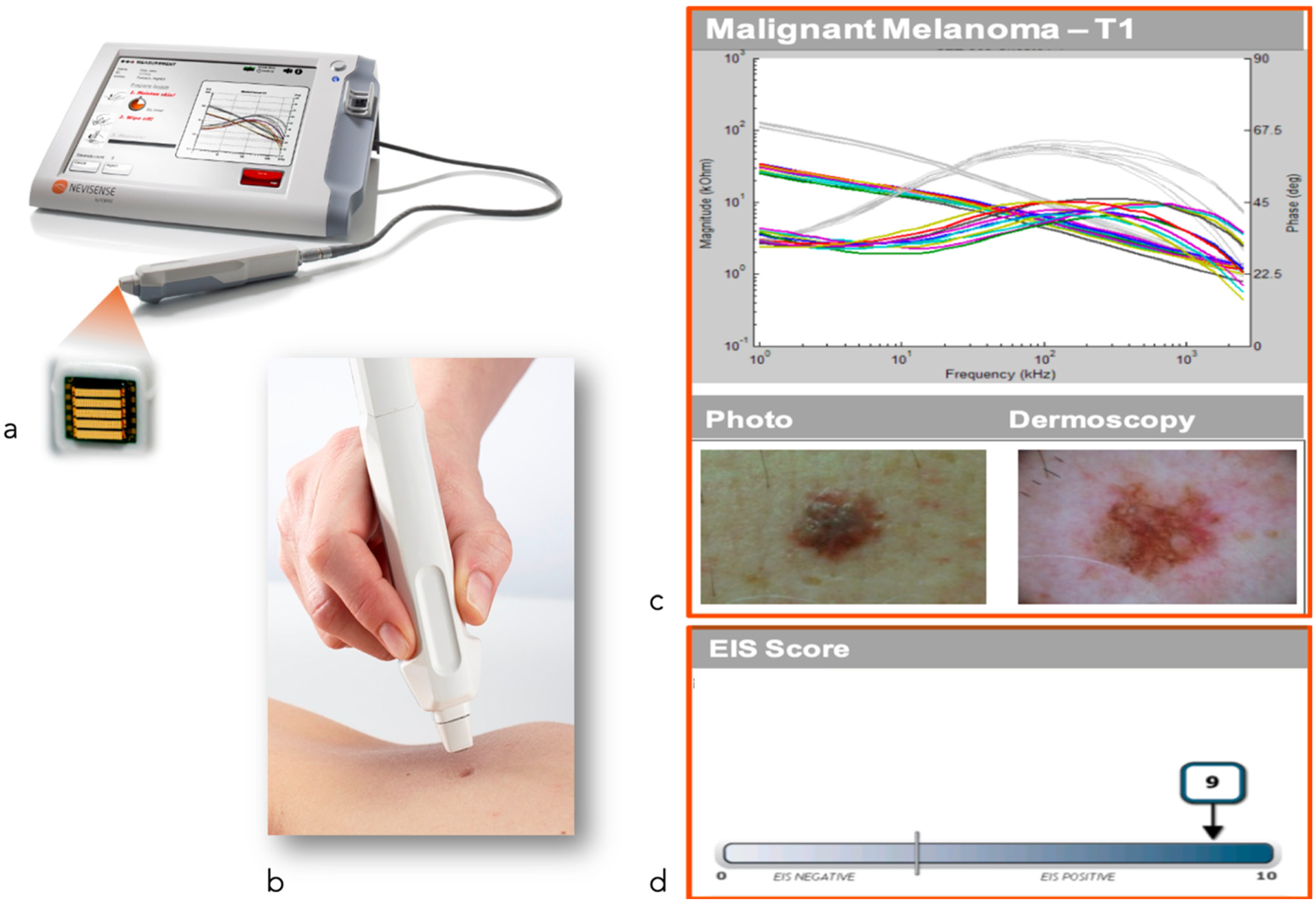
| Electrical impedance spectroscopy | Electrical impedance spectroscopy assumes that neoplastic transformation of cells alters their electrical impedance [58] | High processing speed and sensitivity for the diagnosis of both melanoma and non-melanoma skin cancers but limited specificity [59], only validated in prospective studies for melanocytic tumors | Role as an additive diagnostic tool, assessing resection margins, evaluating the success of a specific therapeutic regime in addition to classical dermoscopy. |
| Multispectral imaging | Provides precise quantification of spectral, colorimetric, and spatial features of the components of the skin using different light emission systems such as halogen lamps and light-emitting diodes [60,61]. The use of a multispectral imaging system based on an indium gallium arsenide camera makes near-infrared optical imaging possible. | Low resolution of indium gallium arsenide camera sensors, difficulties in selecting the near-infrared light-emitting diodes and small field of view of the system, long acquisition time. Lack of clinical validation | Development of miniaturized spectral imaging systems which could be linked to smartphones, allowing a promising quantitative, mobile skin diagnosis [62], improving speed of acquisition. Clinical validation. |
| Multiphoton microscopy (MPM) 5 | Based on the simultaneous excitation of a fluorophore by pulsed packets of photons of near-infrared wavelengths [63,64]. It has mainly been used, in a laboratory setting, to elucidate the mechanisms of the resistance of melanoma to immune and targeted therapy. However, has also proved useful in distinguishing between normal and precancerous epithelia [65] | Mainly used for research purposes. Lengthy time of acquisition | Use in combination with other techniques, such as coherent anti-Stokes Raman scattering, to reach rapid intraoperative assessment |
| Multispectral optoacoustic tomography (MSOT) 6 | Measures the ultrasound wave generated by the expansion of a dye exposed to short laser pulses [66]. It has been used in research studies for the noninvasive detection of sentinel lymph nodes [67] | Very low specificity [67] | New studies are needed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soglia, S.; Pérez-Anker, J.; Lobos Guede, N.; Giavedoni, P.; Puig, S.; Malvehy, J. Diagnostics Using Non-Invasive Technologies in Dermatological Oncology. Cancers 2022, 14, 5886. https://doi.org/10.3390/cancers14235886
Soglia S, Pérez-Anker J, Lobos Guede N, Giavedoni P, Puig S, Malvehy J. Diagnostics Using Non-Invasive Technologies in Dermatological Oncology. Cancers. 2022; 14(23):5886. https://doi.org/10.3390/cancers14235886
Chicago/Turabian StyleSoglia, Simone, Javiera Pérez-Anker, Nelson Lobos Guede, Priscila Giavedoni, Susana Puig, and Josep Malvehy. 2022. "Diagnostics Using Non-Invasive Technologies in Dermatological Oncology" Cancers 14, no. 23: 5886. https://doi.org/10.3390/cancers14235886
APA StyleSoglia, S., Pérez-Anker, J., Lobos Guede, N., Giavedoni, P., Puig, S., & Malvehy, J. (2022). Diagnostics Using Non-Invasive Technologies in Dermatological Oncology. Cancers, 14(23), 5886. https://doi.org/10.3390/cancers14235886







