Retroviral Replicating Vector Toca 511 (Vocimagene Amiretrorepvec) for Prodrug Activator Gene Therapy of Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture
2.2. Plasmid Constructs and RRV Production
2.3. Replication Kinetics of RRV in Lung Cancer Cell Lines
2.4. Cytotoxicity Assay In Vitro
2.5. Animal Studies
2.6. Subcutaneous Tumor Models
2.7. Pleural Dissemination Models
2.8. Bioluminescence Imaging
2.9. Analysis of RRV Systemic Biodistribution
2.10. Statistical Analysis
3. Results
3.1. Evaluation of RRV in Lung Cancer Cells In Vitro
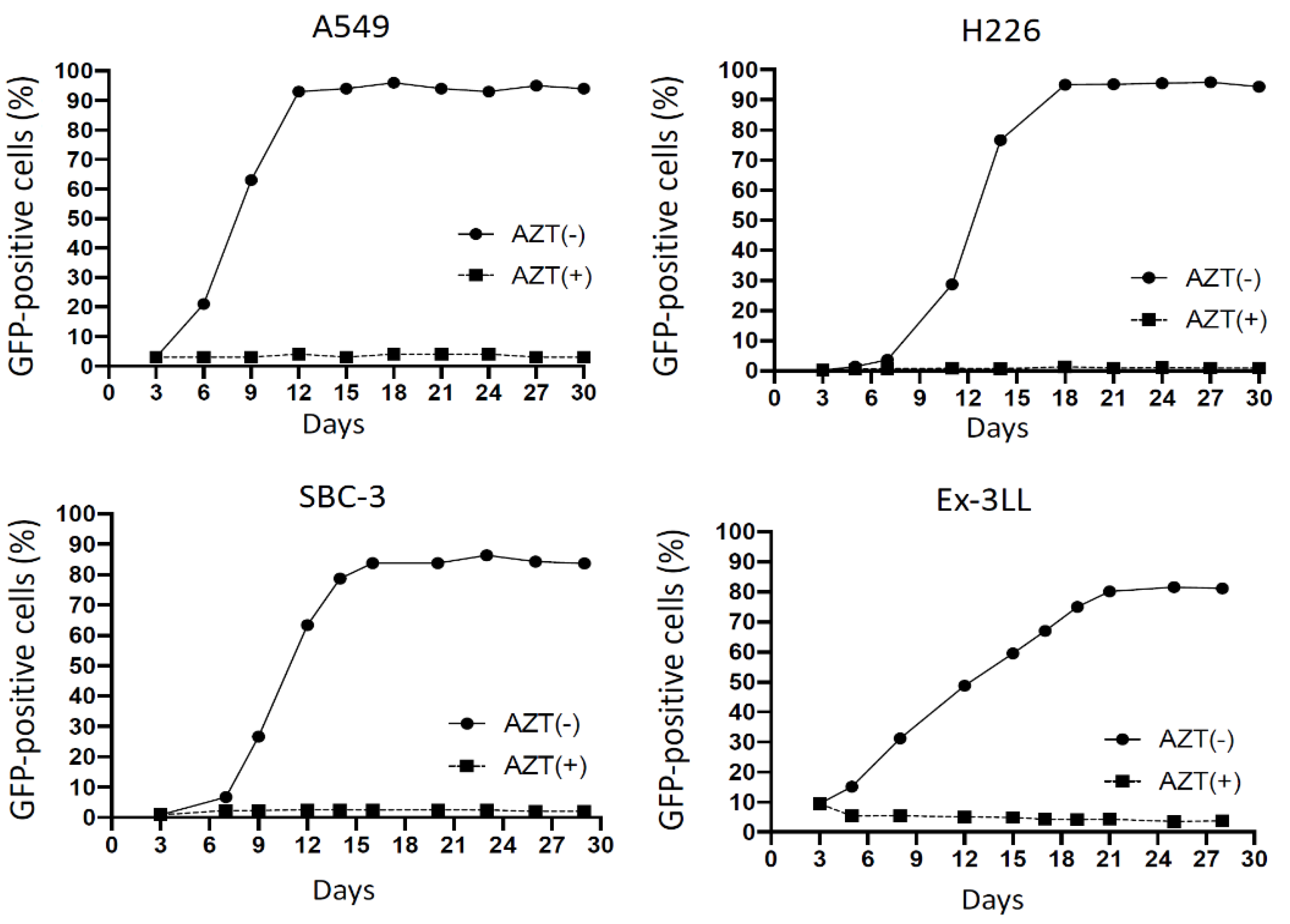
3.1.1. RRV Shows Rapid Viral Replication Resulting in High Levels of Cellular Transduction
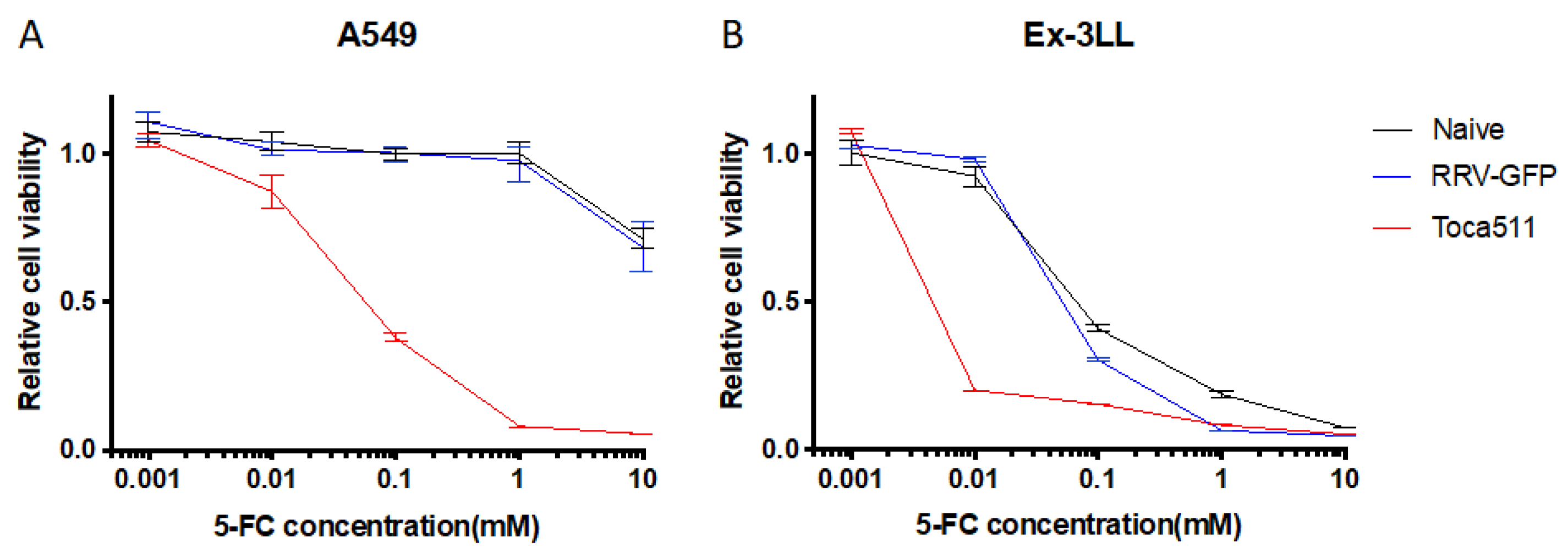
3.1.2. Toca 511/5-FC Treatment Shows Remarkable Toxicity to Lung Cancer Cells In Vitro
3.2. Evaluation of RRV-GFP Tumor Transduction and Toca 511/5-FC Therapeutic Efficacy in Subcutaneous Lung Cancer Models In Vivo
3.2.1. RRV Shows Significant Viral Replication and Tumor Transduction in Both Immunodeficient and Immunocompetent Subcutaneous Tumor Models of Lung Cancer In Vivo
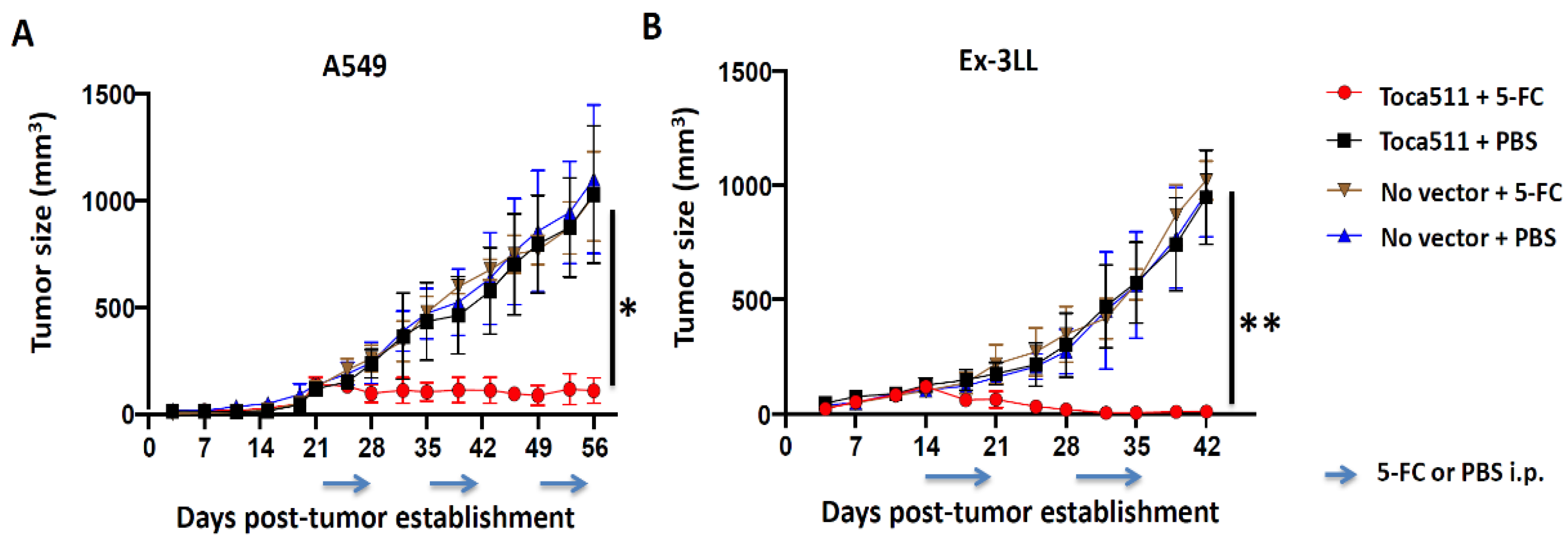
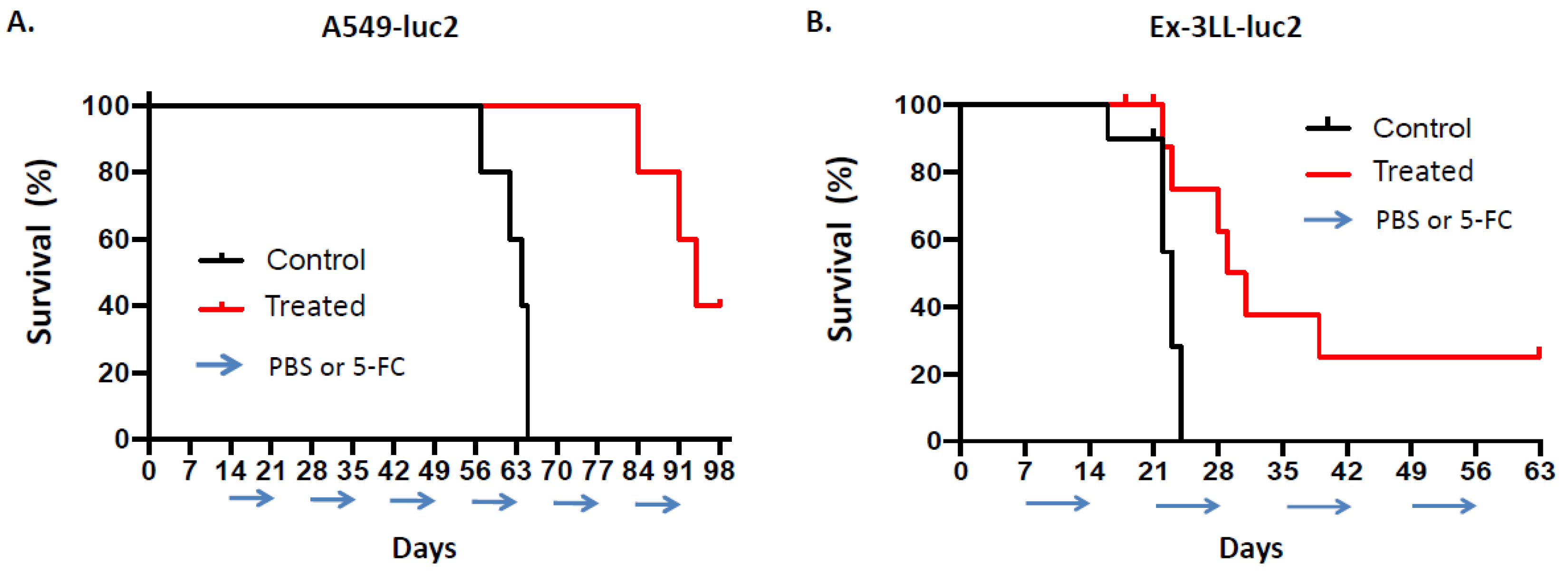
3.2.2. Toca 511/5-FC Treatment Achieves Significant Anti-Tumor Efficacy in Both Immunodeficient and Immunocompetent Subcutaneous Tumor Models of Lung Cancer
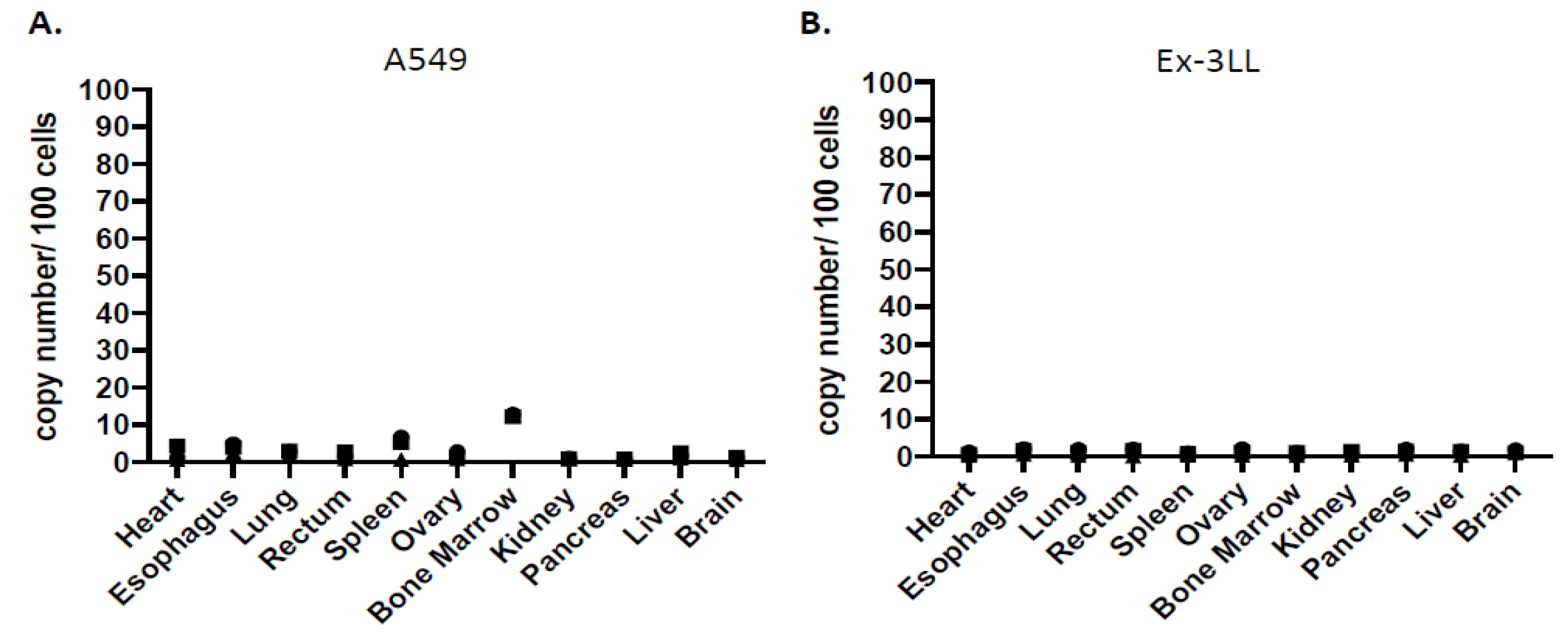
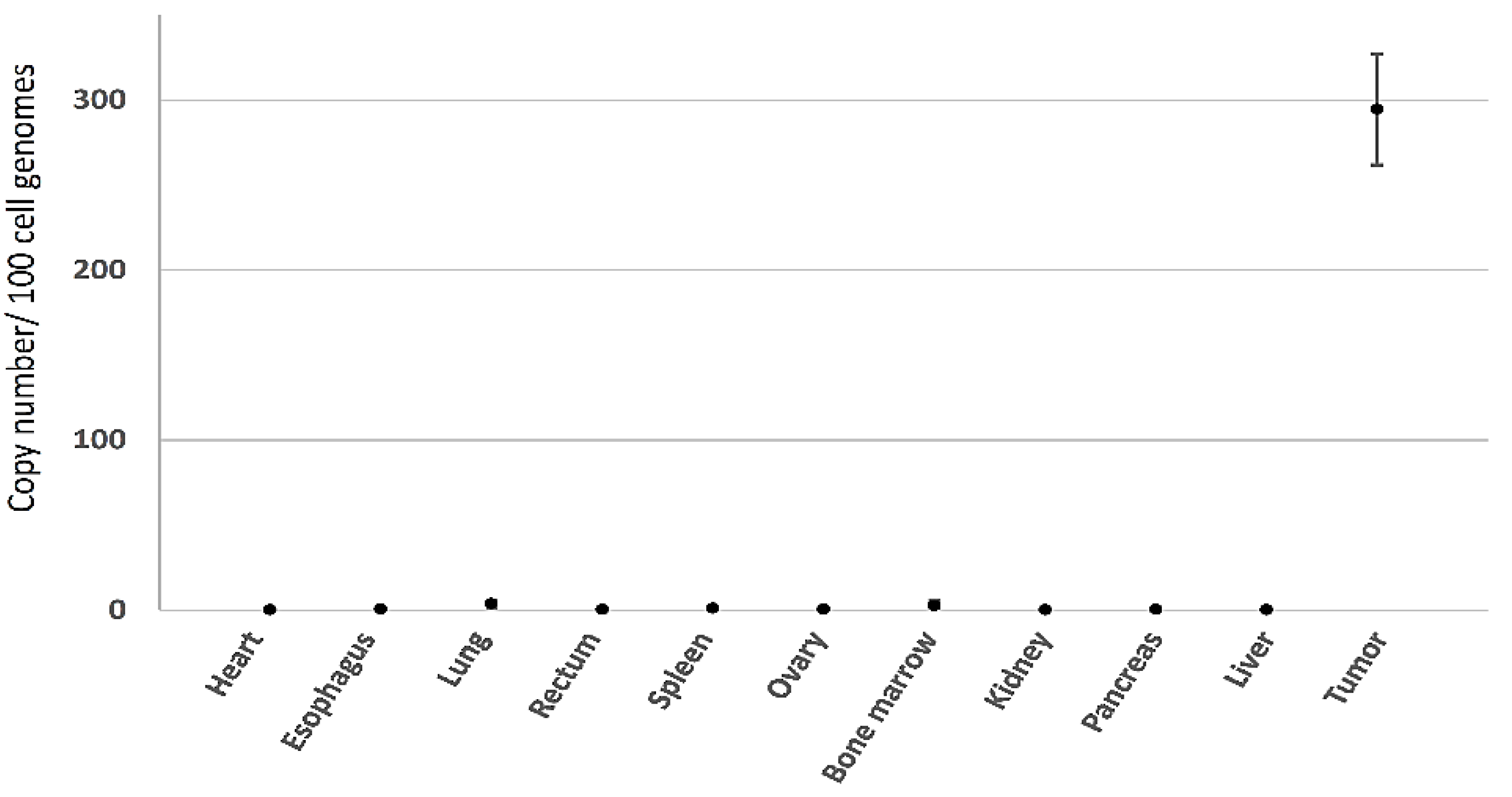
3.2.3. Minimal Systemic Biodistribution of RRV in Normal Tissues after Toca 511/5-FC Treatment in Subcutaneous Tumor Models of Lung Cancer
3.3. Evaluation of RRV in Orthotopic Lung Cancer Pleural Dissemination Models In Vivo
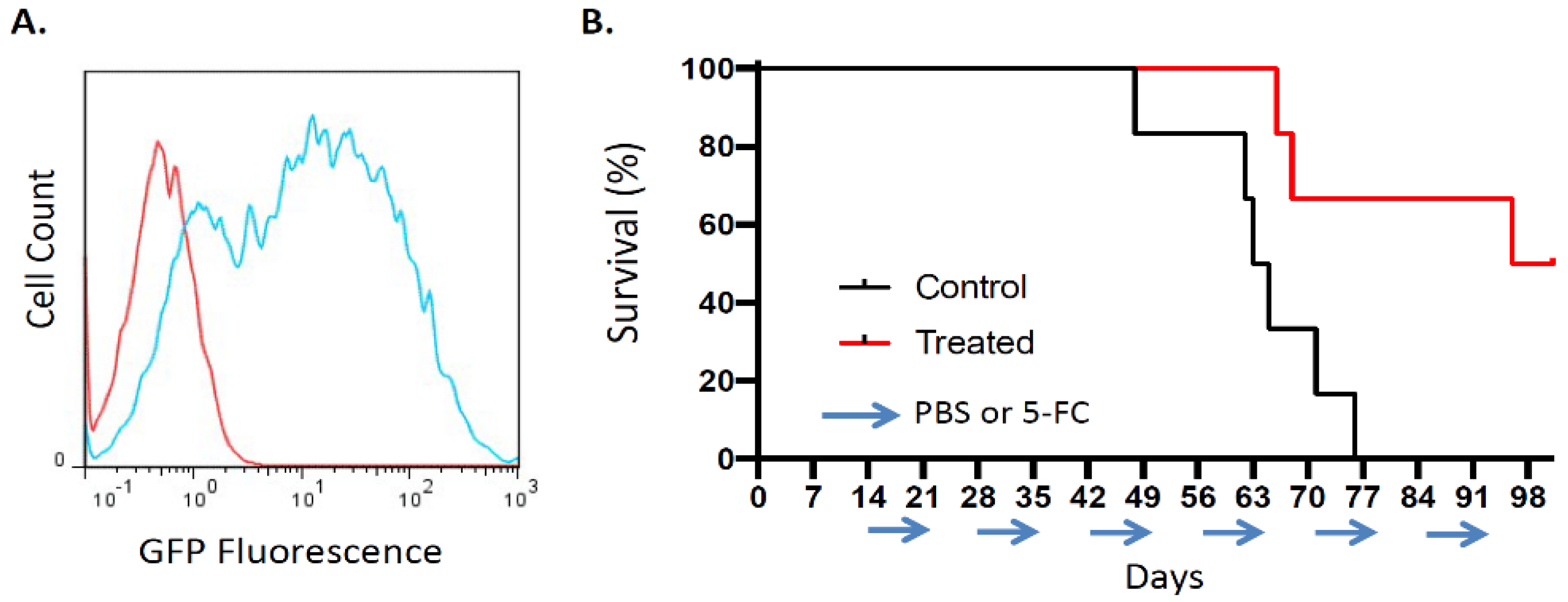
3.3.1. RRV Shows Significant Tumor Transduction after Intrathoracic Injection in an Orthotopic Pleural Dissemination Model of Human Lung Cancer
