Dietary Polyphenol Intake and Gastric Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Research in Context
1.1. Evidence before Study
1.2. Added Value of Study
1.3. Implications of Available Evidence
2. Introduction
3. Methods
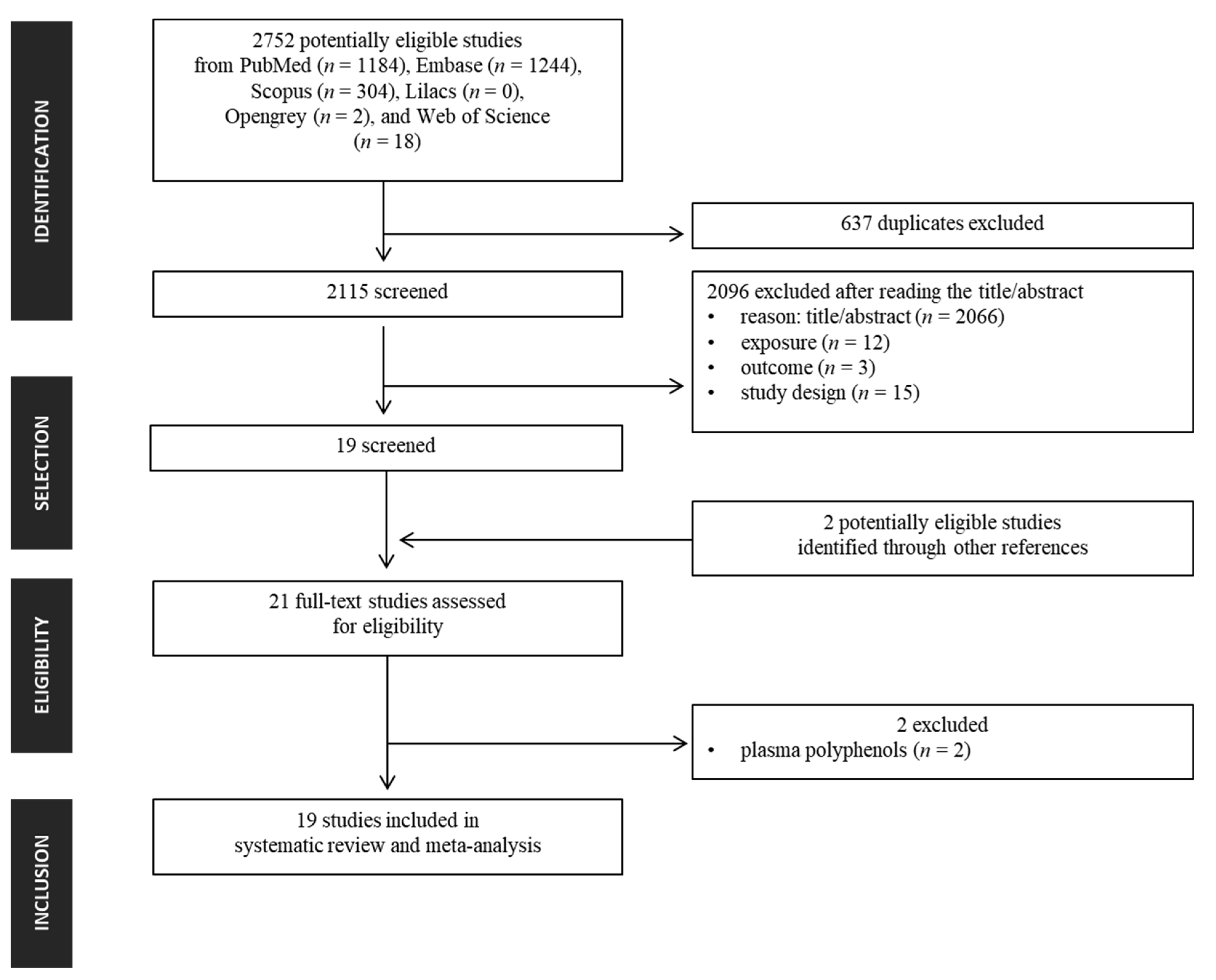
3.1. Search Strategy and Selection Criteria
3.2. Data Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report 2018; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Przegląd Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.H.; Mayne, S.T.; Risch, H.A.; et al. Dietary Intake of Flavonoids and Oesophageal and Gastric Cancer: Incidence and Survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Vitelli-Storelli, F.; Rossi, M.; Pelucchi, C.; Rota, M.; Palli, D.; Ferraroni, M.; Lunet, N.; Morais, S.; López-Carrillo, L.; Zaridze, D.G.; et al. Polyphenol Intake and Gastric Cancer Risk: Findings from the Stomach Cancer Pooling Project (Stop). Cancers 2020, 12, 3064. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary Assessment and Role in the Prevention of Cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef]
- Rambaran, T.F. Nanopolyphenols: A Review of Their Encapsulation and Anti-Diabetic Effects. SN Appl. Sci. 2020, 2, 1–26. [Google Scholar] [CrossRef]
- Garcia-Closas, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. Intake of Specific Carotenoids and Flavonoids and the Risk of Gastric Cancer in Spain. Cancer Causes Control 1999, 10, 71–75. [Google Scholar] [CrossRef]
- Lagiou, P.; Samoli, E.; Lagiou, A.; Peterson, J.; Tzonou, A.; Dwyer, J.; Trichopoulos, D. Flavonoids, Vitamin C and Adenocarcinoma of the Stomach. Cancer Causes Control 2004, 15, 67–72. [Google Scholar] [CrossRef]
- Hara, A.; Sasazuki, S.; Inoue, M.; Iwasaki, M.; Shimazu, T.; Sawada, N.; Yamaji, T.; Tsugane, S. Isoflavone Intake and Risk of Gastric Cancer: A Population-Based Prospective Cohort Study in Japan. Am. J. Clin. Nutr. 2012, 95, 147–154. [Google Scholar] [CrossRef]
- Sun, L.; Subar, A.F.; Bosire, C.; Dawsey, S.M.; Kahle, L.L.; Zimmerman, T.P.; Abnet, C.C.; Heller, R.; Graubard, B.I.; Cook, M.B.; et al. Dietary Flavonoid Intake Reduces the Risk of Head and Neck but Not Esophageal or Gastric Cancer in US Men and Women. J. Nutr. 2017, 147, 1729–1738. [Google Scholar] [CrossRef]
- Hirvonen, T.; Virtamo, J.; Korhonen, P.; Albanes, D.; Pietinen, P. Flavonol and Flavone Intake and the Risk of Cancer in Male Smokers (Finland). Cancer Causes Control 2001, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Lee, J.; Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kwon, O.; Kim, J. Dietary Flavonoids and Gastric Cancer Risk in a Korean Population. Nutrients 2014, 6, 4961–4973. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Rosato, V.; Bosetti, C.; Lagiou, P.; Parpinel, M.; Bertuccio, P.; Negri, E.; la Vecchia, C. Flavonoids, Proanthocyanidins, and the Risk of Stomach Cancer. Cancer Causes Control 2010, 21, 1597–1604. [Google Scholar] [CrossRef]
- Rubín-García, M.; Vitelli-Storelli, F.; José Molina, A.; Zamora-Ros, R.; Aragonés, N.; Adarnaz, E.; Castaño-Vinyals, G.; Obón-Santacana, M.; Gómez-Acebo, I.; Molina-Barceló, A.; et al. Association between Polyphenol Intake and Gastric Cancer Risk by Anatomic and Histologic Subtypes: MCC-Spain. Nutrients 2020, 12, 3281. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid Intake and Risk of Chronic Diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Sun, J.; Wang, M.; Ding, J.; Lu, Q.; Yuan, L. Dietary Flavonoid Intake and the Risk of Digestive Tract Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary Flavonoid Intake and Risk of Stomach and Colorectal Cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Yu, F.; Tian, Y.; Wu, Y.; Cui, L.; Liu, L.E. The Association between Soy-Based Food and Soy Isoflavone Intake and the Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. J. Sci. Food Agric. 2021, 101, 5314–5324. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwel, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 April 2022).
