The Role of [68Ga]PSMA PET/CT for Clinical Suspicion of Prostate Cancer in Patients with or without Previous Negative Biopsy: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy and Study Selection
2.2. Data Extraction
2.3. Quality Assessment
3. Results
3.1. Literature Search
3.2. Basic Characteristics
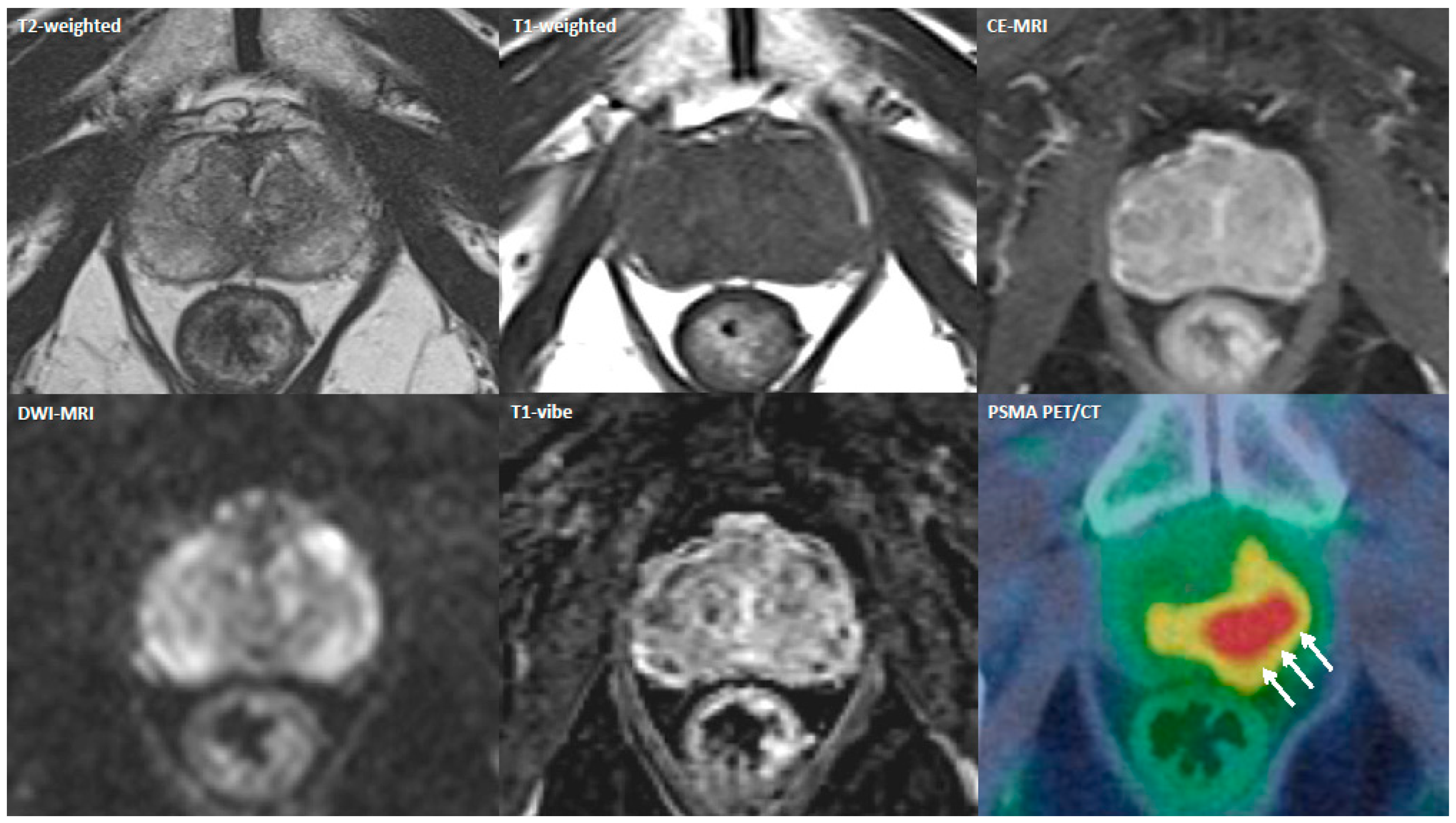
3.3. Imaging and Technical Aspects
3.4. Main Findings
3.5. Assessment for the Risk of Bias
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Carlsson, S.; Benfante, N.; Alvim, R.; Sjoberg, D.D.; Vickers, A.; Reuter, V.E.; Fine, S.W.; Vargas, H.A.; Wiseman, M.; Mamoor, M.; et al. Long-Term Outcomes of Active Surveillance for Prostate Cancer: The Memorial Sloan Kettering Cancer Center Experience. J. Urol. 2020, 203, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Vesprini, D.; Sethukavalan, P.; Jethava, V.; Zhang, L.; Jain, S.; Yamamoto, T.; Mamedov, A.; Loblaw, A. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol. 2015, 33, 272–277. [Google Scholar] [CrossRef] [PubMed]
- European Health Union: A New EU Approach on Cancer Detection–Screening More and Screening Better. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_5562 (accessed on 12 September 2022).
