Approaches to the Management of Metastatic Adenoid Cystic Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Biology of ACC
2.1. Cellular Origin and Histopathology
2.2. Genomic Landscape
2.2.1. MYB/MYBL1
2.2.2. Notch Signaling
2.2.3. Chromatin State Regulators
2.3. Immune Microenvironment
3. Adjuvant Chemoradiation
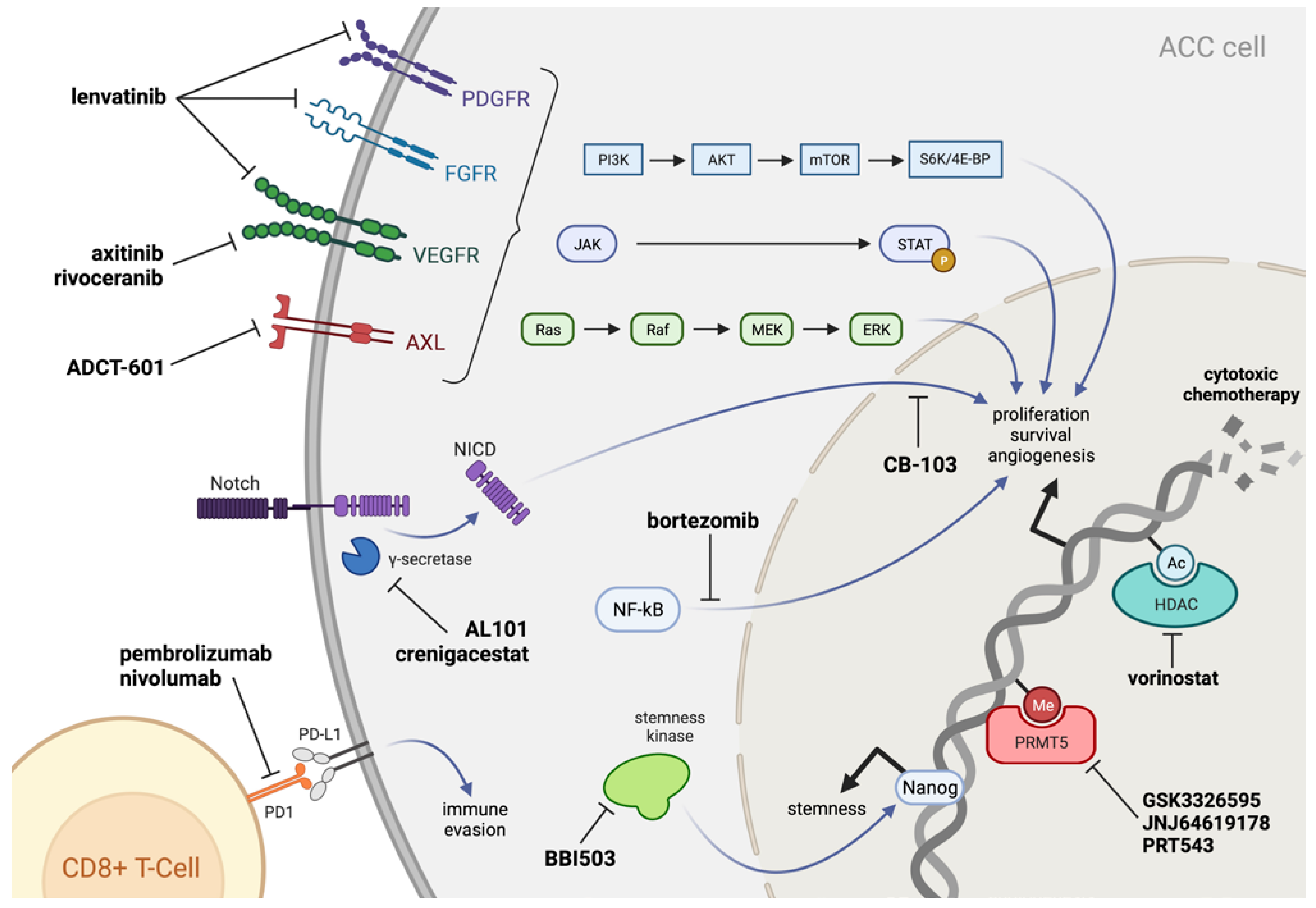
4. Systemic Agents for Recurrent/Metastatic ACC
4.1. Cytotoxic Chemotherapy
4.2. Tyrosine Kinase Inhibitors
4.3. Immunotherapy
4.4. Biological Therapy (Non-TKI)
5. Emerging Therapies for ACC
5.1. Notch Signaling Pathway
5.2. Stemness Inhibitors
5.3. PRMT5 Inhibitors
5.4. Axl Pathway Inhibitors
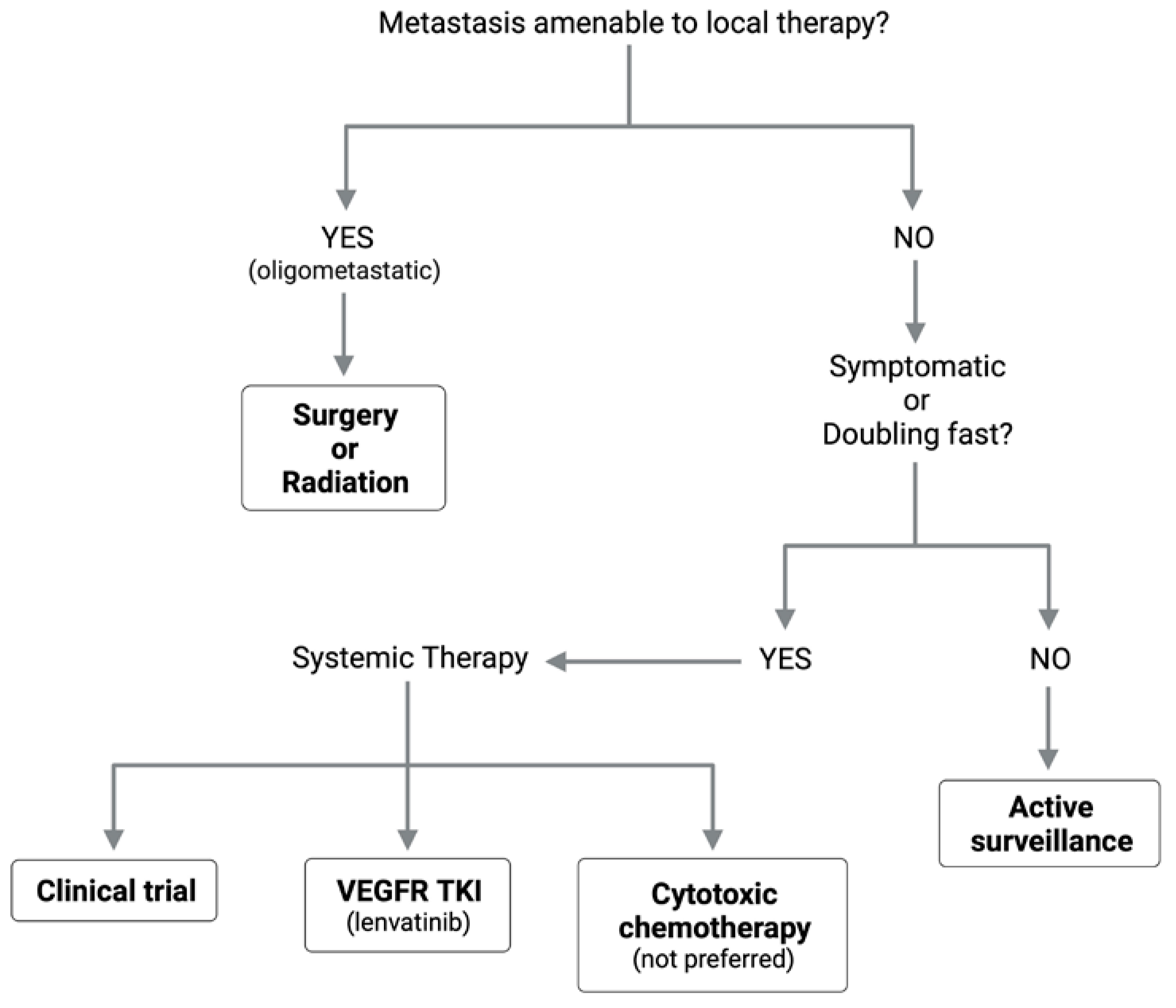
6. Local Therapy for Oligometastatic Lung Disease
6.1. Burden of Lung Metastasis in ACC
6.2. Pulmonary Metastasectomy
6.3. Radiation for Pulmonary Metastases
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | adenoid cystic carcinoma |
| CAP | cyclophosphamide-doxorubicin-cisplatin |
| CR | complete response |
| DFI | disease-free interval |
| DFS | disease-free survival |
| DM | distant metastasis |
| HNC | head and neck cancer |
| HNSCC | head and neck squamous cell carcinoma |
| ICI | immune checkpoint inhibitor |
| OS | overall survival |
| PD-1 | programmed death-1 receptor |
| PD-L | programmed death ligand |
| PFS | progression-free survival |
| PM | pulmonary metastasectomy |
| PR | partial response |
| R/M | recurrent/metastatic |
| RT | radiation therapy |
| SBRT | stereotactic body radiation therapy |
| SCC | squamous cell carcinoma |
| SD | stable disease |
| TKI | tyrosine kinase inhibitor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; vander Poorten, V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M.; Takes, R.P.; et al. Adenoid Cystic Carcinoma of the Head and Neck—An Update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.J.; Migliacci, J.; Karassawa Zanoni, D.; McGill, M.; Patel, S.; Ganly, I. Minor Salivary Gland Tumors of the Head and Neck-Memorial Sloan Kettering Experience: Incidence and Outcomes by Site and Histological Type. Cancer 2019, 125, 3354–3366. [Google Scholar] [CrossRef]
- Jones, A.V.; Craig, G.T.; Speight, P.M.; Franklin, C.D. The Range and Demographics of Salivary Gland Tumours Diagnosed in a UK Population. Oral Oncol. 2008, 44, 407–417. [Google Scholar] [CrossRef]
- Giannini, P.J.; Shetty, K.V.; Horan, S.L.; Reid, W.D.; Litchmore, L.L. Adenoid Cystic Carcinoma of the Buccal Vestibule: A Case Report and Review of the Literature. Oral Oncol. 2006, 42, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Ellington, C.L.; Goodman, M.; Kono, S.A.; Grist, W.; Wadsworth, T.; Chen, A.Y.; Owonikoko, T.; Ramalingam, S.; Shin, D.M.; Khuri, F.R.; et al. Adenoid Cystic Carcinoma of the Head and Neck: Incidence and Survival Trends Based on 1973-2007 Surveillance, Epidemiology, and End Results Data. Cancer 2012, 118, 4444–4451. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Glick, J.; Yang, X.; Lei, D.; Bjoerndal, K.; et al. Elective Neck Dissection in Patients with Head and Neck Adenoid Cystic Carcinoma: An International Collaborative Study. Ann. Surg. Oncol. 2015, 22, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.G.; Amaral, A.L.P.; Prado, L.A.F.; Kligerman, J.; Silveira, T.R.P. Adenoid Cystic Carcinoma of Salivary Glands. A Study of 61 Cases with Clinicopathologic Correlation. Cancer 1986, 57, 312–319. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Zhan, C.; Zhang, Y.; Hou, J.; Yin, X. Perineural Invasion in Adenoid Cystic Carcinoma of the Salivary Glands: Where We Are and Where We Need to Go. Front. Oncol. 2020, 10, 1493. [Google Scholar] [CrossRef]
- Spiro, R.H. Distant Metastasis in Adenoid Cystic Carcinoma of Salivary Origin. Am. J. Surg. 1997, 174, 495–498. [Google Scholar] [CrossRef]
- Terhaard, C.H.J.; Lubsen, H.; van der Tweel, I.; Hilgers, F.J.M.; Eijkenboom, W.M.H.; Marres, H.A.M.; Tjho-Heslinga, R.E.; de Jong, J.M.A.; Roodenburg, J.L.N. Salivary Gland Carcinoma: Independent Prognostic Factors for Locoregional Control, Distant Metastases, and Overall Survival: Results of the Dutch Head and Neck Oncology Cooperative Group. Head Neck 2004, 26, 681–693. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Hoffman, M.P. Anatomy, Biogenesis and Regeneration of Salivary Glands. Monogr. Oral Sci. 2014, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef]
- Chaudhry, A.P.; Leifer, C.; Cutler, L.S.; Satchidanand, S.; Labay, G.R.; Yamane, G.M. Histogenesis of Adenoid Cystic Carcinoma of the Salivary Glands. Light and Electronmicroscopic Study. Cancer 1986, 58, 72–82. [Google Scholar] [CrossRef]
- Moskaluk, C.A. Adenoid Cystic Carcinoma: Clinical and Molecular Features. Head Neck Pathol. 2013, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Azumi, N.; Battifora, H. The Cellular Composition of Adenoid Cystic Carcinoma. An Immunohistochemical Study. Cancer 1987, 60, 1589–1598. [Google Scholar] [CrossRef]
- Van Weert, S.; van der Waal, I.; Witte, B.I.; René Leemans, C.; Bloemena, E. Histopathological Grading of Adenoid Cystic Carcinoma of the Head and Neck: Analysis of Currently Used Grading Systems and Proposal for a Simplified Grading Scheme. Oral Oncol. 2015, 51, 71–76. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Penner, C.; Ho, N.J.; Foss, R.D.; Miettinen, M.; Wieneke, J.A.; Moskaluk, C.A.; Stelow, E.B. Sinonasal Tract and Nasopharyngeal Adenoid Cystic Carcinoma: A Clinicopathologic and Immunophenotypic Study of 86 Cases. Head Neck Pathol. 2014, 8, 88–109. [Google Scholar] [CrossRef]
