Caerin 1.1/1.9 Enhances Antitumour Immunity by Activating the IFN-α Response Signalling Pathway of Tumour Macrophages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cervical Cancer Patient Information
2.2. Mice
2.3. Cell Line, Peptide Synthesis and Antibodies
2.4. Tumour Challenge
2.5. Immunisation of Mice
2.6. Tumour Local Administration of Caerin Peptide
2.7. Depletion of Macrophage
2.8. Flow Cytometry
2.9. Cytokine ELISA for IL-12, IL-10, IL-6 and TNF-α
2.10. Single Cell RNA Sequencing Data Analysis
2.11. Protein-Protein Interaction (PPI) Analysis
2.12. GSEA Analysis
2.13. SCENIC Analysis of Transcription Factor
2.14. Real Time PCR
3. Results
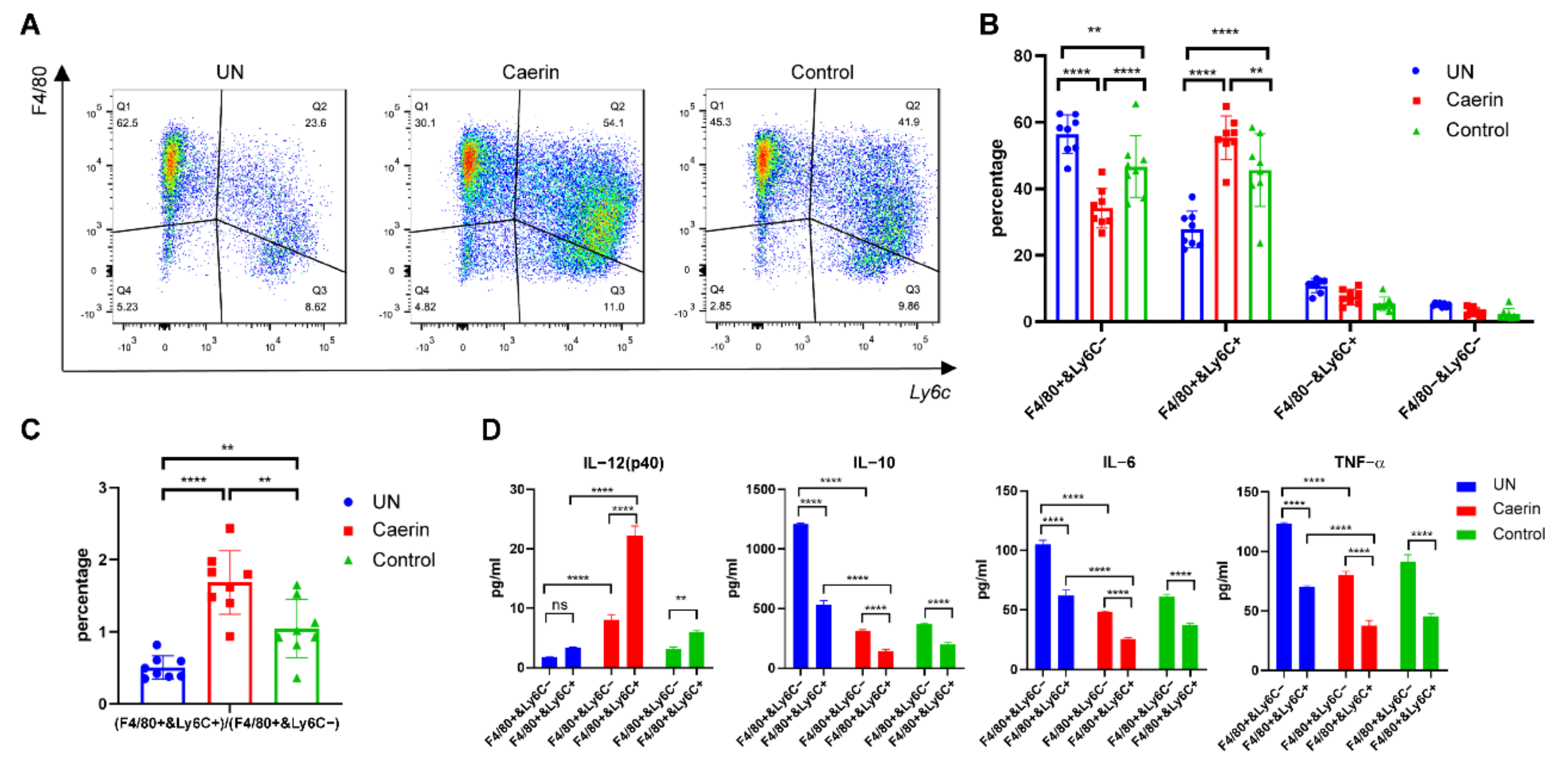
3.1. Immunotherapy plus Caerin 1.1/1.9 Induces Tumour Macrophages to Secrete More IL-12 and Less IL-6 and IL-10
3.2. Depletion of Macrophages Attenuate the Caerin 1.1/1.9 Enhanced Efficacy of Immunotherapy
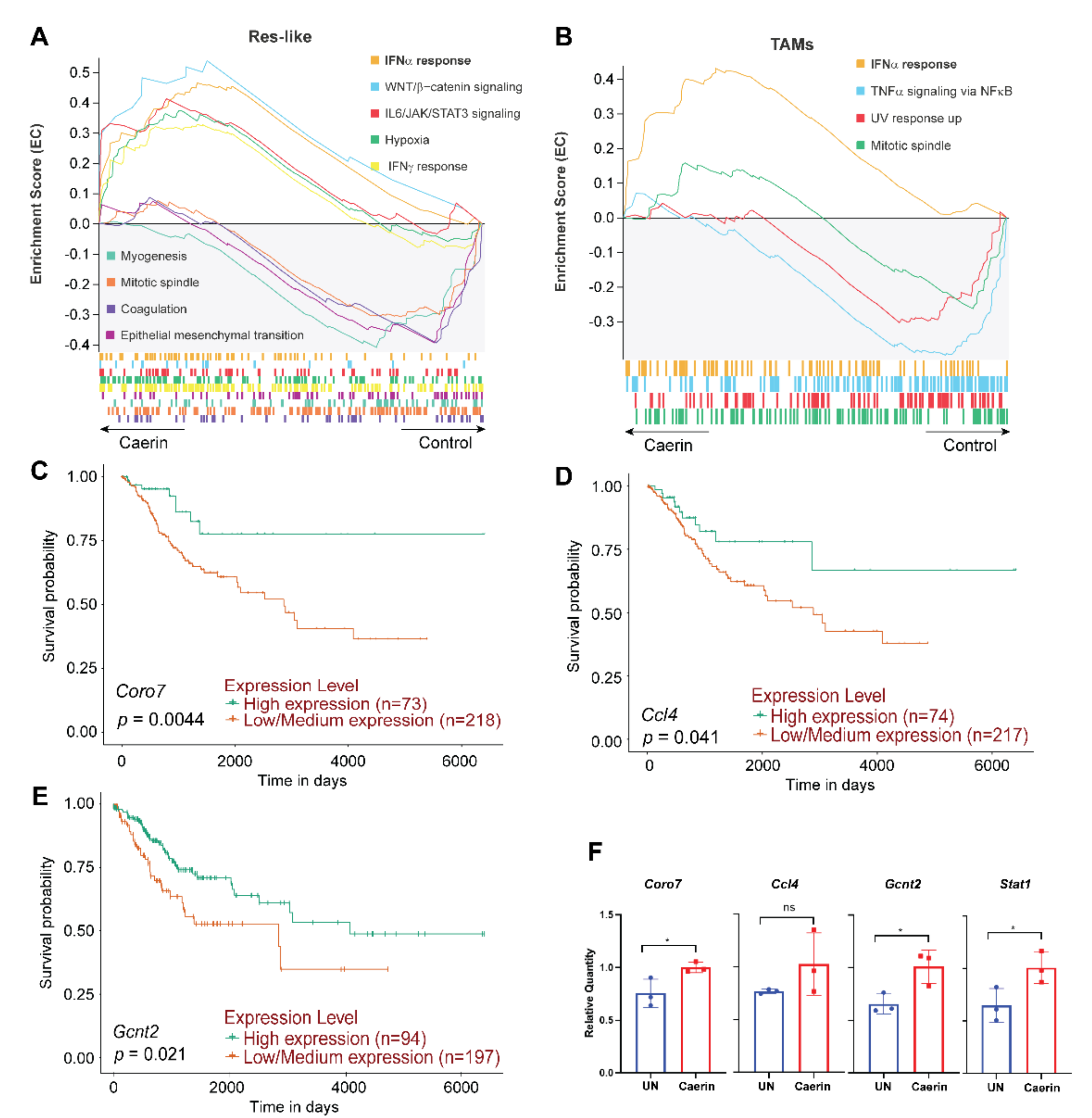
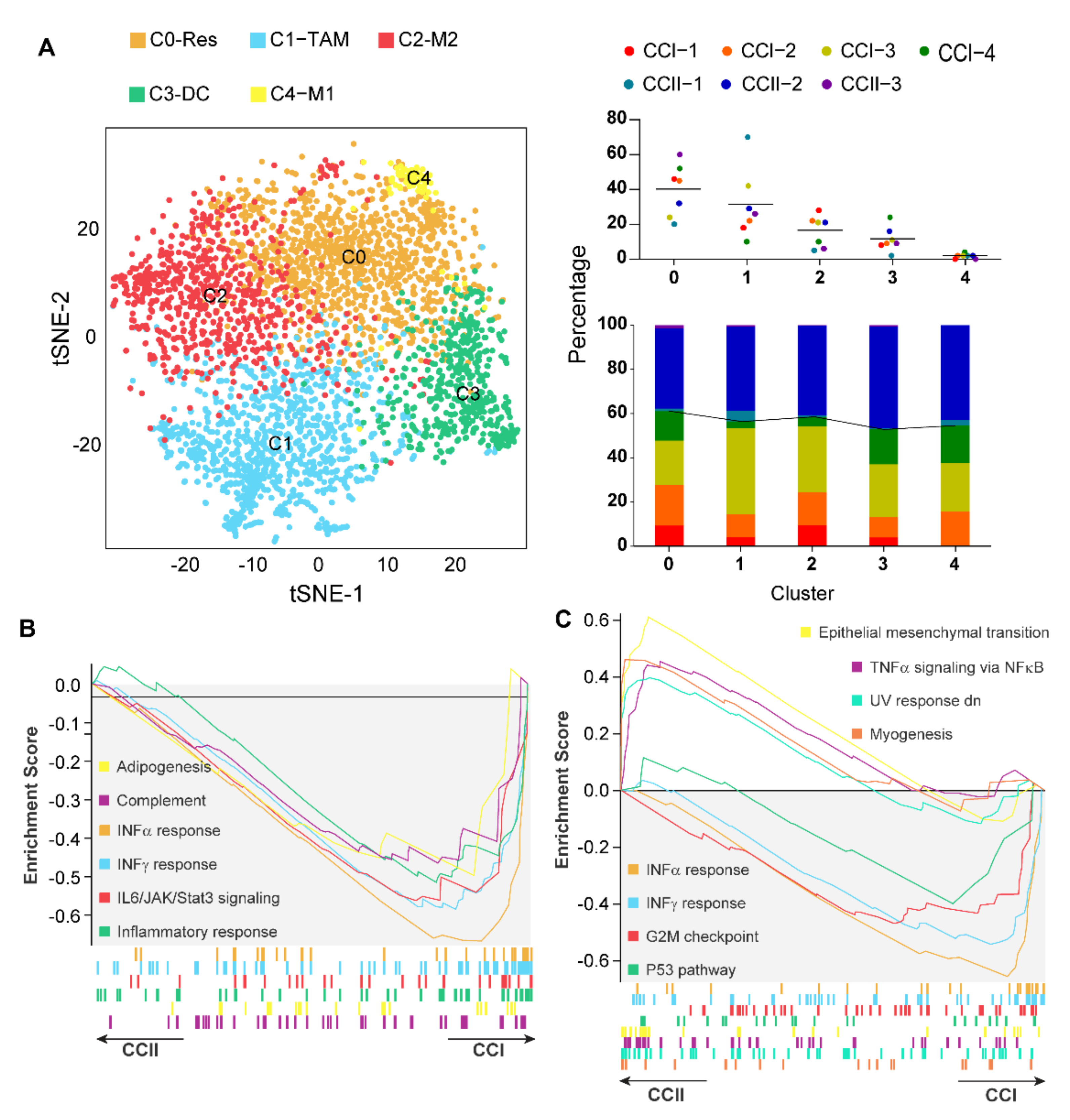
3.3. Caerin 1.1/1.9 Activates IFN-α Response Pathway in Tumour Macrophages
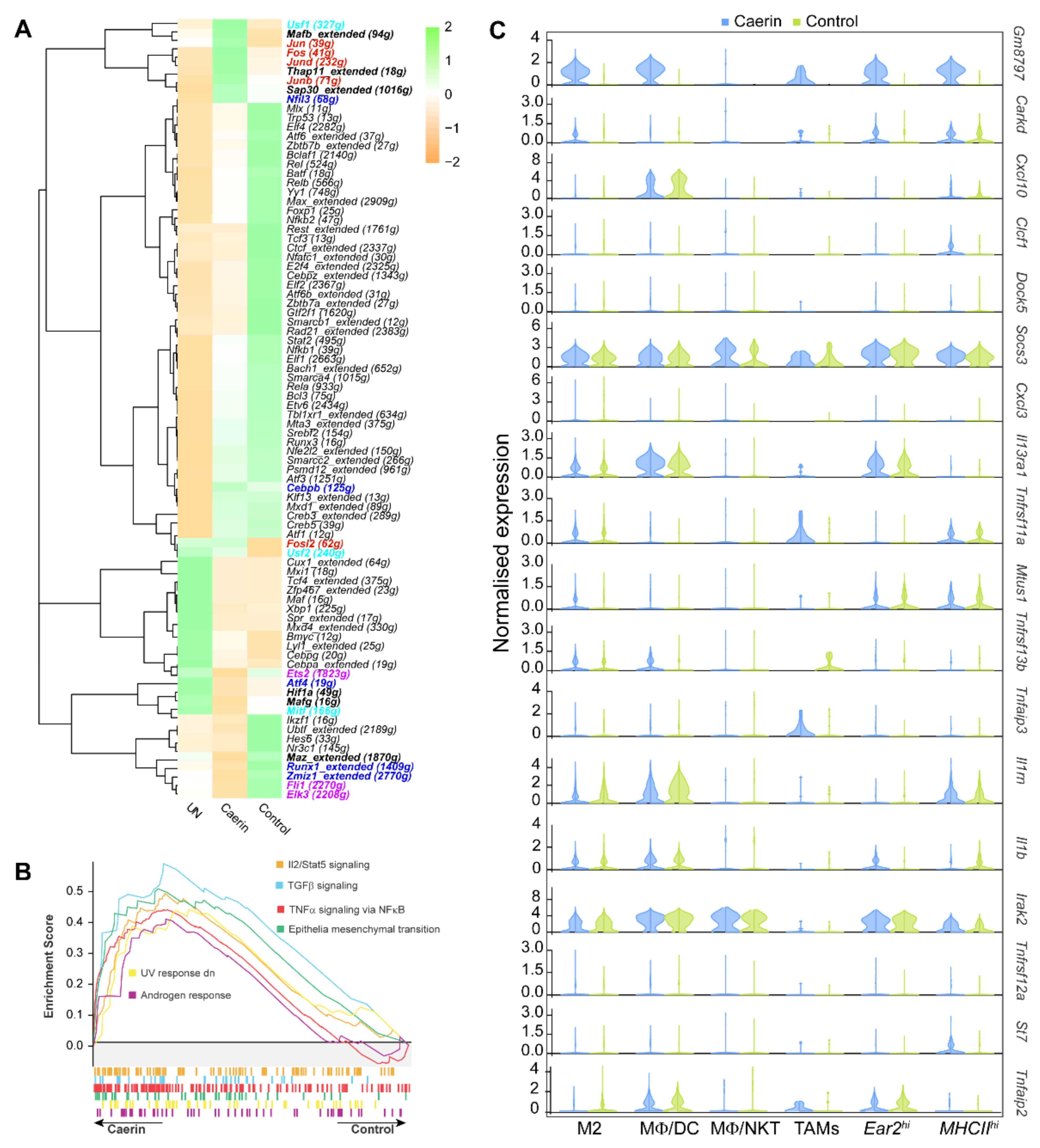
3.4. Caerin plus DT Immunotherapy Activates the Transcription Factors Regulating Pro-Inflammatory Genes of Tumour Macrophages
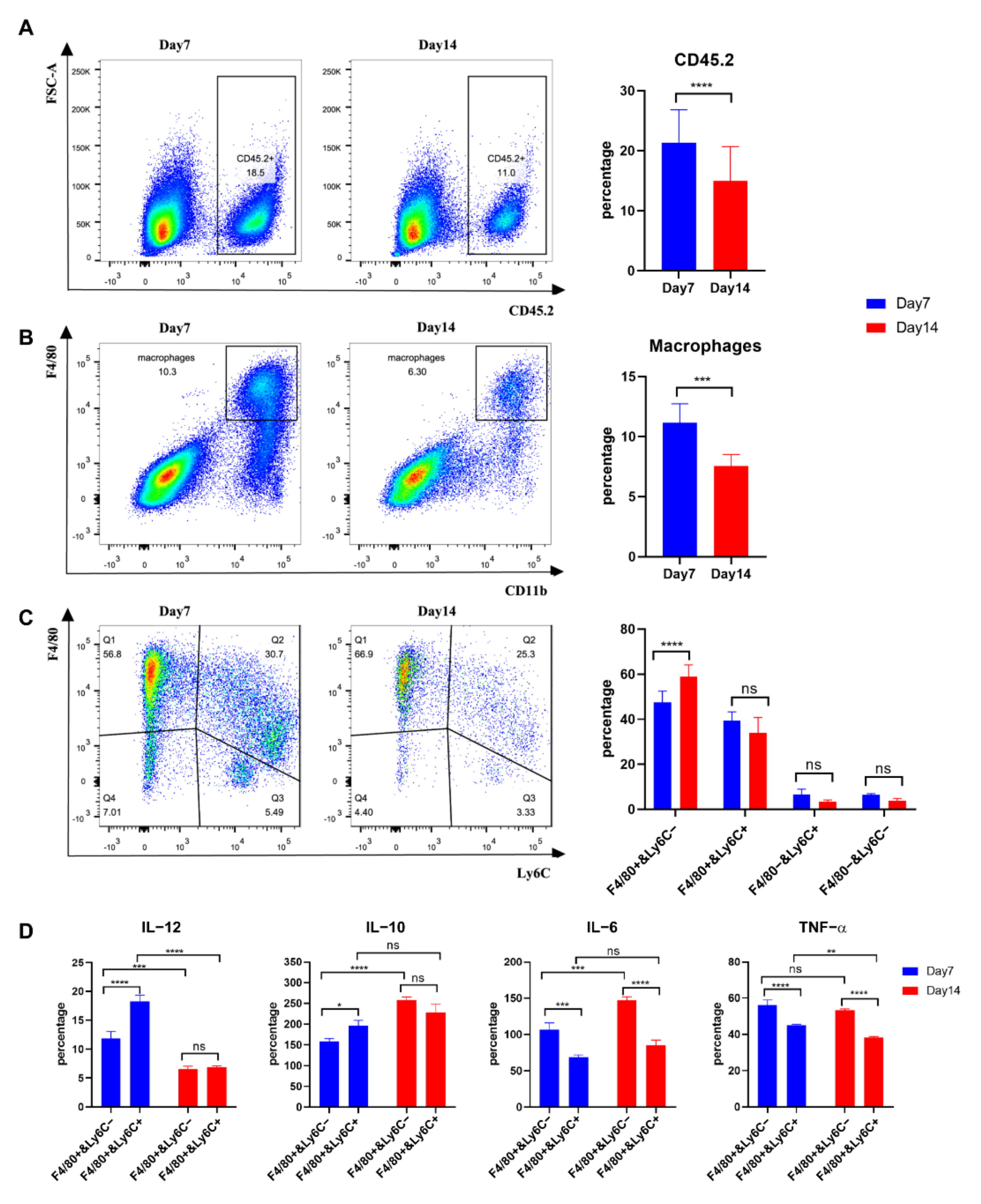
3.5. Tumour Macrophages Isolated 7 Days after Tumour Inoculation from TC-1 Tumours Secreted more IL-12 than Those from Day 14
3.6. IFN-α Response Signalling Pathway Activated Macrophages Are Increased in Early-Stage Cervical Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef]
- van der Sluis, T.C.; van der Burg, S.H.; Arens, R.; Melief, C.J. New approaches in vaccine-based immunotherapy for human papillomavirus-induced cancer. Curr. Opin. Immunol. 2015, 35, 9–14. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Huang, K.; Luan, Y.; Cao, Z.; Chen, S.; Ma, B.; Yuan, J.; Wu, X.; Chen, G.; Wang, T.; et al. Human papillomavirus infection among head and neck squamous cell carcinomas in southern China. PLoS ONE 2019, 14, e0221045. [Google Scholar] [CrossRef] [PubMed]
- Tsikis, S.; Hoefer, L.; Bethimoutis, G.; Nicolaidou, E.; Paparizos, V.; Antoniou, C.; Chardalias, L.; Stavropoulos, G.E.; Sharma, S.; Long, B.C.; et al. Risk factors, prevalence, and site concordance of human papillomavirus in high-risk Greek men. Eur. J. Cancer Prev. 2018, 27, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Maharjan, M.; Shrestha, G.; Maharjan, N.; Petrini, M.A.; Zuo, N.; He, C.; Yang, J.; Xu, M.; Ge, C.; et al. Prevalence and type-specific distribution of human papillomavirus infection among women in mid-western rural, Nepal- A population-based study. BMC Infect. Dis. 2018, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chu, R.; Wang, K.; Zhang, X.; Li, J.; Zhao, Z.; Yao, S.; Wang, Z.; Dong, T.; Yang, X.; et al. Prognostic Assessment of Cervical Cancer Patients by Clinical Staging and Surgical-Pathological Factor: A Support Vector Machine-Based Approach. Front. Oncol. 2020, 10, 1353. [Google Scholar] [CrossRef]