- Yamada, A.; Komaki, Y.; Komaki, F.; Micic, D.; Zullow, S.; Sakuraba, A. Risk of Gastrointestinal Cancers in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis. Lancet Oncol. 2018, 19, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, X.; Yuan, W.; Chen, Z. Intake of Anthocyanins and Gastric Cancer Risk: A Comprehensive Meta-Analysis on Cohort and Case-Control Studies. J. Nutr. Sci. Vitaminol. 2019, 65, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ramírez, R.U.; Galván-Portillo, M.V.; Ward, M.H.; Agudo, A.; González, C.A.; Oñate-Ocaña, L.F.; Herrera-Goepfert, R.; Palma-Coca, O.; López-Carrillo, L. Dietary Intake of Polyphenols, Nitrate and Nitrite and Gastric Cancer Risk in Mexico City. Int. J. Cancer 2009, 125, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Ekström, A.M.; Serafini, M.; Nyrén, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary Quercetin Intake and Risk of Gastric Cancer: Results from a Population-Based Study in Sweden. Ann. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Agudo, A.; Luján-Barroso, L.; Romieu, I.; Ferrari, P.; Knaze, V.; Bueno-de-Mesquita, H.B.; Leenders, M.; Travis, R.C.; Navarro, C.; et al. Dietary Flavonoid and Lignan Intake and Gastric Adenocarcinoma Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Am. J. Clin. Nutr. 2012, 96, 1398–1408. [Google Scholar] [CrossRef]
- Lin, Y.; Wolk, A.; Håkansson, N.; Lagergren, J.; Lu, Y. Dietary Intake of Lignans and Risk of Esophageal and Gastric Adenocarcinoma: A Cohort Study in Sweden. Cancer Epidemiol. Biomark. Prev. 2013, 22, 308–312. [Google Scholar] [CrossRef]
- Wada, K.; Tsuji, M.; Tamura, T.; Konishi, K.; Kawachi, T.; Hori, A.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Soy Isoflavone Intake and Stomach Cancer Risk in Japan: From the Takayama Study. Int. J. Cancer 2015, 137, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Park, Y.; Lee, J.; Choi, I.J.; Kim, Y.W.; Ryu, K.W.; Sung, J.; Kim, J. Effects of Soy Product Intake and Interleukin Genetic Polymorphisms on Early Gastric Cancer Risk in Korea: A Case-Control Study. Cancer Res. Treat. 2017, 49, 1044–1056. [Google Scholar] [CrossRef]
- Vitelli-Storelli, F.; Molina, A.J.; Zamora-Ros, R.; Fernández-Villa, T.; Roussou, V.; Romaguera, D.; Aragonés, N.; Obón-Santacana, M.; Guevara, M.; Gómez-Acebo, I.; et al. Flavonoids and the Risk of Gastric Cancer: An Exploratory Case-Control Study in the MCC-Spain Study. Nutrients 2019, 11, 967. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kim, J. Antioxidant-Rich Diet, GSTP1 Rs1871042 Polymorphism, and Gastric Cancer Risk in a Hospital-Based Case-Control Study. Front. Oncol. 2021, 10, 596355. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, S.; Su, Y. Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies. Nutrients 2016, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—Food Sources and Health Benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Lei, L.; Yang, Y.; He, H.; Chen, E.; Du, L.; Dong, J.; Yang, J. Flavan-3-Ols Consumption and Cancer Risk: A Meta-Analysis of Epidemiologic Studies. Oncotarget 2016, 7, 73573–73592. [Google Scholar] [CrossRef] [PubMed]
- Golpour, A.; Rafie, N.; Safavi, S.M.; Miraghajani, M. Dietary Isoflavones and Gastric Cancer: A Brief Review of Current Studies. J. Res. Med. Sci. 2015, 20, 893–900. [Google Scholar] [CrossRef]
