Feasibility of Intratumoral Anti-PD1 as Treatment of Human Basal Cell Carcinoma: An Explorative Study with Adjuvant Ablative Fractional Laser
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setup
2.2. Interventions
2.3. Tumoral Drug Quantification
2.4. Evaluation of Immunological Response
Histological Tissue Processing, Immunohistochemistry, and Digital Photo Analysis
2.5. Tumor Response
2.6. Safety
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Local Tumor Immune Cell Infiltration
3.3. PD1/PD-L1
3.4. Tumor Response
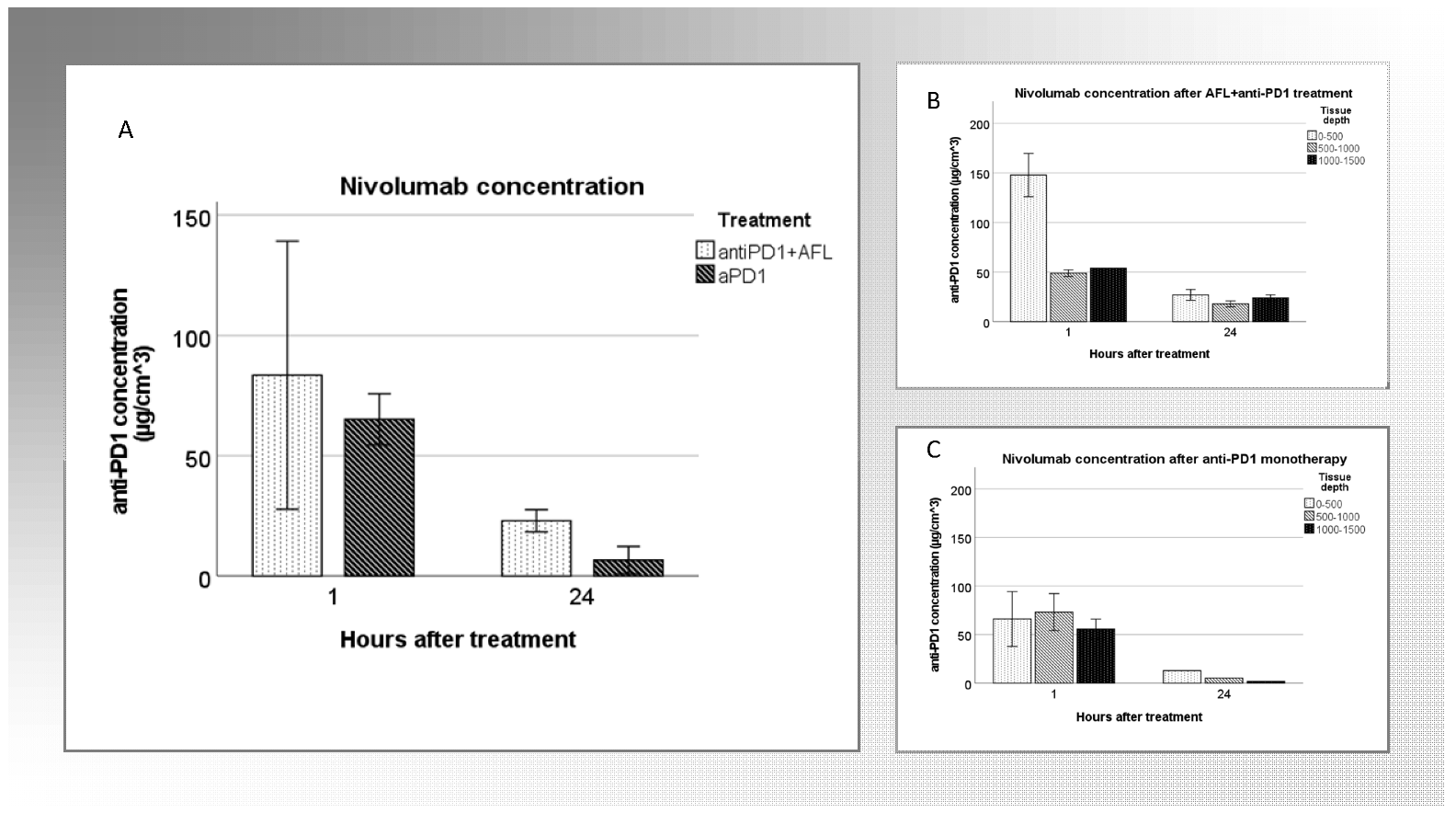
3.5. Tumoral Drug Concentration
3.6. Safety
3.6.1. Local Skin Reactions
3.6.2. Systemic Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICI | immune check point inhibitor |
| AFL | ablative fractional laser |
| BCC | basal cell carcinoma |
| Anti-PD1 | anti-programmed death-1 |
| MM | malignant melanoma |
| TME | tumor microenvironment |
| TILs | tumor infiltrating lymphocytes |
| SCC | squamous cell carcinoma |
| KC | keratinocyte carcinoma |
| IHC | immunohistochemistry |
| CR | complete remission |
| LSR | local skin reactions |
| AE | adverse event |
References
- Holm, A.S.; Nissen, C.V.; Wulf, H.C. Basal cell carcinoma is as common as the sum of all other cancers: Implications for treatment capacity. Acta Derm. Venereol. 2016, 96, 505–509. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Donia, M.; Ellebaek, E.; Øllegaard, T.H.; Duval, L.; Aaby, J.B.; Hoejberg, L.; Køhler, U.H.; Schmidt, H.; Bastholt, L.; Svane, I.M. The real-world impact of modern treatments on the survival of patients with metastatic melanoma. Eur. J. Cancer 2019, 108, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, G.; Capone, M.; Sabbatino, F.; Di Mauro, A.; Cantile, M.; Cerrone, M.; Madonna, G.; Grimaldi, A.M.; Mallardo, D.; Palla, M.; et al. The ratio of grzb+ − foxp3+ over cd3+ t cells as a potential predictor of response to nivolumab in patients with metastatic melanoma. Cancers 2021, 13, 2325. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. Tumor Microenvironment Nicole. Curr. Biol. 2020, 30, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Duggan, S. Cemiplimab: First Global Approval. Drugs 2018, 78, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.N.S.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Olesen, U.H.; Wiinberg, M.; Lerche, C.M.; Jæhger, D.E.; Andresen, T.L.; Haedersdal, M. Anti-PD-1 Therapy with Adjuvant Ablative Fractional Laser Improves Anti-Tumor Response in Basal Cell Carcinomas. Cancers 2021, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fontenete, S.; Lerche, C.M.; Paasch, U.; Perez-Moreno, M.; Olesen, U.H.; Haedersdal, M. Tumor Clearance and Immune Cell Recruitment in UV-Induced Murine Squamous Cell Carcinoma Exposed to Ablative Fractional Laser and Imiquimod Treatment. Lasers Surg. Med. 2021, 53, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.L.; Hendel, K.K.; Persson, D.P.; Husted, S.; Olesen, U.H.; Haedersdal, M. Topical delivery of PD-1 inhibitors with laser-assisted passive diffusion and active intradermal injection: Investigation of cutaneous pharmacokinetics and biodistribution patterns. Lasers Surg. Med. 2022, 54, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Hurkmans, D.P.; Basak, E.A.; Van Dijk, T.; Mercieca, D.; Schreurs, M.W.J.; Wijkhuis, A.J.M.; Bins, S.; Hoop, E.O.; Debets, R.; Joerger, M.; et al. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J. Immunother. Cancer 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaporis, H.G.; Guttman-Yassky, E.; Lowes, M.A.; Haider, A.S.; Fuentes-Duculan, J.; Darabi, K.; Whynot-Ertelt, J.; Khatcherian, A.; Cardinale, I.; Novitskaya, I.; et al. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J. Investig. Dermatol. 2007, 127, 2391–2398. [Google Scholar] [CrossRef]
- Omland, S.H.; Nielsen, P.; Gjerdrum, L.; Gniadecki, R. Immunosuppressive environment in basal cell carcinoma: The role of regulatory T-cells (T-regs). Acta Derm. Venereol. 2016, 96, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Marra, A.; Liguori, L.; Scognamiglio, G.; Fusciello, C.; Botti, G.; Ferrone, S.; Pepe, S. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: A case report and review of the literature. J. Immunother. Cancer 2018, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.; Scheper, W.; Kvistborg, P. Cancer Neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold tumors: A therapeutic challenge for immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Golden, E.B.; Apetoh, L. Radiotherapy and Immunogenic Cell Death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Olesen, U.H.; Clergeaud, G.; Hendel, K.K.; Yeung, K.; Lerche, C.M.; Andresen, T.L.; Haedersdal, M. Enhanced and Sustained Cutaneous Delivery of Vismodegib by Ablative Fractional Laser and Microemulsion Fomulation. J. Investig. Dermatol. 2020, 140, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, M.; Cunningham, T.J.; Demehri, S.; Manstein, D. Fractional Laser Releases Tumor-Associated Antigens in Poorly Immunogenic Tumor and Induces Systemic Immunity. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Shi, L.; Zhang, F.; Zhou, F.; Zhang, L.; Wang, B.; Wang, P.; Zhang, Y.; Zhang, D.; Zhang, G.; et al. Laser immunotherapy for cutaneous squamous cell carcinoma with optimal thermal effects to enhavnce tumour immunogenecity. Int. J. Hyperth. 2018, 34, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yang, J.; Zhang, Y.; Liu, M.; Lang, M.L.; Li, M.; Chen, W.R. Local Phototherapy Synergizes with Immunoadjuvant for Treatment of Pancreatic Cancer through Induced Immunogeneic Tumor Vaccine. Clin. Cancer Res. 2018, 24, 5335–5346. [Google Scholar] [CrossRef]
- Lo, J.A.; Kawakubo, M.; Juneja, V.R.; Su, M.Y.; Erlich, T.H.; LaFleur, M.W.; Kemeny, L.V.; Rashid, M.; Malehmir, M.; Rabi, S.A.; et al. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci. Transl. Med. 2021, 13, eabd8636. [Google Scholar] [CrossRef] [PubMed]