- Du, F.; Zhou, C.X.; Gao, Y. Myoepithelial Differentiation in Cribriform, Tubular and Solid Pattern of Adenoid Cystic Carcinoma: A Potential Involvement in Histological Grading and Prognosis. Ann. Diagn. Pathol. 2016, 22, 12–17. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole Exome Sequencing of Adenoid Cystic Carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Frierson, H.F.; Moskaluk, C.A. Mutation Signature of Adenoid Cystic Carcinoma: Evidence for Transcriptional and Epigenetic Reprogramming. J. Clin. Investig. 2013, 123, 2783–2785. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Andrén, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent Fusion of MYB and NFIB Transcription Factor Genes in Carcinomas of the Breast and Head and Neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744. [Google Scholar] [CrossRef]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.T.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The Mutational Landscape of Adenoid Cystic Carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.B.; Kanner, W.A.; Fehr, A.; Andrén, Y.; Moskaluk, C.A.; Löning, T.; Stenman, G.; Frierson, H.F. Analysis of MYB Expression and MYB-NFIB Gene Fusions in Adenoid Cystic Carcinoma and Other Salivary Neoplasms. Mod. Pathol. 2011, 24, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Lao, X.; Liang, Y. The Value of MYB as a Prognostic Marker for Adenoid Cystic Carcinoma: Meta-Analysis. Head Neck 2019, 41, 1517–1524. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Valero Mayor, C.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic Hallmarks of Recurrent/Metastatic Adenoid Cystic Carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhou, C.X.; Li, T.J. Notch Activation Leads to Loss of Myoepithelial Differentiation and Poor Outcome in Solid Adenoid Cystic Carcinoma. Oral Dis. 2020, 26, 1677–1686. [Google Scholar] [CrossRef]
- Rettig, E.M.; Talbot, C.C.; Sausen, M.; Jones, S.; Bishop, J.A.; Wood, L.D.; Tokheim, C.; Niknafs, N.; Karchin, R.; Fertig, E.J.; et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev. Res. 2016, 9, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Jagielska, B.; Sarnowska, E.; Rusetska, N.; Jancewicz, I.; Durzynska, M.; Kubala, S.; Chmielik, E.; Paul, P.; Rutkowski, T.; Sarnowski, T.J.; et al. Advanced Adenoid Cystic Carcinoma (ACC) Is Featured by SWI/SNF Chromatin Remodeling Complex Aberrations. J. Cancer Res. Clin. Oncol. 2019, 145, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, C.; de Arruda, J.A.A.; de Faria, A.C.R.; Oliveira, G.A.Q.; de Paula, H.M.; Fonseca, F.P.; Mesquita, R.A.; Silva, T.A.; Mendonça, E.F.; Batista, A.C. Immune Microenvironment and Evasion Mechanisms in Adenoid Cystic Carcinomas of Salivary Glands. Oral Oncol. 2019, 88, 95–101. [Google Scholar] [CrossRef]
- Ock, C.Y.; Keam, B.; Kim, S.; Lee, J.S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, M.; Kuo, F.; Katabi, N.; Lee, M.; Nadeem, Z.; Dalin, M.G.; Makarov, V.; Chowell, D.; Dogan, S.; Ganly, I.; et al. The Immune Microenvironment and Neoantigen Landscape of Aggressive Salivary Gland Carcinomas Differ by Subtype. Clin. Cancer Res. 2020, 26, 2859–2870. [Google Scholar] [CrossRef]
- Botticelli, A.; Cirillo, A.; Strigari, L.; Valentini, F.; Cerbelli, B.; Scagnoli, S.; Cerbelli, E.; Zizzari, I.G.; della Rocca, C.; D’Amati, G.; et al. Anti-PD-1 and Anti-PD-L1 in Head and Neck Cancer: A Network Meta-Analysis. Front. Immunol. 2021, 12, 705096. [Google Scholar] [CrossRef] [PubMed]
- Crosta, S.; Boldorini, R.; Bono, F.; Brambilla, V.; Dainese, E.; Fusco, N.; Gianatti, A.; L’Imperio, V.; Morbini, P.; Pagni, F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers 2021, 13, 292. [Google Scholar] [CrossRef]
- Sridharan, V.; Gjini, E.; Liao, X.; Chau, N.G.; Haddad, R.I.; Severgnini, M.; Hammerman, P.; El-Naggar, A.; Freeman, G.J.; Hodi, F.S.; et al. Immune Profiling of Adenoid Cystic Carcinoma: PD-L2 Expression and Associations with Tumor-Infiltrating Lymphocytes. Cancer Immunol. Res. 2016, 4, 679–687. [Google Scholar] [CrossRef]
- Chen, W.; Fung, A.S.; McIntyre, J.B.; Simpson, R.; Afzal, A.R.; Hao, D.; Lau, H. Assessment of Tumour Infiltrating Lymphocytes And Pd-L1 Expression In Adenoid Cystic Carcinoma Of The Salivary Gland. Clin. Investig. Med. 2021, 44, 38–41. [Google Scholar] [CrossRef]
- Chang, H.; Kim, J.S.; Choi, Y.J.; Cho, J.G.; Woo, J.S.; Kim, A.; Kim, J.S.; Kang, E.J. Overexpression of PD-L2 Is Associated with Shorter Relapse-Free Survival in Patients with Malignant Salivary Gland Tumors. Onco Targets Ther. 2017, 10, 2983–2992. [Google Scholar] [CrossRef]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, M.J.; Tanvetyanon, T.; Mccaffrey, J.C.; Otto, K.J.; Padhya, T.A.; Kish, J.; Trotti, A.M.; Harrison, L.B.; Caudell, J.J. Adjuvant Radiotherapy versus Concurrent Chemoradiotherapy for the Management of High-Risk Salivary Gland Carcinomas. Head Neck 2016, 38, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Vempati, P.; Miles, B.; Teng, M.; Genden, E.; Demicco, E.G.; Misiukiewicz, K.; Posner, M.; Gupta, V.; Bakst, R.L. Adjuvant Therapy for Salivary Gland Carcinomas. Anticancer Res. 2016, 36, 4165–4170. [Google Scholar]
