Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
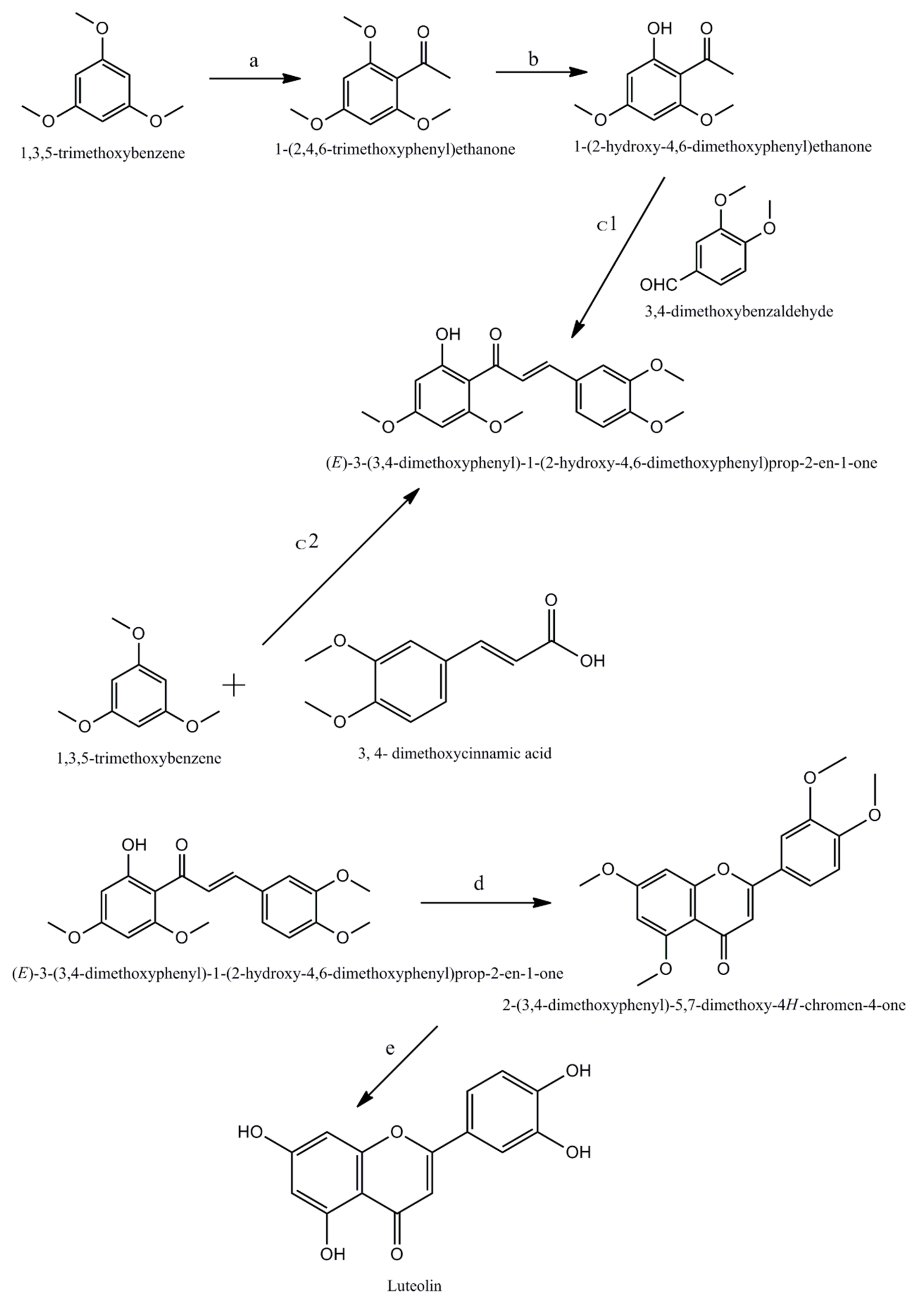
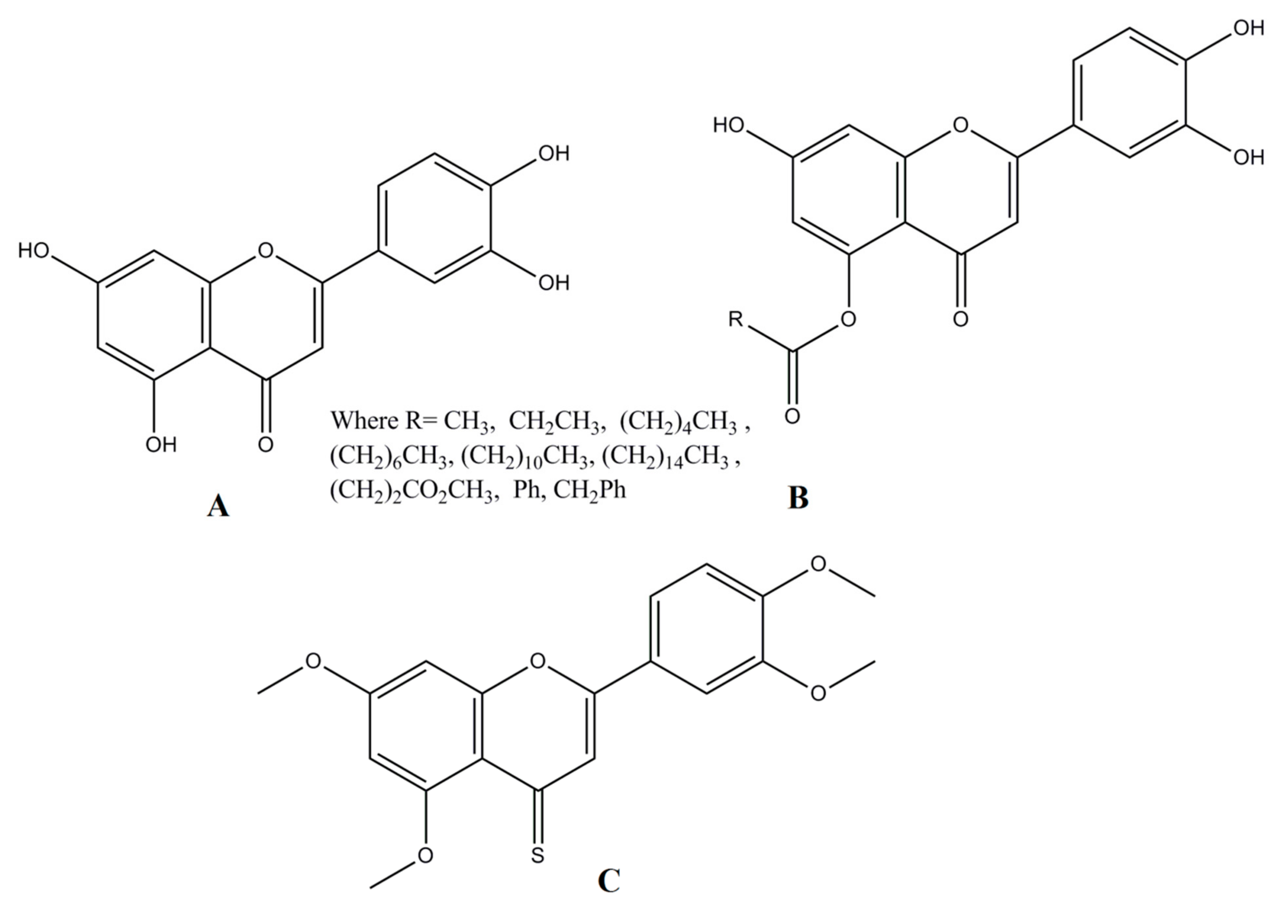
2. Chemistry Associated with Luteolin
3. Absorption and Metabolism of Luteolin
4. Mechanistic Insight into the Anticancer Activity of Luteolin
4.1. Apoptotic and Cell Cycle Arrest Mechanisms of Luteolin
4.2. Autophagy- Inducing Mechanism of Luteolin
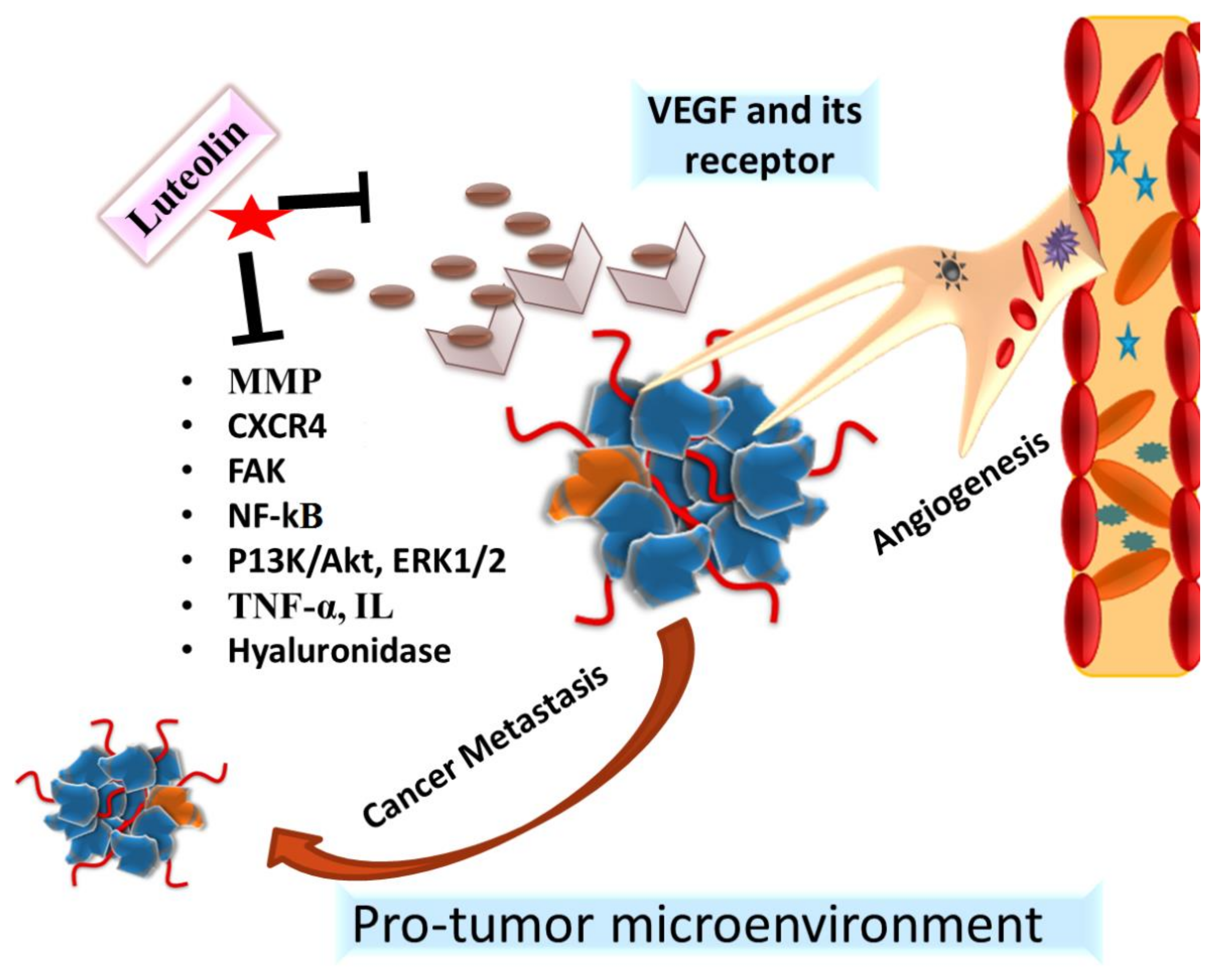
4.3. Antiangiogenic and Antimetastatic Action of Luteolin
4.4. Immunomodulatory Mechanisms of Luteolin
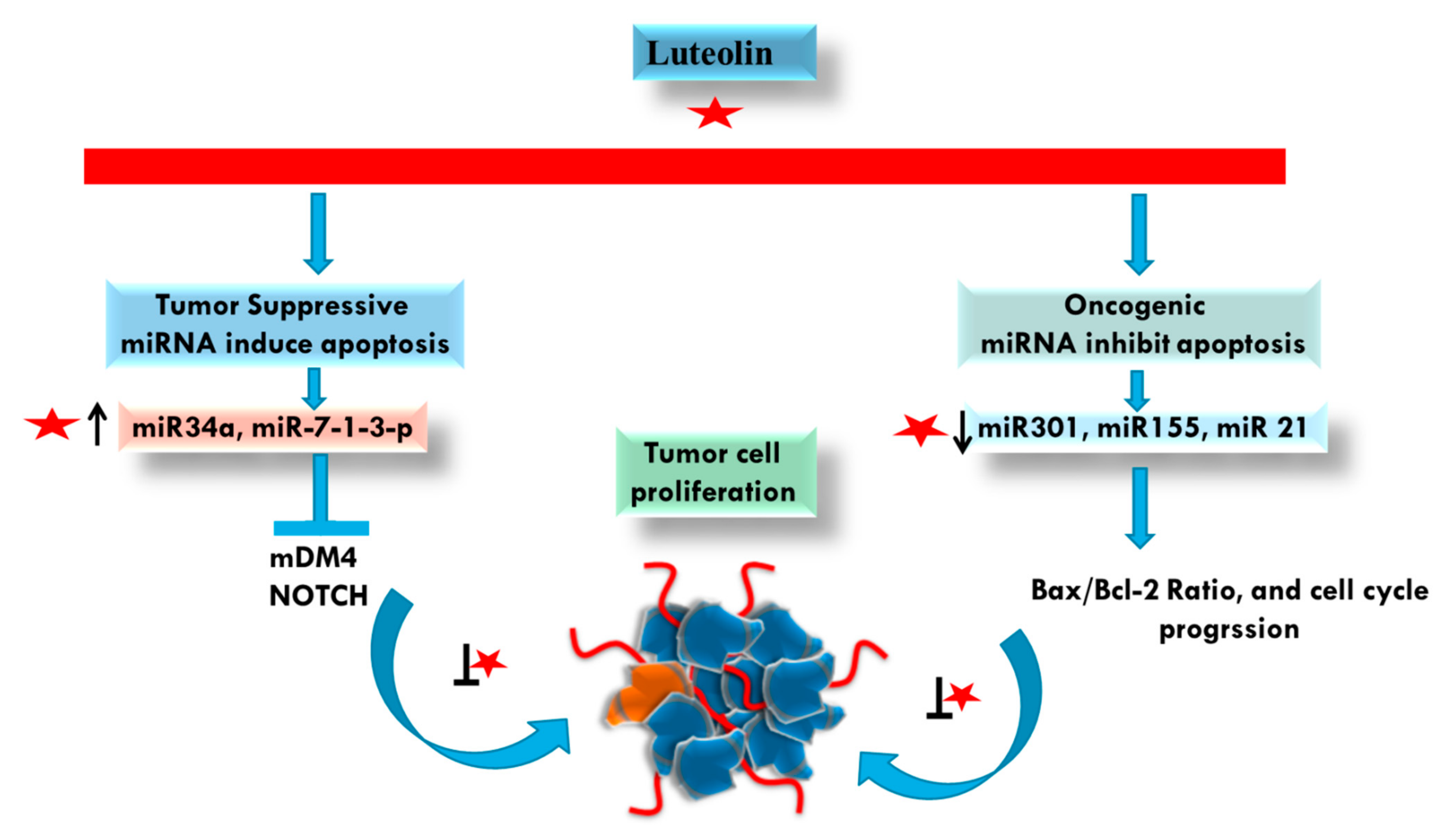
4.5. microRNA (miRNA) Modulations by Luteolin in Cancer
5. Synergistic Effects of Luteolin with Conventional Anti-Cancer Drugs
6. Insight into the Nanodelivery of Luteolin in Cancer
7. Safety Studies Related to Administration of Luteolin
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Anticancer action of plant products: Changing stereotyped attitudes. Explor. Target Antitumor. Ther. 2022, 3, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.S.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar] [CrossRef]
- Ganai, S.A.; Sheikh, F.A.; Baba, Z.A.; Mir, M.A.; Mantoo, M.A.; Yatoo, M.A. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 2021, 35, 3509–3532. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Fratantonio, D.; Hasan, M.M.; Sharifi, S.; Fathi, N.; Ullah, H.; Rastrelli, L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine 2019, 59, 152883. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Syllaios, A.; Schizas, D.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; Ntounis, T.; et al. Breast Cancer Apoptosis and the Therapeutic Role of Luteolin. Chirurgia 2021, 116, 170–177. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Gupta, R.; Kumar, D.; Bhattacharya, S.; Sarkar, A.; Kumar, P. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Brief. Funct. Genomics 2018, 18, 230–239. [Google Scholar] [CrossRef]
- Pan, J.; Cai, X.; Zheng, X.; Zhu, X.; Feng, J.; Wang, X. Luteolin inhibits viability, migration, angiogenesis and invasion of non-small cell lung cancer vascular endothelial cells via miR-133a-3p/purine rich element binding protein B-mediated MAPK and PI3K/Akt signaling pathways. Tissue Cell 2022, 75, 101740. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Huang, Z.; Chen, M.; Mo, Y.; Mo, Z. Luteolin Potentially Treating Prostate Cancer and COVID-19 Analyzed by the Bioinformatics Approach: Clinical Findings and Drug Targets. Front. Endocrinol. 2022, 12, 802447. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 2016, 25, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Gutiérrez-del-Río, I.; Yagüe, P.; Manteca, Á.; Villar, C.J.; Lombó, F. De novo biosynthesis of apigenin, luteolin, and eriodictyol in the actinomycete Streptomyces albus and production improvement by feeding and spore conditioning. Front. Microbiol. 2017, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Cui, W.; Yang, J.; Yang, B. Total Synthesis of Luteolin. J. Chem. Res. 2014, 38, 60–61. [Google Scholar] [CrossRef]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Syntheses of mono-acylated luteolin derivatives, evaluation of their antiproliferative and radical scavenging activities and implications on their oral bioavailability. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Ravishankar, D.; Watson, K.A.; Boateng, S.Y.; Green, R.J.; Greco, F.; Osborn, H.M.I. Exploring quercetin and luteolin derivatives as antiangiogenic agents. Eur. J. Med. Chem. 2015, 97, 259–274. [Google Scholar] [CrossRef]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Shoji, N.; Hatanaka, S.; Tsushida, T.; Miyazawa, T. Metabolic Fate of Luteolin in Rats: Its Relationship to Anti-inflammatory Effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Zhu, L.; Li, Q.; Zeng, X.; Lu, L.; Hu, M.; Wang, X.; Liu, Z. Metabolic Disposition of Luteolin Is Mediated by the Interplay of UDP-Glucuronosyltransferases and Catechol-O-Methyltransferases in Rats. Drug Metab. Dispos. 2017, 45, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Yasuda, M.T.; Fujita, K.; Hosoya, T.; Imai, S.; Shimoi, K. Absorption and Metabolism of Luteolin and Its Glycosides from the Extract of Chrysanthemum morifolium Flowers in Rats and Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 7693–7699. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and Metabolism of Luteolin in Rats and Humans in Relation to in Vitro Anti-inflammatory Effects. J. Agric. Food Chem. 2018, 66, 11320–11329. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Pan, H.; Lu, Y.; Ding, L. An HPLC-MS/MS method for the simultaneous determination of luteolin and its major metabolites in rat plasma and its application to a pharmacokinetic study. J. Sep. Sci. 2018, 41, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, K.; Zhao, C.; Ran, X.; Zhang, Y.; Zhang, T. A rapid HPLC–MS/MS method for the simultaneous determination of luteolin, resveratrol and their metabolites in rat plasma and its application to pharmacokinetic interaction studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1191, 123118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Chen, Y.R.; Chow, J.M.; Chien, M.H.; Yang, S.F.; Wen, Y.C.; Lee, W.J.; Tseng, T.H. Stimulation of Fas/FasL-mediated apoptosis by luteolin through enhancement of histone H3 acetylation and c-Jun activation in HL-60 leukemia cells. Mol. Carcinog. 2018, 57, 866–877. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Oh, J.; Kim, J.S. Luteolin Shifts Oxaliplatin-Induced Cell Cycle Arrest at G0/G1 to Apoptosis in HCT116 Human Colorectal Carcinoma Cells. Nutrients 2019, 11, 770. [Google Scholar] [CrossRef]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin Induces Apoptosis and Autophagy in HCT116 Colon Cancer Cells via p53-Dependent Pathway. Nutr. Cancer 2021, 74, 677–686. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Turktekin, M.; Konac, E.; Onen, H.I.; Alp, E.; Yilmaz, A.; Menevse, S. Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29). J. Med. Food 2011, 14, 1107–1117. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, B.; Gao, F.; Shi, R. Modulation of G2/M cell cycle arrest and apoptosis by luteolin in human colon cancer cells and xenografts. Oncol. Lett. 2018, 15, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.W.; Ma, Y.Y.; Guan, T.P.; Xia, Y.J.; Shao, C.M.; Chen, L.G.; Ren, Y.J.; Yao, H.B.; Yang, Q.; He, X.J. Oridonin induces apoptosis in gastric cancer through Apaf-1, cytochrome c and caspase-3 signaling pathway. World J. Gastroenterol. 2012, 18, 7166–7174. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fu, L.; Wu, Y.; Xiao, H.; Wang, J.; Sun, G. Influence of luteolin on the apoptosis of esophageal cancer Eca109 cells and its mechanism of action. Food Sci. Hum. Wellness 2019, 8, 189–194. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, B.S.; Kang, H.M.; Kim, J.H.; Shin, S.H.; Kim, I.R. Role of Luteolin-Induced Apoptosis and Autophagy in Human Glioblastoma Cell Lines. Medicina 2021, 57, 879. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef]
