New Challenges Facing Systemic Therapies of Advanced HCC in the Era of Different First-Line Immunotherapy-Based Combinations
Abstract
Simple Summary
Abstract
1. Introduction
2. New Standard of Care and Possible Challengers
2.1. Results of the Atezolizumab-Bevacizumab Combination Have Been Disruptive
2.2. Two Different Types of Combination Are Currently Tested in First-Line Versus Sorafenib or Lenvatinib
2.2.1. Immunotherapy Combined with an Anti-Angiogenic Tyrosine-Kinase Inhibitor (TKI)
2.2.2. Immunotherapy Combinations with an Anti-CTLA-4 Antibody
2.3. Potential Criteria of Choice between the Validated Combinations
2.4. Is There Still a Role for Monotherapy in the First-Line Setting?
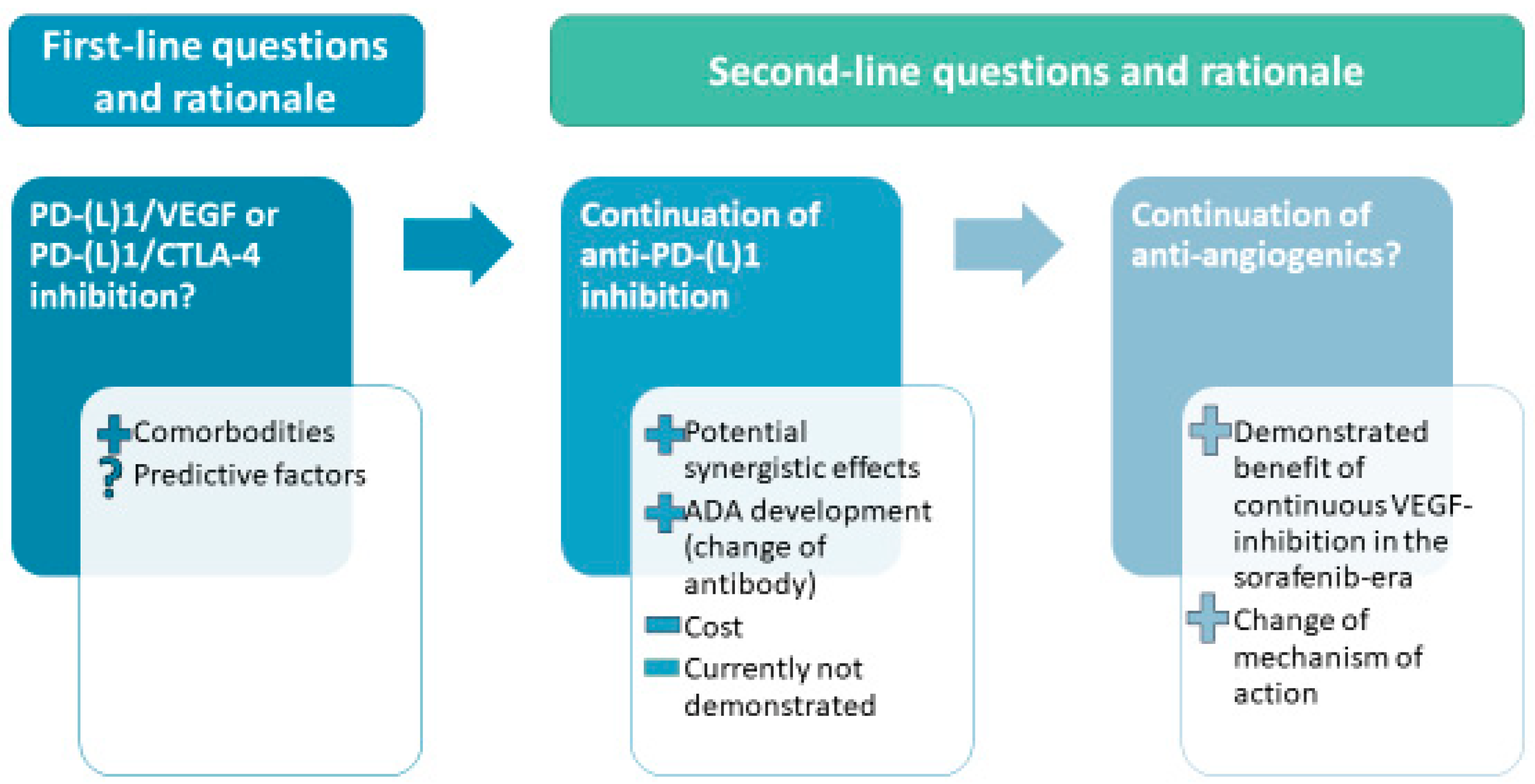
3. Current and Future Second-Line Options in the Area of First-Line Immunotherapy-Based Combination
3.1. Available Guidelines for Treatment after Progression on Atezolizumab-Bevacizumab
3.2. What Will Be the Optimal Sequence of Treatment?
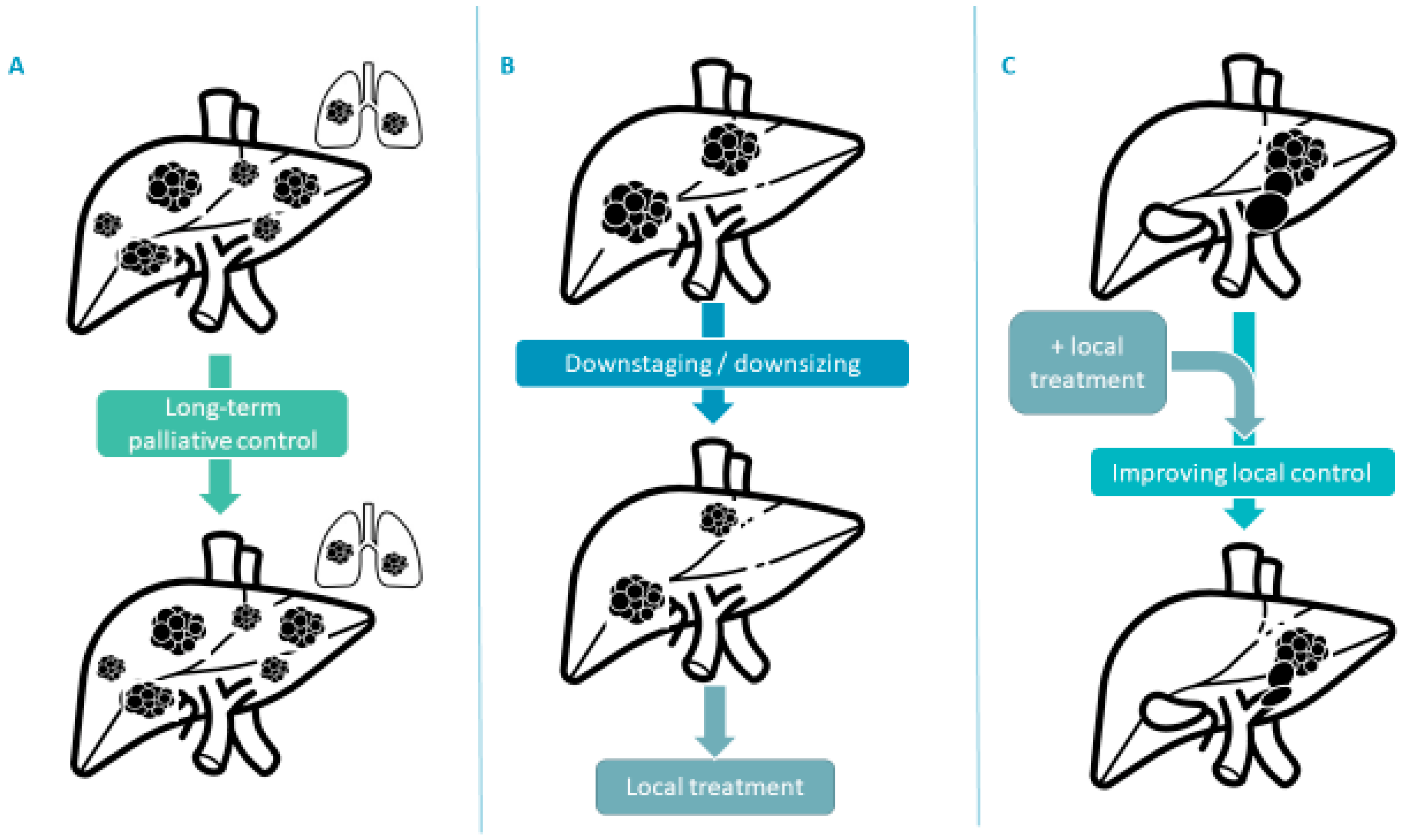
3.3. New Treatment Strategies for Advanced Disease
3.4. Development of Immunotherapy-Combination in Earlier Stages, and Potential Difficulties
4. Challenges for Future Development
4.1. Building Evidence on Sequencial Treatment
4.2. Backbone for New Combinations
4.3. Predictive Biomarkers of Response for Personalized Therapy
4.4. Financial Burden
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular Carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant Sorafenib for Hepatocellular Carcinoma after Resection or Ablation (STORM): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.