- Schoenfeld, J.D.; Sher, D.J.; Norris, C.M.; Haddad, R.I.; Posner, M.R.; Balboni, T.A.; Tishler, R.B. Salivary Gland Tumors Treated with Adjuvant Intensity-Modulated Radiotherapy with or without Concurrent Chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hsieh, M.Y.; Chen, M.K.; Chou, M.C. Adenoid Cystic Carcinoma of Head and Neck: A Retrospective Clinical Analysis of a Single Institution. Auris Nasus Larynx 2018, 45, 831–837. [Google Scholar] [CrossRef]
- Hsieh, C.E.; Lin, C.Y.; Lee, L.Y.; Yang, L.Y.; Wang, C.C.; Wang, H.M.; Chang, J.T.C.; Fan, K.H.; Liao, C.T.; Yen, T.C.; et al. Adding Concurrent Chemotherapy to Postoperative Radiotherapy Improves Locoregional Control but Not Overall Survival in Patients with Salivary Gland Adenoid Cystic Carcinoma-a Propensity Score Matched Study. Radiat. Oncol. 2016, 11, 47. [Google Scholar] [CrossRef]
- Ishida, E.; Ogawa, T.; Rokugo, M.; Ishikawa, T.; Wakamori, S.; Ohkoshi, A.; Usubuchi, H.; Higashi, K.; Ishii, R.; Nakanome, A.; et al. Management of Adenoid Cystic Carcinoma of the Head and Neck: A Single-Institute Study with over 25-Year Follow-Up. Head Face Med. 2020, 16, 14. [Google Scholar] [CrossRef]
- Laurie, S.A.; Ho, A.L.; Fury, M.G.; Sherman, E.; Pfister, D.G. Systemic Therapy in the Management of Metastatic or Locally Recurrent Adenoid Cystic Carcinoma of the Salivary Glands: A Systematic Review. Lancet Oncol. 2011, 12, 815–824. [Google Scholar] [CrossRef]
- Sahara, S.; Herzog, A.E.; Nör, J.E. Systemic Therapies for Salivary Gland Adenoid Cystic Carcinoma. Am. J. Cancer Res. 2021, 11, 4092–4110. [Google Scholar] [PubMed]
- de Sousa, L.G.; Jovanovic, K.; Ferrarotto, R. Metastatic Adenoid Cystic Carcinoma: Genomic Landscape and Emerging Treatments. Curr. Treat. Options Oncol. 2022, 23, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, A.I.; Clark, J.R.; Fallon, B.G.; Posner, M.R.; Norris, C.M.; Miller, D. Cyclophosphamide, Doxorubicin, and Cisplatin Combination Chemotherapy for Advanced Carcinomas of Salivary Gland Origin. Cancer 1987, 60, 2869–2872. [Google Scholar] [CrossRef]
- Ha, H.; Keam, B.; Ock, C.Y.; Heo, D.S. Efficacy of Cyclophosphamide, Doxorubicin, and Cisplatin for Adenoid Cystic Carcinoma, and Their Relationship with the Pre-Chemotherapy Tumor Growth Rate. Chin. Clin. Oncol. 2020, 9, 15. [Google Scholar] [CrossRef]
- Laurie, S.A.; Siu, L.L.; Winquist, E.; Maksymiuk, A.; Harnett, E.L.; Walsh, W.; Tu, D.; Parulekar, W.R. A Phase 2 Study of Platinum and Gemcitabine in Patients with Advanced Salivary Gland Cancer: A Trial of the NCIC Clinical Trials Group. Cancer 2010, 116, 362–368. [Google Scholar] [CrossRef]
- Haddad, R.; Posner, M.R. Palliative Chemotherapy in Patients with Salivary Gland Neoplasms and Preliminary Reports of 2 Recent Phase II Studies with Trastuzumab and Gemcitabine. Clin. Adv. Hematol. Oncol. 2003, 1, 226–228. [Google Scholar]
- Nakano, K.; Sato, Y.; Sasaki, T.; Shimbashi, W.; Fukushima, H.; Yonekawa, H.; Mitani, H.; Kawabata, K.; Takahashi, S. Combination Chemotherapy of Carboplatin and Paclitaxel for Advanced/Metastatic Salivary Gland Carcinoma Patients: Differences in Responses by Different Pathological Diagnoses. Acta Otolaryngol. 2016, 136, 948–951. [Google Scholar] [CrossRef]
- Airoldi, M.; Garzaro, M.; Pedani, F.; Ostellino, O.; Succo, G.; Riva, G.; Sensini, M.; Naqe, N.; Bellini, E.; Raimondo, L.; et al. Cisplatin+Vinorelbine Treatment of Recurrent or Metastatic Salivary Gland Malignancies (RMSGM): A Final Report on 60 Cases. Am. J. Clin. Oncol. 2017, 40, 86–90. [Google Scholar] [CrossRef]
- Airoldi, M.; Pedani, F.; Succo, G.; Gabriele, A.M.; Ragona, R.; Marchionatti, S.; Bumma, C. Phase II Randomized Trial Comparing Vinorelbine versus Vinorelbine plus Cisplatin in Patients with Recurrent Salivary Gland Malignancies. Cancer 2001, 91, 541–547. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, C.G.; Koh, Y.W.; Choi, E.C.; Kim, J.; Yoon, S.O.; Kim, H.R.; Cho, B.C. Efficacy and Safety of Vinorelbine plus Cisplatin Chemotherapy for Patients with Recurrent and/or Metastatic Salivary Gland Cancer of the Head and Neck. Head Neck 2018, 40, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.P.; Martins, R.G.; Baik, C.; Chow, L.Q.; Santana-Davila, R.; Goulart, B.H.; Lee, S.; Eaton, K.D. Phase II Trial of Eribulin Mesylate in Recurrent or Metastatic Salivary Gland Malignancies. Head Neck 2018, 40, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, H.; Takahashi, S.; Hirao, M.; Tahara, M.; Iwasa, S.; Sato, Y.; Hamakawa, T.; Shitara, K.; Horinouchi, H.; Chin, K.; et al. Liposomal Eribulin for Advanced Adenoid Cystic Carcinoma, Gastric Cancer, Esophageal Cancer, and Small Cell Lung Cancer. Cancer Med. 2022. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Park, S.; Nam, S.J.; Keam, B.; Kim, T.M.; Jeon, Y.K.; Lee, S.H.; Hun Hah, J.; Kwon, T.K.; Kim, D.W.; Sung, M.W.; et al. VEGF and Ki-67 Overexpression in Predicting Poor Overall Survival in Adenoid Cystic Carcinoma. Cancer Res. Treat. 2016, 48, 518–526. [Google Scholar] [CrossRef]
- Kondo, S.; Mukudai, Y.; Soga, D.; Nishida, T.; Takigawa, M.; Shirota, T. Differential Expression of Vascular Endothelial Growth Factor in High- and Low-Metastasis Cell Lines of Salivary Gland Adenoid Cystic Carcinoma. Anticancer Res. 2014, 34, 671–677. [Google Scholar]
