Galectins in Esophageal Cancer: Current Knowledge and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
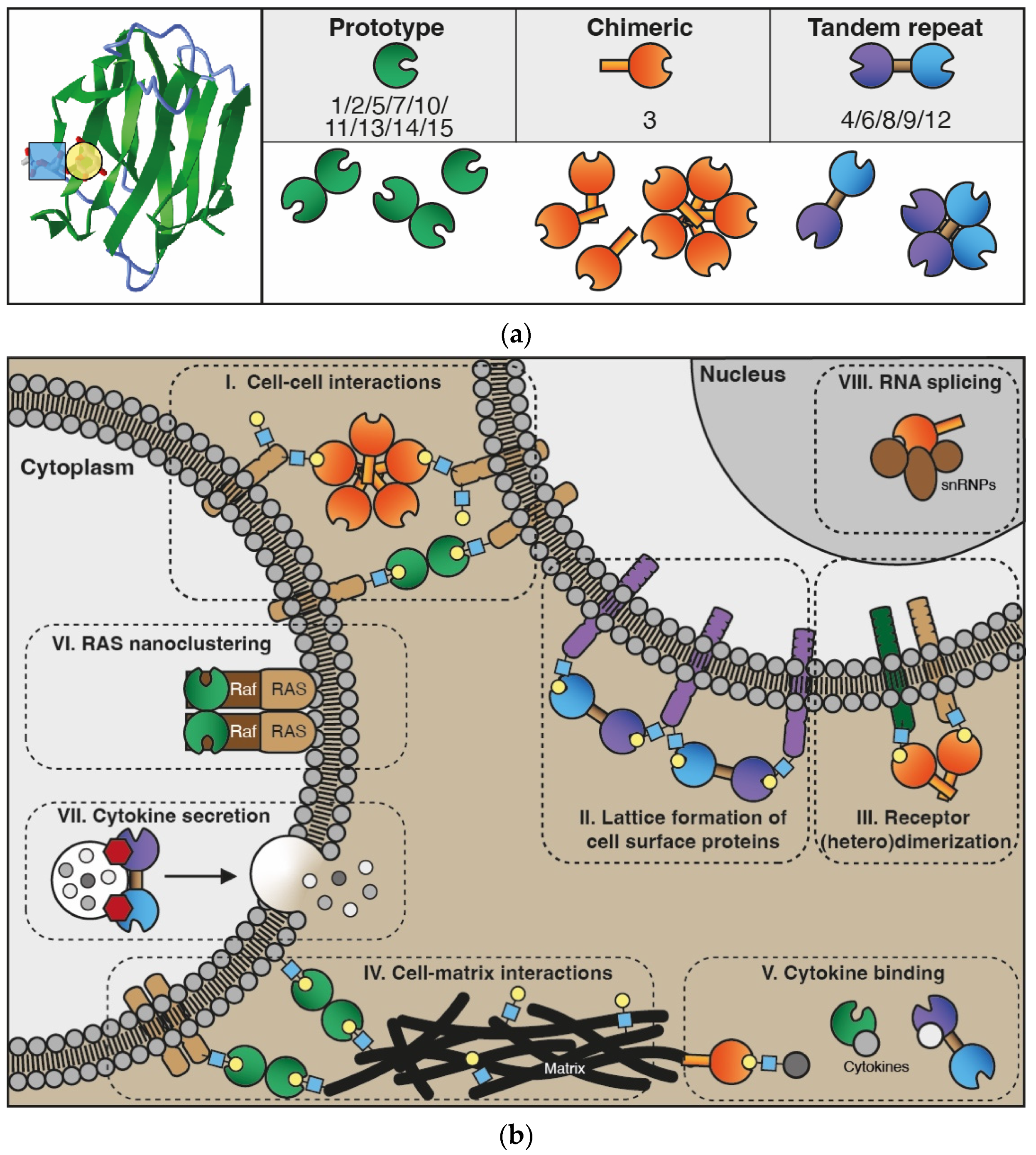
2. The Galectin Protein Family
3. Galectins in Esophageal Cancer
3.1. Galectin-1
3.2. Galectin-3
3.3. Galectin-7
3.4. Galectin-9
4. Summary and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Prim. 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Nowicki-Osuch, K.; Zhuang, L.; Jammula, S.; Bleaney, C.W.; Mahbubani, K.T.; Devonshire, G.; Katz-Summercorn, A.; Eling, N.; Wilbrey-Clark, A.; Madissoon, E.; et al. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science 2021, 373, 760–767. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaabi, A.; van der Post, R.S.; van der Werf, L.R.; Wijnhoven, B.P.L.; Rosman, C.; Hulshof, M.C.C.M.; van Laarhoven, H.W.M.; Verhoeven, R.H.A.; Siersema, P.D. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long-term outcome of esophageal cancer: A population-based study. Acta Oncol. 2021, 60, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; Harrasser, M.; de Klerk, L.K.; van Schooten, T.S.; van Grieken, N.C.T.; Eken, M.; Grifhorst, M.S.; Pocorni, N.; Jordanova, E.S.; van Berge Henegouwen, M.I.; et al. Pre-treatment tumor-infiltrating T cells influence response to neoadjuvant chemoradiotherapy in esophageal adenocarcinoma. Oncoimmunology 2021, 10, 1954807. [Google Scholar] [CrossRef]
- de Klerk, L.K.; Goedegebuure, R.S.A.; van Grieken, N.C.T.; van Sandick, J.W.; Cats, A.; Stiekema, J.; van der Kaaij, R.T.; Farina Sarasqueta, A.; van Engeland, M.; Jacobs, M.A.J.M.; et al. Molecular profiles of response to neoadjuvant chemoradiotherapy in oesophageal cancers to develop personalized treatment strategies. Mol. Oncol. 2021, 15, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Kuo, T.T.; Lo, C.C.; Cheng, W.C.; Chang, W.C.; Tseng, G.C.; Bai, S.T.; Huang, Y.K.; Hsieh, C.Y.; Hsu, H.S.; et al. ADAM9 functions as a transcriptional regulator to drive angiogenesis in esophageal squamous cell carcinoma. Int. J. Biol. Sci. 2021, 17, 3898–3910. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Kaneko, K.; Konishi, K.; Ito, H.; Yamamoto, T.; Katagiri, A.; Muramoto, T.; Yano, Y.; Kobayashi, Y.; Oyama, T.; et al. The onset of angiogenesis in a multistep process of esophageal squamous cell carcinoma. Front Biosci. 2009, 14, 3872–3878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the hallmarks of cancer: Galectins as multifunctional mediators of tumor progression. J. Exp. Med. 2020, 217, e20182041. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Rabinovich, G.A.; Griffioen, A.W. Vascular galectins: Regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013, 24, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Blidner, A.G.; Méndez-Huergo, S.P.; Cagnoni, A.J.; Rabinovich, G.A. Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett. 2015, 589, 3407–3418. [Google Scholar] [CrossRef] [PubMed]