3.3.2. Toca 511/5-FC Treatment Improved Overall Survival in an Orthotopic Pleural Dissemination Model of Human Lung Cancer
3.3.3. Minimal Systemic Biodistribution of RRV after Toca 511/5-FC Treatment in Orthotopic Pleural Dissemination Model of Human Lung Cancer
3.3.4. Tumor Growth Inhibition over Multiple Prodrug Treatment Cycles Visualized in Real Time by Bioluminescence Imaging after Toca 511/5-FC Treatment in Orthotopic Lung Cancer Pleural Dissemination Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Postmus, P.E.; Brambilla, E.; Chansky, K.; Crowley, J.; Goldstraw, P.; Patz, E.F., Jr.; Yokomise, H.; International Association for the Study of Lung Cancer International Staging Committee, Cancer Research and Biostatistics, Observers to the Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2007, 2, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Aisner, D.L.; Wood, D.E.; Akerley, W.; Bauman, J.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; Dilling, T.J.; Dobelbower, M.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Silvestri, G.A. Palliative Care in Lung Cancer: A Review. Semin. Respir. Crit. Care Med. 2016, 37, 750–759. [Google Scholar] [CrossRef]
- Spiro, S.G.; Rudd, R.M.; Souhami, R.L.; Brown, J.; Fairlamb, D.J.; Gower, N.H.; Maslove, L.; Milroy, R.; Napp, V.; Parmar, M.K.; et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: Improved survival without detriment to quality of life. Thorax 2004, 59, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guerra, H.; Roth, J.A. Gene Therapy for Lung Cancer. Crit. Rev. Oncog. 2016, 21, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Tanaka, N.; Kanazawa, S.; Ohtani, S.; Saijo, Y.; Nukiwa, T.; Yoshimura, K.; Sato, T.; Eto, Y.; Chada, S.; et al. Multicenter phase I study of repeated intratumoral delivery of adenoviral p53 in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 1689–1699. [Google Scholar] [CrossRef]