- Ho, A.L.; Dunn, L.; Sherman, E.J.; Fury, M.G.; Baxi, S.S.; Chandramohan, R.; Dogan, S.; Morris, L.G.T.; Cullen, G.D.; Haque, S.; et al. A Phase II Study of Axitinib (AG-013736) in Patients with Incurable Adenoid Cystic Carcinoma. Ann. Oncol. 2016, 27, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J.; Ahn, M.J.; Ock, C.Y.; Lee, K.W.; Kwon, J.H.; Yang, Y.; Choi, Y.H.; Kim, M.K.; Ji, J.H.; Yun, T.; et al. Randomized Phase II Study of Axitinib versus Observation in Patients with Recurred or Metastatic Adenoid Cystic Carcinoma. Clin. Cancer Res. 2021, 27, 5272–5279. [Google Scholar] [CrossRef]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Tran, C.; Baxi, S.; Katabi, N.; Antonescu, C.R.; Ostrovnaya, I.; Haque, S.S.; Pfister, D.G.; et al. Phase II Study of Lenvatinib in Patients with Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2019, 37, 1529–1537. [Google Scholar] [CrossRef]
- Locati, L.D.; Galbiati, D.; Calareso, G.; Alfieri, S.; Singer, S.; Cavalieri, S.; Bergamini, C.; Bossi, P.; Orlandi, E.; Resteghini, C.; et al. Patients with Adenoid Cystic Carcinomas of the Salivary Glands Treated with Lenvatinib: Activity and Quality of Life. Cancer 2020, 126, 1888–1894. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, L.; Dou, S.; Li, R.; Li, J.; Ye, L.; Jiang, W.; Dong, M.; Ruan, M.; Yang, W.; et al. Apatinib in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma of the Head and Neck: A Single-Arm, Phase II Prospective Study. Ther. Adv. Med. Oncol. 2021, 13, 17588359211013626. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Ahn, M.-J.; Muzaffar, J.; Keam, B.; Bowles, D.W.; Wong, D.J.L.; Ho, A.L.; Kim, S.-B.; Worden, F.P.; Yun, T.; et al. A Phase 2 Study of the Oral Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Inhibitor, Rivoceranib, for Recurrent or Metastatic (R/M) Adenoid Cystic Carcinoma (ACC). J. Clin. Oncol. 2022, 40, 6020. [Google Scholar]
- Mahmood, U.; Bang, A.; Chen, Y.H.; Mak, R.H.; Lorch, J.H.; Hanna, G.J.; Nishino, M.; Manuszak, C.; Thrash, E.M.; Severgnini, M.; et al. A Randomized Phase 2 Study of Pembrolizumab with or Without Radiation in Patients With Recurrent or Metastatic Adenoid Cystic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 134–144. [Google Scholar] [CrossRef]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; le Tourneau, C.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Fayette, J.; Even, C.; Digue, L.; Geoffrois, L.; Rolland, F.; Cupissol, D.; Guigay, J.; le Tourneau, C.; Dillies, A.-F.; Zanetta, S.; et al. NISCAHN: A Phase II, Multicenter Nonrandomized Trial Aiming at Evaluating Nivolumab (N) in Two Cohorts of Patients (Pts) with Recurrent/Metastatic (R/M) Salivary Gland Carcinoma of the Head and Neck (SGCHN), on Behalf of the Unicancer Head & Neck Group. J. Clin. Oncol. 2019, 37, 6083. [Google Scholar] [CrossRef]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Fetten, J.V.; Michel, L.S.; Kriplani, A.; Morris, L.; Ostrovnaya, I.; Katabi, N.; Haque, S.; et al. A Phase II Trial Cohort of Nivolumab plus Ipilimumab in Patients (Pts) with Recurrent/Metastatic Adenoid Cystic Carcinoma (R/M ACC). J. Clin. Oncol. 2019, 37, 6084. [Google Scholar] [CrossRef]
- Chae, Y.K.; Othus, M.; Patel, S.P.; Ohr, J.P.; Worden, F.P.; Suga, J.M.; Naing, A.; Fenton, S.E.; Kang, H.; Gurung, S.; et al. Abstract 3418: A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: The Salivary Gland Tumor Cohort. Cancer Res. 2020, 80, 3418. [Google Scholar] [CrossRef]
- Duvic, M.; Talpur, R.; Ni, X.; Zhang, C.; Hazarika, P.; Kelly, C.; Chiao, J.H.; Reilly, J.F.; Ricker, J.L.; Richon, V.M.; et al. Phase 2 Trial of Oral Vorinostat (Suberoylanilide Hydroxamic Acid, SAHA) for Refractory Cutaneous T-Cell Lymphoma (CTCL). Blood 2007, 109, 31–39. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Kummar, S.; Sarantopoulos, J.; Shibata, S.; LoRusso, P.; Yerk, M.; Holleran, J.; Lin, Y.; Beumer, J.H.; Harvey, R.D.; et al. Phase I Study of Vorinostat in Patients with Advanced Solid Tumors and Hepatic Dysfunction: A National Cancer Institute Organ Dysfunction Working Group Study. J. Clin. Oncol. 2010, 28, 4507–4512. [Google Scholar] [CrossRef]
- Goncalves, P.H.; Heilbrun, L.K.; Barrett, M.T.; Kummar, S.; Hansen, A.R.; Siu, L.L.; Piekarz, R.L.; Sukari, A.W.; Chao, J.; Pilat, M.J.; et al. A Phase 2 Study of Vorinostat in Locally Advanced, Recurrent, or Metastatic Adenoid Cystic Carcinoma. Oncotarget 2017, 8, 32918–32929. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Wu, Q.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Robak, T. Bortezomib for the Treatment of Hematologic Malignancies: 15 Years Later. Drugs R&D 2019, 19, 73. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, B.; Chen, X. Expressions of Nuclear Factor KappaB, Inducible Nitric Oxide Synthase, and Vascular Endothelial Growth Factor in Adenoid Cystic Carcinoma of Salivary Glands: Correlations with the Angiogenesis and Clinical Outcome. Clin. Cancer Res. 2005, 11, 7334–7343. [Google Scholar] [CrossRef]
- Argiris, A.; Ghebremichael, M.; Burtness, B.; Axelrod, R.S.; Deconti, R.C.; Forastiere, A.A. A Phase 2 Trial of Bortezomib Followed by the Addition of Doxorubicin at Progression in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma of the Head and Neck: A Trial of the Eastern Cooperative Oncology Group (E1303). Cancer 2011, 117, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. Mechanism of Action of Lenalidomide in Hematological Malignancies. J. Hematol. Oncol. 2009, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Sperling, A.S.; Ebert, B.L. Cancer Therapies Based on Targeted Protein Degradation—Lessons Learned with Lenalidomide. Nat. Rev. Clin. Oncol. 2021, 18, 401–417. [Google Scholar] [CrossRef]