- Sabzichi, M.; Hamishehkar, H.; Ramezani, F.; Sharifi, S.; Tabasinezhad, M.; Pirouzpanah, M.; Ghanbari, P.; Samadi, N. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac. J. Cancer Prev. 2014, 15, 5311–5316. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Tian, J.; Song, M.; Zhao, R.; Liu, K.; Zhu, F.; Shim, J.H.; Dong, Z.; Lee, M.H. Targeting LIMK1 with luteolin inhibits the growth of lung cancer in vitro and in vivo. J. Cell. Mol. Med. 2021, 25, 5560–5571. [Google Scholar] [CrossRef]
- Park, S.H.; Ham, S.; Kwon, T.H.; Kim, M.S.; Lee, D.H.; Kang, J.W.; Oh, S.R.; Yoon, D.Y. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 219–231. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Pi, D.; Wu, Y.; Zuo, Q.; Li, C.; Ouyang, M. Luteolin induces pyroptosis in HT-29 cells by activating the Caspase1/Gasdermin D signalling pathway. Front. Pharmacol. 2022, 13, 952587. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Autophagy regulation using luteolin: New insight into its anti-tumor activity. Cancer Cell Int. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Zhou, J.; Ong, C.N.; Shen, H.M. Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt-GSK-3β-Cyclin D1 pathway. Cancer Lett. 2010, 298, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.O.; Nah, J.; Jung, Y.K. Molecules and their functions in autophagy. Exp. Mol. Med. 2012, 44, 73–80. [Google Scholar] [CrossRef]
- Park, S.H.; Park, H.S.; Lee, J.H.; Chi, G.Y.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Hyun, J.W.; Kim, W.J.; Choi, Y.H. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food Chem. Toxicol. 2013, 56, 100–109. [Google Scholar] [CrossRef]
- Uekita, T.; Fujii, S.; Miyazawa, Y.; Hashiguchi, A.; Abe, H.; Sakamoto, M.; Sakai, R. Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells. Cancer Sci. 2013, 104, 865–870. [Google Scholar] [CrossRef]
- Potočnjak, I.; Šimić, L.; Gobin, I.; Vukelić, I.; Domitrović, R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. Vitro 2020, 66, 104852. [Google Scholar] [CrossRef]
- Xu, H.; Linn, B.S.; Zhang, Y.; Ren, J. A review on the antioxidative and prooxidative properties of luteolin. React. Oxyg. Species 2019, 7, 136–147. [Google Scholar]
- Ren, L.Q.; Li, Q.; Zhang, Y. Luteolin Suppresses the Proliferation of Gastric Cancer Cells and Acts in Synergy with Oxaliplatin. BioMed Res. Int. 2020, 2020, 9396512. [Google Scholar] [CrossRef]
- Cook, M.T. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer 2018, 10, 89. [Google Scholar] [CrossRef]
- Franza, L.; Carusi, V.; Nucera, E.; Pandolfi, F. Luteolin, inflammation and cancer: Special emphasis on gut microbiota. Biofactors 2021, 47, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Lin, J.T.; Mahalakshmi, B.; Chuang, Y.C.; Lin, C.C.; Lo, Y.S.; Hsieh, M.J.; Chen, M.K. Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomolecules 2020, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fristiohady, A.; Nguyen, C.H.; Milovanovic, D.; Huttary, N.; Krieger, S.; Hong, J.; Geleff, S.; Birner, P.; Jäger, W.; et al. Apigenin and Luteolin Attenuate the Breaching of MDA-MB231 Breast Cancer Spheroids through the Lymph Endothelial Barrier in Vitro. Front. Pharmacol. 2018, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Chen, X.; Wu, M.; Kong, H.; Chu, G.; Zhou, Z.; Zhang, C.; Chen, B. Luteolin inhibits angiogenesis of the M2-like TAMs via the downregulation of hypoxia inducible factor-1a and the STAT3 signalling pathway under hypoxia. Mol. Med. Rep. 2018, 18, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Lei, X.; Huang, M.; Ye, W.; Zhang, R.; Zhang, D. Luteolin inhibits angiogenesis by blocking Gas6/Axl signaling pathway. Int. J. Oncol. 2017, 51, 677–685. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Seo, Y.; Ryu, K.; Park, J.; Jeon, D.K.; Jo, S.; Lee, H.K.; Namkung, W. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS ONE 2017, 12, e0174935. [Google Scholar] [CrossRef]
- Zhou, Q.; Yan, B.; Hu, X.; Li, X.B.; Zhang, J.; Fang, J. Luteolin inhibits invasion of prostate cancer PC3 cells through E-cadherin. Mol. Cancer Ther. 2009, 8, 1684–1691. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jung, S.K.; Byun, S.; Son, J.E.; Oh, M.H.; Lee, J.; Kang, M.J.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Raf and PI3K are the molecular targets for the anti-metastatic effect of luteolin. Phytother. Res. 2013, 27, 1481–1488. [Google Scholar] [CrossRef]
- Li, H.; Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Xia, G. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef]
- Lin, H.W.; Shen, T.J.; Yang, N.C.; Wang, M.; Hsieh, W.C.; Chuang, C.J.; Lai, C.Y.; Chang, Y.Y. Luteolin Reduces Aqueous Extract PM2.5-induced Metastatic Activity in H460 Lung Cancer Cells. Int. J. Med. Sci. 2022, 19, 1502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Q.; Shen, S.; Wei, X.; Li, G. HIF-1α/VEGF signaling-mediated epithelial–mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phyther. Res. 2019, 33, 798. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu Rev Immunol 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-alpha-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef]