- Cerliani, J.P.; Blidner, A.G.; Toscano, M.A.; Croci, D.O.; Rabinovich, G.A. Translating the ‘Sugar Code’ into Immune and Vascular Signaling Programs. Trends. Biochem. Sci. 2017, 42, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Aanhane, E.; Schulkens, I.A.; Heusschen, R.; Castricum, K.; Leffler, H.; Griffioen, A.W.; Thijssen, V.L. Different angioregulatory activity of monovalent galectin-9 isoforms. Angiogenesis 2018, 21, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Barkan, B.; Shoji, H.; Aries, I.M.; Mathieu, V.; Deltour, L.; Hackeng, T.M.; Kiss, R.; Kloog, Y.; Poirier, F.; et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010, 70, 6216–6224. [Google Scholar] [CrossRef] [PubMed]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Ilarregui, J.M.; Croci, D.O.; Bianco, G.A.; Toscano, M.A.; Salatino, M.; Vermeulen, M.E.; Geffner, J.R.; Rabinovich, G.A. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 2009, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Yasinska, I.M.; Meyer, N.H.; Schlichtner, S.; Hussain, R.; Siligardi, G.; Casely-Hayford, M.; Fiedler, W.; Wellbrock, J.; Desmet, C.; Calzolai, L.; et al. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front Immunol. 2020, 11, 580557. [Google Scholar] [CrossRef] [PubMed]
- Compagno, D.; Tiraboschi, C.; Garcia, J.D.; Rondón, Y.; Corapi, E.; Velazquez, C.; Laderach, D.J. Galectins as Checkpoints of the Immune System in Cancers, Their Clinical Relevance, and Implication in Clinical Trials. Biomolecules 2020, 10, 750. [Google Scholar] [CrossRef]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Blaževitš, O.; Mideksa, Y.G.; Šolman, M.; Ligabue, A.; Ariotti, N.; Nakhaeizadeh, H.; Fansa, E.K.; Papageorgiou, A.C.; Wittinghofer, A.; Ahmadian, M.R.; et al. Galectin-1 dimers can scaffold Raf-effectors to increase H-ras nanoclustering. Sci. Rep. 2016, 6, 24165. [Google Scholar] [CrossRef]
- Santalla Méndez, R.; Furones, A.R.; Classens, R.; Haverdil, M.; Capdevila, M.C.; van Duffelen, A.; Beest, M.T.; Spruijt, C.G.; Vermeulen, M.; van Spriel, A.B.; et al. Galectin-9 interacts with Vamp-3 to regulate cytokine secretion in dendritic cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim. Biophys. Acta 2010, 1800, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids. Res. 2001, 29, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.J.; Wang, W.; Wang, J.L. Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconj J. 2004, 19, 499–506. [Google Scholar] [CrossRef]
- Miller, M.C.; Ludwig, A.K.; Wichapong, K.; Kaltner, H.; Kopitz, J.; Gabius, H.J.; Mayo, K.H. Adhesion/growth-regulatory galectins tested in combination: Evidence for formation of hybrids as heterodimers. Biochem. J. 2018, 475, 1003–1018. [Google Scholar] [CrossRef]