- Harvey, R.D.; Carthon, B.C.; Lewis, C.; Hossain, M.S.; Zhang, C.; Chen, Z.; Harris, W.B.; Alese, O.B.; Shaib, W.; Bilen, M.A.; et al. Phase 1 Safety and Pharmacodynamic Study of Lenalidomide Combined with Everolimus in Patients with Advanced Solid Malignancies with Efficacy Signal in Adenoid Cystic Carcinoma. Br. J. Cancer 2020, 123, 1228–1234. [Google Scholar] [CrossRef]
- Kim, D.W.; Oh, D.Y.; Shin, S.H.; Kang, J.H.; Cho, B.C.; Chung, J.S.; Kim, H.J.; Park, K.U.; Kwon, J.H.; Han, J.Y.; et al. A Multicenter Phase II Study of Everolimus in Patients with Progressive Unresectable Adenoid Cystic Carcinoma. BMC Cancer 2014, 14, 795. [Google Scholar] [CrossRef]
- Sasso, J.M.; Tenchov, R.; Wang, D.; Johnson, L.S.; Wang, X.; Zhou, Q.A. Molecular Glues: The Adhesive Connecting Targeted Protein Degradation to the Clinic. Biochemistry 2022. [Google Scholar] [CrossRef]
- Miller, L.E.; Au, V.; Mokhtari, T.E.; Goss, D.; Faden, D.L.; Varvares, M.A. A Contemporary Review of Molecular Therapeutic Targets for Adenoid Cystic Carcinoma. Cancers 2022, 14, 992. [Google Scholar] [CrossRef]
- Wai, K.C.; Kang, H.; Ha, P.K. Molecular Markers That Matter in Salivary Malignancy. Otolaryngol. Clin. N. Am. 2021, 54, 613–627. [Google Scholar] [CrossRef]
- Even, C.; Lassen, U.; Merchan, J.; le Tourneau, C.; Soria, J.C.; Ferte, C.; Ricci, F.; Diener, J.T.; Yuen, E.; Smith, C.; et al. Safety and Clinical Activity of the Notch Inhibitor, Crenigacestat (LY3039478), in an Open-Label Phase I Trial Expansion Cohort of Advanced or Metastatic Adenoid Cystic Carcinoma. Investig. New Drugs 2020, 38, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Bowles, D.W.; Even, C.; Hao, D.; Kang, H.; Metcalf, R.; Muzaffar, J.; Oliva, M.; Perez, C.A.; Popovtzer, A.; et al. 904P ACCURACY: A Phase II Trial of AL101, a Selective Gamma Secretase Inhibitor, in Subjects with Recurrent/Metastatic (R/M) Adenoid Cystic Carcinoma (ACC) Harboring Notch Activating Mutations (Notchmut): Results of 6-Mg Cohort. Ann. Oncol. 2021, 32, S803–S804. [Google Scholar] [CrossRef]
- Medinger, M.; Junker, T.; Heim, D.; Tzankov, A.; Jermann, P.M.; Bobadilla, M.; Vigolo, M.; Lehal, R.; Vogl, F.D.; Bauer, M.; et al. CB-103: A Novel CSL-NICD Inhibitor for the Treatment of NOTCH-Driven T-Cell Acute Lymphoblastic Leukemia: A Case Report of Complete Clinical Response in a Patient with Relapsed and Refractory T-ALL. Eur. J. Haematol. 2022, 3, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Lopez Miranda, E.; Stathis, A.; Hess, D.; Racca, F.; Quon, D.; Rodon, J.; Saavedra Santa Gadea, O.; Perez Garcia, J.M.; Nuciforo, P.; Vivancos, A.; et al. Phase 1 Study of CB-103, a Novel First-in-Class Inhibitor of the CSL-NICD Gene Transcription Factor Complex in Human Cancers. J. Clin. Oncol. 2021, 39, 3020. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Yarbrough, W.G.; Panaccione, A.; Chang, M.T.; Ivanov, S.v. Clinical and Molecular Insights into Adenoid Cystic Carcinoma: Neural Crest-like Stemness as a Target. Laryngoscope Investig. Otolaryngol. 2016, 1, 60–77. [Google Scholar] [CrossRef]
- Ahmed, M.; Chaudhari, K.; Babaei-Jadidi, R.; Dekker, L.V.; Shams Nateri, A. Concise Review: Emerging Drugs Targeting Epithelial Cancer Stem-Like Cells. Stem Cells 2017, 35, 839–850. [Google Scholar] [CrossRef]
- Cote, G.M.; Edenfield, W.J.; Laurie, S.A.; Chau, N.G.; Becerra, C.; Spira, A.I.; Li, Y.; Li, W.; Hitron, M.; Li, C. A Phase 1b/2 Study of Amcasertib, a First-in-Class Cancer Stemness Kinase Inhibitor, in Advanced Adenoid Cystic Carcinoma. J. Clin. Oncol. 2017, 35, 6036. [Google Scholar] [CrossRef]
- Feustel, K.; Falchook, G.S. Protein Arginine Methyltransferase 5 (PRMT5) Inhibitors in Oncology Clinical Trials: A Review. J. Immunother. Precis. Oncol. 2022, 5, 58–67. [Google Scholar] [CrossRef]
- Villar, M.V.; Spreafico, A.; Moreno, V.; Braña, I.; Hernandez, T.; Razak, A.A.; Wang, J.; Haddish-Berhane, N.; Mehta, J.; Johnson, A.; et al. 537MO First-in-Human Study of JNJ-64619178, a Protein Arginine Methyltransferase 5 (PRMT5) Inhibitor, in Patients with Advanced Cancers. Ann. Oncol. 2020, 31, S470. [Google Scholar] [CrossRef]
- Siu, L.L.; Rasco, D.W.; Vinay, S.P.; Romano, P.M.; Menis, J.; Opdam, F.L.; Heinhuis, K.M.; Egger, J.L.; Gorman, S.A.; Parasrampuria, R.; et al. 438O METEOR-1: A Phase I Study of GSK3326595, a First-in-Class Protein Arginine Methyltransferase 5 (PRMT5) Inhibitor, in Advanced Solid Tumours. Ann. Oncol. 2019, 30, v159. [Google Scholar] [CrossRef]
- McKean, M.; Patel, M.R.; Wesolowski, R.; Ferrarotto, R.; Stein, E.M.; Shoushtari, A.N.; Mauro, D.; Viscusi, J.; Scherle, P.; Bhagwat, N.; et al. Abstract P039: A Phase 1 Dose Escalation Study of Protein Arginine Methyltransferase 5 (PRMT5) Inhibitor PRT543 in Patients with Advanced Solid Tumors and Lymphoma. Mol. Cancer Ther. 2021, 20, P039. [Google Scholar] [CrossRef]
- Humtsoe, J.O.; Kim, H.S.; Leonard, B.; Ling, S.; Keam, B.; Marchionni, L.; Afsari, B.; Considine, M.; Favorov, A.V.; Fertig, E.J.; et al. Newly Identified Members of FGFR1 Splice Variants Engage in Cross-Talk with AXL/AKT Axis in Salivary Adenoid Cystic Carcinoma. Cancer Res. 2021, 81, 1001–1013. [Google Scholar] [CrossRef]
