Lipocalin 2 Reduces MET Levels by Inhibiting MEK/ERK Signaling to Inhibit Nasopharyngeal Carcinoma Cell Migration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics
2.2. Cells and Cell Culture
2.3. Small Interfering RNA (siRNA) Transfection
2.4. DNA Construction, Transient Transfection
2.5. Cell Growth
2.6. Cell Migration and Invasion Assays
2.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Real-Time Quantitative PCR
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
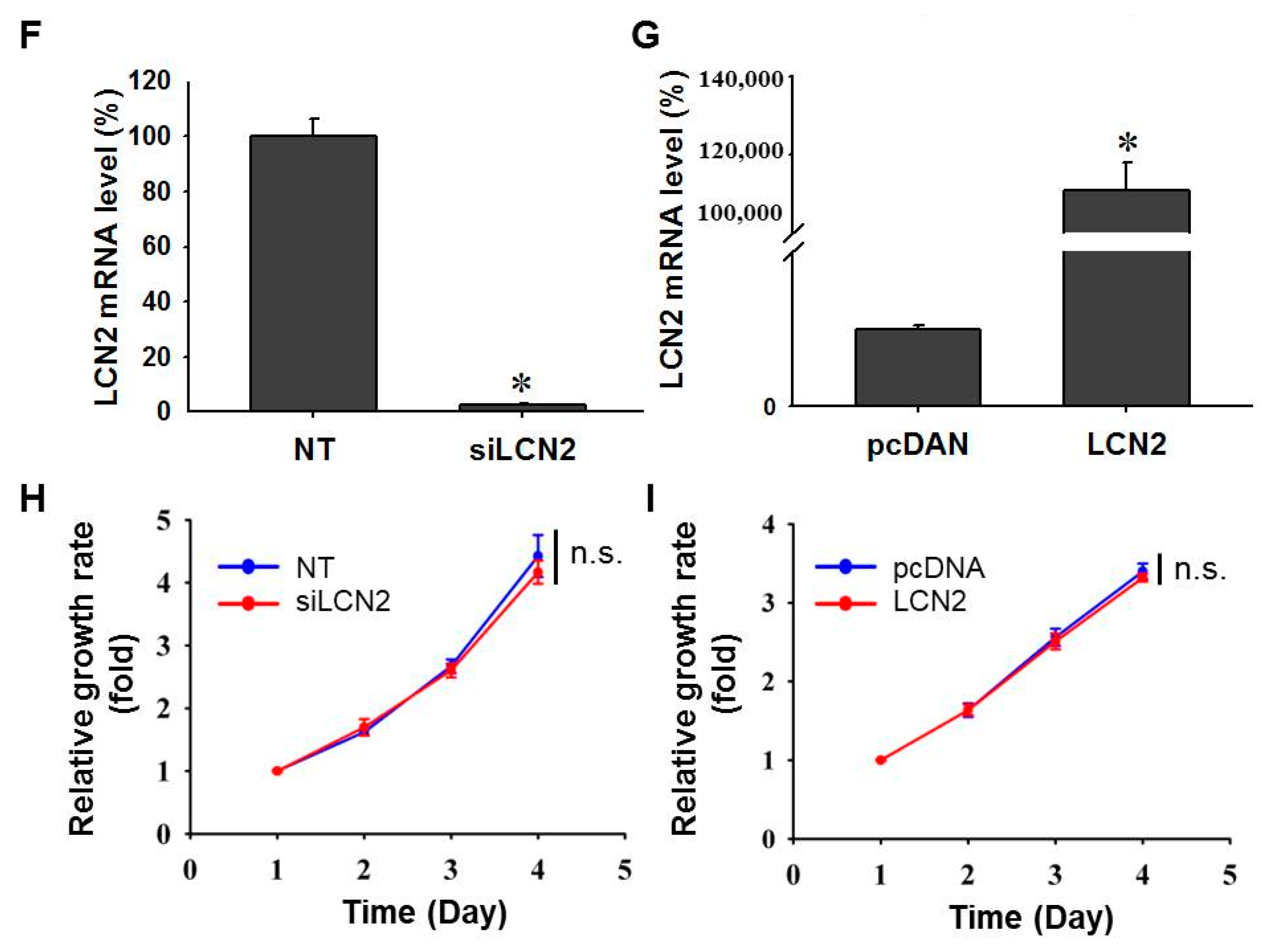
3.1. NPC Cell Growth Is Not Affected by Differential LCN2 Levels
3.2. NPC Cell Migration and Invasion Are Negatively Regulated by LCN2 Levels
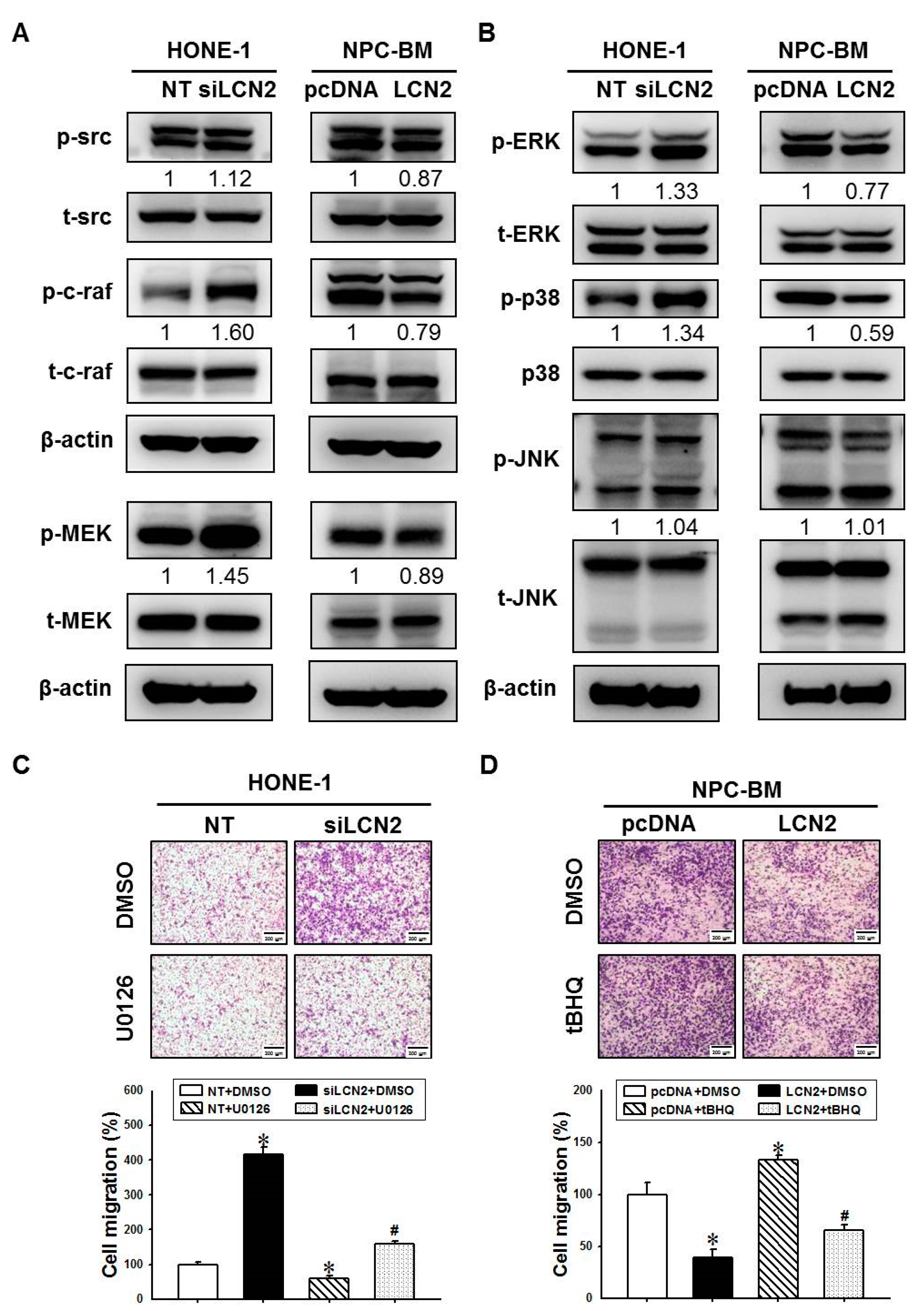
3.3. LCN2 Reduces Cell Migration by Inhibiting the MEK/ERK Signaling
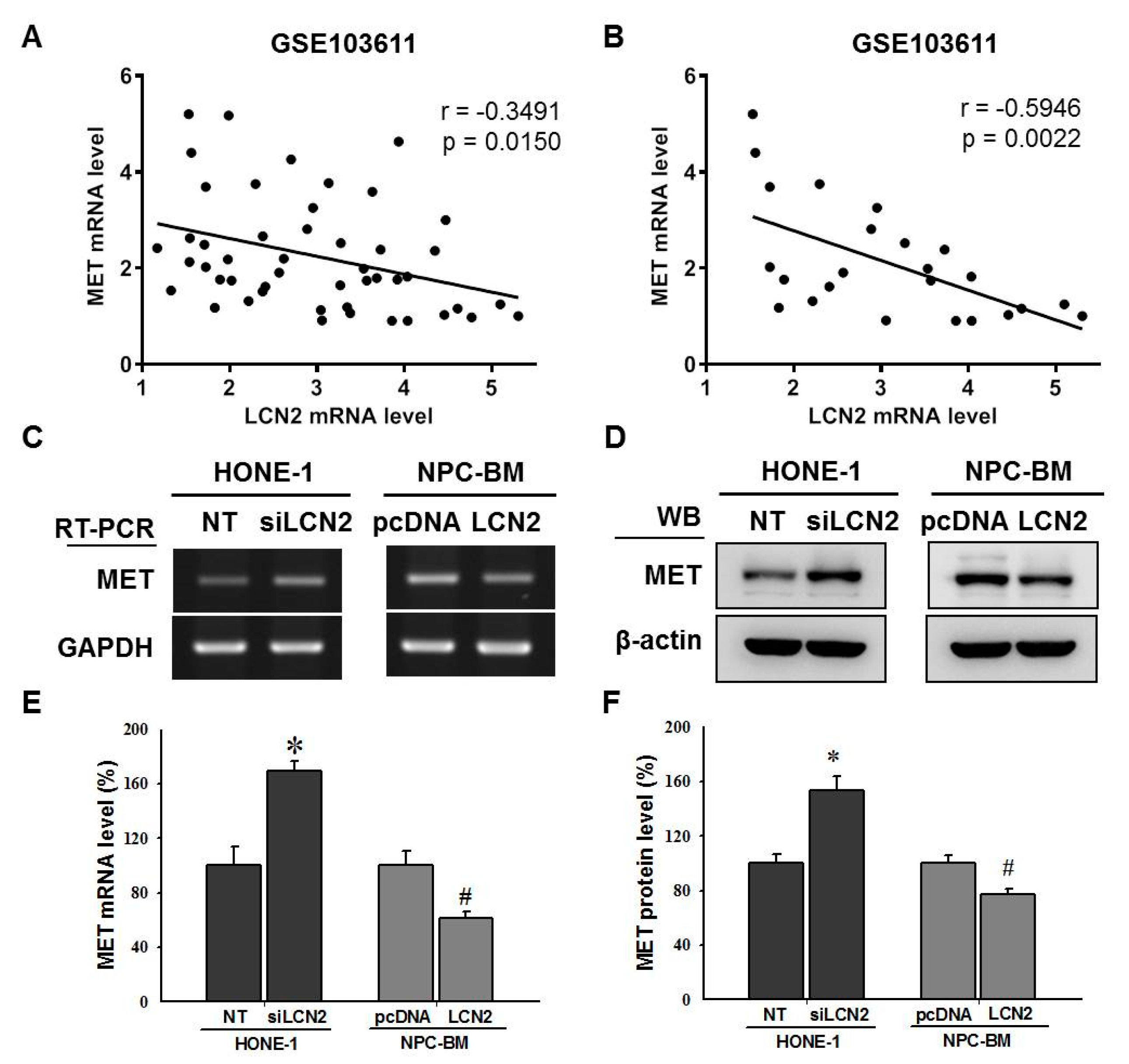
3.4. LCN2 Negatively Regulated MET Levels by Inhibiting the MEK/ERK Signaling Pathway
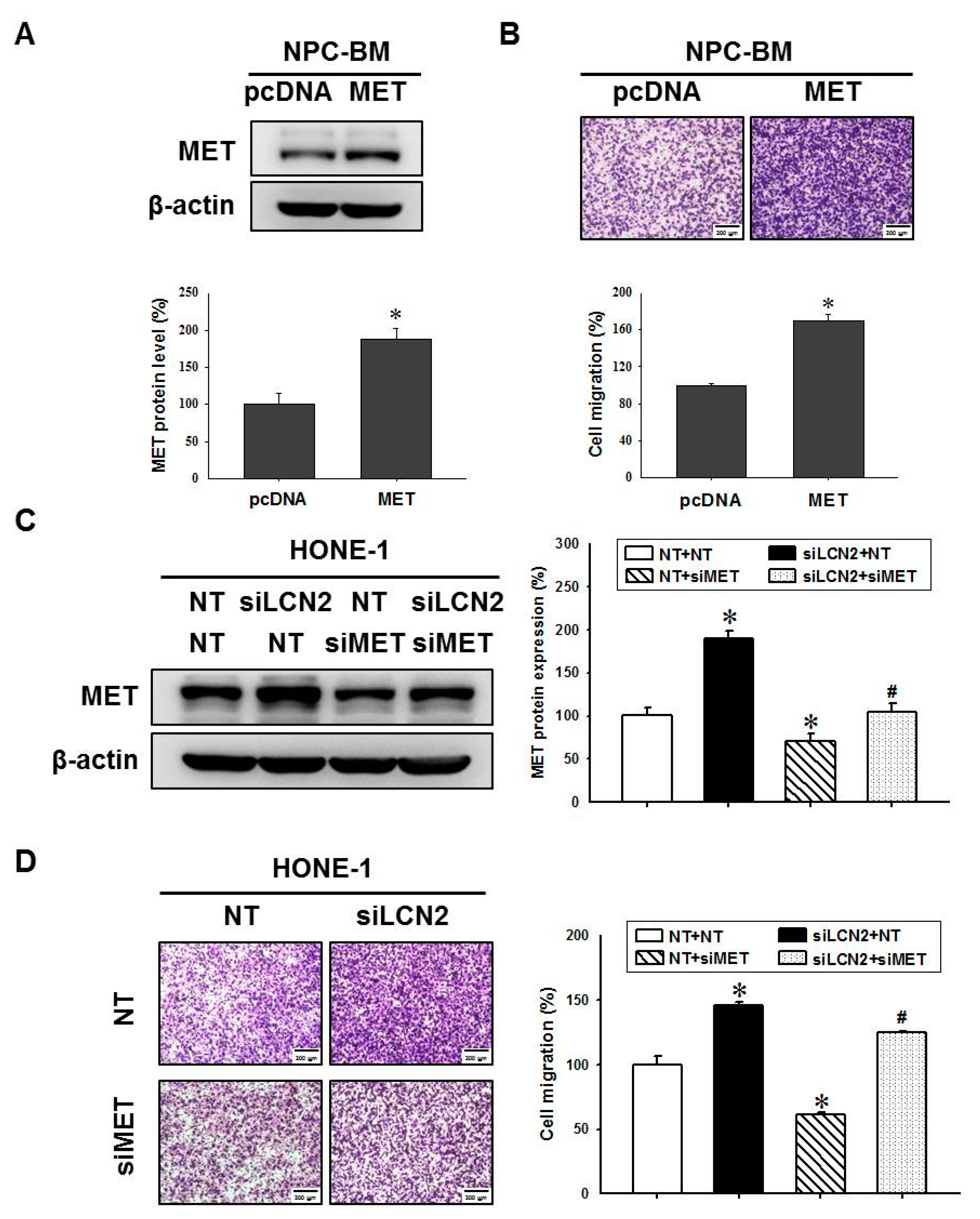
3.5. LCN2 Decreased Cell Migration by Reduced MET Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeyakumar, A.; Brickman, T.M.; Jeyakumar, A.; Doerr, T. Review of nasopharyngeal carcinoma. Ear Nose Throat J. 2006, 85, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Adami, H.-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1765. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Jian, J.J.-M.; Tsai, S.Y.C.; Chan, K.-Y.; Yen, L.K.; Chu, N.-M.; Tan, T.-D.; Tsou, M.-H.; Huang, A.T. Prognostic features and treatment outcome in locoregionally advanced nasopharyngeal carcinoma following concurrent chemotherapy and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 755–762. [Google Scholar] [CrossRef]