- Sanjurjo, L.; Broekhuizen, E.C.; Koenen, R.R.; Thijssen, V.L.J.L. Galectokines: The Promiscuous Relationship between Galectins and Cytokines. Biomolecules 2022, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, N.; Sun, Y.J.; Liu, K.-F.; Gilcrease, M.Z.; Schober, W.; Nangia-Makker, P.; Raz, A.; Bresalier, R.S. Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J. Biol. Chem. 2007, 282, 21337–21348. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Hiremath, G.; Wiktorowicz, J.E.; Soman, K.V.; Straub, C.; Nance, C.; Quintanilla, N.; Pazdrak, K.; Thakkar, K.; Olive, A.P.; et al. Proteomic Analysis in Esophageal Eosinophilia Reveals Differential Galectin-3 Expression and S-Nitrosylation. Digestion 2016, 93, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, J.; Liu, X.; Li, L.; Zheng, J. Cleavage and phosphorylation: Important post-translational modifications of galectin-3. Cancer Metastasis Rev. 2017, 36, 367–374. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kayser, K.; Hauck, E.; André, S.; Bovin, N.V.; Kaltner, H.; Banach, L.; Lancaster, E.; Gabius, H.J. Expression of endogenous lectins (galectins, receptors for ABH-epitopes) and the MIB-1 antigen in esophageal carcinomas and their syntactic structure analysis in relation to post-surgical tumor stage and lymph node involvement. Anticancer. Res. 2001, 21, 1439–1444. [Google Scholar] [PubMed]
- Li, S.H.; Chen, Y.H.; Lu, H.I.; Lo, C.M.; Huang, C.C.; Wang, Y.M.; Huang, E.Y. Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers 2021, 13, 3147. [Google Scholar] [CrossRef]
- Hoshino, I.; Nabeya, Y.; Takiguchi, N.; Gunji, H.; Ishige, F.; Iwatate, Y.; Kuwajima, A.; Shiratori, F.; Okada, R.; Shimada, H. Inducing multiple antibodies to treat squamous cell esophageal carcinoma. BMC Cancer 2020, 20, 1007. [Google Scholar] [CrossRef]
- Nanami, T.; Hoshino, I.; Shiratori, F.; Yajima, S.; Oshima, Y.; Suzuki, T.; Ito, M.; Hiwasa, T.; Kuwajima, A.; Shimada, H. Prevalence of serum galectin-1 autoantibodies in seven types of cancer: A potential biomarker. Mol. Clin. Oncol. 2021, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhou, C.; Lou, X.; Xiao, Z.; Zhu, H.; Wang, Q.; Wang, Y.; Lu, N.; He, S.; Zhan, Q.; et al. PTTG overexpression promotes lymph node metastasis in human esophageal squamous cell carcinoma. Cancer Res. 2009, 69, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yan, M.; Wu, W.; Lv, P.; Wang, J.; Huo, Y.; Lou, Y.; Ma, X.; Chang, J.; Guan, F.; et al. ESCCAL-1 promotes cell-cycle progression by interacting with and stabilizing galectin-1 in esophageal squamous cell carcinoma. NPJ Precis. Oncol. 2022, 6, 12. [Google Scholar] [CrossRef]
- Kang, S.Y.; Han, J.H.; Lee, K.J.; Choi, J.-H.; Park, J.I.; Kim, H.I.; Lee, H.-W.; Jang, J.H.; Park, J.S.; Kim, H.C.; et al. Low expression of Bax predicts poor prognosis in patients with locally advanced esophageal cancer treated with definitive chemoradiotherapy. Clin. Cancer Res. 2007, 13, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Noguchi, T.; Takeno, S.; Takahashi, Y.; Fumoto, S.; Kawahara, K. Impact of nuclear galectin-3 expression on histological differentiation and vascular invasion in patients with esophageal squamous cell carcinoma. Oncol. Rep. 2005, 13, 235–239. [Google Scholar] [PubMed]