- Logg, C.R.; Tai, C.K.; Logg, A.; Anderson, W.F.; Kasahara, N. A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors. Hum. Gene Ther. 2001, 12, 921–932. [Google Scholar] [CrossRef]
- Wang, W.; Tai, C.K.; Kasahara, N.; Chen, T.C. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retrovirus vectors. Hum. Gene Ther. 2003, 14, 117–127. [Google Scholar] [CrossRef]
- Hiraoka, K.; Kimura, T.; Logg, C.R.; Kasahara, N. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin. Cancer Res. 2006, 12, 7108–7116. [Google Scholar] [CrossRef]
- Kikuchi, E.; Menendez, S.; Ozu, C.; Ohori, M.; Cordon-Cardo, C.; Logg, C.R.; Kasahara, N.; Bochner, B.H. Highly efficient gene delivery for bladder cancers by intravesically administered replication-competent retroviral vectors. Clin. Cancer Res. 2007, 13, 4511–4518. [Google Scholar] [CrossRef]
- Logg, C.R.; Kasahara, N. Retrovirus-mediated gene transfer to tumors: Utilizing the replicative power of viruses to achieve highly efficient tumor transduction in vivo. Methods Mol. Biol. 2004, 246, 499–525. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.K.; Wang, W.J.; Chen, T.C.; Kasahara, N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol. Ther. 2005, 12, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Kimura, T.; Logg, C.R.; Tai, C.K.; Haga, K.; Lawson, G.W.; Kasahara, N. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007, 67, 5345–5353. [Google Scholar] [CrossRef][Green Version]
- Ostertag, D.; Amundson, K.K.; Espinoza, F.L.; Martin, B.; Buckley, T.; da Silva, A.P.G.; Lin, A.H.; Valenta, D.T.; Perez, O.D.; Ibanez, C.E.; et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro-Oncology 2012, 14, 145–159. [Google Scholar] [CrossRef]
- Hiraoka, K.; Inagaki, A.; Kato, Y.; Huang, T.T.; Mitchell, L.A.; Kamijima, S.; Takahashi, M.; Matsumoto, H.; Hacke, K.; Kruse, C.A.; et al. Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro-Oncology 2017, 19, 918–929. [Google Scholar] [CrossRef]