- Zhou, F.; Qu, L.; Lv, K.; Chen, H.; Liu, J.; Liu, X.; Li, Y.; Sun, X. Luteolin protects against reactive oxygen species-mediated cell death induced by zinc toxicity via the PI3K-Akt-NF-κB-ERK-dependent pathway. J. Neurosci. Res. 2011, 89, 1859–1868. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Sahin, T.K.; Bilir, B.; Kucuk, O. Modulation of inflammation by phytochemicals to enhance efficacy and reduce toxicity of cancer chemotherapy. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Lee, Y.S. Luteolin suppresses IL-1beta-induced cytokines and MMPs production via p38 MAPK, JNK, NF-κB and AP-1 activation in human synovial sarcoma cell line, SW982. Food Chem. Toxicol. 2010, 48, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, M.; Tian, Y.; Liu, J.; Shang, J. Luteolin inhibits ROS-activated MAPK pathway in myocardial ischemia/reperfusion injury. Life Sci. 2015, 122, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.K.; Ou, Y.C.; Lin, S.Y.; Pan, H.C.; Song, P.J.; Raung, S.L.; Lai, C.Y.; Liao, S.L.; Lu, H.C.; Chen, C.J. Luteolin inhibits cytokine expression in endotoxin/cytokinestimulated microglia. J. Nutr. Biochem. 2011, 22, 612–624. [Google Scholar] [CrossRef]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Scott, H.S.; Goodall, G.J. A network-biology perspective of microRNA function and dysfunction in cancer. Nat. Rev. Genet. 2016, 17, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Manriquez, L.M.; Estrada-Meza, C.; Benavides-Aguilar, J.A.; Ledesma-Pacheco, S.J.; Torres-Copado, A.; Serrano-Cano, F.I.; Bandyopadhyay, A.; Pathak, S.; Chakraborty, S.; Srivastava, A.; et al. Phytochemicals mediated modulation of microRNAs and long non-coding RNAs in cancer prevention and therapy. Phyther. Res. 2021, 36, 705–729. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Huang, M.; Liu, Y.; Shu, Y. Luteolin Induces Apoptosis by Up-regulating miR-34a in Human Gastric Cancer Cells. Technol. Cancer Res. Treat. 2015, 14, 747–755. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Li, M.H.; Qin, Y.M.; Jiang, H.Y.; Zhang, X.; Wu, M.H. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, T.; Wang, J.; Mao, Z.; Duan, B.; Long, Y.; Xue, F.; Liu, D.; Liu, S.; Gao, Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J. Cancer 2018, 9, 3669. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, B.Z.; Lin, Y.P.; Wang, H.B. MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene 2018, 644, 56–65. [Google Scholar] [CrossRef]
- Mishan, M.A.; Khazeei Tabari, M.A.; Mahrooz, A.; Bagheri, A. Role of microRNAs in the anticancer effects of the flavonoid luteolin: A systematic review. Eur. J. Cancer Prev. 2021, 413–421. [Google Scholar] [CrossRef]
- Han, K.; Meng, W.; Zhang, J.J.; Zhou, Y.; Wang, Y.L.; Su, Y.; Lin, S.C.; Gan, Z.H.; Sun, Y.N.; Min, D.L. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. Onco Targets Ther. 2016, 9, 3085. [Google Scholar] [CrossRef]
- Moeng, S.; Son, S.W.; Seo, H.A.; Lee, J.S.; Kim, C.K.; Kuh, H.J.; Park, J.K. Luteolin-regulated MicroRNA-301-3p Targets Caspase-8 and Modulates TRAIL Sensitivity in PANC-1 Cells. Anticancer Res. 2020, 40, 723–731. [Google Scholar] [CrossRef]
- Sun, D.W.; Zhang, H.D.; Mao, L.; Mao, C.F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.X.; Jing, C.W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell. Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef]
- Magura, J.; Moodley, R.; Mackraj, I. The effect of hesperidin and luteolin isolated from Eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2022, 40, 1791–1800. [Google Scholar] [CrossRef]
- Sharma, D.; Tiwari, M.; Pandey, A.; Bhatia, C.; Sharma, A.; Trivedi, P.K. MicroRNA858 Is a Potential Regulator of Phenylpropanoid Pathway and Plant Development. Plant Physiol. 2014, 171, 944. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Butt, G.; El-Zahaby, S.A.; Attar, R.; Uteuliyev, Y.S.; Jovic, J.J.; Tang, K.F.; Naureen, H.; Xu, B. Luteolin mediated targeting of protein network and microRNAs in different cancers: Focus on JAK-STAT, NOTCH, mTOR and TRAIL-mediated signaling pathways. Pharmacol. Res. 2020, 160, 105188. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2016, 21, 312–328. [Google Scholar] [CrossRef]
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019, 42, 131. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Wu, X.; Tang, Z.; Han, Y.; Wang, Q.; Xiao, H. Synergism between luteolin and sulforaphane in anti-inflammation. Food Funct. 2018, 9, 5115–5123. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2013, 29, 819–825. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Ahn, Y.E.; Chung, W.S.; Choi, H.J.; Suh, Y.J. Synergistic effect between celecoxib and luteolin is dependent on estrogen receptor in human breast cancer cells. Tumour Biol. 2015, 36, 6349–6359. [Google Scholar] [CrossRef]
- Shih, Y.L.; Liu, H.C.; Chen, C.S.; Hsu, C.H.; Pan, M.H.; Chang, H.W.; Chang, C.H.; Chen, F.C.; Ho, C.T.; Yang, Y.I.Y.; et al. Combination treatment with luteolin and quercetin enhances antiproliferative effects in nicotine-treated MDA-MB-231 cells by down-regulating nicotinic acetylcholine receptors. J. Agric. Food Chem. 2010, 58, 235–241. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Huang, L.; Liu, A.; Zhang, J. Luteolin enhances the antitumor activity of lapatinib in human breast cancer cells. Biomed. Res. 2017, 28, 4902–4907. [Google Scholar]
- Chakrabarti, M.; Ray, S.K. Synergistic anti-tumor actions of luteolin and silibinin prevented cell migration and invasion and induced apoptosis in glioblastoma SNB19 cells and glioblastoma stem cells. Brain Res. 2015, 1629, 85–93. [Google Scholar] [CrossRef]
- Xu, H.; Yang, T.; Liu, X.; Tian, Y.; Chen, X.; Yuan, R.; Su, S.; Lin, X.; Du, G. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci. 2016, 144, 138–147. [Google Scholar] [CrossRef]
- Soliman, N.A.; Abd-Ellatif, R.N.; ELSaadany, A.A.; Shalaby, S.M.; Bedeer, A.E. Luteolin and 5-flurouracil act synergistically to induce cellular weapons in experimentally induced Solid Ehrlich Carcinoma: Realistic role of P53; a guardian fights in a cellular battle. Chem. Biol. Interact. 2019, 310, 108740. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Lee, J.; Kwon, M.J.; Oh, J.; Kim, J.S. Luteolin Synergistically Enhances Antitumor Activity of Oxaliplatin in Colorectal Carcinoma via AMPK Inhibition. Antioxidants 2022, 11, 626. [Google Scholar] [CrossRef]