- Balasubramanian, K.; Vasudevamurthy, R.; Venkateshaiah, S.U.; Thomas, A.; Vishweshwara, A.; Dharmesh, S.M. Galectin-3 in urine of cancer patients: Stage and tissue specificity. J. Cancer Res. Clin. Oncol. 2009, 135, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Song, X.; Xie, J.; Xu, D.; Liu, F.; Yu, X.; Tian, Y.; Liu, Z.; Qiao, L.; Zhang, J. Effect of galectin-3 on the behavior of Eca-109 human esophageal cancer cells. Mol. Med. Rep. 2015, 11, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, N.; Xie, J.; Luo, H.; Zhang, J.; Deng, G.; Li, Y.; Zhang, J. Gene silencing of galectin-3 changes the biological behavior of Eca109 human esophageal cancer cells. Mol. Med. Rep. 2016, 13, 160–166. [Google Scholar] [CrossRef]
- Cui, G.; Cui, M.; Li, Y.; Liang, Y.; Li, W.; Guo, H.; Zhao, S. Galectin-3 knockdown increases gefitinib sensitivity to the inhibition of EGFR endocytosis in gefitinib-insensitive esophageal squamous cancer cells. Med. Oncol. 2015, 32, 124. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Zheng, C.C.; Huang, Y.N.; Chen, W.Y.; Yang, Q.S.; Ren, J.Y.; Wang, Y.M.; He, Q.Y.; Liao, H.X.; Li, B. Synephrine Hydrochloride Suppresses Esophageal Cancer Tumor Growth and Metastatic Potential through Inhibition of Galectin-3-AKT/ERK Signaling. J. Agric. Food Chem. 2018, 66, 9248–9258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ding, M.; Yu, M.-L.; Feng, M.-X.; Tan, L.-J.; Zhao, F.-K. Identification of galectin-7 as a potential biomarker for esophageal squamous cell carcinoma by proteomic analysis. BMC Cancer 2010, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Ma, J.; Li, W.; Zhao, L.; Gao, Q.; Mai, L. T-cell immunoglobulin and mucin domain-containing protein-3 and galectin-9 protein expression: Potential prognostic significance in esophageal squamous cell carcinoma for Chinese patients. Oncol. Lett. 2017, 14, 8007–8013. [Google Scholar] [CrossRef]
- Akashi, E.; Fujihara, S.; Morishita, A.; Tadokoro, T.; Chiyo, T.; Fujikawa, K.; Kobara, H.; Mori, H.; Iwama, H.; Okano, K.; et al. Effects of galectin-9 on apoptosis, cell cycle and autophagy in human esophageal adenocarcinoma cells. Oncol. Rep. 2017, 38, 506–514. [Google Scholar] [CrossRef]
- Chiyo, T.; Fujita, K.; Iwama, H.; Fujihara, S.; Tadokoro, T.; Ohura, K.; Matsui, T.; Goda, Y.; Kobayashi, N.; Nishiyama, N.; et al. Galectin-9 Induces Mitochondria-Mediated Apoptosis of Esophageal Cancer In Vitro and In Vivo in a Xenograft Mouse Model. Int. J. Mol. Sci. 2019, 20, 2634. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Takemasa, I.; Kaneko, N.; Yokoyama, Y.; Matsuo, E.; Iwasa, S.; Mori, M.; Matsuura, N.; Monden, M.; Nishimura, O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol. Rep. 2011, 25, 1217–1226. [Google Scholar] [CrossRef]