-Y.; et al. Sorafenib or Placebo plus TACE with Doxorubicin-Eluting Beads for Intermediate Stage HCC: The SPACE Trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in Combination with Transarterial Chemoembolisation in Patients with Unresectable Hepatocellular Carcinoma (TACE 2): A Randomised Placebo-Controlled, Double-Blind, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased Alpha-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A Randomized, Multi-Center Phase III Study of Nivolumab (NIVO) vs. Sorafenib (SOR) as First-Line (1L) Treatment in Patients (Pts) with Advanced Hepatocellular Carcinoma (AHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 12. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-Reported Outcomes with Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Unresectable Hepatocellular Carcinoma (IMbrave150): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a Bevacizumab Biosimilar (IBI305) versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT-32): A Randomised, Open-Label, Phase 2–3 Study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Gracián, A.C.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab (NIVO) + Ipilimumab (IPI) + Cabozantinib (CABO) Combination Therapy in Patients (Pts) with Advanced Hepatocellular Carcinoma (AHCC): Results from CheckMate 040. J. Clin. Oncol. 2020, 38, 478. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus Atezolizumab versus Sorafenib for Advanced Hepatocellular Carcinoma (COSMIC-312): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Finn, R.S.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. LBA34 Primary Results from the Phase III LEAP-002 Study: Lenvatinib plus Pembrolizumab versus Lenvatinib as First-Line (1L) Therapy for Advanced Hepatocellular Carcinoma (AHCC). Ann. Oncol. 2022, 33, S1401. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Jia, R.; Yue, C.; Chang, L.; Liu, R.; Zhang, G.; Zhao, C.; Zhang, Y.; Chen, C.; et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-Label, Dose Escalation and Expansion Study. Clin. Cancer Res. 2019, 25, 515–523. [Google Scholar] [CrossRef]
- Qin, S.; Chan, L.S.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. LBA35 Camrelizumab (C) plus Rivoceranib (R) vs. Sorafenib (S) as First-Line Therapy for Unresectable Hepatocellular Carcinoma (UHCC): A Randomized, Phase III Trial. Ann. Oncol. 2022, 33, S1401–S1402. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A Clinical Trial of CTLA-4 Blockade with Tremelimumab in Patients with Hepatocellular Carcinoma and Chronic Hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Kelley, R.K. Efficacy, Tolerability, and Biologic Activity of a Novel Regimen of Tremelimumab (T) in Combination with Durvalumab (D) for Patients (Pts) with Advanced Hepatocellular Carcinoma (AHCC); ASCO Virtual Scientific Program, Robin Kate Kelley, B.S., Eds.; American Society of Clinical Oncology: Alexandria, VA, USA, 2020. [Google Scholar]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- FDA Approves Tremelimumab in Combination with Durvalumab for Unresectable Hepatocellular Carcinoma. 2022. Available online: Https://Www.Fda.Gov/Drugs/Resources-Information-Approved-Drugs/Fda-Approves-Tremelimumab-Combination-Durvalumab-Unresectable-Hepatocellular-Carcinoma (accessed on 24 November 2022).
