Challenging Endocrine Sensitivity of Hormone Receptor-Positive/HER2-Negative Advanced Breast Cancer with the Combination of Eribulin and Endocrine Therapy: The REVERT Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. Randomization and Masking
2.3. Treatment
2.4. Study Assessments
2.5. Study Endpoints
2.6. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Patient Disposition and Interim Analysis
3.2. Patient Characteristics
3.3. Treatment
3.4. Efficacy
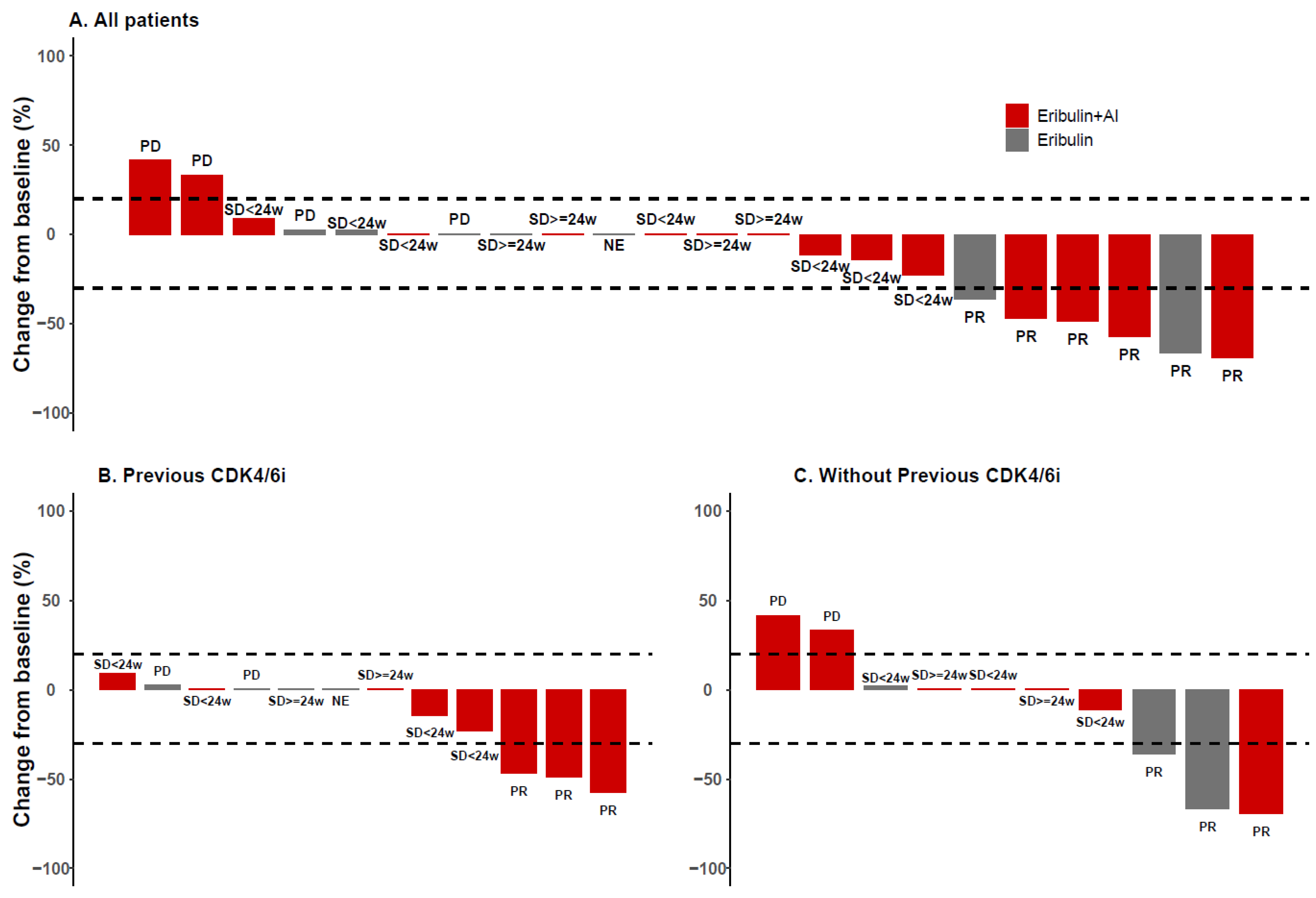
3.5. Subgroup Analyses
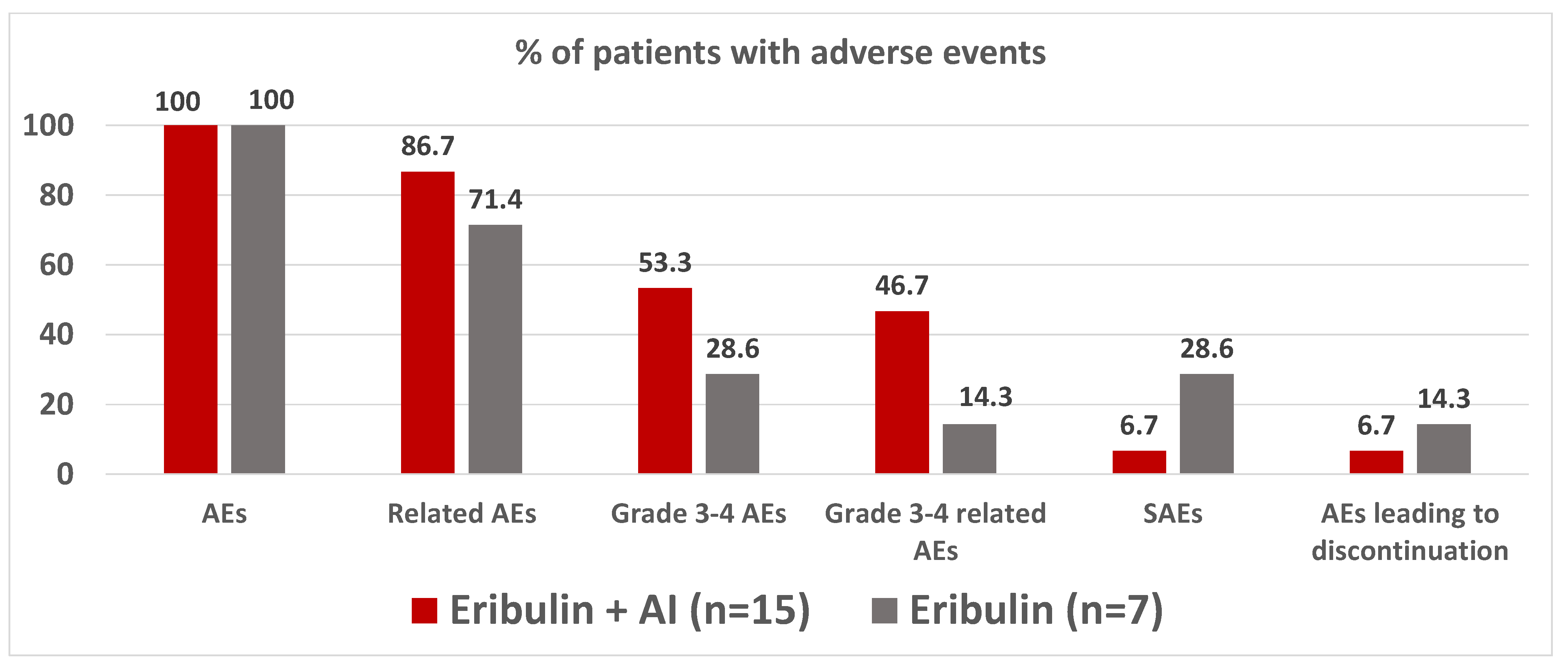
3.6. Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Pérez-Carrión, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Jaenicke, F.; et al. Phase III Study of Letrozole Versus Tamoxifen as First-Line Therapy of Advanced Breast Cancer in Postmenopausal Women: Analysis of Survival and Update of Efficacy From the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2003, 21, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Nabieva, N.; Fasching, P.A. Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers 2021, 13, 5643. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.; Buzdar, A.; Pollak, M.; Harwin, W.; Burton, G.; Mangalik, A.; Steinberg, M.; Webster, A.; Von Euler, M. Anastrozole Is Superior to Tamoxifen as First-Line Therapy for Advanced Breast Cancer in Postmenopausal Women: Results of a North American Multicenter Randomized Trial. J. Clin. Oncol. 2000, 18, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Wander, S.A.; Andre, F.; Moy, B.; Turner, N.C.; Bardia, A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future. Lancet 2020, 395, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Dybdal-Hargreaves, N.F.; Risinger, A.L.; Mooberry, S.L. Eribulin Mesylate: Mechanism of Action of a Unique Microtubule-Targeting Agent. Clin. Cancer Res. 2015, 21, 2445–2452. [Google Scholar] [CrossRef]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.P.; Funahashi, Y.; et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial–mesenchymal transition (EMT) to mesenchymal–epithelial transition (MET) states. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y.; Okamoto, K.; Adachi, Y.; Semba, T.; Uesugi, M.; Ozawa, Y.; Tohyama, O.; Uehara, T.; Kimura, T.; Watanabe, H.; et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014, 105, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Diéras, V.; Delozier, T.; et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Twelves, C.C.; Cortes, J.; Vahdat, L.; Olivo, M.M.; He, Y.Y.; Kaufman, P.P.; Awada, A. Efficacy of eribulin in women with metastatic breast cancer: A pooled analysis of two phase 3 studies. Breast. Cancer Res. Treat. 2014, 148, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.A.; Awada, A.; Twelves, C.; Yelle, L.; Perez, E.A.; Velikova, G.; Olivo, M.S.; He, Y.; Dutcus, C.E.; Cortes, J. Phase III Open-Label Randomized Study of Eribulin Mesylate Versus Capecitabine in Patients With Locally Advanced or Metastatic Breast Cancer Previously Treated With an Anthracycline and a Taxane. J. Clin. Oncol. 2015, 33, 594–601. [Google Scholar] [CrossRef]
- Pascual, T.; Oliveira, M.; Villagrasa, P.; Ortega, V.; Paré, L.; Bermejo, B.; Morales, S.; Amillano, K.; López, R.; Galván, P.; et al. Neoadjuvant eribulin in HER2-negative early-stage breast cancer (SOLTI-1007-NeoEribulin): A multicenter, two-cohort, non-randomized phase II trial. npj Breast. Cancer 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, G.J.; Fernando, T.M.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.; McCune, S.; Armstrong, A.; Shannon, C.; et al. VERONICA: Randomized Phase II Study of Fulvestrant and Venetoclax in ER-Positive Metastatic Breast Cancer Post-CDK4/6 Inhibitors—Efficacy, Safety, and Biomarker Results. Clin. Cancer Res. 2022, 28, 3256–3267. [Google Scholar] [CrossRef]
- Bardia, A.; Aftimos, P.; Bihani, T.; Anderson-Villaluz, A.T.; Jung, J.; Conlan, M.G.; Kaklamani, V.G. EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019, 15, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Mougalian, S.S.; Feinberg, B.A.; Wang, E.; Alexis, K.; Chatterjee, D.; Knoth, R.L.; Nero, D.; Miller, T.; Liassou, D.; Kish, J.K. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019, 15, 3935–3944. [Google Scholar] [CrossRef]