- Gay, C.M.; Balaji, K.; Byers, L.A. Giving AXL the Axe: Targeting AXL in Human Malignancy. Br. J. Cancer 2017, 116, 415–423. [Google Scholar] [CrossRef]
- Humtsoe, J.O.; Jones, L.; Naara, S.; Zammarchi, F.; Burr, N.S.; van Berkel, P.H.; Ha, P.K.; Kang, H. Abstract LB084: AXL as a Therapeutic Target in Adenoid Cystic Carcinoma: Preclinical Evaluation of AXL Targeting Antibody-Drug Conjugate (ADCT-601). Cancer Res. 2022, 82, LB084. [Google Scholar] [CrossRef]
- Seok, J.; Lee, D.Y.; Kim, W.S.; Jeong, W.J.; Chung, E.J.; Jung, Y.H.; Kwon, S.K.; Kwon, T.K.; Sung, M.W.; Ahn, S.H. Lung Metastasis in Adenoid Cystic Carcinoma of the Head and Neck. Head Neck 2019, 41, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.W.; Kim, K.H.; Kim, J.W.; Min, Y.G.; Seong, W.J.; Roh, J.L.; Lee, S.J.; Kwon, T.K.; Park, S.W. Clinicopathologic Predictors and Impact of Distant Metastasis from Adenoid Cystic Carcinoma of the Head and Neck. Arch Otolaryngol. Head Neck Surg. 2003, 129, 1193–1197. [Google Scholar] [CrossRef]
- Umeda, M.; Nishimatsu, N.; Masago, H.; Ishida, Y.; Yokoo, S.; Fujioka, M.; Shibuya, Y.; Komori, T. Tumor-Doubling Time and Onset of Pulmonary Metastasis from Adenoid Cystic Carcinoma of the Salivary Gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 473–478. [Google Scholar] [CrossRef]
- Collins, V.P.; Loeffler, R.K.; Tivey, H. Observations on Growth Rates of Human Tumors. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1956, 76, 988–1000. [Google Scholar]
- Jang, S.; Patel, P.N.; Kimple, R.J.; McCulloch, T.M. Clinical Outcomes and Prognostic Factors of Adenoid Cystic Carcinoma of the Head and Neck. Anticancer Res. 2017, 37, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.Q.; Liang, L.Z.; Zheng, G.S.; Ke, Z.F.; Weng, D.S.; Yang, W.F.; Su, Y.X.; Liao, G.Q. Risk Factors and Prognosis for Salivary Gland Adenoid Cystic Carcinoma in Southern China: A 25-Year Retrospective Study. Medicine 2017, 96, e5964. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hao, Y.; Huang, M.X.; Ma, D.Q.; Luo, H.Y.; Gao, Y.; Peng, X.; Yu, G.Y. Clinicopathological Study of Distant Metastases of Salivary Adenoid Cystic Carcinoma. Int. J. Oral Maxillofac. Surg. 2013, 42, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chai, Y.; Wei, Y.; Qi, F.; Dong, M. Survival and Prognosis of Metastatic Head and Neck Adenoid Cystic Carcinoma. Head Neck 2022, 44, 2407–2416. [Google Scholar] [CrossRef]
- Shingaki, S.; Kanemaru, S.; Oda, Y.; Niimi, K.; Mikami, T.; Funayama, A.; Saito, C. Distant Metastasis and Survival of Adenoid Cystic Carcinoma after Definitive Treatment. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 312–316. [Google Scholar] [CrossRef]
- Xu, M.J.; Wu, T.J.; van Zante, A.; El-Sayed, I.H.; Algazi, A.P.; Ryan, W.R.; Ha, P.K.; Yom, S.S. Mortality Risk after Clinical Management of Recurrent and Metastatic Adenoid Cystic Carcinoma. J. Otolaryngol. Head Neck Surg. 2018, 47, 28. [Google Scholar] [CrossRef]
- Tyan, K.; Bae, J.E.; Lorch, J.H.; Margalit, D.N.; Tishler, R.B.; Huynh, M.A.; Jo, V.Y.; Haddad, R.I.; Chau, N.G.; Hanna, G.J.; et al. Oligometastatic Adenoid Cystic Carcinoma: Correlating Tumor Burden and Time to Treatment with Outcomes. Head Neck 2022, 44, 722–734. [Google Scholar] [CrossRef]
- Schlachtenberger, G.; Doerr, F.; Menghesha, H.; Heldwein, M.B.; Lauinger, P.; Wolber, P.; Klussmann, J.P.; Wahlers, T.; Hekmat, K. Pulmonary Metastasectomy for Metastatic Head and Neck Cancer Prolongs Survival Significantly Compared to Non-Surgical Therapy. Eur. J. Cardiothorac. Surg. 2022, 62, ezac098. [Google Scholar] [CrossRef]
- Yotsukura, M.; Kinoshita, T.; Kohno, M.; Asakura, K.; Kamiyama, I.; Emoto, K.; Hayashi, Y.; Ohtsuka, T. Survival Predictors after Resection of Lung Metastases of Head or Neck Cancers. Thorac. Cancer 2015, 6, 579–583. [Google Scholar] [CrossRef]
- Chen, F.; Sonobe, M.; Sato, K.; Fujinaga, T.; Shoji, T.; Sakai, H.; Miyahara, R.; Bando, T.; Okubo, K.; Hirata, T.; et al. Pulmonary Resection for Metastatic Head and Neck Cancer. World J. Surg. 2008, 32, 1657–1662. [Google Scholar] [CrossRef]
- Wedman, J.; Balm, A.J.M.; Hart, A.A.M.; Loftus, B.M.; Hilgers, F.J.M.; Theo Gregor, R.; van Zandwijk, N.; Zoetmulder, F.A.N. Value of Resection of Pulmonary Metastases in Head and Neck Cancer Patients. Head Neck 1996, 18, 311–316. [Google Scholar] [CrossRef]
- Oki, T.; Hishida, T.; Yoshida, J.; Goto, M.; Sekihara, K.; Miyoshi, T.; Aokage, K.; Ishii, G.; Tsuboi, M. Survival and Prognostic Factors after Pulmonary Metastasectomy of Head and Neck Cancer: What Are the Clinically Informative Prognostic Indicators? Eur. J. Cardiothorac. Surg. 2019, 55, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Hasegawa, Y.; Hanai, N.; Ozawa, T.; Hirakawa, H.; Suzuki, A.; Okamoto, H.; Harata, I. Survival Impact of Pulmonary Metastasectomy for Patients with Head and Neck Cancer. Head Neck 2013, 35, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Iijima, Y.; Kinoshita, H.; Akiyama, H.; Beppu, T.; Uramoto, H.; Hirata, T. Surgical Treatment for Pulmonary Metastasis of Head and Neck Cancer: Study of 58 Cases. Ann. Thorac. Cardiovasc. Surg. 2017, 23, 169–174. [Google Scholar] [CrossRef][Green Version]
- Yamazaki, K.; Shodo, R.; Ueki, Y.; Matsuyama, H.; Takahashi, S. Therapeutic Outcome after Resection of Pulmonary Metastasis from Head and Neck Carcinomas. Indian J. Otolaryngol. Head Neck Surg. 2015, 67, 124–128. [Google Scholar] [CrossRef][Green Version]