- Willemse, P.-P.M.; Davis, N.F.; Grivas, N.; Zattoni, F.; Lardas, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Dell’Oglio, P.; Donaldson, J.F.; et al. Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur. Urol. 2022, 81, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Eur. Assoc. Urol. 2022, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Chan, V.W.-S.; Clement, K.D.; Levis, B.; Haider, M.; Agarwal, R.; Emberton, M.; Pond, G.R.; Takwoingi, Y.; Klotz, L.; et al. A protocol for the VISION study: An indiVidual patient data meta-analysis of randomised trials comparing MRI-targeted biopsy to standard transrectal ultraSound guided bIopsy in the detection of prOstate cancer. PLoS ONE 2022, 17, e0263345. [Google Scholar] [CrossRef] [PubMed]
- Kızılay, F.; Çelik, S.; Sözen, S.; Özveren, B.; Eskiçorapçı, S.; Özgen, M.; Özen, H.; Akdoğan, B.; Aslan, G.; Narter, F.; et al. Correlation of Prostate-Imaging Reporting and Data Scoring System scoring on multiparametric prostate magnetic resonance imaging with histopathological factors in radical prostatectomy material in Turkish prostate cancer patients: A multicenter study of t. Prostate Int. 2020, 8, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Sasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Bergh, R.C.V.D.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Lopci, E.; Lughezzani, G.; Fasulo, V.; Lazzeri, M. Re: Stefano Fanti, Alberto Briganti, Louise Emmett, et al. EAU-EANM Consensus Statements on the Role of Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography in Patients with Prostate Cancer and with Respect to [177Lu]Lu-PSMA Radioligand Therapy. Eur Urol Oncol. 2022;5:530-6: Extended Use of PSMA PET/CT in Candidates for Focal Therapy. Eur. Urol. Oncol. 2022, 5, 601–602. [Google Scholar] [CrossRef]
- Le, J.D.; Tan, N.; Shkolyar, E.; Lu, D.Y.; Kwan, L.; Marks, L.S.; Huang, J.; Margolis, D.J.; Raman, S.S.; Reiter, R.E. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: Correlation with whole-mount histopathology. Eur. Urol. 2015, 67, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Sonn, G.A.; Fan, R.E.; Ghanouni, P.; Wang, N.N.; Brooks, J.D.; Loening, A.M.; Daniel, B.L.; To’O, K.J.; Thong, A.E.; Leppert, J.T. Prostate Magnetic Resonance Imaging Interpretation Varies Substantially Across Radiologists. Eur. Urol. Focus 2019, 5, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. Pet imaging with a [68ga]gallium-labelled psma ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, M.J.; Chang, J.I.; Stricker, P.D.; Van Leeuwen, P.J.; Nguyen, Q.A.; Ho, B.; Delprado, W.; Lee, J.; Thompson, J.E.; Cusick, T.; et al. Diagnostic accuracy of 68 Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: Impact of the addition of 68 Ga-PSMA PET to mpMRI. BJU Int. 2019, 124 (Suppl. S1), 42–49. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Simpson, B.S.; Parry, M.A.; Allen, C.; Ball, R.; Freeman, A.; Kelly, D.; Kim, H.L.; Kirkham, A.; You, S.; et al. Genetic Landscape of Prostate Cancer Conspicuity on Multiparametric Magnetic Resonance Imaging: A Systematic Review and Bioinformatic Analysis. Eur. Urol. Open Sci. 2020, 20, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Nuo, Y.; Li, A.; Yang, L.; Xue, H.; Wang, F.; Wang, L. Efficacy of 68Ga-PSMA-11 PET/CT with biparametric MRI in diagnosing prostate cancer and predicting risk stratification: A comparative study. Quant. Imaging Med. Surg. 2022, 12, 53–65. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study[Formul. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Sood, R.; Faridi, M.S.; Goel, H.; Sharma, U. Role of 68Ga-PSMA-PET/CT for the detection of primary prostate cancer prior to biopsy: A prospective study. Cent. Eur. J. Urol. 2021, 74, 315–320. [Google Scholar] [CrossRef]