- Olthof, E.P.; van der Aa, M.A.; Adam, J.A.; Stalpers, L.J.A.; Wenzel, H.H.B.; van der Velden, J.; Mom, C.H. The role of lymph nodes in cervical cancer: Incidence and identification of lymph node metastases-a literature review. Int. J. Clin. Oncol. 2021, 26, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Tuyaerts, S.; Van Nuffel, A.M.T.; Naert, E.; Van Dam, P.A.; Vuylsteke, P.; De Caluwe, A.; Aspeslagh, S.; Dirix, P.; Lippens, L.; De Jaeghere, E.; et al. PRIMMO study protocol: A phase II study combining PD-1 blockade, radiation and immunomodulation to tackle cervical and uterine cancer. BMC Cancer 2019, 19, 506. [Google Scholar] [CrossRef] [PubMed]
- Saglam, O.; Conejo-Garcia, J. PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr. Cancer Sci. Ther. 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Borcoman, E.; Le Tourneau, C. Pembrolizumab in cervical cancer: Latest evidence and clinical usefulness. Ther. Adv. Med. Oncol. 2017, 9, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Andersson, A.C.; Mariya, S.; Pedersen, A.G.; Burk, R.D.; Folgori, A.; Colloca, S.; Cortese, R.; Nicosia, A.; Pamungkas, J.; et al. Therapeutic Vaccine Against Primate Papillomavirus Infections of the Cervix. J. Immunother. 2017, 40, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Llopiz, D.; Aranda, F.; Diaz-Valdes, N.; Ruiz, M.; Infante, S.; Belsue, V.; Lasarte, J.J.; Sarobe, P. Vaccine-induced but not tumor-derived Interleukin-10 dictates the efficacy of Interleukin-10 blockade in therapeutic vaccination. Oncoimmunology 2016, 5, e1075113. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Chen, S.; Yuan, J.; Cavezza, S.F.; Wei, M.Q.; Li, H.; Pan, X.; Liu, X.; Wang, T. Comparative proteomic study reveals the enhanced immune response with the blockade of interleukin 10 with anti-IL-10 and anti-IL-10 receptor antibodies in human U937 cells. PLoS ONE 2019, 14, e0213813. [Google Scholar] [CrossRef]
- Tondini, E.; Arakelian, T.; Oosterhuis, K.; Camps, M.; van Duikeren, S.; Han, W.; Arens, R.; Zondag, G.; van Bergen, J.; Ossendorp, F. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology 2019, 8, 1652539. [Google Scholar] [CrossRef]
- Chandra, J.; Dutton, J.L.; Li, B.; Woo, W.P.; Xu, Y.; Tolley, L.K.; Yong, M.; Wells, J.W.; Leggatt, G.R.; Finlayson, N.; et al. DNA Vaccine Encoding HPV16 Oncogenes E6 and E7 Induces Potent Cell-mediated and Humoral Immunity Which Protects in Tumor Challenge and Drives E7-expressing Skin Graft Rejection. J. Immunother. 2017, 40, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Glisson, B.S. New Hope for Therapeutic Cancer Vaccines in the Era of Immune Checkpoint Modulation. Annu. Rev. Med. 2019, 70, 409–424. [Google Scholar] [CrossRef]