- Gray, A.L.; Stephens, C.A.; Bigelow, R.L.H.; Coleman, D.T.; Cardelli, J.A. The polyphenols (-)-epigallocatechin-3-gallate and luteolin synergistically inhibit TGF-β-induced myofibroblast phenotypes through RhoA and ERK inhibition. PLoS ONE 2014, 9, e109208. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Qiao, T.; Wu, Z.; Huang, Z. Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion. J. Ovarian Res. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, R.; Zhang, P.; Wen, J.; Luo, Y.; Zhao, Z.; You, L.; Ho, C.T. Combination Effects of Polyphenols Present in Sugarcane on Proliferation in MCF-7 Human Breast Cancer Cells. Sugar Tech 2022, 24, 832–840. [Google Scholar] [CrossRef]
- Shi, M.L.; Chen, Y.F.; Wu, W.Q.; Lai, Y.; Jin, Q.; Qiu, W.L.; Yu, D.L.; Li, Y.Z.; Liao, H.F. Luteolin inhibits the proliferation, adhesion, migration and invasion of choroidal melanoma cells in vitro. Exp. Eye Res. 2021, 210, 108643. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pugel, E.P.; Zagorac, G.B.; Mahmutefendić, H.; Škoda, M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 2013, 310, 115–123. [Google Scholar] [CrossRef]
- Li, T.; Fu, X.; Liu, B.; Wang, X.; Li, J.; Zhu, P.; Niu, X.; Bai, J.; Liu, Y.; Lu, X.; et al. Luteolin binds Src, promotes STAT3 protein ubiquitination and exerts anti-melanoma effects in cell and mouse models. Biochem. Pharmacol. 2022, 200, 115044. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the Remedial Potential of Nano Delivery-Based Flavonoids for Breast Cancer Treatment. Molecules 2021, 26, 5163. [Google Scholar] [CrossRef]
- Xian, D.; Guo, M.; Xu, J.; Yang, Y.; Zhao, Y.; Zhong, J. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021, 26, 134. [Google Scholar] [CrossRef]
- Wruck, C.J.; Claussen, M.; Fuhrmann, G.; Römer, L.; Schulz, A.; Pufe, T.; Waetzig, V.; Peipp, M.; Herdegen, T.; Götz, M.E. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. In Neuropsychiatric Disorders an Integrative Approach; Springer: Vienna, Austria, 2007; pp. 57–67. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Aromokeye, R.; Si, H. Combined Curcumin and Luteolin Synergistically Inhibit Colon Cancer Associated with Notch1 and TGF-β Signaling Pathways in Cultured Cells and Xenograft Mice. Cancers 2022, 14, 3001. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, B.; Liu, X.; Zhang, X.; Wu, L.; Xing, D.; Ma, L.; Liu, J. Myocyte enhancer factor 2D promotes hepatocellular carcinoma through AMOTL2/YAP signaling that inhibited by luteolin. Int. J. Clin. Exp. Pathol. 2022, 15, 206. [Google Scholar]
- Han, S.; Lin, F.; Qi, Y.; Liu, C.; Zhou, L.; Xia, Y.; Chen, K.; Xing, J.; Liu, Z.; Yu, W.; et al. HO-1 Contributes to Luteolin-Triggered Ferroptosis in Clear Cell Renal Cell Carcinoma via Increasing the Labile Iron Pool and Promoting Lipid Peroxidation. Oxid. Med. Cell. Longev. 2022, 2022, 3846217. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Li, L.L.; Wang, L.; Dong, L.; Xi, G.; Lu, Y.J.; Li, Z. Luteolin impacts deoxyribonucleic acid repair by modulating the mitogen-activated protein kinase pathway in colorectal cancer. Bioengineered 2022, 13, 10998. [Google Scholar] [CrossRef]
- Sun, F.; Li, B.; Guo, Y.; Wang, Y.; Cheng, T.; Yang, Q.; Liu, J.; Fan, Z.; Guo, Z.; Wang, Z. Effects of ultrasonic pretreatment of soybean protein isolate on the binding efficiency, structural changes, and bioavailability of a protein-luteolin nanodelivery system. Ultrason. Sonochem. 2022, 88, 106075. [Google Scholar] [CrossRef]
- Abbas, H.; El Sayed, N.S.; Youssef, N.A.H.A.; Gaafar, P.M.E.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Hussain, A.; Alyahya, K.H.; Mahdi, W.A.; Qamar, W. Formulation and Evaluation of Luteolin-Loaded Nanovesicles: In Vitro Physicochemical Characterization and Viability Assessment. ACS Omega 2022, 7, 1048–1056. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Yuan, C.; Xing, R.; Ni, J.; Hu, G.; Chen, F.; Wang, X. Inflammaluteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017, 39, 113–125. [Google Scholar] [CrossRef]
- Kanai, K.; Nagata, S.; Hatta, T.; Sugiura, Y.; Sato, K.; Yamashita, Y.; Kimura, Y.; Itoh, N. Therapeutic anti-inflammatory effects of luteolin on endotoxin-induced uveitis in Lewis rats. J. Vet. Med. Sci. 2016, 78, 1381–1384. [Google Scholar] [CrossRef]
- De Leo, E.; Elmonem, M.A.; Berlingerio, S.P.; Berquez, M.; Festa, B.P.; Raso, R.; Bellomo, F.; Starborg, T.; Janssen, M.J.; Abbaszadeh, Z.; et al. Cell-Based Phenotypic Drug Screening Identifies Luteolin as Candidate Therapeutic for Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2020, 31, 1522–1537. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Chiao, M.T.; Liang, Y.J.; Yang, Y.C.; Shen, C.C.; Yang, C.Y. Luteolin inhibits migration of human glioblastoma U-87 MG and T98G cells through downregulation of Cdc42 expression and PI3K/AKT activity. Mol. Biol. Rep. 2013, 40, 5315–5326. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Ying, C.; Ma, B.; Wang, B.; Long, F.; Wang, R.; Fang, L.; Wang, Y. Combination of oncolytic adenovirus and luteolin exerts synergistic antitumor effects in colorectal cancer cells and a mouse model. Mol. Med. Rep. 2017, 16, 9375–9382. [Google Scholar] [CrossRef]
- Qin, T.; Zhu, W.; Kan, X.; Li, L.; Wu, D. Luteolin attenuates the chemoresistance of osteosarcoma through inhibiting the PTN/β-catenin/MDR1 signaling axis by upregulating miR-384. J. Bone Oncol. 2022, 34, 100429. [Google Scholar] [CrossRef]
- Monti, E.; Marras, E.; Prini, P.; Gariboldi, M.B. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur. J. Pharmacol. 2020, 881, 173210. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef]
- Kollur, S.P.; Prasad, S.K.; Pradeep, S.; Veerapur, R.; Patil, S.S.; Amachawadi, R.G.; Rajendra Prasad, S.; Lamraoui, G.; Al-Kheraif, A.A.; Elgorban, A.M.; et al. Luteolin-Fabricated ZnO Nanostructures Showed PLK-1 Mediated Anti-Breast Cancer Activity. Biomolecules 2021, 11, 385. [Google Scholar] [CrossRef]
- Wu, H.T.; Liu, Y.E.; Hsu, K.W.; Wang, Y.F.; Chan, Y.C.; Chen, Y.; Chen, D.R. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/mTOR Pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Wang, W.J.; Xu, C.; Xie, Y.J.; Wang, X.R.; Zhang, Y.Z.; Huang, J.M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef]