- You, J.; Sun, Y.; Bo, Y.; Zhu, Y.; Duan, D.; Cui, H.; Lu, Q. The Association between Dietary Isoflavones Intake and Gastric Cancer Risk: A Meta-Analysis of Epidemiological Studies. BMC Public Health 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; del Rio, D.; Galvano, F. A Comprehensive Meta-Analysis on Dietary Flavonoid and Lignan Intake and Cancer Risk: Level of Evidence and Limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.; Malathi, L.; Krupaa, J.R. Naturally Occurring Products in Cancer Therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.-M.; Zhang, P.-Y.; Liu, Y.; Wu, Y.-M.; Zhang, P.-Y. Protective Effects of Curcumin and Quercetin during Benzo(a)Pyrene Induced Lung Carcinogenesis in Mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Middleton, E. Effect of Plant Flavonoids on Immune and Inflammatory Cell Function; Springer: Berlin/Heidelberg, Germany, 1998; pp. 175–182. [Google Scholar]
- de Melo, E.L.; Pinto, A.M.; Baima, C.L.B.; da Silva, H.R.; da Silva Sena, I.; Sanchez-Ortiz, B.L.; de Lima Teixeira, A.V.T.; Pereira, A.C.M.; da Silva Barbosa, R.; Carvalho, H.O.; et al. Evaluation of the in Vitro Release of Isoflavones from Soybean Germ Associated with Kefir Culture in the Gastrointestinal Tract and Anxiolytic and Antidepressant Actions in Zebrafish (Danio Rerio). J. Funct. Foods 2020, 70, 103986. [Google Scholar] [CrossRef]
- Freedman, N.D.; Chow, W.H.; Gao, Y.T.; Shu, X.O.; Ji, B.T.; Yang, G.; Lubin, J.H.; Li, H.L.; Rothman, N.; Zheng, W.; et al. Menstrual and Reproductive Factors and Gastric Cancer Risk in a Large Prospective Study of Women. Gut 2007, 56, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Ekström, A.M.; Serafini, M.; Nyrén, O.; Hansson, L.-E.; Ye, W.; Wolk, A. Dietary Antioxidant Intake and the Risk of Cardia Cancer and Noncardia Cancer of the Intestinal and Diffuse Types: A Population-Based Case-Control Study in Sweden. Int. J. Cancer 2000, 87, 133–140. [Google Scholar] [CrossRef] [PubMed]
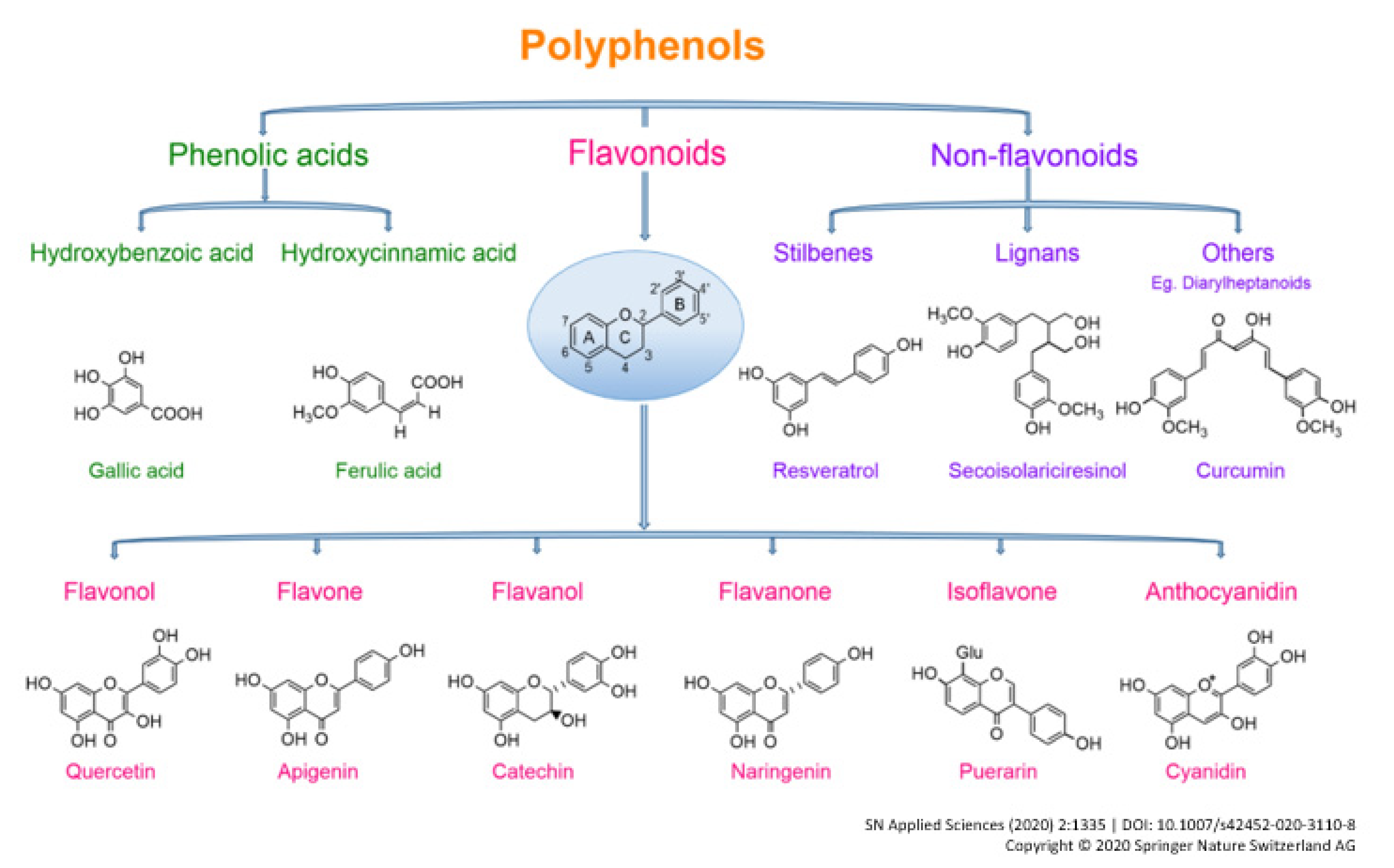
| Author/Country | Study Type and Period | Sample | Diet Assessment Method | Source of Polyphenols | Classes of Polyphenols | RR/OR (95% CI) | Intake Comparison | Adjustment Variables |
|---|---|---|---|---|---|---|---|---|
| Garcia-Closas et al. [8] Spain | Case-control 1987–1989 | Cases = 354 Controls = 354 | Past year’s diet history | Fruit, vegetables, fruit juices, wines and tea infusions | Total Flavonoids Quercetin Kaempferol Myricetin | 0.44 (0.25–0.78) 0.62 (0.35–1.10) 0.48 (0.26–0.88) 1.12 (0.67–1.85) | Highest quartile X Lowest quartile | Intake of nitrites, nitrosamines, vitamin C, total energy, total carotenoids, and other specific flavonoids. |
| Hirvonen et al. [12] Finland | Prospective cohort 1985–1993 | 27,110 GC = 111 | Past year’s diet history | Fruit, vegetables, teas, wines and sweets | Flavonoids and Flavones | 1.2 (0.71–1.9) | Highest quartile X Lowest quartile | Age and supplementation group. |
| Knekt et al. [16] Finland | Prospective cohort 1966–1972 | 9865 GC = 74 | Past year’s diet history | Fruit, vegetables, sweets and beverages (including tea and wines) | Total Flavonoids Quercetin Kaempferol Myricetin Hesperetin Naringenin | 0.87 (0.44–1.75) 1.03 (0.52–2.07) 1.14 (0.59–2.22) 1.16 (0.59–2.26) 0.88 (0.43–1.80) 0.94 (0.47–1.88) | Highest quartile X Lowest quartile | Sex, age, geographic area, occupation, smoking, and BMI. |