- Ni, G.; Yang, X.; Li, J.; Wu, X.; Liu, Y.; Li, H.; Chen, S.; Fogarty, C.E.; Frazer, I.H.; Chen, G.; et al. Intratumoral injection of caerin 1.1 and 1.9 peptides increases the efficacy of vaccinated TC-1 tumor-bearing mice with PD-1 blockade by modulating macrophage heterogeneity and the activation of CD8(+) T cells in the tumor microenvironment. Clin. Transl. Immunol. 2021, 10, e1335. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Liang, D.; Cummins, S.F.; Walton, S.F.; Chen, S.; Wang, Y.; Mounsey, K.; Wei, M.Q.; Yuan, J.; Pan, X.; et al. Comparative Proteomic Study of the Antiproliferative Activity of Frog Host-Defence Peptide Caerin 1.9 and Its Additive Effect with Caerin 1.1 on TC-1 Cells Transformed with HPV16 E6 and E7. Biomed. Res. Int. 2018, 2018, 7382351. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ma, B.; You, X.; Chen, S.; Wu, J.; Wang, T.; Walton, S.F.; Yuan, J.; Wu, X.; Chen, G.; et al. Synthesized natural peptides from amphibian skin secretions increase the efficacy of a therapeutic vaccine by recruiting more T cells to the tumour site. BMC Complement. Altern. Med. 2019, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Chen, S.; Chen, M.; Wu, J.; Yang, B.; Yuan, J.; Walton, S.F.; Li, H.; Wei, M.Q.; Wang, Y.; et al. Host-Defense Peptides Caerin 1.1 and 1.9 Stimulate TNF-Alpha-Dependent Apoptotic Signals in Human Cervical Cancer HeLa Cells. Front. Cell Dev. Biol 2020, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; He, T.; Xiao, Z.; Du, J.; Zhu, K.; Liu, X.; Chen, T.; Liu, W.; Ni, G.; Liu, X.; et al. (131)I-Caerin 1.1 and (131)I-Caerin 1.9 for the treatment of non-small-cell lung cancer. Front. Oncol. 2022, 12, 861206. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yuan, J.; Chen, S.; Huang, K.; Wang, Q.; Ma, J.; Lin, R.; Zhang, L.; Zhou, Y.; Wang, T.; et al. Topical application of temperature-sensitive caerin 1.1 and 1.9 gel inhibits TC-1 tumor growth in mice. Am J. Transl. Res. 2020, 12, 191–202. [Google Scholar]
- Ni, G.; Liu, X.; Li, H.; Fogarty, C.E.; Chen, S.; Zhang, P.; Liu, Y.; Wu, X.; Wei, M.Q.; Chen, G.; et al. Topical Application of Temperature-Sensitive Gel Containing Caerin 1.1 and 1.9 Peptides on TC-1 Tumour-Bearing Mice Induced High-Level Immune Response in the Tumour Microenvironment. Front. Oncol. 2021, 11, 754770. [Google Scholar] [CrossRef]