- Kong, F.-F.; Ying, H.; Du, C.-R.; Huang, S.; Zhou, J.-J.; Hu, C.-S. Effectiveness and toxicities of intensity-modulated radiation therapy for patients with t4 nasopharyngeal carcinoma. PLoS ONE 2014, 9, e91362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henderson, B.E.; Louie, E.; SooHoo Jing, J.; Buell, P.; Gardner, M.B. Risk factors associated with nasopharyngeal carcinoma. N. Engl. J. Med. 1976, 295, 1101–1106. [Google Scholar] [CrossRef]
- Hui, A.B.; Lo, K.W.; Leung, S.F.; Teo, P.; Fung, M.K.; To, K.F.; Wong, N.; Choi, P.H.; Lee, J.C.; Huang, D.P. Detection of recurrent chromosomal gains and losses in primary nasopharyngeal carcinoma by comparative genomic hybridisation. Int. J. Cancer 1999, 82, 498–503. [Google Scholar] [CrossRef]
- Young, L.S.; Murray, P.G. Epstein-barr virus and oncogenesis: From latent genes to tumours. Oncogene 2003, 22, 5108–5121. [Google Scholar] [CrossRef]
- Yang, J.; Moses, M.A. Lipocalin 2: A multifaceted modulator of human cancer. Cell Cycle 2009, 8, 2347–2352. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Wang, L.; Zeng, L.; Tu, Z.-W. Lcn2 is a potential biomarker for radioresistance and recurrence in nasopharyngeal carcinoma. Front. Oncol. 2021, 10, 3292. [Google Scholar] [CrossRef]
- Guo, Y.; Zhai, J.; Zhang, J.; Zhou, H. Ngal protects in nasopharyngeal carcinoma by inducing apoptosis and blocking epithelial-mesenchymal transition. Oncol. Lett. 2020, 19, 3711–3718. [Google Scholar] [CrossRef]