| Lagiou et al. [9] Greece | Case-control 1981–1984 | Cases = 110 Controls = 100 | FFQ last 5 years | Items not described | Model 1/2 | Per one standard deviation increment: | Model 1: Age, gender, place of birth, BMI, height, years of education, smoking habits and duration of smoking, alcohol consumption, total energy intake. Model 2: Model 1 + fruit and vegetable consumption. | |
| Flavanones | 0.49 (0.32–0.76)/0.55 (0.31–0.96) | per 19.8 mg/day | ||||||
| Flavan-3-ols | 1.10 (0.76–1.60)/1.04 (0.68–1.58) | per 135.1 mg/day | ||||||
| Flavonols | 0.40 (0.25–0.64)/0.77 (0.42–1.40) | per 10.0 mg/day | ||||||
| Flavones | 0.60 (0.40–0.89)/0.70 (0.43–1.14) | per 0.3 mg/day | ||||||
| Anthocyanidins | 0.88 (0.60–1.28)/1.14 (0.72–1.80) | per 40.4 mg/day | ||||||
| Isoflavones | 1.27 (0.84–1.93)/1.16 (0.73–1.84) | per 2.0 mg/day | ||||||
| Hernández-Ramírez et al. [25] Mexico | Case control 2004–2005 | Cases = 248 Controls = 478 | FFQ last 3 years | Fruit, vegetables, noodle soup, hot sauce, beans, orange juice, red wine | Model 1/2/3/4 | Highest tertile X Lowest tertile | Model 1: Energy, age, gender, H. pylori CagA status, schooling and consumption of salt, chili, alcohol. Model 2: Model 1 + vitamins C and E. Model 3: Model 2 + fruits and vegetables. Model 4: Model 3 + polyphenols. | |
| Cinnamic Acids | 0.52 (0.34–0.81)/0.49 (0.31–0.78)/ 0.61 (0.38–0.97)/0.80 (0.49–1.31). | |||||||
| Secoisolariciresinol | 0.42 (0.27–0.65)/0.41 (0.26–0.64)/0.47 (0.30–0.74)/0.57 (0.32–0.99). | |||||||
| Coumestrol | 0.45 (0.29–0.70)/0.45 (0.29–0.71)/0.42 (0.27–0.65)/0.67 (0.39–1.16). | |||||||
| Ekström et al. [26] Sweden | Case control 1989–1995 | Cases = 505 Controls = 1116 | FFQ last 20 years | Fruit, vegetables, wine, tea, coffee and fruit juices. | Quercetin | Male: 0.66 (0.42–1.04) Female: 0.49 (0.25–0.94) Cardia: 0.76 (0.40–1.44) Noncardia: 0.57 (0.40–0.83) Intestinal: 0.51 (0.32–0.82) Diffuse: 0.54 (0.31–0.92) | Highest quintile X Lowest quintile | Age, gender, socioeconomic status, number of siblings, body mass index, smoking, and energy and salt intake. |
| Rossi et al. [14] Italy | Case control 1997–2007 | Cases = 230 Controls = 547 | FFQ last 2 years | 78 items such as fruit, vegetables, soup, tea, wine and chocolate. | Isoflavones | 0.88 (0.53–1.46) | Highest quintile X Lowest quintile | Sex, age, education, year of interview, body mass index, tobacco smoking, and total energy intake. |
| Anthocyanidins | 0.91 (0.56–1.47) | |||||||
| Flavan-3-ols | 0.75 (0.45–1.23) | |||||||
| Flavanones | 0.91 (0.54–1.51) | |||||||
| Flavones | 0.83 (0.50–1.39) | |||||||
| Flavonols | 0.62 (0.38–1.02) | |||||||
| Proanthocyanidins | 0.34 (0.20–0.58) | |||||||
| Hara et al. [10] Japan | Prospective cohort 1990–2006 | 84,881 GC = 1249 | FFQ past year | Miso soup, food and soy milk | Isoflavones | Model 1/2 Male 0.98 (0.80, 1.20)/1.00 (0.81, 1.24) Female 0.99 (0.71, 1.37)/1.07 (0.77, 1.50) Model 2 only Cardia: 2.00 (0.97, 4.12) Noncardia: 0.97 (0.74, 1.26) | Highest quartile X Lowest quartile | Model 1: Age and public center area. Model 2: BMI, smoking status, ethanol intake, family history of gastric cancer, vegetable intake, fruit intake, fish intake, salt intake, and total energy intake. |