- Hosokawa, S.; Funai, K.; Sugiyama, K.; Takahashi, G.; Okamura, J.; Takizawa, Y.; Yamatodani, T.; Mineta, H. Survival Outcomes after Surgical Resection of Pulmonary Metastases of Head and Neck Tumours. J. Laryngol. Otol. 2016, 130, 291–295. [Google Scholar] [CrossRef]
- Haro, A.; Yano, T.; Yoshida, T.; Ito, K.; Morodomi, Y.; Shoji, F.; Nakashima, T.; Maehara, Y. Results of a Surgical Resection of Pulmonary Metastasis from Malignant Head and Neck Tumor. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 700–703. [Google Scholar] [CrossRef][Green Version]
- Mimica, X.; McGill, M.; Hay, A.; Karassawa Zanoni, D.; Shah, J.P.; Wong, R.J.; Ho, A.; Cohen, M.A.; Patel, S.G.; Ganly, I. Distant Metastasis of Salivary Gland Cancer: Incidence, Management, and Outcomes. Cancer 2020, 126, 2153–2162. [Google Scholar] [CrossRef]
- Ochi, T.; Wada, H.; Nakajima, T.; Tanaka, K.; Yamamoto, T.; Sakairi, Y.; Suzuki, H.; Yonekura, S.; Hanazawa, T.; Yoshino, I. Surgical Outcomes of Pulmonary Metastasectomy for Head and Neck Cancer in the Current Era of Advances in Chemotherapy and Immunotherapy. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1214–1221. [Google Scholar] [CrossRef]
- Ohta, K.; Matsuda, S.; Okada, A.; Sasaki, M.; Imamura, Y.; Yoshimura, H. Adenoid Cystic Carcinoma of the Sublingual Gland Developing Lung Metastasis 20 Years after Primary Treatment: A Case Report and Literature Review. Medicine 2021, 100, e28098. [Google Scholar] [CrossRef]
- Ito, M.; Okita, R.; Tsutani, Y.; Mimura, T.; Kawasaki, Y.; Miyata, Y.; Okada, M. Lung Metastasis of Adenoid Cystic Carcinoma, Which Mimicked Primary Lung Cancer. Thorac Cancer 2013, 4, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.M.; Howard, D.J. Should We Treat Lung Metastases from Adenoid Cystic Carcinoma of the Head and Neck in Asymptomatic Patients? Ear Nose Throat J. 2009, 88, 969–973. [Google Scholar] [CrossRef]
- Sali, P.A.; Yadav, K.S.; Bushan, K.; Rajpurohit, V.; Varty, P.P.; Sharma, S. A Rare Case of Lacrimal Adenoid Cystic Carcinoma with Large Hepatic and Multiple Pulmonary Metastases with Successful Surgical Treatment. Int. J. Surg. Case Rep. 2016, 20, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Girelli, L.; Locati, L.; Galeone, C.; Scanagatta, P.; Duranti, L.; Licitra, L.; Pastorino, U. Lung Metastasectomy in Adenoid Cystic Cancer: Is It Worth It? Oral Oncol. 2017, 65, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Han, S.; Jeong, W.J.; Jung, Y.H.; Sung, M.W.; Ahn, S.H. Oncological Outcomes after Pulmonary Metastasectomy for Head and Neck Squamous-Cell Carcinoma and Adenoid Cystic Carcinoma. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Guzzo, M.; Bossi, P.; Massone, P.P.B.; Conti, B.; Fumagalli, E.; Bareggi, C.; Cantù, G.; Licitra, L. Lung Metastasectomy in Adenoid Cystic Carcinoma (ACC) of Salivary Gland. Oral Oncol. 2005, 41, 890–894. [Google Scholar] [CrossRef]
- Liu, D.; Labow, D.M.; Dang, N.; Martini, N.; Bains, M.; Burt, M.; Downey, R.; Rusch, V.; Shah, J.; Ginsberg, R.J. Pulmonary Metastasectomy for Head and Neck Cancers. Ann. Surg. Oncol. 1999, 6, 572–578. [Google Scholar] [CrossRef]
- Mazer, T.M.; Robbins, K.T.; McMurtrey, M.J.; Byers, R.M. Resection of Pulmonary Metastases from Squamous Carcinoma of the Head and Neck. Am. J. Surg. 1988, 156, 238–242. [Google Scholar] [CrossRef]
- AlShammari, A.; Almasri, T.; Sarraj, J.; AlAshgar, O.; Ahmed, M.H.; AlKattan, K.; Saleh, W. Pulmonary Metastasis of Head and Neck Cancer: Surgical Removal Outcomes from a Tertiary Care Center. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 199–206. [Google Scholar] [CrossRef]
- Bobbio, A.; Copelli, C.; Ampollini, L.; Bianchi, B.; Carbognani, P.; Bettati, S.; Sesenna, E.; Rusca, M. Lung Metastasis Resection of Adenoid Cystic Carcinoma of Salivary Glands. Eur. J. Cardiothorac. Surg. 2008, 33, 790–793. [Google Scholar] [CrossRef]
- Winter, H.; Meimarakis, G.; Hoffmann, G.; Hummel, M.; Rüttinger, D.; Zilbauer, A.; Stelter, K.; Spelsberg, F.; Jauch, K.W.; Hatz, R.; et al. Does Surgical Resection of Pulmonary Metastases of Head and Neck Cancer Improve Survival? Ann. Surg. Oncol. 2008, 15, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.J.; Hsieh, C.C.; Yeh, C.C.; Yeh, Y.C.; Wu, C.C.; Wang, F.S.; Lai, J.M.; Yang, M.H.; Wang, C.H.; Huang, C.Y.F.; et al. Clinical, Pathophysiologic, and Genomic Analysis of the Outcomes of Primary Head and Neck Malignancy after Pulmonary Metastasectomy. Sci. Rep. 2019, 9, 12913. [Google Scholar] [CrossRef] [PubMed]
- Florescu, C.; Thariat, J. Local Ablative Treatments of Oligometastases from Head and Neck Carcinomas. Crit. Rev. Oncol. Hematol. 2014, 91, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Roberge, D.; Schellenberg, D.; Purdie, T.G.; Swaminath, A.; Pantarotto, J.; Filion, E.; Gabos, Z.; Butler, J.; Letourneau, D.; et al. The Canadian Association of Radiation Oncology Scope of Practice Guidelines for Lung, Liver and Spine Stereotactic Body Radiotherapy. Clin. Oncol. 2012, 24, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sutera, P.; Clump, D.A.; Kalash, R.; D’Ambrosio, D.; Mihai, A.; Wang, H.; Petro, D.P.; Burton, S.A.; Heron, D.E. Initial Results of a Multicenter Phase 2 Trial of Stereotactic Ablative Radiation Therapy for Oligometastatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 116–122. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Kanzaki, R.; Suzuki, O.; Kanou, T.; Ose, N.; Funaki, S.; Shintani, Y.; Minami, M.; Tamari, K.; Otani, K.; Seo, Y.; et al. The Short-Term Outcomes of Pulmonary Metastasectomy or Stereotactic Body Radiation Therapy for Pulmonary Metastasis from Epithelial Tumors. J. Cardiothorac. Surg. 2020, 15. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, R.; Wu, G.; Dong, X. Adenoid Cystic Carcinoma of Submandibular Salivary Gland with Late Metastases to Lung and Choroid: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2014, 72, 1744–1755. [Google Scholar] [CrossRef]