- Tabassum, D.P.; Polyak, K. Tumorigenesis: It takes a village. Nat. Rev. Cancer 2015, 15, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, E.K.; Lee, K.J.; Hong, S.W.; Yoon, Y.; Kim, J.S. Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int. J. Cancer 2006, 118, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Hanai, J.; Mammoto, T.; Seth, P.; Mori, K.; Karumanchi, S.A.; Barasch, J.; Sukhatme, V.P. Lipocalin 2 diminishes invasiveness and metastasis of ras-transformed cells. J. Biol. Chem. 2005, 280, 13641–13647. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.M.; Der, C.J. Oncogenic ras and its role in tumor cell invasion and metastasis. Semin. Cancer Biol. 2004, 14, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Pan, W.-W.; Liu, S.-B.; Shen, Z.-F.; Xu, Y.; Hu, L.-L. Erk/mapk signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; He, W.; Ren, C.; Qiao, J.; Guo, Q.; Hu, J.; Xu, H.; Jiang, X.; Wang, L. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct. Target. Ther. 2020, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single–cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar]
- Ho, H.Y.; Lin, C.W.; Chien, M.H.; Reiter, R.J.; Su, S.C.; Hsieh, Y.H.; Yang, S.F. Melatonin suppresses tpa-induced metastasis by downregulating matrix metalloproteinase-9 expression through jnk/sp-1 signaling in nasopharyngeal carcinoma. J. Pineal Res. 2016, 61, 479–492. [Google Scholar] [CrossRef]
- Chien, M.H.; Ying, T.H.; Yang, S.F.; Yu, J.K.; Hsu, C.W.; Hsieh, S.C.; Hsieh, Y.H. Lipocalin-2 induces apoptosis in human hepatocellular carcinoma cells through activation of mitochondria pathways. Cell Biochem. Biophys. 2012, 64, 177–186. [Google Scholar] [CrossRef]