- Yagiz, K.; Rodriguez-Aguirre, M.E.; Espinoza, F.L.; Montellano, T.T.; Mendoza, D.; Mitchell, L.A.; Ibanez, C.E.; Kasahara, N.; Gruber, H.E.; Jolly, D.J.; et al. A Retroviral Replicating Vector Encoding Cytosine Deaminase and 5-FC Induces Immune Memory in Metastatic Colorectal Cancer Models. Mol. Ther. Oncolytics 2018, 8, 14–26. [Google Scholar] [CrossRef]
- Inoko, K.; Hiraoka, K.; Inagaki, A.; Takahashi, M.; Kushibiki, T.; Hontani, K.; Takano, H.; Sato, S.; Takeuchi, S.; Nakamura, T.; et al. Therapeutic activity of retroviral replicating vector-mediated prodrug activator gene therapy for pancreatic cancer. Cancer Gene Ther. 2018, 25, 184–195. [Google Scholar] [CrossRef]
- Perez, O.D.; Logg, C.R.; Hiraoka, K.; Diago, O.; Burnett, R.; Inagaki, A.; Jolson, D.; Amundson, K.; Buckley, T.; Lohse, D.; et al. Design and selection of Toca 511 for clinical use: Modified retroviral replicating vector with improved stability and gene expression. Mol. Ther. 2012, 20, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra375. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.J.; Zhu, J.J.; Diago, O.R.; Gammon, D.; Haghighi, A.; Lu, G.; Das, A.; Gruber, H.E.; Jolly, D.J.; Ostertag, D. Molecular Analyses Support the Safety and Activity of Retroviral Replicating Vector Toca 511 in Patients. Clin. Cancer Res. 2018, 24, 4680–4693. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Petrecca, K.; Walbert, T.; Butowski, N.; Salacz, M.; Perry, J.; Damek, D.; Bota, D.; Bettegowda, C.; Zhu, J.J.; et al. Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1939–1946. [Google Scholar] [CrossRef]
- Collins, S.A.; Shah, A.; Ostertag, D.; Kasahara, N.; Jolly, D.J. Clinical Development of Retroviral Replicating Vector Toca 511 for Gene Therapy of Cancer. Expert Opin. Biol. Ther. 2021, 21, 1199–1214, online ahead of print. [Google Scholar] [CrossRef]