- Wu, B.; Xiong, J.; Zhou, Y.; Wu, Y.; Song, Y.; Wang, N.; Chen, L.; Zhang, J. Luteolin enhances TRAIL sensitivity in non-small cell lung cancer cells through increasing DR5 expression and Drp1-mediated mitochondrial fission. Arch. Biochem. Biophys. 2020, 692, 108539. [Google Scholar] [CrossRef]
- De Zang, M.; Hu, L.; Fan, Z.Y.; Wang, H.X.; Zhu, Z.L.; Cao, S.; Wu, X.Y.; Li, J.F.; Su, L.P.; Li, C.; et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 2017, 15, 52. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; He, K.; Jiang, W.; Xu, C.; Li, Z.; Wang, H.; Wang, W.; Wang, H.; Teng, X.; et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J. Transl. Med. 2015, 13, 42. [Google Scholar] [CrossRef]
- Kato, H.; Naiki-Ito, A.; Suzuki, S.; Inaguma, S.; Komura, M.; Nakao, K.; Naiki, T.; Kachi, K.; Kato, A.; Matsuo, Y.; et al. DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer. Carcinogenesis 2021, 42, 940–950. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Chen, L.; Li, H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018, 9, 3018–3027. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, Y.H. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochem. Biophys. Res. Commun. 2019, 517, 617–622. [Google Scholar] [CrossRef]
- Im, E.; Yeo, C.; Lee, E.O. Luteolin induces caspase-dependent apoptosis via inhibiting the AKT/osteopontin pathway in human hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018, 209, 259–266. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef]
- Selvendiran, K.; Koga, H.; Ueno, T.; Yoshida, T.; Maeyama, M.; Torimura, T.; Yano, H.; Kojiro, M.; Sata, M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: An implication for the antitumor potential of flavonoids. Cancer Res. 2006, 66, 4826–4834. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, B. Targeted PD-L1 PLGA/liposomes-mediated luteolin therapy for effective liver cancer cell treatment. J. Biomater. Appl. 2021, 36, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Q.; Xiao, B.; Fang, H.; Huang, B.; Huang, F.; Wang, Y. Luteolin enhances the antitumor efficacy of oncolytic vaccinia virus that harbors IL-24 gene in liver cancer cells. J. Clin. Lab. Anal. 2021, 35, 23677. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.D.; Park, S.Y. Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int. J. Oncol. 2019, 54, 665–672. [Google Scholar] [CrossRef]
- Iida, K.; Naiki, T.; Naiki-Ito, A.; Suzuki, S.; Kato, H.; Nozaki, S.; Nagai, T.; Etani, T.; Nagayasu, Y.; Ando, R.; et al. Luteolin suppresses bladder cancer growth via regulation of mechanistic target of rapamycin pathway. Cancer Sci. 2020, 111, 1165–1179. [Google Scholar] [CrossRef]
- Chian, S.; Li, Y.Y.; Wang, X.J.; Tang, X.W. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac. J. Cancer Prev. 2014, 15, 2911–2916. [Google Scholar] [CrossRef]
- Lim, W.; Yang, C.; Bazer, F.W.; Song, G. Luteolin Inhibits Proliferation and Induces Apoptosis of Human Placental Choriocarcinoma Cells by Blocking the PI3K/AKT Pathway and Regulating Sterol Regulatory Element Binding Protein Activity. Biol. Reprod. 2016, 95, 141556. [Google Scholar] [CrossRef]
- Pramodh, S.; Raina, R.; Hussain, A.; Bagabir, S.A.; Haque, S.; Raza, S.T.; Ajamal, M.R.; Behl, S.; Bhagavatula, D. Luteolin Causes 5’CpG Demethylation of the Promoters of TSGs and Modulates the Aberrant Histone Modifications, Restoring the Expression of TSGs in Human Cancer Cells. Int. J. Mol. Sci. 2022, 23, 4067. [Google Scholar] [CrossRef]
- Wu, H.T.; Lin, J.; Liu, Y.E.; Chen, H.F.; Hsu, K.W.; Lin, S.H.; Peng, K.Y.; Lin, K.J.; Hsieh, C.C.; Chen, D.R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, G.Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.J.; Peng, S.X.; Chen, L.W.; Li, Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Wang, Z.; Li, L.; He, M.; Han, S.; Dong, Y.; Liu, X.; Zhao, W.; Ke, Y.; et al. Luteolin attenuates cancer cell stemness in PTX-resistant oesophageal cancer cells through mediating SOX2 protein stability. Pharmacol. Res. 2021, 174, 105939. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.W.; Lu, Z.Y.; Pan, Q.; Chen, T.T.; Feng, X.J.; Wang, S.M.; Pan, Y.C.; Zhu, M.H.; Zhang, S.H. MicroRNA-6809-5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1. Phytomedicine 2019, 57, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Chian, S.; Thapa, R.; Chi, Z.; Wang, X.J.; Tang, X. Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochem. Biophys. Res. Commun. 2014, 447, 602–608. [Google Scholar] [CrossRef]




| S. No. | Combination of Drug Molecules | Type of Cancer | Model System (Cell Lines) | Physiological Effect and Mechanism(s) | Dose | Ref. |
|---|---|---|---|---|---|---|
| 1 | luteolin and oxaliplatin | tumor | HCT116 cells | reduced the expression of p21 protein | - | [101] |
| 2 | luteolin and oxaliplatin | Tumor | gastric adenocarcinoma cell line (SGC-7901) | blocked cell progression in the G0/G1 phase and induced apoptosis; increased cyclin D1 levels | LUT (40 μM) and OXA (30 μM); 24 h | [92] |
| 3 | celecoxib and luteolin | Malignant tumors | breast cancer cells (MCF-7 and MDA-MB-231) | increased cell proliferation, cell death, apoptosis; decreased levels of Akt phosphorylation (pAkt); | 10, 25, 50, 75, 100 μM for 72 h | [94] |
| 4 | quercetin and luteolin | malignant | MDA-MB-231 cell | downregulation of nicotinic acetylcholine receptors and R9-nAChR expression | 0.5 μM | [122] |
| 5 | luteolin and silibinin | malignant | glioblastoma SNB19 cells and glioblastoma stem cells | prevented cell migration and invasion and induced apoptosis; targeted PKCα and iNOS | 20 µM LUT and 50 µM SIL | [35] |
| 6 | apigenin and luteolin | malignant | MDA-MB231 cell | inhibited CCID, MMP1-induced calcium increase and phosphorylation of FAK essential for FAK activation; p53 signaling pathway was activated; NF-κB pathway inhibition | - | [123] |
| 7 | luteolin and cyclophosphamide | Tumor cells | human breast cancer cell | increasing Bcl-2 protein level and antioxidant activity; downregulation of Akt phosphorylation | Lut 30 mg/kg + cyclophosphamide 10 mg/kg | [40] |