- Verschuere, T.; Van Woensel, M.; Fieuws, S.; Lefranc, F.; Mathieu, V.; Kiss, R.; Van Gool, S.W.; De Vleeschouwer, S. Altered galectin-1 serum levels in patients diagnosed with high-grade glioma. J. Neurooncol. 2013, 115, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stroes, C.I.; Schokker, S.; Khurshed, M.; van der Woude, S.O.; Mathôt, R.A.; Slingerland, M.; de Vos-Geelen, J.; Zucchetti, M.; Matteo, C.; van Dijk, E.; et al. A phase Ib/II study of regorafenib and paclitaxel in patients with beyond first-line advanced esophagogastric carcinoma (REPEAT). Adv. Med. Oncol. 2022, 14, 17588359221109196. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Shimada, Y.; Kan, T.; David, S.; Cheng, Y.; Mori, Y.; Agarwal, R.; Paun, B.; Jin, Z.; Olaru, A.; et al. Pituitary tumor-transforming 1 increases cell motility and promotes lymph node metastasis in esophageal squamous cell carcinoma. Cancer Res. 2008, 68, 3214–3224. [Google Scholar] [CrossRef]
- Chen, F.F.; Zhang, S.R.; Peng, H.; Chen, Y.Z.; Cui, X.B. Integrative genomics analysis of hub genes and their relationship with prognosis and signaling pathways in esophageal squamous cell carcinoma. Mol. Med. Rep. 2019, 20, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Baum, L.G. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Investig. 2006, 86, 578–590. [Google Scholar] [CrossRef]
- Qi, Y.; Chiu, J.F.; Wang, L.; Kwong, D.L.; He, Q.Y. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics 2005, 5, 2960–2971. [Google Scholar] [CrossRef]
- Ogura, M.; Takeuchi, H.; Kawakubo, H.; Nishi, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Saikawa, Y.; Omori, T.; et al. Clinical significance of CXCL-8/CXCR-2 network in esophageal squamous cell carcinoma. Surgery 2013, 154, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, K.C.; Jeon, S.M.; Ohn, T.B.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Abrogation of galectin-4 expression promotes tumorigenesis in colorectal cancer. Cell Oncol. 2013, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Rao, P.S.; Thirumala, S.; Rao, U.S. Galectin-4 functions as a tumor suppressor of human colorectal cancer. Int. J. Cancer 2011, 129, 799–809. [Google Scholar] [CrossRef]
- Hippo, Y.; Yashiro, M.; Ishii, M.; Taniguchi, H.; Tsutsumi, S.; Hirakawa, K.; Kodama, T.; Aburatani, H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001, 61, 889–895. [Google Scholar]