- Xu, L.; Xie, X.; Luo, Y. The role of macrophage in regulating tumour microenvironment and the strategies for reprogramming tumour-associated macrophages in antitumour therapy. Eur. J. Cell Biol. 2021, 100, 151153. [Google Scholar] [CrossRef]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Kolliniati, O.; Ieronymaki, E.; Vergadi, E.; Tsatsanis, C. Metabolic Regulation of Macrophage Activation. J. Innate Immun. 2022, 14, 51–68. [Google Scholar] [CrossRef]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Q.; Gao, W.; Yu, S. Recent advancement on development of drug-induced macrophage polarization in control of human diseases. Life Sci. 2021, 284, 119914. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.E.; Enos, R.T.; Velázquez, K.T.; Carson, M.S.; Nagarkatti, M.; Nagarkatti, P.S.; Chatzistamou, I.; Davis, J.M.; Carson, J.A.; Robinson, C.M.; et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G22–G31. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Jassar, A.; Mishalian, I.; Wang, L.C.; Kapoor, V.; Cheng, G.; Sun, J.; Singhal, S.; Levy, L.; Albelda, S.M. Using macrophage activation to augment immunotherapy of established tumours. Br. J. Cancer 2013, 108, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Nolan, K.; Strait, A.A.; Bian, L.; Nguyen, K.A.; Wang, J.H.; Jimeno, A.; Zhou, H.M.; Young, C.D.; Wang, X.J. Macrophages Promote Growth of Squamous Cancer Independent of T cells. J. Dent. Res. 2019, 98, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lepique, A.P.; Daghastanli, K.R.; Cuccovia, I.M.; Villa, L.L. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin. Cancer Res. 2009, 15, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; de Carvalho-Barbosa, M.; Kavelaars, A.; Heijnen, C.J.; Albrecht, P.J.; Dougherty, P.M. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J. Pain 2016, 17, 775–786. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.A.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-cell regulatory network inference and clustering. Nat. Methods 2017, 14, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Thu, V.A.; Irrthum, A.; Wehenkel, L.; Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 2010, 5, e12776. [Google Scholar] [CrossRef]
- Diez-Fernandez, C.; Rüfenacht, V.; Gemperle, C.; Fingerhut, R.; Häberle, J. Mutations and common variants in the human arginase 1 (ARG1) gene: Impact on patients, diagnostics, and protein structure considerations. Hum. Mutat. 2018, 39, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Arginine Arms T Cells to Thrive and Survive. Cell Metab. 2016, 24, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Q.; Qin, L.; Zhao, S.; Wang, J.; Chen, X. Transition of tumor-associated macrophages from MHC class II(hi) to MHC class II(low) mediates tumor progression in mice. BMC Immunol. 2011, 12, 43. [Google Scholar] [CrossRef]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Zimmermann, H.; Degenkolbe, R.; Bernard, H.U.; O’Connor, M.J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 1999, 73, 6209–6219. [Google Scholar] [CrossRef] [PubMed]