| 8 | luteolin and lapatinib | - | BT474 breast cancer cells | inhibited expression of ERBB1, phosphorylation level of Akt, ERK1/2 | - | [124] |
| 9 | CD55-TRAIL and luteolin | Tumor cells | colorectal cancer (CRC), HT-29 cells | displayed greater chromatin condensation, nuclear fragmentation and apoptotic body formation | CD55-TRAIL (15 MOI), luteolin (25 µM), 72 h | [125] |
| 10 | epigallocatechin-3-gallate and luteolin | - | prostate cancer cells | inhibited TGF-β and ERK inhibition pathways, decreased levels of HGF and VEGF | - | [101] |
| 11 | luteolin and paclitaxel | malignant | breast cancer cell lines | regulated Caspase 8, 3, Fas | - | [35] |
| 12 | luteolin and cisplatin | malignant | ovarian cancer, CAOV3/DDP cells | induction of apoptosis and inhibition of cell migration and invasion, downregulation of Bcl-2 expression | 10–40 mg/kg, 5 days | [106] |
| 13 | luteolin and hesperidin | malignant | breast cancerous cell line MCF-7 | downregulated miR21 expression levels while upregulated miR-16 expression levels, caused a significant accumulation of apoptotic cells into the G0/G1 | 20, 60, 100 and 140 mg/mL, for 24 h and 48 h. | [35] |
| 14 | luteolin and 5-fluorouracil | human hepatocellular carcinoma cells (HepG2 and Bel7402 cells) | enhanced bax/bcl-2 ratios and p53 expressions, and induced PARP cleavage | dose ratios (luteolin: 5-fluorouracil = 10:1, 20:1, 40:1) | [100] |
| Type of Cancer | Cell Lines | Effects | Mechanisms | Concentration | References |
|---|---|---|---|---|---|
| Osteosarcoma | MG63 and U2OS | Induces apoptosis | ↓ chemoresistance to doxorubicin and cisplatin, ↓ cancer cell viability and proliferation, ↑ miR-384 level, ↓ PTN expression, ↓ PTN/b-catenin/MDR1 signaling axis, ↑ doxorubicin response in doxorubicin-resistant MG63/DOX cells, ↑ miR-384 in exosomes derived from luteolin-treated MG63 cells | 0, 1, 2, 3, 4, 5 μM | [126] |
| Colon | HCT116 | Induces apoptosis and autophagy | ↓ cell migration, ↓ HIF-1α-dependent transcription, ↓ G1 and G2/M cells, ↑ cells in S phase, ↑ apoptotic frequency, ↑ necrotic cell death, ↑ LC3-II, Luteolin treatment reversed increase of CD44 and CD47 | 2.5–200 μM | [127] |
| SW620 | Induces apoptosis and autophagy | ↓ viability and proliferation of cancer cells, ↑ HO-1, ↑ SOD2, ↓ Bcl-2, ↑ Bax, ↑ Cleaved caspase-3, ↑ PARP cleavage, ↑ Beclin-1, ↑ Atg5, ↑ LC3B-I/II, ↑ LC3B-I, reversal of the epithelial-mesenchymal transition, ↑ FOXO3a, ↑ apoptosis, ↑ TUNEL-positive cells, ↑ p21, ↑ phospho-ERK1/2, phospho-JNK1/2 and phospho-p38 expression | 0.2, 5, 10, 20, 50, 100 μM | [47] | |
| HT-29, SNU-407 | Induces apoptosis | ↓ viability of cancer cells, ↑ Bax, ↑active caspase-9 and 3, ↓ Bcl-2, ↑ protein expression of GCLc, GSS, catalase and HO-1, ↑ DNA demethylation, ↑ mRNA expression of Nrf2 | 0, 5, 10, 20, 30, 40, 50, 60, 70 and 80 μM | [128] | |
| Breast | MCF-7 | Induces apoptosis | ↑ Cytotoxicity for cancer cell lines, ↑ anti-breast cancer activity of L-ZnONPs was mediated by polo-like kinase 1 (PLK1) proteins (In silico studies) | 2.5, 5, 10, 20, and 40 µM concentrations of luteolin, zinc oxide nanoparticles, and L-ZnONPs. | [129] |
| MCF7-TamR | Induces apoptosis | Cell cycle arrest at the G2/M phase, ↓ mitochondrial membrane potential, ↓ PI3K/AKT/mTOR signaling pathway, ↑ p27, ↑ cleaved-Caspase 7, 8, 9, and poly (ADP-ribose) polymerase (PARP), ↑BAX and BIM, ↓ Bcl-2, ↓ p-p85, p-AKT, and p-mTOR, ↑ MLL3 and Mono methylation of H3K4, ↑ K-Ras, H-Ras, and N-Ras mRNA | 0, 10, 20, and 30 μM | [130] | |
| MDA-MB231 | Induces apoptosis and autophagy | ↓ cell migration, ↓ HIF-1α-dependent transcription, ↓ G1 and G2/M cells, ↑ cells in S phase, ↑ apoptotic frequency, ↑ necrotic cell death, ↑ LC3-II, Luteolin treatment reversed increase of CD44 and CD47 | 2.5–200 μM | [127] | |
| MDA-MB-453 and MCF-7 | Induces apoptosis | ↓ cells viability, ↑ apoptosis frequency, ↑ Bax, ↓ Bcl-2, ↓ Vimentin, ↓ Zeb1 ↓ N-cadherin, ↑ E-cadherin, ↑ miR-203 level, ↓ Ras/Raf/MEK/ERK signaling | 0, 5, 10 and 20 μM | [131] | |
| Lung | NCI-H1975 and NCI-H1650 | Induces apoptosis | ↓ proliferation of cancer cells, ↓ LIMK1 activity, ↑ cell cycle arrest at G1 phase, ↑ apoptosis frequency, ↓ cyclin D1 and ↓ cyclin D3, ↑ Bax, ↑ cleaved caspase 3, ↑ cleaved caspase-7, ↑ cleaved PARP expression, ↓ caspase-3, ↓ caspase-7, ↓ p-LIMK1/2 and p-cofilin | 0, 5, 10, 20 or 40 μmol/L | [38] |
| BEAS-2B, KRAS-mutant human lung cell lines H358, H460, H2122, and A549 | Induces apoptosis | ↓ growth and proliferation of cancer cells, ↓ MUC1-C and PD-L1, ↓ p-STAT1 or STAT3, ↑ IL-2, ↓ IFN-γ-induced PD-L1 expression | Apigenin and luteolin—0, 10, 20, 30, 40, 50 μM | [132] | |
| Non-Small Cell Lung | A549 and NCI–H1975 | Induces apoptosis | ↓ cancer cell viability, ↑ apoptosis, ↑ caspase-8, caspase-3 and caspase-9, ↑ DR5 expression, ↓ mitochondrial length, ↑ Drp1 from the cytoplasm onto mitochondria, ↑p- Drp1(Serine616 residue), ↑ cytochrome c release from mitochondria, ↑ cytochrome c in the cytosolic fraction, ↓ mitochondrial cytochrome c content | 0, 5, 10, 20, 30, 40 μM Luteolin + TRAIL (25 ng/mL) | [133] |
| A549 and H460 | Induces apoptosis | ↓ proliferation of cancer cells, ↑ apoptosis frequency, ↑ P53 and P21, ↓ MDM4, ↑ Caspase 3 and 9 | 0, 5, 10, 20, 30, 40, 60, 80, and 100 µM | [81] | |
| Gastric | NCI-N87 and MKN28, Hs-746T | Induces apoptosis | ↓ cell proliferation, invasion, and migration of cancer cells, reversed EMT by shrinking the cytoskeleton, ↑ E-cadherin, ↓N-cadherin, ↓ vimentin, ↓ Snail, ↓ β-catenin levels, ↓ Notch1, ↓ cyclin-D1, ↓ Hes-1 | 0, 10, 20 and 30 μM | [134] |
| MKN45, MKN28, BGC823, AGS and SGC7901 | Induces apoptosis | ↓ Proliferation and invasiveness of cancer cells, ↑ apoptosis frequency, ↓ MMP9, ↓ p-cMet, ↓ p-Akt, ↓ p-ERK, ↑ cleaved caspase-3 and PARP-1 | 0–80 μM | [135] | |
| Pancreatic | MIAPaCa2, PANC1, BxPC3, KP4, HuPT3, PK1, PA-TU-8988T, TCCPAN2 and AsPC1 | Induces apoptosis | ↓ cancer cell proliferation, ↓ STAT3 activity, ↓ phospho-AMPK (Thr172), ↓ phospho-p38 MAPK (Thr180/Tyr182), ↓ phospho-STAT3 (Tyr 705), ↑ phospho-GSK3β (Ser 9), ↓ DPYD expression | 25 or 50 μM | [136] |