- Nagy, N.; Bronckart, Y.; Camby, I.; Legendre, H.; Lahm, H.; Kaltner, H.; Hadari, Y.; Van Ham, P.; Yeaton, P.; Pector, J.C.; et al. Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor. Gut 2002, 50, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Legendre, H.; Engels, O.; Andre, S.; Kaltner, H.; Wasano, K.; Zick, Y.; Pector, J.C.; Decaestecker, C.; Gabius, H.J.; et al. Refined prognostic evaluation in colon carcinoma using immunohistochemical galectin fingerprinting. Cancer 2003, 97, 1849–1858. [Google Scholar] [CrossRef]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Cardenas Delgado, V.M.; Nugnes, L.G.; Colombo, L.L.; Troncoso, M.F.; Fernandez, M.M.; Malchiodi, E.L.; Frahm, I.; Croci, D.O.; Compagno, D.; Rabinovich, G.A.; et al. Modulation of endothelial cell migration and angiogenesis: A novel function for the “tandem-repeat” lectin galectin-8. Faseb. J. 2011, 25, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Méndez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; García-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-Dependent Lectin-Receptor Interactions Preserve Angiogenesis in Anti-VEGF Refractory Tumors. Cell 2014, 156, 744–758. [Google Scholar] [CrossRef]
- Dos Santos, S.N.; Sheldon, H.; Pereira, J.X.; Paluch, C.; Bridges, E.M.; El-Cheikh, M.C.; Harris, A.L.; Bernardes, E.S. Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget 2017, 8, 49484–49501. [Google Scholar] [CrossRef]
- Shatz-Azoulay, H.; Vinik, Y.; Isaac, R.; Kohler, U.; Lev, S.; Zick, Y. The Animal Lectin Galectin-8 Promotes Cytokine Expression and Metastatic Tumor Growth in Mice. Sci. Rep. 2020, 10, 7375. [Google Scholar] [CrossRef]
- Colomb, F.; Wang, W.; Simpson, D.; Zafar, M.; Beynon, R.; Rhodes, J.M.; Yu, L.G. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 2017, 292, 8381–8389. [Google Scholar] [CrossRef]
- Zhao, Q.; Barclay, M.; Hilkens, J.; Guo, X.; Barrow, H.; Rhodes, J.M.; Yu, L.G. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 2010, 9, 154. [Google Scholar] [CrossRef]
- Liu, F.-T.; Yang, R.-Y.; Saegusa, J.; Chen, H.-Y.; Hsu, D.K. Galectins in regulation of apoptosis. Adv. Exp. Med. Biol. 2011, 705, 431–442. [Google Scholar] [CrossRef]
- Barkan, B.; Cox, A.D.; Kloog, Y. Ras inhibition boosts galectin-7 at the expense of galectin-1 to sensitize cells to apoptosis. Oncotarget 2013, 4, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Biron-Pain, K.; Grosset, A.-A.; Poirier, F.; Gaboury, L.; St-Pierre, Y. Expression and functions of galectin-7 in human and murine melanomas. PLoS ONE 2013, 8, e63307. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Shao, J.; Qiao, M.; Luo, Z.; Deng, X.; Ke, Q.; Dong, X.; Shen, L. Identification of LCA-binding Glycans as a Novel Biomarker for Esophageal Cancer Metastasis using a Lectin Array-based Strategy. J. Cancer 2020, 11, 4736–4745. [Google Scholar] [CrossRef] [PubMed]
- Jouve, N.; Despoix, N.; Espeli, M.; Gauthier, L.; Cypowyj, S.; Fallague, K.; Schiff, C.; Dignat-George, F.; Vély, F.; Leroyer, A.S. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J. Biol. Chem. 2013, 288, 2571–2579. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, X.; Qiu, L.; Peng, F.; Geng, S.; Shen, L.; Luo, Z. Knockdown of C1GalT1 inhibits radioresistance of human esophageal cancer cells through modifying β1-integrin glycosylation. J. Cancer 2018, 9, 2666–2677. [Google Scholar] [CrossRef]