| Zamora-Ros et al. [27] EPIC study (Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden and UK) | Prospective cohort 1992–2010 | 477,312 GC = 683 | Several validated FFQs | Items not described | Total flavonoids | Male: 0.97 (0.67, 1.41) Female: 0.49 (0.30, 0.80) Cardia: 0.84 (0.64, 1.11) Noncardia: 0.85 (0.70, 1.03) Intestinal: 0.70 (0.52, 0.94) Diffuse: 0.94 (0.74, 1.19) | Male/Female: Highest quartile X Lowest quartile Cardia/Noncardia; Intestinal/Diffuse: log2 | Age, educational level, physical activity, BMI, alcohol and energy intake, and daily consumption of fruit, vegetables, and red and processed meat. |
| Anthocyanidins | Male: 0.98 (0.68, 1.41) Female: 0.71 (0.44, 1.16) Cardia: 0.89 (0.69, 1.15) Noncardia: 0.90 (0.79, 1.04) Intestinal: 0.92 (0.73, 1.16) Diffuse: 0.86 (0.75, 0.99) | |||||||
| Flavonols | Male: 0.93 (0.63, 1.37) Female: 0.45 (0.27, 0.75) Cardia: 0.85 (0.60, 1.20) Noncardia: 0.90 (0.71, 1.13) Intestinal: 0.72 (0.49, 1.06) Diffuse: 1.04 (0.78, 1.40) | |||||||
| Flavanones | Male: 0.91 (0.64, 1.28) Female: 1.01 (0.68, 1.50) Cardia: 0.92 (0.81, 1.05) Noncardia: 0.99 (0.90, 1.09) Intestinal: 1.10 (0.92, 1.32) Diffuse: 0.96 (0.87, 1.07) | |||||||
| Flavones | Male: 0.86 (0.60, 1.23) Female: 0.59 (0.38, 0.93) Cardia: 0.83 (0.65, 1.07) Noncardia: 0.94 (0.82, 1.07) Intestinal: 0.97 (0.76, 1.24) Diffuse: 0.92 (0.78, 1.09) | |||||||
| Flavanols | Male: 0.93 (0.64, 1.34) Female: 0.52 (0.32, 0.83) Cardia: 0.89 (0.70, 1.11) Noncardia: 0.90 (0.78, 1.05) Intestinal: 0.78 (0.65, 0.94) Diffuse: 0.98 (0.81, 1.19) | |||||||
| Flavan-3-ol monomers | Male: 0.98 (0.68, 1.40) Female: 0.55 (0.34, 0.88) Cardia: 0.91 (0.79, 1.05) Noncardia: 0.93 (0.84, 1.02) Intestinal: 0.83 (0.72, 0.95) Diffuse: 1.01 (0.89, 1.14) | |||||||
| Proanthocyanidins | Male: 0.84 (0.55, 1.27) Female: 0.71 (0.44, 1.15) Cardia: 1.05 (0.71, 1.56) Noncardia: 0.92 (0.78, 1.09) Intestinal: 0.86 (0.71, 1.04) Diffuse: 0.98 (0.78, 1.24) | |||||||
| Theaflavins | Male: 1.06 (0.73, 1.54) Female: 0.57 (0.36, 0.91) Cardia: 0.98 (0.95, 1.02) Noncardia: 0.99 (0.97, 1.01) Intestinal: 0.95 (0.92, 0.98) Diffuse:1.02 (0.99, 1.05) | |||||||
| Isoflavones | Male: 0.77 (0.50, 1.18) Female: 1.05 (0.61, 1.82) Cardia: 1.13 (0.85, 1.50) Noncardia: 1.00 (0.83, 1.19) Intestinal: 1.08 (0.80, 1.47) Diffuse: 0.90 (0.71, 1.13) | |||||||
| Lignans | Male: 0.99 (0.63, 1.55) Female: 0.94 (0.54, 1.64) Cardia: 0.61 (0.33, 1.13) Noncardia: 0.85 (0.59, 1.23) Intestinal: 1.17 (0.65, 2.12) Diffuse: 0.79 (0.50, 1.25) | |||||||
| Lin et al. [28] Sweden | Prospective cohort 1987–2009 | 81.670 GC = 128 | FFQ | 65 unspecified items | Lignans | Model 1/2 0.78 (0.48–1.28)/0.89 (0.52–1.55) Men only 0.81 (0.43–1.55)/0.83 (0.40–1.76) | Highest quartile X Lowest quartile | Model 1: Sex, age, and energy intake. Model 2: Model 1 + education, BMI, alcohol intake, smoking status, gastric ulcer, duodenal ulcer, and diabetes. P.S: For men only, adjustment for sex was not included. |
| Woo et al. [13] Korea | Case-control 2011–2014 | Cases = 334 Controls = 334 | FFQ past year | 144 unspecified items | Model 1/2 | Highest tertile X Lowest tertile | Model 1: Total energy intake, H. pylori, age, sex, education, smoking status, alcohol consumption, BMI, physical activity, consumption of pickled vegetables, red and processed meat. Model 2: Model 1 + fruit and vegetable consumption. | |
| Total flavonoids | Total 0.49 (0.31–0.76)/0.62 (0.36–1.09) Male 0.70 (0.39–1.24)/0.80 (0.39–1.63) Female 0.33 (0.15–0.73)/0.68 (0.25–1.86) | |||||||
| Flavonols | Total 0.51 (0.32–0.82)/0.69 (0.39–1.20) Male 0.59 (0.32–1.10)/0.65 (0.32–1.35) Female 0.51 (0.24–1.10)/1.22 (0.47–3.16) | |||||||