- Ho, H.Y.; Lin, F.C.; Chen, P.N.; Chen, M.K.; Hsin, C.H.; Yang, S.F.; Lin, C.W. Tricetin suppresses migration and presenilin-1 expression of nasopharyngeal carcinoma through akt/gsk-3β pathway. Am. J. Chin. Med. 2020, 48, 1203–1220. [Google Scholar] [CrossRef]
- Hsin, C.H.; Huang, C.C.; Chen, P.N.; Hsieh, Y.S.; Yang, S.F.; Ho, Y.T.; Lin, C.W. Rubus idaeus inhibits migration and invasion of human nasopharyngeal carcinoma cells by suppression of mmp-2 through modulation of the erk1/2 pathway. Am. J. Chin. Med. 2017, 45, 1557–1572. [Google Scholar] [CrossRef]
- Su, S.C.; Yeh, C.M.; Lin, C.W.; Hsieh, Y.H.; Chuang, C.Y.; Tang, C.H.; Lee, Y.C.; Yang, S.F. A novel melatonin-regulated lncrna suppresses tpa-induced oral cancer cell motility through replenishing prune2 expression. J. Pineal Res. 2021, 71, e12760. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jacobson, K.; Schaller, M.D. Map kinases and cell migration. J. Cell Sci 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Favata, M.F.; Horiuchi, K.Y.; Manos, E.J.; Daulerio, A.J.; Stradley, D.A.; Feeser, W.S.; Van Dyk, D.E.; Pitts, W.J.; Earl, R.A.; Hobbs, F.; et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998, 273, 18623–18632. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Tan, T.H.; Kong, A.N. Butylated hydroxyanisole and its metabolite tert-butylhydroquinone differentially regulate mitogen-activated protein kinases. The role of oxidative stress in the activation of mitogen-activated protein kinases by phenolic antioxidants. J. Biol. Chem. 1997, 272, 28962–28970. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Yang, J.-S.; Hsieh, Y.-H.; Chu, H.-J.; Chou, C.-H.; Lu, E.W.-H.; Lin, C.-W.; Yang, S.-F. Lipocalin-2 inhibits osteosarcoma cell metastasis by suppressing met expression via the mek–erk pathway. Cancers 2021, 13, 3181. [Google Scholar] [CrossRef]