- Falchook, G.S.; Ahnert, J.R.; Venkat, S.; Donahue, A.; Horner, P.; Thomassen, A.; Accomando, W.; Rodriguez-Aguirre, M.; Bentley, C.; Hogan, D.; et al. Immune modulation after Toca 511 and Toca FC treatment of colorectal cancer patients. J. Clin. Oncol. 2020, 38, 186. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Willett, F.M.; Foye, L.V., Jr.; Roth, M.; Hall, B.E. Combined therapy of inoperable lung carcinoma with 5-fluorouracil and irradiation. Dis. Chest 1961, 39, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef]
- Mendiola, C.; Vaz, M.A. Is capecitabine a new choice of treatment for lung adenocarcinoma? A case report involving partial response in second line of treatment and hypothesis of the biological basis. Clin. Transl. Oncol. 2009, 11, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Paydas, S.; Bicakci, K.; Yavuz, S. Dramatic response with capecitabine after cranial radiation to the brain parenchymal and leptomeningeal metastases from lung cancer. Eur. J. Intern. Med. 2009, 20, 96–99. [Google Scholar] [CrossRef]
- Han, J.Y.; Lee, D.H.; Lee, S.Y.; Park, C.G.; Kim, H.Y.; Lee, H.G.; Lee, J.J.; Kim, H.T.; Lee, J.S. Phase II study of weekly irinotecan plus capecitabine for chemotherapy-naive patients with advanced nonsmall cell lung carcinoma. Cancer 2005, 104, 2759–2765. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Morse, B.; Strosberg, J. Capecitabine and Temozolomide in Advanced Lung Neuroendocrine Neoplasms. Oncologist 2020, 25, e48–e52. [Google Scholar] [CrossRef] [PubMed]
- Kajita, J.; Fuse, E.; Kuwabara, T.; Kobayashi, H. The contribution of cytochrome P450 to the metabolism of tegafur in human liver. Drug Metab. Pharmacokinet. 2003, 18, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, D.C.; Moran, G.R. Mammalian dihydropyrimidine dehydrogenase. Arch. Biochem. Biophys. 2021, 714, 109066. [Google Scholar] [CrossRef] [PubMed]