- Kelley, R.K.; Mollon, P.; Blanc, J.-F.; Daniele, B.; Yau, T.; Cheng, A.-L.; Valcheva, V.; Marteau, F.; Guerra, I.; Abou-Alfa, G.K. Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma. Adv. Ther. 2020, 37, 2678–2695. [Google Scholar] [CrossRef]
- Proskorovsky, I.; Krotneva, M.; Ozgurdal, K.; Jaeger, S.; Su, Y. Anchored Matching-Adjusted Indirect Comparison (MAIC) of Regorafenib (REG) versus Cabozantinib (CAB) in Advanced Hepatocellular Carcinoma (HCC). J. Clin. Oncol. 2021, 39, e16188. [Google Scholar] [CrossRef]
- Boige, V.; Malka, D.; Bourredjem, A.; Dromain, C.; Baey, C.; Jacques, N.; Pignon, J.-P.; Vimond, N.; Bouvet-Forteau, N.; De Baere, T.; et al. Efficacy, Safety, and Biomarkers of Single-Agent Bevacizumab Therapy in Patients with Advanced Hepatocellular Carcinoma. Oncologist 2012, 17, 1063–1072. [Google Scholar] [CrossRef]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.-L.; Yau, T.; Furuse, J.; Park, J.-W.; Boyd, Z.; et al. Association of Inflammatory Biomarkers with Clinical Outcomes in Nivolumab-Treated Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [CrossRef]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef]
- Haber, P.K.; Torres-Martin, M.; Dufour, J.-F.; Verslype, C.; Marquardt, J.; Galle, P.R.; Vogel, A.; Meyer, T.; Labgaa, I.; Roberts, L.R.; et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2021, 39, 4100. [Google Scholar] [CrossRef]
- McCoon, P.; Lee, Y.S.; Kelley, R.K.; Guthrie, V.B.; Wu, S.; Bien, S.A.; Negro, A.; He, P.; Kurland, J.; Barrett, J.C.; et al. T-Cell Receptor Pharmacodynamics Associated with Survival and Response to Tremelimumab (T) in Combination with Durvalumab (D) in Patients (Pts) with Unresectable Hepatocellular Carcinoma (UHCC). J. Clin. Oncol. 2021, 39, 4087. [Google Scholar] [CrossRef]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical Activity and Molecular Correlates of Response to Atezolizumab Alone or in Combination with Bevacizumab versus Sunitinib in Renal Cell Carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.-H.; He, A.R.; Ryoo, B.-Y.; et al. Molecular Correlates of Clinical Response and Resistance to Atezolizumab in Combination with Bevacizumab in Advanced Hepatocellular Carcinoma. Nat. Med. 2022, 28, 1599–1611. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, J.H.; Ryu, M.-H.; Park, S.R.; Lee, D.; Kim, K.M.; Shim, J.H.; Lim, Y.-S.; Lee, H.C.; Lee, J.; et al. Clinical Outcomes with Multikinase Inhibitors after Progression on First-Line Atezolizumab plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma: A Multinational Multicenter Retrospective Study. Liver Cancer 2021, 10, 107–114. [Google Scholar] [CrossRef]
- Chen, C.-T.; Feng, Y.-H.; Yen, C.-J.; Chen, S.-C.; Lin, Y.-T.; Lu, L.-C.; Hsu, C.-H.; Cheng, A.-L.; Shao, Y.-Y. Prognosis and Treatment Pattern of Advanced Hepatocellular Carcinoma after Failure of First-Line Atezolizumab and Bevacizumab Treatment. Hepatol. Int. 2022, 16, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic Treatment of Hepatocellular Carcinoma: An EASL Position Paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Pires da Silva, I.; Ahmed, T.; Reijers, I.L.M.; Weppler, A.M.; Betof Warner, A.; Patrinely, J.R.; Serra-Bellver, P.; Allayous, C.; Mangana, J.; Nguyen, K.; et al. Ipilimumab Alone or Ipilimumab plus Anti-PD-1 Therapy in Patients with Metastatic Melanoma Resistant to Anti-PD-(L)1 Monotherapy: A Multicentre, Retrospective, Cohort Study. Lancet Oncol. 2021, 22, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Enrico, D.; Paci, A.; Chaput, N.; Karamouza, E.; Besse, B. Antidrug Antibodies Against Immune Checkpoint Blockers: Impairment of Drug Efficacy or Indication of Immune Activation? Clin. Cancer Res. 2020, 26, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.-L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Kim, C.; Yang, H.; Kim, I.; Kang, B.; Kim, H.; Kim, H.; Lee, W.S.; Jung, S.; Lim, H.Y.; Cheon, J.; et al. Association of High Levels of Antidrug Antibodies Against Atezolizumab With Clinical Outcomes and T-Cell Responses in Patients With Hepatocellular Carcinoma. JAMA Oncol. 2022, 2022, 4733. [Google Scholar] [CrossRef]