- Niwa, Y.; Asano, M.; Nakagawa, T.; France, D.; Semba, T.; Funahashi, Y. Antitumor Activity of Eribulin After Fulvestrant Plus CDK4/6 Inhibitor in Breast Cancer Patient-derived Xenograft Models. Anticancer Res. 2020, 40, 6699–6712. [Google Scholar] [CrossRef] [PubMed]
- Tobin, N.P.; Sims, A.H.; Lundgren, K.L.; Lehn, S.; Landberg, G. Cyclin D1, Id1 and EMT in breast cancer. BMC Cancer 2011, 11, 417. [Google Scholar] [CrossRef]


| Characteristics, n (%) | All Patients (n = 22) | Eribulin + AI (n = 15) | Eribulin (n = 7) |
|---|---|---|---|
| Age in years, median (range) | 65 (56–77) | 60 (50–77) | 63 (50–77) |
| Race | |||
| Black | 2 (9.1) | 1 (6.7) | 1 (14.3) |
| Hispanic or Latino | 2 (9.1) | 2 (13.3) | 0 (0.0) |
| White | 18 (82.8) | 12 (80.0) | 6 (85.7) |
| ECOG PS score | |||
| 0 | 18 (81.8) | 13 (86.7) | 5 (71.4) |
| 1 | 4 (18.2) | 2 (13.3) | 2 (28.6) |
| Visceral involvement | |||
| Yes | 22 (100) | 15 (100) | 7 (100) |
| Liver involvement | |||
| Yes | 14 (63.6) | 10 (66.7) | 4 (57.1) |
| Number of metastatic sites | |||
| <3 | 14 (63.6) | 10 (66.7) | 4 (57.1) |
| ≥3 | 8 (36.4) | 5 (33.3) | 3 (42.9) |
| Prior lines of ET for ABC | |||
| 0 | 9 (40.9) | 6 (40.0) | 3 (42.9) |
| 1 | 7 (31.8) | 5 (33.3) | 2 (28.6) |
| 2 | 5 (22.7) | 3 (20.0) | 2 (28.6) |
| 3 | 1 (4.5) | 1 (6.7) | 0 (0.0) |
| AI administered in the last regimen | |||
| Letrozole | 15 (68.2) | 10 (66.7) | 5 (71.4) |
| Exemestane | 6 (27.3) | 5 (33.3) | 1 (14.3) |
| Anastrozole | 1 (4.5) | 0(0.0) | 1 (14.3) |
| Previous treatment with CDK4/6i | |||
| Yes | 12 (54.5) | 8 (53.3) | 4 (57.1) |
| No | 10 (45.5) | 7 (46.7) | 3 (42.9) |
| Treatment with CDK4/6i in the immediate line of therapy | |||
| Yes | 9 (40.9) | 6 (40.0) | 3 (42.9) |
| No | 13 (59.1) | 9 (60.0) | 4 (57.1) |
| Eribulin + AI (N = 15) | Eribulin(N = 7) | p | |
|---|---|---|---|