- Jiao, J.; Kang, F.; Zhang, J.; Quan, Z.; Wen, W.; Zhao, X.; Ma, S.; Wu, P.; Yang, F.; Guo, W.; et al. Establishment and prospective validation of an SUVmax cutoff value to discriminate clinically significant prostate cancer from benign prostate diseases in patients with suspected prostate cancer by 68Ga-PSMA PET/CT: A real-world study. Theranostics 2021, 11, 8396–8411. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Chinnappan, S.; Kumar, J.S.; Chandran, G.; Nath, S. SUVmax/ADC Ratio as a Molecular Imaging Biomarker for Diagnosis of Biopsy-Naïve Primary Prostate Cancer. Indian J. Nucl. Med. 2021, 36, 377–384. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, Z.; Zhang, N.; Du, P.; Yang, Y.; Liu, Y.; Yu, W.; Li, N.; Gorin, M.A.; et al. 68Ga-Psma Pet/Ct Combined With Pet/Ultrasound-Guided Prostate Biopsy Can Diagnose Clinically Significant Prostate Cancer in Men With Previous Negative Biopsy Results. J. Nucl. Med. 2020, 61, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, F.; Yang, L.; Zang, S.; Xue, H.; Yin, X.; Guo, H.; Sun, H.; Wang, F. 68Ga-PSMA-11 PET/CT combining ADC value of MRI in the diagnosis of naive prostate cancer: Perspective of radiologist. Medicine 2020, 99, e20755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Li, W.-C.; Xu, Z.; Jiang, N.; Zang, S.-M.; Xu, L.-W.; Huang, W.-B.; Wang, F.; Sun, H.-B. 68Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Lughezzani, G.; Castello, A.; Colombo, P.; Casale, P.; Saita, A.; Buffi, N.M.; Guazzoni, G.; Chiti, A.; Lazzeri, M. PSMA-PET and micro-ultrasound potential in the diagnostic pathway of prostate cancer. Clin. Transl. Oncol. 2021, 23, 172–178. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Wu, P.; Ren, J.; Ma, S.; Zhang, J.; Song, W.; Lin, X.; Jiao, D.; Shi, S.; et al. Comparison of 68Ga-PSMA-617 PET/CT with mpMRI for the detection of PCa in patients with a PSA level of 4–20 ng/mL before the initial biopsy. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Lopci, E.; Lughezzani, G.; Castello, A.; Saita, A.; Colombo, P.; Hurle, R.; Peschechera, R.; Benetti, A.; Zandegiacomo, S.; Pasini, L.; et al. Prospective Evaluation of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography in Primary Prostate Cancer Diagnosis. Eur. Urol. Focus 2021, 7, 764–771. [Google Scholar] [CrossRef]
- Chandra, P.; Rajaian, S.; Krishnamurthy, K.; Murugasen, L.; Chandran, G.; Kumar, J.S.; Nath, S. Diagnostic Accuracy of Prebiopsy Ga-68 PSMA PET/CT in Detecting Primary Prostate Carcinomas with Prostate-Specific Antigen <50 ng/Ml. Indian J. Nucl. Med. 2020, 35, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Q.; Zhang, C.; Zhao, X.; Marra, G.; Gao, J.; Lv, X.; Fu, Y.; Wang, F.; Qiu, X.; et al. Combination of 68Ga-PSMA PET/CT and multiparametric MRI improves the detection of clinically significant prostate cancer: A lesion-by-lesion analysis. J. Nucl. Med. 2019, 60, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, N.; Kumar, S.; Saurav, K.; Prasad, V.; Vasudeva, P. Comparison of percentage free psa, mri and gapsma pet scan for diagnosing cancer prostate in men with psa between 4 and 20 ng/mL. Indian J. Urol. 2019, 35, 202–207. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, S.; Wu, P.; Liu, D.; Yang, B.; Han, D.; Li, Y.; Lin, X.; Song, W.; Cao, M.; et al. Diagnostic performance of 68 Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: Comparison with cancer-predicting nomograms. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Pillai, A.M.; Oommen, K.E.; Somarajan, S.; Raman, V.K.; Thomas, R.; Dinesh, D. Gallium 68-PSMA PET/CT for lesion characterization in suspected cases of prostate carcinoma. Nucl. Med. Commun. 2018, 39, 1013–1021. [Google Scholar] [CrossRef]