- Dinwiddie, D.L.; Harrod, K.S. Human metapneumovirus inhibits IFN-alpha signaling through inhibition of STAT1 phosphorylation. Am. J. Respir. Cell Mol. Biol. 2008, 38, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Labrecque, S.; Gauzzi, M.C.; Cuddihy, A.R.; Wong, A.H.; Pellegrini, S.; Matlashewski, G.J.; Koromilas, A.E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene 1999, 18, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Senft, A.P.; Taylor, R.H.; Lei, W.; Campbell, S.A.; Tipper, J.L.; Martinez, M.J.; Witt, T.L.; Clay, C.C.; Harrod, K.S. Respiratory syncytial virus impairs macrophage IFN-alpha/beta- and IFN-gamma-stimulated transcription by distinct mechanisms. Am. J. Respir. Cell Mol. Biol. 2010, 42, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Du, J.; Yang, J.; Hu, X.; Li, L. Changes of serum levels of tumor necrosis factor (TNF-α) and soluble interleukin-2 receptor (SIL 2R) in patients with cervical cancer and their clinical significance. Am. J. Transl. Res. 2021, 13, 6599–6604. [Google Scholar]
- Musella, M.; Manic, G.; De Maria, R.; Vitale, I.; Sistigu, A. Type-I-interferons in infection and cancer: Unanticipated dynamics with therapeutic implications. Oncoimmunology 2017, 6, e1314424. [Google Scholar] [CrossRef] [PubMed]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Demaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef]
- Weiss, J.M.; Guérin, M.V.; Regnier, F.; Renault, G.; Galy-Fauroux, I.; Vimeux, L.; Feuillet, V.; Peranzoni, E.; Thoreau, M.; Trautmann, A.; et al. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. Oncoimmunology 2017, 6, e1346765. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021, 184, 5338–5356.e5321. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Miyabe, H.; Hyodo, M.; Sato, Y.; Hayakawa, Y.; Harashima, H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control. Release 2015, 216, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Hirai, H.; Sato, R.; Adachi, H.; Sato, F.; Hayashi, Y.; Sato, A.; Kamio, N.; Miura, Y.; Nakano, M.; et al. C/EBPβ is a critical mediator of IFN-α-induced exhaustion of chronic myeloid leukemia stem cells. Blood Adv. 2019, 3, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Abelson, M.R.; Childs, E.E.; Ferreira, M.C.; Bishu, S.; Conti, H.R.; Gaffen, S.L. C/EBPβ Promotes Immunity to Oral Candidiasis through Regulation of β-Defensins. PLoS ONE 2015, 10, e0136538. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Abelson, M.R.; Hernandez-Mir, G.; Childs, E.E.; Cruz, J.A.; Poholek, A.C.; Chattopadhyay, A.; Gaffen, S.L.; McGeachy, M.J. CCAAT/Enhancer-binding protein β promotes pathogenesis of EAE. Cytokine 2017, 92, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Qie, C.; Jiang, J.; Liu, W.; Hu, X.; Chen, W.; Xie, X.; Liu, J. Single-cell RNA-Seq reveals the transcriptional landscape and heterogeneity of skin macrophages in Vsir(-/-) murine psoriasis. Theranostics 2020, 10, 10483–10497. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, T.C.; Sluijter, M.; van Duikeren, S.; West, B.L.; Melief, C.J.; Arens, R.; van der Burg, S.H.; van Hall, T. Therapeutic Peptide Vaccine-Induced CD8 T Cells Strongly Modulate Intratumoral Macrophages Required for Tumor Regression. Cancer Immunol. Res. 2015, 3, 1042–1051. [Google Scholar] [CrossRef]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage targeting in cancer. Ann. N. Y. Acad. Sci. 2021, 1499, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Wang, X.; Yu, J.; Ma, F.; Li, Z.; Zhou, Y.; Zeng, S.; Ma, X.; Li, Y.R.; Neal, A.; et al. Targeting monoamine oxidase A-regulated tumor-associated macrophage polarization for cancer immunotherapy. Nat. Commun. 2021, 12, 3530. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.C.; Ruffell, B.; Oei, Y.; Bissell, M.J.; Coussens, L.M.; Pryer, N.; Daniel, D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology 2013, 2, e26968. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Günthner, R.; Anders, H.J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013, 2013, 731023. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Behmoaras, J.; Bhangal, G.; Smith, J.; McDonald, K.; Mutch, B.; Lai, P.C.; Domin, J.; Game, L.; Salama, A.; Foxwell, B.M.; et al. Jund is a determinant of macrophage activation and is associated with glomerulonephritis susceptibility. Nat. Genet. 2008, 40, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.F.; Baccarella, A.; Pancholi, N.; Pufall, M.A.; Herbert, D.R.; Kim, C.C. JUNB is a key transcriptional modulator of macrophage activation. J. Immunol. 2015, 194, 177–186. [Google Scholar] [CrossRef]
- Renoux, F.; Stellato, M.; Haftmann, C.; Vogetseder, A.; Huang, R.; Subramaniam, A.; Becker, M.O.; Blyszczuk, P.; Becher, B.; Distler, J.H.W.; et al. The AP1 Transcription Factor Fosl2 Promotes Systemic Autoimmunity and Inflammation by Repressing Treg Development. Cell Rep. 2020, 31, 107826. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Z.; Li, N.; Jiang, W.; Gao, P.; Yang, M.; Yu, X.; Wang, G.; Zhang, Y. Ets2 suppresses inflammatory cytokines through MAPK/NF-κB signaling and directly binds to the IL-6 promoter in macrophages. Aging 2019, 11, 10610–10625. [Google Scholar] [CrossRef]
- Ohanna, M.; Cheli, Y.; Bonet, C.; Bonazzi, V.F.; Allegra, M.; Giuliano, S.; Bille, K.; Bahadoran, P.; Giacchero, D.; Lacour, J.P.; et al. Secretome from senescent melanoma engages the STAT3 pathway to favor reprogramming of naive melanoma towards a tumor-initiating cell phenotype. Oncotarget 2013, 4, 2212–2224. [Google Scholar] [CrossRef]