| Overall response rate, (95% CI) | 26.7 (7.8–55.1) p = 0.0541 | 28.6 (3.7–71.0) | 0.918 |
| Best response, n (%) | - | ||
| Complete response | 0 (0.0) | 0 (0.0) | |
| Partial response | 4 (26.7) | 2 (28.6) | |
| Stable disease ≥ 24 weeks | 3 (20.0) | 1 (14.3) | |
| Stable disease < 24 weeks | 6 (40.0) | 1 (14.3) | |
| Progressive disease | 2 (13.3) | 2 (28.6) | |
| Not evaluable | 0 (0.0) | 1 (0.0) | |
| Clinical benefit rate, (95% CI) | 46.7% (21.3–73.4%) | 42.9% (9.9–81.6%) | 0.878 |
| Median duration of response, months (IQR) * | 3.6 (3.4–4.0) | 6.9 (4.9–8.8) | 0.800 |
| Median time to response, months (IQR) * | 1.9 (1.8–2.5) | 2.1 (2.1–2.1) | 0.481 |
| Progression-free survival rate at 6 months (95% CI) | 52.0% (22.3–75.2%) | 50.0% (11.1–80.4%) | 0.959 |
| Overall survival rate at 12 months (95% CI) | 92.9% (59.1–99.0%) | 80.0% (20.4–96.0%) | 0.888 |
| Eribulin + AI | Eribulin | |||
|---|---|---|---|---|
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| All AEs | 15 (100.0%) | 8 (53.3%) | 7 (100.0%) | 2 (28.6%) |
| Hematological | 8 (53.3%) | 4 (26.7%) | 2 (28.6%) | 1 (4.3%) |
| Neutropenia | 5 (33.3%) | 4 (26.7%) | 2 (28.6%) | 1 (14.3%) |
| Anemia | 3 (20.0%) | 0 (0.0%) | 1 (14.3%) | 0 (0.0%) |
| Febrile neutropenia | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 1 (14.3%) |
| Leukopenia | 1 (6.7%) | 0 (0.0%) | 1 (14.3%) | 0 (0.0%) |
| Thrombocytopenia | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 0 (0.0%) |
| Non-hematological | 15 (100.0%) | 6 (40.0%) | 7 (100.0%) | 2 (28.6%) |
| Fatigue | 6 (40.0%) | 0 (0.0%) | 2 (28.6%) | 0 (0.0%) |
| Peripheral neuropathy | 5 (33.3%) | 2 (13.3%) | 1 (14.3%) | 0 (0.0%) |
| Nausea | 4 (26.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hepatotoxicity | 3 (20.0%) | 2 (13.3%) | 1 (14.3%) | 1 (14.3%) |
| Alopecia | 3 (20.0%) | 0 (0%) | 3 (42.9%) | 0 (0.0%) |
| Headache | 3 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Pyrexia | 3 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Chest pain | 2 (13.3%) | 1 (6.7%) | 1 (14.3%) | 0 (0.0%) |