| Flavones | Total 0.51 (0.31–0.82)/0.72 (0.38–1.35) Male 0.70 (0.38–1.29)/0.84 (0.37–1.89) Female 0.15 (0.06–0.38)/0.22 (0.07–0.67) | |||||||
| Flavanones | Total 0.66 (0.43–1.02)/0.92 (0.55–1.52) Male 0.90 (0.52–1.56)/1.12 (0.58–2.17) Female 0.39 (0.18–0.86)/0.64 (0.27–1.52) | |||||||
| Flavan-3-ols | Total 0.58 (0.38–0.88)/0.73 (0.45–1.18) Male 0.70 (0.41–1.21)/0.78 (0.41–1.49) Female 0.36 (0.17–0.77)/0.65 (0.27–1.57) | |||||||
| Anthocyanidins | Total 0.73 (0.46–1.15)/1.06 (0.62–1.80) Male 0.92 (0.51–1.67)/1.16 (0.57–2.34) Female 0.58 (0.27–1.25)/1.22 (0.49–3.01) | |||||||
| Isoflavones | Total 0.72 (0.46–1.12)/0.85 (0.54–1.35) Male 0.90 (0.52–1.54)/0.98 (0.56–1.73) Female 0.51 (0.24–1.08)/0.67 (0.31–1.47) | |||||||
| Petrick et al. [4] USA | Multi-center Case-control 1993–1995–2000 | Cases = 589 Controls = 662 | FFQ last 3–5 years | Fruit, vegetables, juices, wine, tea, coffee, pizza, bread, cake, soups, chicken | Total flavonoids | GCA: 1.32 (0.87, 2.00) OGA: 1.08 (0.73, 1.58) | Highest quartile X Lowest quartile | Age, sex, race, geographic center, cigarette smoking, and dietary energy intake. |
| Anthocyanidins | GCA: 0.71 (0.46, 1.10) OGA: 0.70 (0.47, 1.03) | |||||||
| Flavan-3-ols | GCA: 1.17 (0.77, 1.78) OGA: 1.30 (0.88, 1.92) | |||||||
| Flavanones | GCA: 1.23 (0.81, 1.87) OGA: 0.88 (0.60, 1.28) | |||||||
| Flavones | GCA: 1.09 (0.71, 1.67) OGA: 1.01 (0.69, 1.50) | |||||||
| Flavonols | GCA: 1.42 (0.93, 2.17) OGA: 0.98 (0.67, 1.46) | |||||||
| Isoflavones | GCA: 1.56 (0.93, 2.60) OGA: 1.50 (0.96, 2.37) | |||||||
| Lignans | GCA: 1.01 (0.65, 1.58) OGA: 0.73 (0.48, 1.11) | |||||||
| Wada et al. [29] Japan | Prospective cohort 1992–2008 | 30,792 GC = 678 | Diet record-12 days and FFQ (past year) | Miso soup, tofu (soy bean curd), deep-fried tofu, fried tofu, freeze-dried tofu, natto, houba-miso, soymilk and boiled soy beans | Isoflavones | Male: 0.81 (0.60–1.09) Female: 0.60 (0.37–0.98) | Highest quartile X Lowest quartile | Age, BMI, physical activity score, smoking status, alcohol consumption, salt intake and education years for men, and menopausal status for women. |
| Sun et al. [11] USA | Prospective cohort 1995–2011 | 469,008 GC = 1297 | FFQ past year | 116 unspecified items | Total flavonoids | Cardia: 1.02 (0.78, 1.34) Noncardia: 1.11 (0.86, 1.44) | Highest quintile X Lowest quintile | Age, sex, race, education, smoking status, BMI, alcohol intake, self-reported health, vigorous physical activity of ≥20 min and total energy intake. |
| Anthocyanidins | Cardia: 1.05 (0.80, 1.39) Noncardia: 0.94 (0.72, 1.23) | |||||||
| Flavan-3-ols | Cardia: 1.04 (0.80, 1.36) Noncardia: 1.19 (0.92, 1.54) | |||||||
| Flavanones | Cardia: 0.87 (0.68, 1.13) Noncardia: 0.99 (0.76, 1.30) | |||||||
| Flavones | Cardia: 0.99 (0.73, 1.34) Noncardia: 1.06 (0.80, 1.40) | |||||||
| Flavonols | Cardia: 1.08 (0.80, 1.45) Noncardia: 1.25 (0.94, 1.65) | |||||||
| Isoflavones | Cardia: 0.99 (0.73, 1.34) Noncardia: 0.73 (0.54, 0.98) | |||||||
| Yang et al. [30] Korea | Case control 2011–2014 | Cases = 377 Controls = 754 | FFQ past year | Legumes, tofu, soymilk, sprouts, and doenjang (Korean traditional fermented soybean paste and soybeans) | Isoflavones | 0.70 (0.49–1.00) Male: 0.63 (0.40–0.99) Female: 0.82 (0.45–1.49) | Highest tertile X Lowest tertile | Education, alcohol consumption, smoking status, Helicobacter pylori infection, and regular exercise. |