- Ohanna, M.; Giuliano, S.; Bonet, C.; Imbert, V.; Hofman, V.; Zangari, J.; Bille, K.; Robert, C.; Bressac-de Paillerets, B.; Hofman, P.; et al. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS). Genes Dev. 2011, 25, 1245–1261. [Google Scholar] [CrossRef]
- Bellissimo, D.C.; Chen, C.H.; Zhu, Q.; Bagga, S.; Lee, C.T.; He, B.; Wertheim, G.B.; Jordan, M.; Tan, K.; Worthen, G.S.; et al. Runx1 negatively regulates inflammatory cytokine production by neutrophils in response to Toll-like receptor signaling. Blood Adv. 2020, 4, 1145–1158. [Google Scholar] [CrossRef]
- Tsoyi, K.; Geldart, A.M.; Christou, H.; Liu, X.; Chung, S.W.; Perrella, M.A. Elk-3 is a KLF4-regulated gene that modulates the phagocytosis of bacteria by macrophages. J. Leukoc. Biol. 2015, 97, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Solimando, A.G.; Pezzella, F. The Anti-VEGF(R) Drug Discovery Legacy: Improving Attrition Rates by Breaking the Vicious Cycle of Angiogenesis in Cancer. Cancers 2021, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.B.; Zaorsky, N.G.; Deng, L.; Wang, H.H.; Chao, J.; Zhao, L.J.; Yuan, Z.Y.; Ping, W. Pericytes: A double-edged sword in cancer therapy. Future Oncol. 2015, 11, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Spidlen, J.; Breuer, K.; Rosenberg, C.; Kotecha, N.; Brinkman, R.R. FlowRepository: A resource of annotated flow cytometry datasets associated with peer-reviewed publications. Cytometry A 2012, 81, 727–731. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Direction | Sequence (5′–3′) | Tm (°C) |
|---|---|---|---|
| Stat1 | Forward primer | ACCACCTCTCTTCCTGTCGT | 55.6 |
| Reverse primer | GATTCCTGGGCTCTGTCACC | 59 | |
| Ccl4 | Forward primer | CCTTCTGTGCTCCAGGGTTC | 59.3 |
| Reverse primer | TGCCTCTTTTGGTCAGGAATAC | 58.7 | |
| Cav1 | Forward primer | GCAGACGAGGTGACTGAGAA | 55.5 |
| Reverse primer | AAATGCCCCAGATGAGTGCC | 62 | |
| Coro7 | Forward primer | CAGCAGCCCTTTACCCCTAC | 59.1 |
| Reverse primer | CACCATCACACACCCAGACA | 57.2 | |
| Gnt2 | Forward primer | GCAATGGGCGACAAAGGAA | 61.6 |
| Reverse primer | TGCGAAGCGAGTCTATCAGC | 59 | |
| Actb | Forward primer | GCTGTCCCTGTATGCCTCTG | 57.8 |
| Reverse primer | ATGTCACGCACGATTTCCCT | 60.1 |
| Avg. Exp | Patient | Topical Gel | Triple Therapy (DT + Control/Caerin) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CCI | CCII | MΦ Type | Control | Caerin | MΦ Type | Control | Caerin | MΦ Type | |
| Fn1 | 3.958464 | 0.756023 | MΦ/DC | 8.651744 | 6.683293 | Arg1B | 1.509254 | 0.796691 | M2 |
| 1.104985 | 3.562443 | M2 | |||||||
| Vegfa | 2.032601 | 3.077626 | M2 | 1.381038 | 1.730747 | MΦ/DC | 0.436143 | 0.254142 | MΦ/DC |
| 1.530725 | 2.29818 | TAM | |||||||
| 0.781789 | 2.755229 | MΦ/DC | |||||||