- Qian, C.N.; Guo, X.; Cao, B.; Kort, E.J.; Lee, C.C.; Chen, J.; Wang, L.M.; Mai, W.Y.; Min, H.Q.; Hong, M.H.; et al. Met protein expression level correlates with survival in patients with late-stage nasopharyngeal carcinoma. Cancer Res. 2002, 62, 589–596. [Google Scholar]
- Li, Y.; Zhang, S.; Tang, Z.; Chen, J.; Kong, W. Silencing of c-met by rna interference inhibits the survival, proliferation, and invasion of nasopharyngeal carcinoma cells. Tumour Biol. 2011, 32, 1217–1224. [Google Scholar] [CrossRef]
- Lee, B.S.; Kang, S.; Kim, K.A.; Song, Y.J.; Cheong, K.H.; Cha, H.Y.; Kim, C.H. Met degradation by sait301, a met monoclonal antibody, reduces the invasion and migration of nasopharyngeal cancer cells via inhibition of egr-1 expression. Cell Death Dis. 2014, 5, e1159. [Google Scholar] [CrossRef]
- Liao, S.K.; Perng, Y.P.; Shen, Y.C.; Chung, P.J.; Chang, Y.S.; Wang, C.H. Chromosomal abnormalities of a new nasopharyngeal carcinoma cell line (npc-bm1) derived from a bone marrow metastatic lesion. Cancer Genet. Cytogenet. 1998, 103, 52–58. [Google Scholar] [CrossRef]
- Rahimi, S.; Roushandeh, A.M.; Ahmadzadeh, E.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Implication and role of neutrophil gelatinase-associated lipocalin in cancer: Lipocalin-2 as a potential novel emerging comprehensive therapeutic target for a variety of cancer types. Mol. Biol. Rep. 2020, 47, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Sanchez, G.S.; Pita-Grisanti, V.; Quinones-Diaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejia, P.E. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; Susin, S.A. Revisiting neutrophil gelatinase-associated lipocalin (ngal) in cancer: Saint or sinner? Cancers 2018, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Roli, L.; Pecoraro, V.; Trenti, T. Can ngal be employed as prognostic and diagnostic biomarker in human cancers? A systematic review of current evidence. Int. J. Biol. Markers 2017, 32, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Yang, W.E.; Lee, W.J.; Hua, K.T.; Hsieh, F.K.; Hsiao, M.; Chen, C.C.; Chow, J.M.; Chen, M.K.; Yang, S.F.; et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase ix inhibition and is associated with favourable prognosis. Carcinogenesis 2016, 37, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Maestro, R.; Polesel, J.; Catania, A.; Maira, F.; Signorelli, S.S.; McCubrey, J.A.; Libra, M. Roles of neutrophil gelatinase-associated lipocalin (ngal) in human cancer. Oncotarget 2014, 5, 1576–1594. [Google Scholar] [CrossRef]
- Leng, X.; Wu, Y.; Arlinghaus, R.B. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J. Cell. Physiol. 2011, 226, 309–314. [Google Scholar] [CrossRef]
- Volpe, V.; Raia, Z.; Sanguigno, L.; Somma, D.; Mastrovito, P.; Moscato, F.; Mellone, S.; Leonardi, A.; Pacifico, F. Ngal controls the metastatic potential of anaplastic thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2013, 98, 228–235. [Google Scholar] [CrossRef]