- Hamada, C.; Tsuboi, M.; Ohta, M.; Fujimura, S.; Kodama, K.; Imaizumi, M.; Wada, H. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: An exploratory analysis from a meta-analysis of six randomized controlled trials. J. Thorac. Oncol. 2009, 4, 1511–1516. [Google Scholar] [CrossRef]
- Kawahara, M. Efficacy of S-1 in non-small cell lung cancer. Expert Opin. Pharmacother. 2014, 15, 1927–1942. [Google Scholar] [CrossRef]
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int. J. Clin. Oncol. 2019, 24, 731–770. [Google Scholar] [CrossRef]
- Nakazawa, S.; Shimizu, K.; Mogi, A.; Kuwano, H. VATS segmentectomy: Past, present, and future. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 81–90. [Google Scholar] [CrossRef]
- Vannucci, F.; Gonzalez-Rivas, D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer 2016, 100, 114–119. [Google Scholar] [CrossRef]
- Kuriyama, S.; Masui, K.; Sakamoto, T.; Nakatani, T.; Kikukawa, M.; Tsujinoue, H.; Mitoro, A.; Yamazaki, M.; Yoshiji, H.; Fukui, H.; et al. Bystander effect caused by cytosine deaminase gene and 5-fluorocytosine in vitro is substantially mediated by generated 5-fluorouracil. Anticancer Res. 1998, 18, 3399–3406. [Google Scholar]
- Pierrefite-Carle, V.; Baqué, P.; Gavelli, A.; Mala, M.; Chazal, M.; Gugenheim, J.; Bourgeon, A.; Milano, G.; Staccini, P.; Rossi, B. Cytosine deaminase/5-fluorocytosine-based vaccination against liver tumors: Evidence of distant bystander effect. J. Natl. Cancer Inst. 1999, 91, 2014–2019. [Google Scholar] [CrossRef]
- Takeuchi, H.; Matano, T. Host factors involved in resistance to retroviral infection. Microbiol. Immunol. 2008, 52, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.; Aguilera, A.N.; Blouch, K.; Ross, S.R. DDX41 Recognizes RNA/DNA Retroviral Reverse Transcripts and Is Critical for In Vivo Control of Murine Leukemia Virus Infection. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Littwitz-Salomon, E.; Schimmer, S.; Dittmer, U. Natural killer T cells contribute to the control of acute retroviral infection. Retrovirology 2017, 14, 5. [Google Scholar] [CrossRef]