| SW1990 andAspc-1 | Induces apoptosis | ↓ cell proliferation, ↓ BCL-2, ↑ apoptotic frequency of cells, ↑ loss of mitochondrial membrane potential, ↑ activation of pro caspase-3 and PARP | 50 μM and 100 μM | [137] | |
| Hepatocellular | HepG2 (p53 wild type) and Hep3B (p53 null type) | Induces apoptosis | ↓ cancer cell numbers, ↑ Protein levels of PARP cleaved, ↓ PCNA, ↓ catalase protein levels, ↑ mRNA levels of both Bip and spliced Xbp-1, ↑ p53 protein levels, ↑ p21 gene expression↓ TAp63 mRNA levels, ↑ LC3-II, ↓ p62 | 0, 5, 10 μM | [138] |
| SK-Hep-1 and AML12 | Induces apoptosis | ↓viability of cancer cells, ↑ apoptotic cell population, ↑ sub-G1 population, ↑ cleaved-caspase 8, -9 and -3, cleaved-PARP, ↓ XIAP, ↓ Mcl-1, ↓ cleaved Bid, ↓ p- AKT | 20, 40, 60 and 80 μM | [139] | |
| SMMC-7721 | Induces apoptosis | ↑ G0/G1-phase arrest, ↓ % age of cell s in G2/M-phase, ↑ %age of early apoptosis, late apoptosis, and total apoptosis, ↑ caspase 8, ↓ Bcl-2, ↑ intracellular autophagosomes, ↑ LC3B ↑ BECN1 mRNA, ↑ conversion of LC3B-I to LC3B-II, ↑ Beclin1 | 0, 12.5, 25, 50, 100, and 200 µM | [140] | |
| HepG2, HLF, and HAK-1B | Induces apoptosis | ↓ cancer cell proliferation, ↑ cleaved caspase-8, caspase-3, caspase-7 and PARP, ↑ Fas/CD95 expression, ↓ p-STAT3s, ↓ Tyr705-phosphorylated STAT3, ↓ Ser727-phosphorylated STAT3, ↓ cyclin D1, ↓ survivin, ↓ Bcl-xL, ↓ VEGF, ↓ Tyr-phosphorylated CDK5 | 0, 10, 20, 50 μM | [141] | |
| Liver | HepG2 | Induces apoptosis | ↑ frequency inhibiting HepG2 cell proliferation than free luteolin, ↑ enhance the uptake of drugs by cells, ↓ Bcl-2 and ↑ LDH | Luteolin-loaded PD-L1 targeted stealth PLGA/Liposomes (5.0 mg luteolin) | [142] |
| MHCC97-H, HepG2,PLC/PRF/5, Hep3B, HEK293 | Induces apoptosis | ↑ inhibitory impact of VVIL-24 on liver cancer cells viability, ↑ IL-24 gene expression, ↑ apoptosis frequency, ↑ cleaved PARP, cleaved caspase-3, cleaved caspase-8, ↓ procaspase-3 and procaspase-8, ↓ XIAP | VV-IL-24 (4 MOI) and Luteolin (5 µg/mL) | [143] | |
| Huh7 andHep3B | Induces apoptosis and autophagy | ↓ cell viability, ↑ apoptotic bodies, ↑ LC3-II, ↓ p62, ↑ DR5, ↑ cleaved caspase-3 and cleaved caspase-8 | 0, 5, 10 and 20 µM | [144] | |
| Bladder | T24, 5637 with a p53 mutation and RT-4 with wild-type p53 | Induces apoptosis | ↑ G2/M arrest, ↑ p21Waf1/Cip1, ↑ p27Kip1, ↓ cyclin A and D1, ↓ phospho(p)-Akt, ↓ phospho(p)-p70S6K, ↓ phospho(p)-S6, ↑ TRX1, ↓ Intracellular ROS | 0, 1, 10, 25, 50, 100 µM | [145] |
| Colorectal | HCT 116 and SW 620 (Oxaliplatin resistant) | Induces apoptosis | ↑ Nrf2, ↑ NQO1, ↑ HO-1, ↑ GST α1/2, ↓ reduced glutathione, ↑ chemotherapeutic potential of cisplatin, oxaliplatin and doxorubicin | 1, 5 and 10 μM | [146] |
| Choriocarcinoma | JAR and JEG-3 | Induces apoptosis | ↓ Proliferation and viability of cancer cells ↑ apoptosis frequency, ↑ loss of mitochondrial membrane potential, ↓ p-AKT, ↓ p-P70S6K, ↑p-GSK3β, ↓ AKT, ↑ERK1/2, ↓ PI3K/AKT and ERK1/2 signaling pathways ↓ SREBP1, ↓ SREBP2, ↓ SCAP mRNAs, ↓ p-mTOR, ↓ lipogenic genes | 0, 5, 10 and 20 μM | [147] |
| Cervical | HeLa | Induces apoptosis | ↓ methylation of crucial tumor suppressor genes like APC, BRCA1, CDH13, CDKN2, MGMT, MLH1, RARB, RASSF1 and TIMP3, ↓ global DNA methylation, ↓ DNMT activity, ↓ Histone deacetylation activity, modifies the expression of various chromatin-modifying enzymes, ↓histone methyl transferases such as ASH1L, WHSC1, SU2V40H1, ↓ HAT activity | 5, 10, and 20 µM | [148] |
| Type of Cancer | Cell Line | Effects | Mechanism | Concentration | References |
|---|---|---|---|---|---|
| Melanoma | C918 and OCM-1 | Suppress metastasis | ↓ proliferation, adhesion, migration and invasion, ↓ MMP-2, ↓ MMP-9, ↓ PI3K/Akt signaling pathway, ↓ fluorescence intensity of F-actin, ↓ inhibit cellular F-actin aggregation, ↓ p-PI3K P85, ↓ p-Akt expression | 0, 2.5, 5, 10, 20, 40 µM | [105] |
| A375 | Suppress metastasis | ↓ MMP-2, ↓ MMP-9, ↑ TIMP-1 and TIMP-2, ↓ p-AKT1, ↓ p-PI3K, ↓ PI3K/AKT pathway | 0, 10, 15 and 20 μM | [124] | |
| A375 and B16-F10 | Suppress metastasis | ↓ migratory, invasive, adhesive, and tube-forming potential, ↓ EMT, ↑ E-cadherin, ↓ N-cadherin and vimentin, ↓ p-Akt, ↓ HIF-1α, ↓ VEGF-A, ↓ p-VEGFR-2, ↓ MMP-2, ↓ MMP-9 | 5, 10, and 20 μM | [63] | |
| Glioblastoma | U-87 MG and T98G | Inhibits migration | ↓ Cdc42 (cell division cycle 42), ↓reduced PI3K/AKT activation, ↓ proteaosome pathway, ↑ Cdc42 proteolysis | 15 and 30 μM | [123] |
| Breast | MDA-MB-231, MDA-MB-486, 4T1 and BT-549 | Inhibits metastasis | ↓ proliferation and metastasis, ↓ AKT/mTOR signaling pathway, reversed the epithelial-mesenchymal transition (EMT), ↓ MMP9, ↓ AKT/mTOR, ↑ H3K27Ac and H3K56A, ↑ p-AKT and p-mTOR proteins | 0, 10, 20, and 30 μM | [149] |
| MDA-MB-231, MCF10A, 4T1 | Inhibits metastasis, and recurrence | ↓ cell migration proliferation and colony formation, ↓ YAP/TAZ transcriptional activity and nuclear localization, ↓ EMT, ↓ fibronectin, N-cadherin, and vimentin, ↑ E-cadherin, ↓ CTGF and CYR61 | 0, 5, 10, 20, 40, 80 μM | [150] | |
| MDA-MB-231 | Suppress metastasis | ↓C-X-C, chemokine receptor type 4 (CXCR4), ↓ MMP-2, ↓ MMP-9 | -- | [122] | |
| MDA-MA-231 and BT5-49 | Suppress metastasis | ↓ cell invasion, ↓ β-catenin expression, reorganization of cytoskeletal protein F-actin in the cytoplasm, ↑ E-cadherin, ↑ claudin, ↓ N-cadherin ↓ vimentin, ↓ Snail, ↓ Slug, | 0, 10, 30 and 100 μM | [61] | |
| Lung | A549 | Inhibition of cell migration | ↓ cell motility and migration, suppression of MEK-ERK pathway by PD98059 significantly reversed luteolin-inhibited cell migration, ↑ E cadherin, ↓ N-cadherin | 0–100 μM | [151] |
| Oesophageal | TE-1 | Suppress metastasis | ↓ stem-like properties of PTX-resistant cancer cells, ↓ SOX2, ↓ PI3K/AKT, ↓p-AKT(S473), ↓ UBR5 expression (ubiquitin E3 ligase that promotes SOX2 degradation), ↓ PTX-resistant cancer cell migration and invasion by blocking epithelial-mesenchymal transition (EMT) | -- | [152] |
| Hepatocellular | HepG2, Huh7 | miRNA regulation | ↑ miR-6809-5p (miR-6809-5p targets flotillin 1 (FLOT1) in HCC), ↑ FLOT1 prevented miR-6809-5p-mediated growth suppression. Multiple signaling pathways including Erk1/2, p38, JNK, and NF-κB/p65 were inactivated by miR-6809-5p overexpression or FLOT1 downregulation | 10 μM | [153] |