- Tung, M.-C.; Hsieh, S.-C.; Yang, S.-F.; Cheng, C.-W.; Tsai, R.-T.; Wang, S.-C.; Huang, M.-H.; Hsieh, Y.-H. Knockdown of lipocalin-2 suppresses the growth and invasion of prostate cancer cells. Prostate 2013, 73, 1281–1290. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Li, F.; Hua, Q.; Chen, S.; Xiao, B.; Dai, M.; Li, M.; Zheng, A.; Yu, D.; et al. Down-regulation of neutrophil gelatinase-associated lipocalin in head and neck squamous cell carcinoma correlated with tumorigenesis, not with metastasis. Int. J. Clin. Exp. Pathol. 2015, 8, 8857–8868. [Google Scholar]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Luan, T.; Yu, Y. Increased hepatocyte growth factor and c-met receptor expression in nasopharyngeal carcinoma. Int. J. Clin. Exp. Med. 2014, 7, 5583–5587. [Google Scholar] [PubMed]
- Zheng, P.-C.; Chen, X.; Zhu, H.-W.; Zheng, W.; Mao, L.-H.; Lin, C.; Liu, J.-N.; Zheng, M. Capn4 is a marker of poor clinical outcomes and promotes nasopharyngeal carcinoma metastasis via nuclear factor-κb-induced matrix metalloproteinase 2 expression. Cancer Sci. 2014, 105, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Xiang, Y.; Guo, X.; Lu, J.; Xia, W.; Yu, Y.; Peng, Y.; Wang, L.; Wang, G.; Ye, Y.; et al. C-src activation promotes nasopharyngeal carcinoma metastasis by inducing the epithelial-mesenchymal transition via pi3k/akt signaling pathway: A new and promising target for npc. Oncotarget 2016, 7, 28340–28355. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xie, X.; Yang, M.; Wang, Y.; Wu, H.; Deng, T.; Weng, X.; Wen, W.; Nie, G. Ybx3 mediates the metastasis of nasopharyngeal carcinoma via pi3k/akt signaling. Front. Oncol. 2021, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guan, Y.; Zeng, L.; Liu, G.; Zhu, Y.; Xu, H.; Lu, Y.; Liu, J.; Guo, J.; Feng, X.; et al. High cox-2 expression contributes to a poor prognosis through the inhibition of chemotherapy-induced senescence in nasopharyngeal carcinoma. Int. J. Oncol. 2018, 53, 1138–1148. [Google Scholar] [CrossRef]