- Cornetta, K.; Moen, R.C.; Culver, K.; Morgan, R.A.; McLachlin, J.R.; Sturm, S.; Selegue, J.; London, W.; Blaese, R.M.; Anderson, W.F. Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum. Gene Ther. 1990, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, V.; Hilbert, D.; Wolff, L. Susceptibility and resistance to Moloney murine leukemia virus-induced promonocytic leukemia. Virology 1994, 205, 479–485. [Google Scholar] [CrossRef]
- Li, S.X.; Barrett, B.S.; Heilman, K.J.; Messer, R.J.; Liberatore, R.A.; Bieniasz, P.D.; Kassiotis, G.; Hasenkrug, K.J.; Santiago, M.L. Tetherin promotes the innate and adaptive cell-mediated immune response against retrovirus infection in vivo. J. Immunol. 2014, 193, 306–316. [Google Scholar] [CrossRef]
- Okeoma, C.M.; Petersen, J.; Ross, S.R. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J. Virol. 2009, 83, 3029–3038. [Google Scholar] [CrossRef]
- Nowinski, R.C.; Doyle, T. Decreased immunity to viral antigens and increased expression of endogenous leukemia viruses in athymic (nude) mice. Virology 1977, 77, 429–432. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Espinoza, F.L.; Mendoza, D.; Kato, Y.; Inagaki, A.; Hiraoka, K.; Kasahara, N.; Gruber, H.E.; Jolly, D.J.; Robbins, J.M. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro-Oncology 2017, 19, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Ready, N.; Hellmann, M.D.; Awad, M.M.; Otterson, G.A.; Gutierrez, M.; Gainor, J.F.; Borghaei, H.; Jolivet, J.; Horn, L.; Mates, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J. Clin. Oncol. 2019, 37, 992–1000. [Google Scholar] [CrossRef]