- Lopci, E.; Saita, A.; Lazzeri, M.; Lughezzani, G.; Colombo, P.; Buffi, N.M.; Hurle, R.; Marzo, K.; Peschechera, R.; Benetti, A.; et al. 68Ga-PSMA Positron Emission Tomography/Computerized Tomography for Primary Diagnosis of Prostate Cancer in Men with Contraindications to or Negative Multiparametric Magnetic Resonance Imaging: A Prospective Observational Study. J. Urol. 2018, 200, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Miederer, M.; Wieler, H.J.; Ruf, C.; Jakobs, F.M.; Schreckenberger, M. Diagnostic performance of 68Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with 18FEC PET/CT. Oncotarget 2017, 8, 111073–111083. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Eskicorapci, S.Y.; Guliyev, F.; Islamoglu, E.; Ergen, A.; Ozen, H. The effect of prior biopsy scheme on prostate cancer detection for repeat biopsy population: Results of the 14-core prostate biopsy technique. Int. Urol. Nephrol. 2007, 39, 189–195. [Google Scholar] [CrossRef]
- Syer, T.J.; Godley, K.C.; Cameron, D.; Malcolm, P.N. The diagnostic accuracy of high b-value diffusion- and T2-weighted imaging for the detection of prostate cancer: A meta-analysis. Abdom. Radiol. 2018, 43, 1787–1797. [Google Scholar] [CrossRef]
- Kwak, J.T.; Sankineni, S.; Xu, S.; Turkbey, B.; Choyke, P.L.; Pinto, P.A.; Moreno, V.; Merino, M.; Wood, B.J. Prostate cancer: A correlative study of Multiparametric MR imaging and digital histopathology. Radiology 2017, 285, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; Donaldson, I.; Emberton, M.; Ehdaie, B.; Hadaschik, B.A.; Marks, L.S.; Mozer, P.; Rastinehad, A.R.; Ahmed, H.U. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: A systematic review. Eur. Urol. 2015, 68, 8–19. [Google Scholar] [CrossRef]
- Roberts, M.J.; Morton, A.; Donato, P.; Kyle, S.; Pattison, D.A.; Thomas, P.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT tumour intensity pre-operatively predicts adverse pathological outcomes and progression-free survival in localised prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 477–482. [Google Scholar] [CrossRef]
- Petersen, L.J.; Zacho, H.D. PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: An expedited systematic review. Cancer Imaging 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Surasi, D.S. Editorial comment on diagnostic accuracy of 68Ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: A systematic review and meta-analysis. Am. J. Roentgenol. 2021, 216, 607. [Google Scholar] [CrossRef]
- Zhao, Y.; Simpson, B.S.; Morka, N.; Freeman, A.; Kirkham, A.; Kelly, D.; Whitaker, H.C.; Emberton, M.; Norris, J.M. Comparison of Multiparametric Magnetic Resonance Imaging with Prostate-Specific Membrane Antigen Positron-Emission Tomography Imaging in Primary Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3497. [Google Scholar] [CrossRef]
- Simopoulos, D.N.; Natarajan, S.; Jones, T.A.; Fendler, W.P.; Sisk, A.E.; Marks, L.S. Targeted Prostate Biopsy Using 68Gallium PSMA-PET/CT for Image Guidance. Urol. Case Rep. 2017, 14, 11–14. [Google Scholar] [CrossRef]
- Lopci, E.; Guazzoni, G.; Lazzeri, M. 68Ga Prostate-specific Membrane Antigen PET/CT for Primary Diagnosis of Prostate Cancer: Complementary or Alternative to Multiparametric MR Imaging–Letters to the Editor. Radiology 2018, 287, 725–726. [Google Scholar] [CrossRef]