- Breder, V.V. IMbrave150: Exploratory Efficacy and Safety Results of Hepatocellular Carcinoma (HCC) Patients (Pts) with Main Trunk and/or Contralateral Portal Vein Invasion (Vp4) Treated with Atezolizumab (Atezo) + Bevacizumab (Bev) versus Sorafenib (Sor) in a Global Ph III Study. In Proceedings of the ASCO Annual Meeting, Online, 4–8 June 2021; Valeriy Vladimirovich Breder, A.V., Ed.; American Society of Clinical Oncology: Alexandria, VA, USA, 2021. [Google Scholar]
- Sové, R.J.; Verma, B.K.; Wang, H.; Ho, W.J.; Yarchoan, M.; Popel, A.S. Virtual Clinical Trials of Anti-PD-1 and Anti-CTLA-4 Immunotherapy in Advanced Hepatocellular Carcinoma Using a Quantitative Systems Pharmacology Model. J. Immunother. Cancer 2022, 10, e005414. [Google Scholar] [CrossRef]
- Sung, W.; Hong, T.S.; Poznansky, M.C.; Paganetti, H.; Grassberger, C. Mathematical Modeling to Simulate the Effect of Adding Radiation Therapy to Immunotherapy and Application to Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1055–1062. [Google Scholar] [CrossRef]
- Watkins, P.B. DILIsym: Quantitative Systems Toxicology Impacting Drug Development. Curr. Opin. Toxicol. 2020, 23–24, 67–73. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D. PD-L1, TMB, and Other Potential Predictors of Response to Immunotherapy for Hepatocellular Carcinoma: How Can They Assist Drug Clinical Trials? Expert Opin. Investig. Drugs 2022, 31, 415–423. [Google Scholar] [CrossRef]
- Li, L.; Yang, S.; Chen, Y.; Tian, L.; He, Y.; Wu, B.; Dong, D. Immune Checkpoint Inhibitors Plus an Anti-VEGF Antibody as the First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Network Meta-Analysis and Cost-Effectiveness Analysis. Front. Pharmacol. 2022, 13, 891008. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Shi, J.; Jia, X.; Dang, S.; Wang, W. Cost-Effectiveness of Atezolizumab Plus Bevacizumab vs. Sorafenib for Patients With Unresectable or Metastatic Hepatocellular Carcinoma. JAMA Netw. Open 2021, 4, e214846. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Chan, S.-K.; Lee, S.-F.; Choi, H.C.-W. First-Line Atezolizumab Plus Bevacizumab versus Sorafenib in Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Cancers 2021, 13, 931. [Google Scholar] [CrossRef]
| Combination | Control Arm | Trial Name and Reference | Selected Characteristics Population Included | n Main Comparison | OS Experimental vs. Control | PFS Experimental vs. Control | RECIST 1.1 ORR Experimental vs. Control | Treatment-Emergent Grade 3/4 Toxicities Experimental vs. Control |
|---|---|---|---|---|---|---|---|---|
| Atezolizumab-bevacizumab | sorafenib | IMbrave150 [17,18] | MVI: 40% (14% Vp4) Non-viral: 30.5% | 501 | Median 19.2 vs. 13.4 months, HR = 0.66, p < 0.001 | Median 6.8 vs. 4.3 months, HR = 0.29, p < 0.001 | 30% vs. 11%, p < 0.001 | 63% vs. 57% |
| Lenvatinib-pembrolizumab | lenvatinib | LEAP-002 [24] | MVI: 17% (0% Vp4) Non-viral: 39% | 794 | Median 21.2 vs. 19.0, HR = 0.840, p = 0.0227 * | Median 8.2 vs. 8.0 months, HR = 0.87, p = 0.0466 * | 26% vs. 18% | 62% vs. 57% |
| Camrelizumab-rivoceranib | sorafenib | Qin et al. [26] | MVI: 17% (0% Vp4) Non-viral: 19.2% | 543 | Median 22.1 vs. 15.2 months, HR = 0.62, p < 0.001 | Median 5.6 vs. 3.7 months, HR = 0.52 p < 0.001 | 35.2% vs. 8.9% | 81% vs. 52% |
| Cabozantinib-atezolizumab | sorafenib | COSMIC-312 [22] | MVI: 30% (18% Vp4) Non-viral: 39% | 649 | Median 15.4 vs. 15.5 months, HR = 0.90, p = 0.44 | Median 6.8 vs. 4.2 months, HR = 0.63, p = 0.0012 | 11% vs. 4% | 64% vs. 46% |