- Lipson, E.J.; Forde, P.M.; Hammers, H.J.; Emens, L.A.; Taube, J.M.; Topalian, S.L. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin. Oncol. 2015, 42, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Longo, D.L.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B., 2nd; Jones, J.A. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann. Palliat Med. 2013, 2, 178–188. [Google Scholar] [CrossRef]
- Group, NSCLC Meta-Analyses Collaborative. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushiya, H.; Hiraoka, K.; Suzuki, T.; Inoko, K.; Inagaki, A.; Niwa, H.; Sasaki, K.; Nakamura, T.; Tsuchikawa, T.; Shichinohe, T.; et al. Retroviral Replicating Vector Toca 511 (Vocimagene Amiretrorepvec) for Prodrug Activator Gene Therapy of Lung Cancer. Cancers 2022, 14, 5820. https://doi.org/10.3390/cancers14235820
Kushiya H, Hiraoka K, Suzuki T, Inoko K, Inagaki A, Niwa H, Sasaki K, Nakamura T, Tsuchikawa T, Shichinohe T, et al. Retroviral Replicating Vector Toca 511 (Vocimagene Amiretrorepvec) for Prodrug Activator Gene Therapy of Lung Cancer. Cancers. 2022; 14(23):5820. https://doi.org/10.3390/cancers14235820
Chicago/Turabian StyleKushiya, Hiroki, Kei Hiraoka, Tomohiro Suzuki, Kazuho Inoko, Akihito Inagaki, Hiroki Niwa, Katsunori Sasaki, Toru Nakamura, Takahiro Tsuchikawa, Toshiaki Shichinohe, and et al. 2022. "Retroviral Replicating Vector Toca 511 (Vocimagene Amiretrorepvec) for Prodrug Activator Gene Therapy of Lung Cancer" Cancers 14, no. 23: 5820. https://doi.org/10.3390/cancers14235820
APA StyleKushiya, H., Hiraoka, K., Suzuki, T., Inoko, K., Inagaki, A., Niwa, H., Sasaki, K., Nakamura, T., Tsuchikawa, T., Shichinohe, T., Jolly, D. J., Kasahara, N., & Hirano, S. (2022). Retroviral Replicating Vector Toca 511 (Vocimagene Amiretrorepvec) for Prodrug Activator Gene Therapy of Lung Cancer. Cancers, 14(23), 5820. https://doi.org/10.3390/cancers14235820