- Meyer, A.R.; Joice, G.A.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Integration of PSMA-targeted PET imaging into the armamentarium for detecting clinically significant prostate cancer. Curr. Opin. Urol. 2018, 28, 493–498. [Google Scholar] [CrossRef]
- Donato, P.; Roberts, M.J.; Morton, A.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; Yaxley, J. Improved specificity with 68Ga PSMA PET/CT to detect clinically significant lesions ‘invisible’ on multiparametric MRI of the prostate: A single institution comparative analysis with radical prostatectomy histology. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 20–30. [Google Scholar] [CrossRef]
- Kalapara, A.A.; Nzenza, T.; Pan, H.Y.; Ballok, Z.; Ramdave, S.; O’Sullivan, R.; Ryan, A.; Cherk, M.; Hofman, M.S.; Konety, B.R.; et al. Detection and localisation of primary prostate cancer using 68gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int. 2020, 126, 83–90. [Google Scholar] [CrossRef]
- Berger, I.; Annabattula, C.; Lewis, J.; Shetty, D.V.; Kam, J.; MacLean, F.; Arianayagam, M.; Canagasingham, B.; Ferguson, R.; Khadra, M.; et al. 68Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: Correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018, 21, 204–211. [Google Scholar] [CrossRef]
- Awenat, S.; Piccardo, A.; Carvoeiras, P.; Signore, G.; Giovanella, L.; Prior, J.; Treglia, G. Diagnostic role of18f-psma-1007 pet/ct in prostate cancer staging: A systematic review. Diagnostics 2021, 11, 552. [Google Scholar] [CrossRef]
- Urso, L.; Castello, A.; Rocca, G.C.; Lancia, F.; Panareo, S.; Cittanti, C.; Uccelli, L.; Florimonte, L.; Castellani, M.; Ippolito, C.; et al. Role of PSMA-ligands imaging in Renal Cell Carcinoma management: Current status and future perspectives. J. Cancer Res. Clin. Oncol. 2022, 148, 1299–1311. [Google Scholar] [CrossRef]
- Zettinig, O.; Shah, A.; Hennersperger, C.; Eiber, M.; Kroll, C.; Kübler, H.; Maurer, T.; Milletarì, F.; Rackerseder, J.; zu Berge, C.S.; et al. Multimodal image-guided prostate fusion biopsy based on automatic deformable registration. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1997–2007. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Rüschoff, J.H.; Ferraro, D.A.; Muehlematter, U.J.; Laudicella, R.; Hermanns, T.; Rodewald, A.-K.; Moch, H.; Eberli, D.; Burger, I.A.; Rupp, N.J. What’s behind 68Ga-PSMA-11 uptake in primary prostate cancer PET? Investigation of histopathological parameters and immunohistochemical PSMA expression patterns. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4042–4053. [Google Scholar] [CrossRef]
- Zeng, Y.; Leng, X.; Liao, H.; Jiang, G.; Chen, P. Diagnostic value of integrated 18F-PSMA-1007 PET/MRI compared with that of biparametric MRI for the detection of prostate cancer. Prostate Int. 2022, 10, 108–116. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Cassarino, G.; Artioli, P.; Cecchin, D.; Dal Moro, F.; Zucchetta, P. PET/MRI in prostate cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 859–873. [Google Scholar] [CrossRef]
- Ghafoor, S.; Burger, I.A.; Vargas, A.H. Multimodality Imaging of Prostate Cancer. J. Nucl. Med. 2019, 60, 1350–1358. [Google Scholar] [CrossRef]
- Cardet, R.E.D.F.; Hofman, M.S.; Segard, T.; Yim, J.; Williams, S.; Francis, R.J.; Frydenberg, M.; Lawrentschuk, N.; Murphy, D.G.; Lourenco, R.D.A. Is Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging Cost-effective in Prostate Cancer: An Analysis Informed by the proPSMA Trial. Eur. Urol. 2021, 79, 413–418. [Google Scholar] [CrossRef]