- Omland, S.H.; Wenande, E.C.; Svane, I.M.; Tam, J.; Olesen, U.H.; Hædersdal, M. Laser immunotherapy: A potential treatment modality for keratinocyte carcinoma. Cancers 2021, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Omland, S.H.; Hamrouni, A.; Gniadecki, R. High diversity of the T-cell receptor repertoire of tumor-infiltrating lymphocytes in basal cell carcinoma. Exp. Dermatol. 2017, 26, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Schröter, U.; Höxtermann, S.; Susok, L.; Stockfleth, E.; Becker, J.C. Decline of programmed death-1-positive circulating T regulatory cells predicts more favourable clinical outcome of patients with melanoma under immune checkpoint blockade. Br. J. Dermatol. 2020, 182, 1214–1220. [Google Scholar] [CrossRef]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 immunotherapy does not deplete Foxp3 þ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 2019, 25, 1233–1238. [Google Scholar] [CrossRef]
- Topalian, S.; Stephen, H.F.; Brahmer, J.; Gettinger, S.N.; Schmith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]



| Baseline, Week 0 | Visit 2, Week 3 | Visit 3, Week 4 | Visit 4, Week 15 |
|---|---|---|---|
|
|
|
|
| Patients/Tumors | Age, Mean | Sex | BCC Subtype | Anatomical Localization |
|---|---|---|---|---|
| Overall, Patients, n = 28 BCC, n = 39 | 72 | F: 10, M: 18 | Nodular, n =15 Superficial, n = 13 Mixed, n = 11 | Trunk, n = 28 Extremities, n = 9 Neck, n = 2 |
| Anti-PD1+AFL, Patients, n = 10 BCC, n = 14 | 73 | F: 3, M: 7 | Nodular, n = 7 Superficial, n = 3 Mixed, n = 4 | Trunk, n = 9 Extremities, n = 4 Neck, n = 1 |
| Anti-PD1, Patients, n = 9 BCC, n = 15 | 72 | F: 2, M: 7 | Nodular, n = 2 Superficial, n = 8 Mixed, n = 5 | Trunk, n = 11 Extremities, n = 3 Neck, n = 1 |
| AFL, Patients, n = 9 BCC, n = 10 | 70 | F: 5, M: 4 | Nodular, n = 6 Superficial, n = 2 Mixed, n = 2 | Trunk, n = 8 Extremities, n = 2 Neck, n = 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omland, S.H.; Ejlertsen, J.S.; Krustrup, D.; Christensen, R.L.; Svane, I.M.; Olesen, U.H.; Hædersdal, M. Feasibility of Intratumoral Anti-PD1 as Treatment of Human Basal Cell Carcinoma: An Explorative Study with Adjuvant Ablative Fractional Laser. Cancers 2022, 14, 5815. https://doi.org/10.3390/cancers14235815
Omland SH, Ejlertsen JS, Krustrup D, Christensen RL, Svane IM, Olesen UH, Hædersdal M. Feasibility of Intratumoral Anti-PD1 as Treatment of Human Basal Cell Carcinoma: An Explorative Study with Adjuvant Ablative Fractional Laser. Cancers. 2022; 14(23):5815. https://doi.org/10.3390/cancers14235815
Chicago/Turabian StyleOmland, Silje Haukali, Jacob Secher Ejlertsen, Dorrit Krustrup, Rikke Louise Christensen, Inge Marie Svane, Uffe Hoegh Olesen, and Merete Hædersdal. 2022. "Feasibility of Intratumoral Anti-PD1 as Treatment of Human Basal Cell Carcinoma: An Explorative Study with Adjuvant Ablative Fractional Laser" Cancers 14, no. 23: 5815. https://doi.org/10.3390/cancers14235815
APA StyleOmland, S. H., Ejlertsen, J. S., Krustrup, D., Christensen, R. L., Svane, I. M., Olesen, U. H., & Hædersdal, M. (2022). Feasibility of Intratumoral Anti-PD1 as Treatment of Human Basal Cell Carcinoma: An Explorative Study with Adjuvant Ablative Fractional Laser. Cancers, 14(23), 5815. https://doi.org/10.3390/cancers14235815