- Moiseeva, E.P.; Williams, B.; Goodall, A.H.; Samani, N.J. Galectin-1 interacts with beta-1 subunit of integrin. Biochem. Biophys. Res. Commun. 2003, 310, 1010–1016. [Google Scholar] [CrossRef]
- Margadant, C.; van den Bout, I.; van Boxtel, A.L.; Thijssen, V.L.; Sonnenberg, A. Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration. J. Biol. Chem. 2012, 287, 44684–44693. [Google Scholar] [CrossRef]
- Carcamo, C.; Pardo, E.; Oyanadel, C.; Bravo-Zehnder, M.; Bull, P.; Caceres, M.; Martinez, J.; Massardo, L.; Jacobelli, S.; Gonzalez, A.; et al. Galectin-8 binds specific beta1 integrins and induces polarized spreading highlighted by asymmetric lamellipodia in Jurkat T cells. Exp. Cell Res. 2006, 312, 374–386. [Google Scholar] [CrossRef]
- Furtak, V.; Hatcher, F.; Ochieng, J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 289, 845–850. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer. Glycoconj J. 2017, 34, 147–156. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, L.G. Expression and Impact of C1GalT1 in Cancer Development and Progression. Cancers 2021, 13, 6305. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, V.; Miller, M.C.; Blanchet, X.; Duan, R.; Leberzammer, J.; Duchene, J.; Soehnlein, O.; Megens, R.T.; Ludwig, A.K.; Dregni, A.; et al. Chemokines and galectins form heterodimers to modulate inflammation. EMBO Rep. 2020, 21, e47852. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo, L.; Schulkens, I.A.; Touarin, P.; Heusschen, R.; Aanhane, E.; Castricum, K.C.M.; De Gruijl, T.D.; Nilsson, U.J.; Leffler, H.; Griffioen, A.W.; et al. Chemokines modulate glycan binding and the immunoregulatory activity of galectins. Commun. Biol. 2021, 4, 1415. [Google Scholar] [CrossRef]
- Palumbo, A.; Meireles Da Costa, N.; Pontes, B.; Leite de Oliveira, F.; Lohan Codeço, M.; Ribeiro Pinto, L.F.; Nasciutti, L.E. Esophageal Cancer Development: Crucial Clues Arising from the Extracellular Matrix. Cells 2020, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Elola, M.T.; Ferragut, F.; Méndez-Huergo, S.P.; Croci, D.O.; Bracalente, C.; Rabinovich, G.A. Galectins: Multitask signaling molecules linking fibroblast, endothelial and immune cell programs in the tumor microenvironment. Cell Immunol. 2018, 333, 34–45. [Google Scholar] [CrossRef]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef]
- Martin-Saldaña, S.; Chevalier, M.T.; Pandit, A. Therapeutic potential of targeting galectins—A biomaterials-focused perspective. Biomaterials 2022, 286, 121585. [Google Scholar] [CrossRef]
| Subtype 1 | Sample Type 2 | Main Finding | Ref. |
|---|---|---|---|
| Galectin-1 | |||
| ESCC | Patients (n = 43) | Frequency of galectin-1 positive cells is lower in lymph node positive vs. lymph node negative patients. | [41] |