| Gastric | MKN45 and BGC823 | Inhibits metastasis | ↓ cell migration and invasion, ↓ lung metastasis, ↓ Cyclin D1, ↓ Cyclin E, ↓ Bcl2, ↓ MMP2, ↓ MMP9, ↓ N-cadherin, ↓ Vimentin ↓ Notch1, ↓p-PI3K, ↓ p-AKT, ↓ p-mTOR, ↓ p-ERK, ↓p-STAT3 ↑ p-P38 signaling, ↑ p21, ↑ Bax, ↑ E-cadherin, | 20 and 40 μM | [82] |
| MKN45 and BGC823 | miRNA regulation | ↑ miR-139, ↑ miR-34a, ↑ miR-422a, ↑ miR-107 (tumour suppressor), ↓ miR-21, ↓ miR-155, ↓ miR-224, ↓ miR-340 (oncogenes) | 20 and 40 μM | [82] | |
| Colorectal | HT-29, SW480, SW620 and LoVo | Suppress metastasis | ↓cells migration and invasion, ↓ MMP-2, ↓ MMP-3, ↓MMP-9, ↓ MMP-16 | 0, 10, 50 and 100 µM | [92] |
| HT-29, SW480, SW620 and LoVo | miRNA regulation | ↑ miR-384 and ↓ PTN expressions, miR-384 inhibitor partially reversed the inhibition of cells migration and invasion induced by luteolin | 0, 10, 50 and 100 µM | [92] |
| Type of Cancer | Animal Models | Effects | Mechanisms | Dosage | Duration | References |
|---|---|---|---|---|---|---|
| Osteosarcoma | BALB/c nude mice xenografted with MG 63 5 × 106 cells | Inhibited tumor growth | ↓ tumor size and growth, ↑ anti-tumor effect in combination with doxorubicin, ↑ miR-384, ↓ PTN, β-catenin and P-glycoprotein | 2 mg/kg doxorubicin + 30 mg/kg luteolin | 28 days | [126] |
| Breast | BALB/c nude mice xenografted with 4T1 cells | Inhibited the tumor growth | ↓ final tumor volume and weight, ↓ YAP/TAZ expression | 40 mg/kg | 18 days | [150] |
| Lung | Patient-derived xenograft mouse model with SCID mice | Inhibited tumor growth | ↓ tumor growth and weight, ↓ Ki-67, ↓ p-Limk1/2 and p-cofilin expression | 100 mg/kg | 59 days | [38] |
| Nude mice model with H358 xenografts | Inhibited tumor growth | ↓ tumor volume and size,↓ tumor weight, ↓ lunglesions, ↑ %age CD8+ T cells in blood, spleen, or tumor was increased, ↑ IFN-γ, TNFα,and Granzyme B | 30 mg/kg of apigenin or luteolin | 21 days | [132] | |
| Lewis lung carcinoma model with C57BL/6J mice | Inhibited tumor growth | ↓ tumor volume and size, ↓ tumor weight, ↓ lung lesions, ↑ %age CD8+ T cells in blood, spleen, or tumor was increased, ↑ IFN-γ, TNFα,and Granzyme B | 30 mg/kg of apigenin or luteolin + anti-PD-L1 mAb (10 mg/kg) | 21 days | [132] | |
| KRASLA2 mice model | Inhibited tumor growth | ↓ tumor volume and size, ↓ tumor weight, ↓ lunglesions, ↑ %age CD8+ T cells in blood, spleen, or tumor was increased, ↑ IFN-γ, TNFα, and Granzyme B | 30 mg/kg of apigenin or luteolin | 21 days | [132] | |
| Nude mice model with H460 xenografts | Suppressed tumor growth | ↓ tumor volumes and tumor weights, ↑ inflammatory cell infiltration, ↑ clear cell death characteristics and phenotype, ↑ TUNEL-positive cells were, ↓ Ki67-labeling index, ↑ miR-34a-5p, ↑P53 and P21, ↓ MDM4 | 50, 100, and 200 mg/kg/day) | 15 days | [81] | |
| C57BL/6 Nrf2+/+ and Nrf2/ mice xenografted with A549 tumor cells (1 × 107 cells) | Inhibited tumor growth | ↓ NQO-1 expression, ↓ protein level of NQO1 AKR1C, HO-1, and GSTm1 | cisplatin only (5 mg/kg), luteolin only (40 mg/kg), or a combination of cisplatin (5 mg/kg) and luteolin (40 mg/kg). | 35 days | [154] | |
| Gastric | BALB/c male nude mice xenografted with MKN28 cells | Inhibited the tumor growth | ↓ tumor volume and tumor eight, ↓ β-catenin, ↓ Notch1, ↓ Ki-67 expression, ↑ TUNEL staining | -- | 4 weeks | [134] |
| Human tumor xenograft (PDTX) models of gastric cancer (BALB/c nude mice) | Inhibited the tumor growth | ↓ cMet protein, ↓ MMP9, ↓ p-cMet | 10 mg/kg | 30 days | [135] | |
| Hepatocellular | BALB/c nude mice xenografted with 4 × 106 MHCC97-H cells | Inhibited tumor growth | ↓ tumor growth, ↑ IL-24 protein, ↓ CD31, ↓ Ki67 staining, ↑ protein level cleaved caspase-3, ↑ cytopathic effect | luteolin (50 mg/kg) alone, intraperitoneal injection; VV-IL-24 (2 × 107 plaque-forming units) and their combination | 35 days | [143] |
| BALB/c athymic nude mice injected with HAK-1B cells | Inhibited the tumor growth | ↓ tumor volume, ↓ Tyr705-phosphorylated STAT3, ↓ Ser727-phosphorylated STAT3, ↓ cyclin D1, ↓ VEGF, ↑ Fas/CD95, ↑ cleavage in caspase-7 | 50 or 200 ppm | 6 weeks | [141] | |
| Pancreatic | Female Syrian golden hamsters injected with subcutaneousinjections of BOP | Inhibited pancreatic carcinogenesis | ↓total cholesterol, ↑ amylase, ↓ incidence and multiplicity of PDACs, ↓ progression of neoplastic lesions, ↓ Ki-67 labeling index ↓ lesions, ↓ DPYD ↓ pSTAT3 signaling | 100 ppm | 6 weeks | [136] |
| SCID mice xenografted with 1.65 × 106 SW1990 cells | Inhibited tumor growth | No pathological changes in these normal tissues compared with the vehicle-treated group, did not produce remarkable weight loss of mice | 75 mg/kg and 150 mg/kg | 2 weeks | [137] | |
| Bladder | KSN nude mice xenografted with 5 × 104 BC31 cells | Inhibited the tumor growth | ↓ toxic effect, tumor volumes, ↓ Ki67-labeling index, ↑ TUNEL-positive cells, ↓ proliferation of cancer, ↑ apoptosis frequency, ↑ p21-positive cells | 100 ppm | 5 weeks | [145] |
| Colorectal | C57BL/6 Nrf2+/+ and Nrf2−/− mice | Inhibited the tumor growth | ↑ Nrf2 and NQO1, HO-1, GST α1/2, ↓ reduced glutathione | 40 mg/kg | 14 days | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373. https://doi.org/10.3390/cancers14215373
Singh Tuli H, Rath P, Chauhan A, Sak K, Aggarwal D, Choudhary R, Sharma U, Vashishth K, Sharma S, Kumar M, et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers. 2022; 14(21):5373. https://doi.org/10.3390/cancers14215373
Chicago/Turabian StyleSingh Tuli, Hardeep, Prangya Rath, Abhishek Chauhan, Katrin Sak, Diwakar Aggarwal, Renuka Choudhary, Ujjawal Sharma, Kanupriya Vashishth, Sheetu Sharma, Manoj Kumar, and et al. 2022. "Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives" Cancers 14, no. 21: 5373. https://doi.org/10.3390/cancers14215373
APA StyleSingh Tuli, H., Rath, P., Chauhan, A., Sak, K., Aggarwal, D., Choudhary, R., Sharma, U., Vashishth, K., Sharma, S., Kumar, M., Yadav, V., Singh, T., Yerer, M. B., & Haque, S. (2022). Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers, 14(21), 5373. https://doi.org/10.3390/cancers14215373







Manoj_Kumar.jpg)