| Durvalumab-tremelimumab | sorafenib | HIMALAYA [30] | MVI: 26% (0%Vp4) Non-viral: 42% | 782 | Median 16.4 vs. 13.8 months, HR = 0.78, p = 0.0035 | Median 3.8 vs. 4.1 months, HR = 0.90 | 20% vs. 5% | 51% vs. 52% |
| Anti-Angiogenics-Based | Anti-CTLA-4-Based | ||
|---|---|---|---|
| Bevacizumab-Based | TKIs-Based | ||
| Strong criteria of choice | Pre-existing untreated auto-immune disorder (psoriasis…) | Severe vascular comorbidities Significant Portal Hypertension | |
| Criteria of choice subject to interpretation | Better toxicity profile | Non-severe vascular comorbidities Equilibrated arterial hypertension | |
| Low-risk auto-immune disorder (diabetes, thyroid disorder…) | |||
| Second-Line Options | Further-Line Options | |
|---|---|---|
| BCLC [3] | Clinical trials | |
| EASL [44] | Multi-TKI and VEGFR2 inhibitor as per off-label availability | |
| ILCA, https://ilca-online.org/education/ilca-guidances/ (accessed on 24 November 2022) | sorafenib, lenvatinib, cabozantinib | regorafenib |
| NCCN preferred treatments | sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab | |
| ESMO https://www.esmo.org/guidelines/gastrointestinal-cancers/hepatocellular-carcinoma (accessed on 24 November 2022) | sorafenib, lenvatinib, cabozantinib, ramucirumab | regorafenib (for TKI-experienced patients) |
| French recommendation (TNCD) | sorafenib, lenvatinib | regorafenib, cabozantinib, ramucirumab |
| Trial Name | Context | Treatment | Planned Number of Patients | Clinicaltrials.gov Identifier |
|---|---|---|---|---|
| IMbrave 050 | After curative-intent resection or ablation | Atezolizumab-bevacizumab vs. surveillance | 668 | NCT04102098 |
| EMERALD-2 | After curative-intent resection or ablation | Durvalumab +/− bevacizumab vs. placebo | 908 | NCT03847428 |
| Checkmate-9DX | After curative-intent resection or ablation | Nivolumab vs. placebo | 545 | NCT03383458 |
| Keynote-937 | After curative-intent resection or ablation | Pembrolizumab vs. placebo | 950 | NCT03867084 |
| ABLATE-2 | Before and after ablation | Atezolizumab-bevacizumab + ablation vs. ablation alone | 202 | NCT04727307 |
| ML42612 | With TACE | Atezolizumab-bevacizumab + TACE vs. TACE alone | 342 | NCT04712643 |
| EMERALD-1 | With TACE | Durvalumab +/− bevacizumab + TACE vs. placebo + TACE | 724 | NCT03778957 |
| EMERALD-3 | With TACE | Durvalumab-tremelimumab +/− lenvatinib + TACE vs. TACE alone | 525 | NCT05301842 |
| TACE-3 | Before and after TACE | nivolumab + TACE vs. TACE alone | 522 | NCT04268888 |
| LEAP-012 | With TACE | pembrolizumab-lenvatinib + TACE vs. placebo + TACE | 950 | NCT04246177 |
| ROWAN | With SIRT | durvalumab-tremelimumab vs. SIRT alone | 150 | NCT05063565 |
| ABC-HCC | In lieu of TACE | atezolizumab-bevacizumab vs. TACE | 434 | NCT04803994 |
| INTRATACE | In lieu of TACE | regorafenib-tislelizumab vs. TACE | 496 | NCT04777851 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edeline, J.; Meyer, T.; Blanc, J.-F.; Raoul, J.-L. New Challenges Facing Systemic Therapies of Advanced HCC in the Era of Different First-Line Immunotherapy-Based Combinations. Cancers 2022, 14, 5868. https://doi.org/10.3390/cancers14235868
Edeline J, Meyer T, Blanc J-F, Raoul J-L. New Challenges Facing Systemic Therapies of Advanced HCC in the Era of Different First-Line Immunotherapy-Based Combinations. Cancers. 2022; 14(23):5868. https://doi.org/10.3390/cancers14235868
Chicago/Turabian StyleEdeline, Julien, Tim Meyer, Jean-Frédéric Blanc, and Jean-Luc Raoul. 2022. "New Challenges Facing Systemic Therapies of Advanced HCC in the Era of Different First-Line Immunotherapy-Based Combinations" Cancers 14, no. 23: 5868. https://doi.org/10.3390/cancers14235868
APA StyleEdeline, J., Meyer, T., Blanc, J.-F., & Raoul, J.-L. (2022). New Challenges Facing Systemic Therapies of Advanced HCC in the Era of Different First-Line Immunotherapy-Based Combinations. Cancers, 14(23), 5868. https://doi.org/10.3390/cancers14235868