| Vitelli-Storelli et al. [31] Spain | Multi-center Case-control 2008–2013 | Cases = 329 Controls = 2700 | FFQ | Vegetables and legumes, fruit, cereals, sweets and snacks, and alcoholic and other beverages. | Total flavonoids | 0.76 (0.65, 0.89) Male: 0.51 (0.31–0.82) Female: 0.89 (0.42–1.90) Cardia: 0.67 (0.33, 1.39) Noncardia: 0.55 (0.35, 0.87) Intestinal: 0.74 (0.38, 1.42) Diffuse: 0.38 (0.17, 0.84) | All cases: log2 Male/Female; Cardia/Noncardia; Intestinal/Diffuse: Highest quartile X Lowest quartile | Age, gender, socioeconomic status, area of residence, GC family history, BMI, smoking, physical activity, energy, sodium, red meat, vegetables and past alcohol intake. |
| Anthocyanidins | 0.88 (0.80, 0.96) Male: 0.47 (0.30–0.74) Female: 1.14 (0.59–2.22) Cardia: 0.62 (0.29, 1.31) Noncardia: 0.67 (0.42, 1.06) Intestinal: 0.61 (0.32, 1.15) Diffuse: 0.92 (0.42, 1.98) | |||||||
| Chalcones | 0.89 (0.83, 0.95) Male: 0.48 (0.31–0.74) Female: 0.90 (0.80, 1.03) Cardia: 0.40 (0.2, 0.79) Noncardia: 0.60 (0.37, 0.98) Intestinal: 0.39 (0.19, 0.82) Diffuse: 0.89 (0.4, 1.98) | |||||||
| Dihydrochalcones | 1.02 (0.95, 1.11) Male: 1.35 (0.87–2.09) Female: 0.96 (0.45–2.05) Cardia: 1.92 (0.92, 4.02) Noncardia: 1.38 (0.87, 2.2) Intestinal: 1.4 (0.73, 2.67) Diffuse: 1.23 (0.57, 2.67) | |||||||
| Dihydroflavonols | 0.89 (0.84, 0.95) Male: 0.38 (0.24–0.59) Female: 0.89 (0.38–2.06) Cardia: 0.60 (0.29, 1.23) Noncardia: 0.47 (0.29, 0.76) Intestinal: 0.54 (0.29, 1.01) Diffuse: 0.49 (0.21, 1.11) | |||||||
| Flavan-3-ols | 0.82 (0.73, 0.92) Male: 0.49 (0.32–0.77) Female: 0.61 (0.29–1.28) Cardia: 0.65 (0.28, 1.48) Noncardia: 0.59 (0.35, 0.98) Intestinal: 0.57 (0.28, 1.16) Diffuse: 0.42 (0.18, 1.01) | |||||||
| Flavanones | 0.92 (0.85, 1.00) Male: 0.66 (0.43–1.00 Female: 0.88 (0.42–1.83) Cardia: 0.60 (0.29, 1.23) Noncardia: 0.79 (0.51, 1.22) Intestinal: 0.89 (0.49, 1.64) Diffuse: 0.64 (0.30, 1.36) | |||||||
| Flavones | 0.99 (0.89, 1.11) Male: 0.75 (0.47–1.20) Female: 1.42 (0.65–3.15) Cardia: 0.70 (0.32, 1.5) Noncardia: 1.27 (0.8, 2.02) Intestinal: 1.36 (0.72, 2.56) Diffuse: 1.64 (0.74, 3.62) | |||||||
| Flavonols | 0.93 (0.78, 1.10) Male: 0.62 (0.37–1.02) Female: 1.71 (0.74–3.92) Cardia: 0.84 (0.36, 1.98) Noncardia: 1.13 (0.68, 1.88) Intestinal: 1.28 (0.63, 2.56) Diffuse: 1.46 (0.63, 3.4) | |||||||
| Isoflavonoids | 1.05 (0.98, 1.12) Male: 1.11 (0.65–1.91) Female: 0.99 (0.89, 1.11) Cardia: 1.27 (0.54, 2.98) Noncardia: 1.36 (0.78, 2.38) Intestinal: 1.81 (0.8, 4.08) Diffuse: 1.22 (0.51, 2.93) | |||||||
| Proanthocyanidins | 0.82 (0.71, 0.94) Male: 0.57 (0.36–0.93) Female: 1.22 (0.57–2.58) Cardia: 1.1 (0.52, 2.35) Noncardia: 0.84 (0.51, 1.38) Intestinal: 1.01 (0.5, 2.07) Diffuse: 0.87 (0.39, 1.98) | |||||||
| Vitelli-Storelli et al. [5] Stop Project (Italy, Greece, Spain, Portugal, Mexico, Russia) | 10 Case-control studies 1998–2015 | Cases = 3471 Controls = 8344 | FFQ | Vegetables, fruit, sweets, cereals, alcohol, juices and other drinks. | Total polyphenolsTotal flavonoids Anthocyanidins Flavanols FlavonolsFlavanones Total phenolic acids Hydroxybenzoic acids Hydroxycinnamic acids | 0.67 (0.54–0.81) 0.73 (0.55–0.90) 0.74 (0.56–0.92) 0.77 (0.66–0.88) 0.76 (0.51–1.01) 0.57 (0.44–0.69) 0.75 (0.55–0.94) 0.73 (0.57–0.89) 0.82 (0.58–1.06) | Highest quartile X Lowest quartile | Age, sex, social class, alcohol consumption, BMI, family history of gastric cancer, smoking status, and salt consumption. |