- Parihar, A.; Vadi, S.; Mittal, B.; Kumar, R.; Sood, A.; Singh, H.; Bahl, A. Adenoid Cystic Carcinoma of Buccal Mucosa: Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Detection and Biopsy of Pulmonary Metastases and Assessment of Treatment Response. Indian J. Nucl. Med. 2019, 34, 71–73. [Google Scholar] [CrossRef]
- Li, D.; Pang, X.; Zhu, X.; Shanzhou, Q.; Wen, G.; Ma, D. Low-Dose Radiotherapy Combined with Immunotherapy for Suboral Adenoid Cystic Carcinoma with Bilateral Lung Metastasis: A Case Report and Literature Review. Oncol. Lett. 2022, 24, 279. [Google Scholar] [CrossRef]
- Kobayashi, D.; Abe, T.; Saitoh, J.; Oike, T.; Sato, H.; Musha, A.; Mizukami, T.; Shimizu, T.; Nakano, T.; Ohno, T. Stereotactic Body Radiotherapy for Adenoid Cystic Carcinoma Metastatic to the Lung: A Case Report. J. Med. Case Rep. 2021, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, E.; Sánchez, I.; Díaz, O.; Valles, A.; Balderrama, R.; Fuentes, J.; Lara, B.; Olimón, C.; Ruiz, V.; Rodríguez, J.; et al. Current Evidence for Stereotactic Body Radiotherapy in Lung Metastases. Curr. Oncol. 2021, 28, 2560–2578. [Google Scholar] [CrossRef] [PubMed]
- Lischalk, J.W.; Malik, R.M.; Collins, S.P.; Collins, B.T.; Matus, I.A.; Anderson, E.D. Stereotactic Body Radiotherapy (SBRT) for High-Risk Central Pulmonary Metastases. Radiat. Oncol. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chmura, S.; Winter, K.A.; Robinson, C.; Pisansky, T.M.; Borges, V.; Al-Hallaq, H.; Matuszak, M.; Park, S.S.; Yi, S.; Hasan, Y.; et al. Evaluation of Safety of Stereotactic Body Radiotherapy for the Treatment of Patients with Multiple Metastases: Findings From the NRG-BR001 Phase 1 Trial. JAMA Oncol. 2021, 7, 845. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Correa, R.J.M.; Schneiders, F.; Haasbeek, C.J.A.; Rodrigues, G.B.; Lock, M.; Yaremko, B.P.; Bauman, G.S.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of 4-10 Oligometastatic Tumors (SABR-COMET-10): Study Protocol for a Randomized Phase III Trial. BMC Cancer 2019, 19. [Google Scholar] [CrossRef]
- Franzese, C.; Ingargiola, R.; Tomatis, S.; Iacovelli, N.A.; Beltramo, G.; Franco, P.; Bonomo, P.; Zanetti, I.B.; Argenone, A.; Cante, D.; et al. Metastatic Salivary Gland Carcinoma: A Role for Stereotactic Body Radiation Therapy? A Study of AIRO-Head and Neck Working Group. Oral Dis. 2022, 28, 345–351. [Google Scholar] [CrossRef]
- Franzese, C.; Badalamenti, M.; Teriaca, A.; de Virgilio, A.; Mercante, G.; Cavina, R.; Ferrari, D.; Santoro, A.; Spriano, G.; Scorsetti, M. Metastasis-Directed Stereotactic Body Radiation Therapy in the Management of Oligometastatic Head and Neck Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 1307–1313. [Google Scholar] [CrossRef]
- Pasalic, D.; Betancourt-Cuellar, S.L.; Taku, N.; Ludmir, E.B.; Lu, Y.; Allen, P.K.; Tang, C.; Antonoff, M.B.; Fuller, C.D.; Rosenthal, D.I.; et al. Outcomes and Toxicities Following Stereotactic Ablative Radiotherapy for Pulmonary Metastases in Patients with Primary Head and Neck Cancer. Head Neck 2020, 42, 1939–1953. [Google Scholar] [CrossRef]

| Study | n (ACC PMs) | Intervention | Outcomes | Ref. |
|---|---|---|---|---|
| Girelli et al. (2017) | 109 | PM: 83.5% CR 16.5% IR | 5-year OS: 66.8% 10-year OS: 40.5% | [134] |
| Park et al. (2022) | 18 | PM: 100% CR | 1-year DFS: 88.9% 3-year DFS: 38.9% 5-year DFS: 32.4% 8-year DFS: 0% | [135] |
| Locati et al. (2005) | 20 | PM: 55% CR 45% IR | Median OS: 78 mo. (CR) vs. 52 mo. (IR) Median PFS: 30 mo. (CR) vs. 15 mo. (IR) | [136] |
| Liu et al. (1999) | 16 | PM: 81% CR 19% IR | 5-year OS: 84% | [137] |
| Mazer et al. (1988) | 13 | PM | 5-year OS: 63% | [138] |
| AlShammari et al. (2020) | 11 | PM | 5-year OS: 100% | [139] |
| Bobbio et al. (2008) | 9 | PM | Median OS: 72 mo. | [140] |
| Winter et al. (2008) | 6 | PM | 5-year OS: 33.3% Median OS: 43.5 mo. | [141] |
| Ishida et al. (2020) | 5 | PM | 5-year OS: 100% | [50] |
| Lu et al. (2019) | 3 | PM | 2-year OS: 100% 5-year OS: 100% | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, R.H.; Wai, K.C.; Chan, J.W.; Ha, P.K.; Kang, H. Approaches to the Management of Metastatic Adenoid Cystic Carcinoma. Cancers 2022, 14, 5698. https://doi.org/10.3390/cancers14225698
Lee RH, Wai KC, Chan JW, Ha PK, Kang H. Approaches to the Management of Metastatic Adenoid Cystic Carcinoma. Cancers. 2022; 14(22):5698. https://doi.org/10.3390/cancers14225698
Chicago/Turabian StyleLee, Rex H., Katherine C. Wai, Jason W. Chan, Patrick K. Ha, and Hyunseok Kang. 2022. "Approaches to the Management of Metastatic Adenoid Cystic Carcinoma" Cancers 14, no. 22: 5698. https://doi.org/10.3390/cancers14225698
APA StyleLee, R. H., Wai, K. C., Chan, J. W., Ha, P. K., & Kang, H. (2022). Approaches to the Management of Metastatic Adenoid Cystic Carcinoma. Cancers, 14(22), 5698. https://doi.org/10.3390/cancers14225698