- Huang, T.; Yin, L.; Wu, J.; Gu, J.J.; Ding, K.; Zhang, N.; Du, M.Y.; Qian, L.X.; Lu, Z.W.; He, X. Tnfaip3 inhibits migration and invasion in nasopharyngeal carcinoma by suppressing epithelial mesenchymal transition. Neoplasma 2017, 64, 389–394. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-P.; Lin, C.-W.; Huang, C.-C.; Lu, Y.-T.; Ho, Y.-T.; Yang, S.-F.; Hsin, C.-H. Lipocalin 2 Reduces MET Levels by Inhibiting MEK/ERK Signaling to Inhibit Nasopharyngeal Carcinoma Cell Migration. Cancers 2022, 14, 5707. https://doi.org/10.3390/cancers14225707
Li J-P, Lin C-W, Huang C-C, Lu Y-T, Ho Y-T, Yang S-F, Hsin C-H. Lipocalin 2 Reduces MET Levels by Inhibiting MEK/ERK Signaling to Inhibit Nasopharyngeal Carcinoma Cell Migration. Cancers. 2022; 14(22):5707. https://doi.org/10.3390/cancers14225707
Chicago/Turabian StyleLi, Ju-Pi, Chiao-Wen Lin, Cheng-Chen Huang, Yen-Ting Lu, Yu-Ting Ho, Shun-Fa Yang, and Chung-Han Hsin. 2022. "Lipocalin 2 Reduces MET Levels by Inhibiting MEK/ERK Signaling to Inhibit Nasopharyngeal Carcinoma Cell Migration" Cancers 14, no. 22: 5707. https://doi.org/10.3390/cancers14225707
APA StyleLi, J.-P., Lin, C.-W., Huang, C.-C., Lu, Y.-T., Ho, Y.-T., Yang, S.-F., & Hsin, C.-H. (2022). Lipocalin 2 Reduces MET Levels by Inhibiting MEK/ERK Signaling to Inhibit Nasopharyngeal Carcinoma Cell Migration. Cancers, 14(22), 5707. https://doi.org/10.3390/cancers14225707