| Rubín-García et al. [15] Spain | Multi-center Case-control 2008–2013 | Cases = 329 Controls = 2700 | FFQ past year | Legumes, vegetables, fruit, cereals, sweets and snacks, as well as alcoholic beverages and others. | Stilbenes Lignans Hydroxybenzaldehydes Hydroxycoumarin Tyrosols Other polyphenols | 0.47 (0.32–0.69) 0.53 (0.36–0.84) 0.41 (0.28–0.61) 0.49 (0.34–0.71) 0.56 (0.39–0.80) 1.49 (1.06–2.10) | Highest quartile X Lowest quartile | Age; sex; socioeconomic status; smoking status; first-degree family history of GC; physical activity; BMI; alcohol consumption; and vegetables, red meat, salt, and total energy intake. |
| Kim et al. [32] Korea | Case-control 2011–2014 | Cases = 415 Controls = 830 | FFQ | Mostly fruit and vegetables | Total phenolics | Model 1/2 0.52 (0.37–0.75)/0.57 (0.39–0.83) | Highest tertile X Lowest tertile | Model 1: Age, BMI, education level, income, physical activity, smoking status, first-degree family history of GC, and total energy intake. Model 2: Model 1 + H. pylori infection status. |
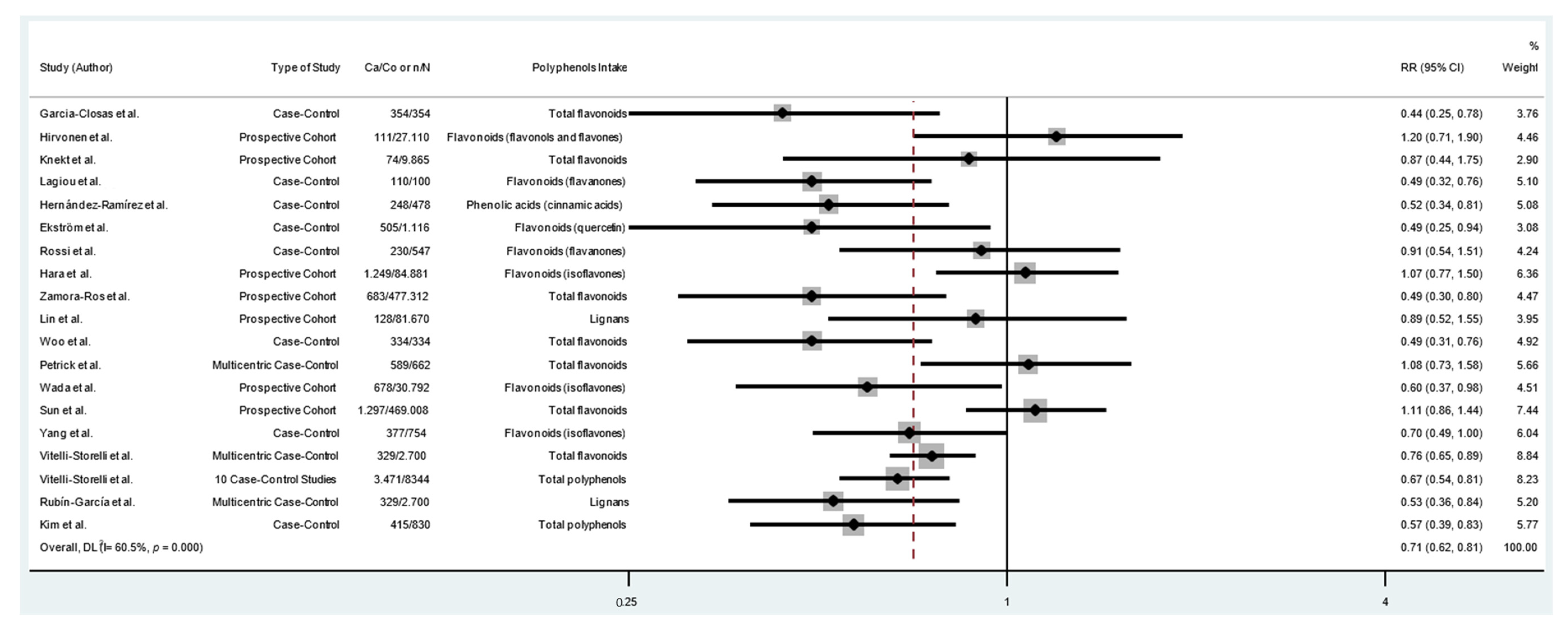
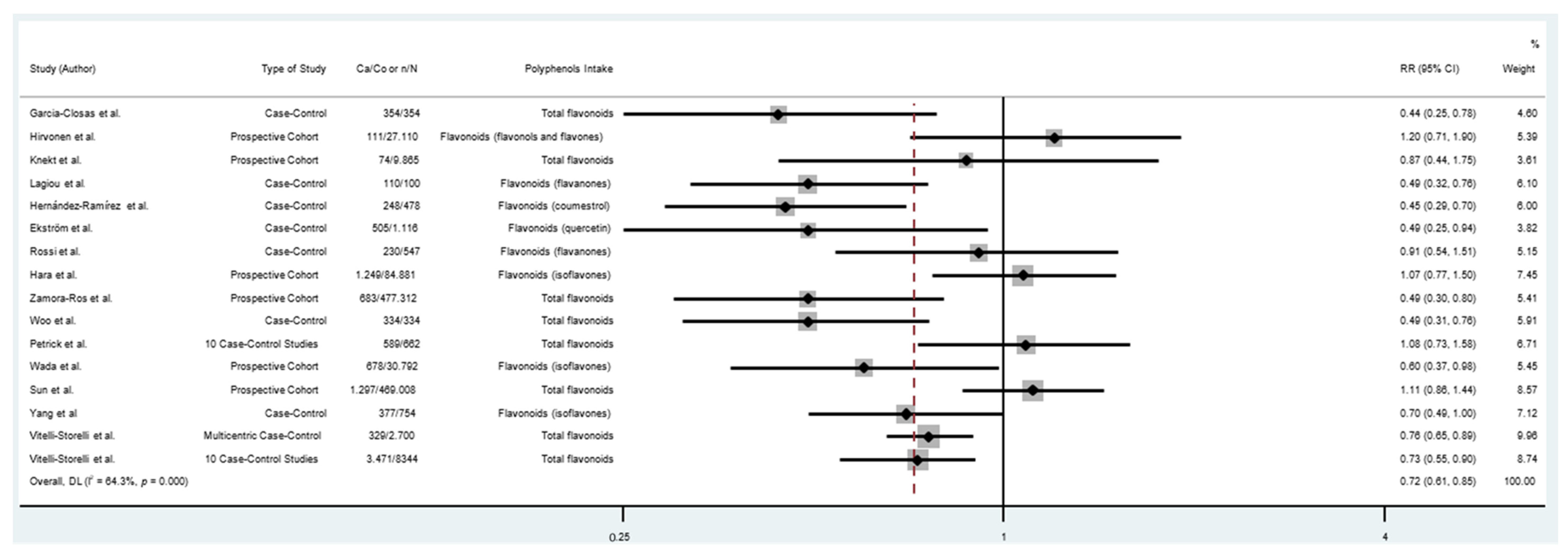
| Subgroup | Number of Participants | Number of Studies | RR (95% CI) | Heterogeneity Test | |
|---|---|---|---|---|---|
| I2 (%) | p | ||||
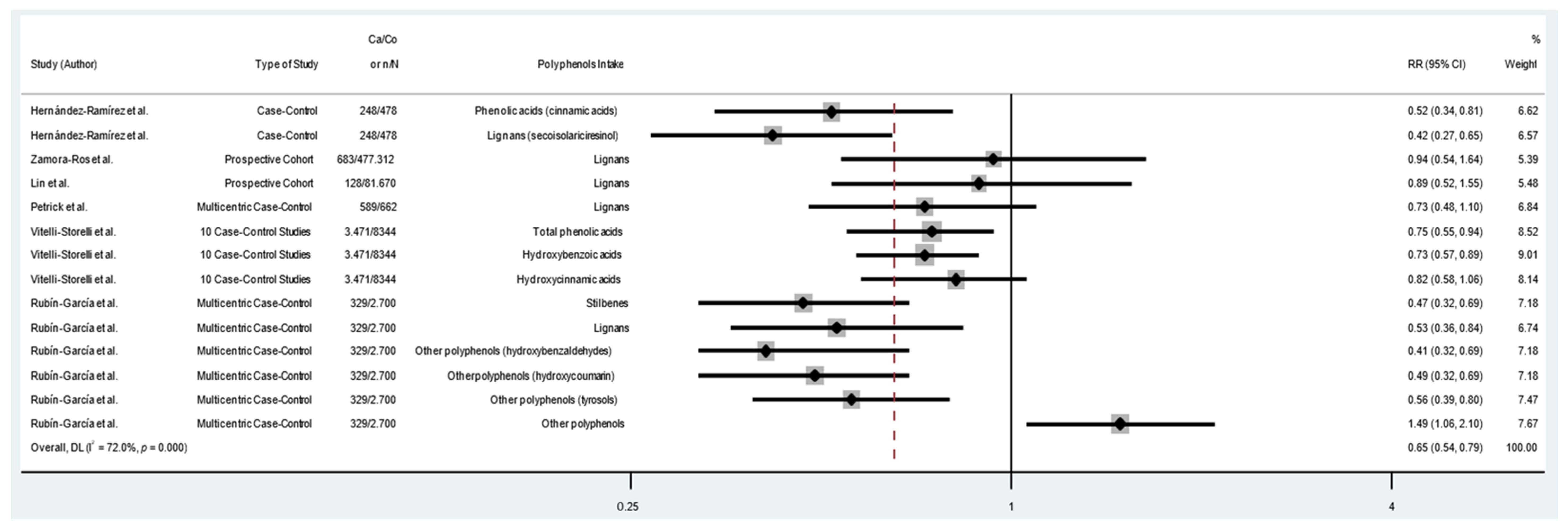
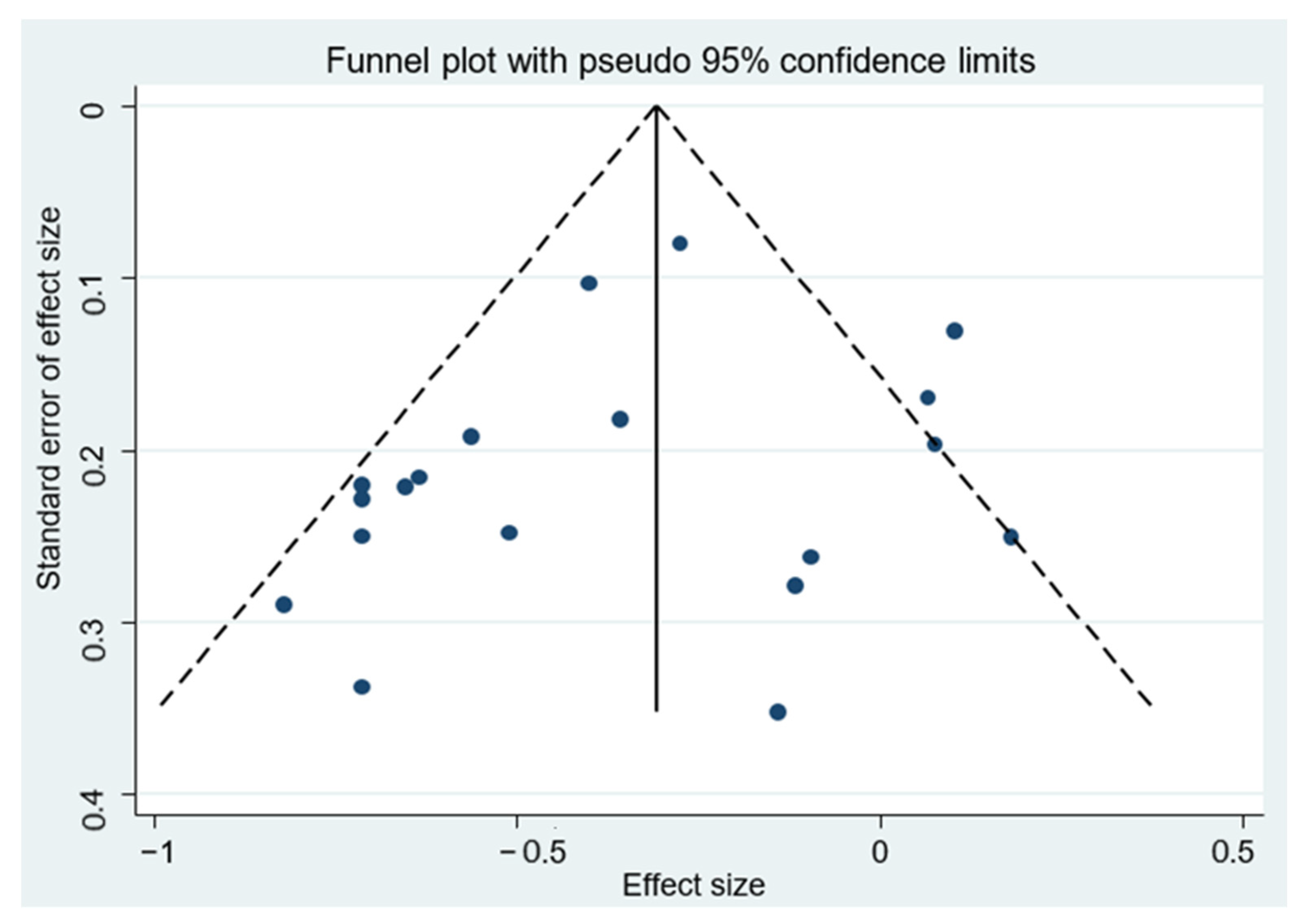
| All studies | 1,197,857 | 19 | 0.71 (0.62–0.81) * | 60.5 | <0.001 |
| Study design | |||||
| Cohort | 1,171,647 | 7 | 0.88 (0.69–1.12) | 54.7 | 0.039 |
| Case–control | 26,210 | 12 | 0.64 (0.56–0.74) * | 44.4 | 0.049 |
| Sex ¹ | |||||
| Male | 264,991 | 8 | 0.79 (0.67–0.94) * | 31.6 | 0.176 |
| Female | 399,416 | 7 | 0.65 (0.48–0.87) * | 49.7 | 0.064 |
| Anatomical type ¹ | |||||
| Cardia | 1343 | 6 | 1.01 (0.79–1.27) | 42.3 | 0.123 |
| Noncardia | 2603 | 6 | 0.85 (0.69–1.05) | 65.2 | 0.013 |
| Histological type ¹ | |||||
| Diffuse | 476 | 3 | 0.63 (0.37–1.09) | 71.8 | 0.029 |
| Intestinal | 662 | 3 | 0.65 (0.52–0.82) * | 0.0 | 0.494 |
| Geographical area | |||||
| Asia | 118,717 | 5 | 0.67 (0.51–0.89) * | 60.7 | 0.038 |
| America | 470,985 | 3 | 0.87 (0.56–1.36) | 78.3 | 0.010 |
| Europe | 608,155 | 11 | 0.67 (0.57–0.79) * | 44.2 | 0.056 |
| Adjustments | |||||
| Family history of gastric cancer, Yes | 103,999 | 5 | 0.71 (0.59–0.86) * | 59.3 | 0.044 |
| No | 1,093,858 | 14 | 0.70 (0.57–0.86) * | 63.5 | 0.001 |
| Fruit and/or vegetables intake 2, Yes | 569,855 | 7 | 0.68 (0.55–0.83) * | 49.5 | 0.065 |
| No | 638,597 | 16 | 0.72 (0.61–0.85) * | 61.2 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagundes, M.d.A.; Silva, A.R.C.; Fernandes, G.A.; Curado, M.P. Dietary Polyphenol Intake and Gastric Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5878. https://doi.org/10.3390/cancers14235878
Fagundes MdA, Silva ARC, Fernandes GA, Curado MP. Dietary Polyphenol Intake and Gastric Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(23):5878. https://doi.org/10.3390/cancers14235878
Chicago/Turabian StyleFagundes, Marcela de Araújo, Alex Richard Costa Silva, Gisele Aparecida Fernandes, and Maria Paula Curado. 2022. "Dietary Polyphenol Intake and Gastric Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 23: 5878. https://doi.org/10.3390/cancers14235878
APA StyleFagundes, M. d. A., Silva, A. R. C., Fernandes, G. A., & Curado, M. P. (2022). Dietary Polyphenol Intake and Gastric Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(23), 5878. https://doi.org/10.3390/cancers14235878