| ESCC | Patients (n = 93) | High galectin-1 expression is an independent prognostic factor for OS and DFS in ESSC. | [42] |
| ESCC | Patients (n = 85) | No prognostic value of circulating autoantibodies against galectin-1. | [43] |
| ESCC | Patients (n = 172) | No prognostic value of circulating autoantibodies against galectin-1 | [44] |
| ESCC | Cell lines (EC9706/KYSE150) | Galectin-1 is induced by PTTG and stimulates cell motility. | [45] |
| ESCC | Patients (n = 41) Cell lines (EC9706/EC109/KYSE70/KYSE150/KYSE450), Xenografts | Interaction with long non-coding RNA ESCCAL-1 stabilizes galectin-1 protein and promotes cell cycle progression. | [46] |
| Galectin-3 | |||
| ESCC | Patients (n = 43) | No association of galectin-3 with nodal involvement or tumor stage. | [41] |
| ESCC | Patients (n = 63) | High galectin-3 expression is not prognostic for survival outcome. | [47] |
| ESCC | Patients (n = 154) | Nuclear or cytoplasmic galectin-3 expression is not prognostic in ESCC. | [48] |
| NS | Patients (n = 52) | Urinary galectin-3 is a potential diagnostic tool to monitor or follow up the disease stage. | [49] |
| ESCC | Cell line (Eca-109) | galectin-3 overexpression increases malignant behavior. | [50] |
| ESCC | Cell line (Eca-109) | Galectin-3 knockdown represents a therapeutic strategy for ESCC | [51] |
| ESCC | Cell lines (KYSE-450/TE-8) | Galectin-3 knockdown increases gefitinib sensitivity. | [52] |
| ESCC | Cell lines, Xenografts (KYSE30/KYSE270) | Galectin-3 inhibition of hampers esophageal tumor growth and metastasis. | [53] |
| Galectin-7 | |||
| ESCC | Patients (n = 50) | Galectin-7 as a potential biomarker for ESCC. | [54] |
| Galectin-9 | |||
| ESCC | Patients (n = 45) | Low galectin-9 expression is associated with a poor prognosis. | [55] |
| EAC | Cell lines (OE19/OE33/SK-GT4/OACM5.1c) | Galectin-9 suppresses proliferation and induces apoptosis in EAC cells. | [56] |
| ESCC | Cell lines, Xenografts (KYSE-150/KYSE-180) | Galectin-9 induces mitochondria-mediated apoptosis of esophageal cancer. | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godefa, T.M.; Derks, S.; Thijssen, V.L.J.L. Galectins in Esophageal Cancer: Current Knowledge and Future Perspectives. Cancers 2022, 14, 5790. https://doi.org/10.3390/cancers14235790
Godefa TM, Derks S, Thijssen VLJL. Galectins in Esophageal Cancer: Current Knowledge and Future Perspectives. Cancers. 2022; 14(23):5790. https://doi.org/10.3390/cancers14235790
Chicago/Turabian StyleGodefa, Tesfay M., Sarah Derks, and Victor L. J. L. Thijssen. 2022. "Galectins in Esophageal Cancer: Current Knowledge and Future Perspectives" Cancers 14, no. 23: 5790. https://doi.org/10.3390/cancers14235790
APA StyleGodefa, T. M., Derks, S., & Thijssen, V. L. J. L. (2022). Galectins in Esophageal Cancer: Current Knowledge and Future Perspectives. Cancers, 14(23), 5790. https://doi.org/10.3390/cancers14235790