| Pneumonitis | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 1 (14.3%) |
| Pulmonary embolism | 0 (0.0%) | 0 (0.0%) | 1 (14.3%) | 1 (14.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López González, A.; Del Barco Berrón, S.; Grau, I.; Galan, M.; Castelo Fernández, B.; Cortés, A.; Sánchez Rovira, P.; Martinez-Bueno, A.; Gonzalez, X.; García, A.; et al. Challenging Endocrine Sensitivity of Hormone Receptor-Positive/HER2-Negative Advanced Breast Cancer with the Combination of Eribulin and Endocrine Therapy: The REVERT Study. Cancers 2022, 14, 5880. https://doi.org/10.3390/cancers14235880
López González A, Del Barco Berrón S, Grau I, Galan M, Castelo Fernández B, Cortés A, Sánchez Rovira P, Martinez-Bueno A, Gonzalez X, García A, et al. Challenging Endocrine Sensitivity of Hormone Receptor-Positive/HER2-Negative Advanced Breast Cancer with the Combination of Eribulin and Endocrine Therapy: The REVERT Study. Cancers. 2022; 14(23):5880. https://doi.org/10.3390/cancers14235880
Chicago/Turabian StyleLópez González, Ana, Sonia Del Barco Berrón, Isabel Grau, Maria Galan, Beatriz Castelo Fernández, Alfonso Cortés, Pedro Sánchez Rovira, Alejandro Martinez-Bueno, Xavier Gonzalez, Almudena García, and et al. 2022. "Challenging Endocrine Sensitivity of Hormone Receptor-Positive/HER2-Negative Advanced Breast Cancer with the Combination of Eribulin and Endocrine Therapy: The REVERT Study" Cancers 14, no. 23: 5880. https://doi.org/10.3390/cancers14235880
APA StyleLópez González, A., Del Barco Berrón, S., Grau, I., Galan, M., Castelo Fernández, B., Cortés, A., Sánchez Rovira, P., Martinez-Bueno, A., Gonzalez, X., García, A., Gener, P., Mina, L., Alcalá-López, D., Sampayo, M., Cortés, J., Pérez-Garcia, J. M., Llombart-Cussac, A., & López-Miranda, E. (2022). Challenging Endocrine Sensitivity of Hormone Receptor-Positive/HER2-Negative Advanced Breast Cancer with the Combination of Eribulin and Endocrine Therapy: The REVERT Study. Cancers, 14(23), 5880. https://doi.org/10.3390/cancers14235880