| 0.159604 | 0.844889 | M1 | |||||||
| Aig1 | 1.03329 | 0.633123 | M2 | 0.151279 | 0.272457 | Resident | 0.371028 | 0.246382 | Ear2hi |
| 1.174192 | 0.529877 | MΦ/DC | |||||||
| 1.376729 | 0.733273 | TAM | |||||||
| Slamf7 | 1.039648 | 0.477852 | M2 | 0.931226 | 1.191878 | Arg1A | 2.951016 | 1.864526 | MHCIIhi |
| 0.752496 | 0.360924 | Resident | |||||||
| Igsf6 | 0.899768 | 0.273307 | MΦ/DC | 0.5863 | 0.481483 | M1 | 0.439447 | 1.820253 | MΦ/NKT |
| 0.889448 | 0.69455 | Arg1A | |||||||
| Rnf130 | 4.960039 | 3.241792 | TAM | 0.492294 | 0.612842 | M1 | 0.632459 | 1.614695 | MΦ/NKT |
| Dock5 | 2.297257 | 3.45598 | TAM | 0.183134 | 0.150017 | Arg1B | 0.11796 | 0.218823 | MΦ/DC |
| Rilpl2 | 0.712195 | 0.269815 | MΦ/DC | 0.51496 | 0.389841 | Resident | 0.424038 | 0.229098 | MΦ/DC |
| 0.408273 | 0.320012 | MΦ/DC | |||||||
| Pim3 | 0.414601 | 0.833308 | MΦ/DC | 0.499359 | 0.838651 | Ear2hi | 0.194824 | 0.325949 | MΦ/DC |
| 0.40287 | 0.641915 | Resident | |||||||
| 0.250678 | 1.079287 | TAM | |||||||
| Spint1 | 0.301725 | 0.978266 | TAM | 1.392293 | 1.701847 | Arg1B | 0.832327 | 1.36062 | Ear2hi |
| 0.43993 | 0.53739 | Arg1A | |||||||
| Cd151 | 0.240536 | 0.750399 | TAM | 0.475691 | 0.340939 | Arg1A | 0.360751 | 0.55949 | MΦ/DC |
| 0.200822 | 0.594903 | M2 | |||||||
| 0.182115 | 0.54708 | MΦ/DC | |||||||
| 0.160279 | 0.403624 | Resident | |||||||
| Hivep2 | 1.929148 | 3.051844 | MΦ/DC | 0.103692 | 0.182033 | M1 | 0.16374 | 0.106456 | M2 |
| Tnfaip3 | 1.254977 | 2.087381 | MΦ/DC | 0.574416 | 0.315302 | Arg1A | 0.151357 | 0.334904 | Ear2hi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, X.; Li, J.; Zhang, P.; Li, H.; Chen, G.; Zhang, W.; Wang, T.; Frazer, I.; Ni, G. Caerin 1.1/1.9 Enhances Antitumour Immunity by Activating the IFN-α Response Signalling Pathway of Tumour Macrophages. Cancers 2022, 14, 5785. https://doi.org/10.3390/cancers14235785
Yang X, Liu X, Li J, Zhang P, Li H, Chen G, Zhang W, Wang T, Frazer I, Ni G. Caerin 1.1/1.9 Enhances Antitumour Immunity by Activating the IFN-α Response Signalling Pathway of Tumour Macrophages. Cancers. 2022; 14(23):5785. https://doi.org/10.3390/cancers14235785
Chicago/Turabian StyleYang, Xiaodan, Xiaosong Liu, Junjie Li, Pingping Zhang, Hejie Li, Guoqiang Chen, Wei Zhang, Tianfang Wang, Ian Frazer, and Guoying Ni. 2022. "Caerin 1.1/1.9 Enhances Antitumour Immunity by Activating the IFN-α Response Signalling Pathway of Tumour Macrophages" Cancers 14, no. 23: 5785. https://doi.org/10.3390/cancers14235785
APA StyleYang, X., Liu, X., Li, J., Zhang, P., Li, H., Chen, G., Zhang, W., Wang, T., Frazer, I., & Ni, G. (2022). Caerin 1.1/1.9 Enhances Antitumour Immunity by Activating the IFN-α Response Signalling Pathway of Tumour Macrophages. Cancers, 14(23), 5785. https://doi.org/10.3390/cancers14235785