- Alberts, I.; Mingels, C.; Zacho, H.D.; Lanz, S.; Schöder, H.; Rominger, A.; Zwahlen, M.; Afshar-Oromieh, A. Comparing the clinical performance and cost efficacy of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 in the diagnosis of recurrent prostate cancer: A Markov chain decision analysis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4252–4261. [Google Scholar] [CrossRef]
- Rezapour, A.; Alipour, V.; Moradi, N.; Arabloo, J. Cost-Effectiveness of Multiparametric Magnetic Resonance Imaging and Targeted Biopsy Versus Systematic Transrectal Ultrasound-Guided Biopsy for Prostate Cancer Diagnosis: A Systematic Review. Value Health Reg. Issues 2022, 30, 31–38. [Google Scholar] [CrossRef]
- Salameh, J.-P.; Bossuyt, P.M.; A McGrath, T.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; A Korevaar, D.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef]

| Author | Year | Patients | Study Design | Age (Median) | PSA (ng/mL) | Imaging | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| Hoffmann [39] | 2017 | 25 | Retrospective | 67 | 20.4 ± 33.5 mean | SUVmax 5.4 | 94% | 100% |
| Lopci [38] | 2018 | 45 | Prospective | 64 | 10.46 mean | SUVmax 5.4 | 100% | 76% |
| SUVratio 2 | 100% | 88% | ||||||
| Sasikumar [37] | 2018 | 118 | Prospective | 67 | 11.56 (0.85–4156) median | NR | NR | NR |
| Zhang J [36] | 2019 | 58 | Retrospective | 70 | 19.46 (1.31–49.07) median | PET/CT | 91.67% | 81.82% |
| Kumar [35] | 2019 | 15 | Prospective | 66.2 | 9.9 (5.1–19.5) mean | PET/CT | 88.8% | 66.65% |
| MRI | 62.5% | 76.4% | ||||||
| Chen [34] | 2019 | 54 | Retrospective | 65 | 13.53 (4.04–110) median | PET/CT | 89% | 71% |
| MRI | 76% | 89% | ||||||
| Chandra [33] | 2020 | 64 | Retrospective | 70 | 15.67 (1.74–44) mean | PET/CT | 74% | 92% |
| SUVmax 5.6 | 95% | 90.9% | ||||||
| Lopci [32] | 2020 | 97 | Prospective | 74.7 | 7.6 (1.86–32.6) median | SUVmax 5.4/SUVratio 2.2 | 60% | 97% |
| MRI | 81% | 26% | ||||||
| Li [31] | 2020 | 67 | Retrospective | 68 | 10.48 (3.15–19.76) mean | PET/CT | 87.9% | 88.2% |
| MRI | 84.9% | 52.9% | ||||||
| Lopci [30] | 2020 | 20 | Prospective | 74.7 | 7.6 (1.86–32.6) median | SUVmax 5.4 | 60% | 93% |
| SUVratio 2.2 | 80% | 93% | ||||||
| Zhang L [29] | 2020 | 120 | Prospective | 71.1 | 28.2 ± 26 mean | PET/CT vs. TRUS | NR | NR |
| Wang [28] | 2020 | 63 | Retrospective | 69.56 | 4.15–1298 mean | ADC 1.02 × 10−3 | 58.1% | 90.6% |
| SUVmax 11.7 | 67.2% | 97.7% | ||||||
| Liu [27] | 2020 | 31 | Prospective | 65 | 18.0 (5.48–49.77) median | PET/CT | 100% | 68.4% |
| Chinnappan [26] | 2021 | 67 | Retrospective | 70 | 23.2 (2.97–45.6) mean | PET/CT | 76.3% | 96.5% |
| MRI | 92.1% | 65.5% | ||||||
| Jiao [25] | 2021 | 58 | Retrospective | 70.6 | 40.84 (13.66–89.96) median | SUVmax 5.3 | 85.8% | 86.2% |
| Jain [24] | 2021 | 81 | Prospective | 68.4 | 10.5 ± 4.6 mean | SUVmax 6.15 | 84% | 80% |
| Emmett [23] | 2022 | 291 | Prospective | 64 | 5.6 (4.2–7.5) median | PET + MRI | 97% | 40% |
| MRI | 83% | 53% | ||||||
| Nuo [22] | 2017 | 105 | Retrospective | 68.4 | 3.45–1000 mean | SUVmax 12.9 | 74% | 94% |
| bpMRI | 62% | 88% | ||||||
| bpMRI/PET | 80% | 88% |
| Study | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Hoffmann [39] |  |  |  |  |  |  |  |
| Lopci [38] |  |  |  |  |  |  |  |
| Sasikumar [37] |  |  |  |  |  |  |  |
| Zhang J [36] |  |  |  |  |  |  |  |
| Kumar [35] |  |  |  |  |  |  |  |
| Chen [34] |  |  |  |  |  |  |  |
| Chandra [33] |  |  |  |  |  |  |  |
| Lopci [32] |  |  |  |  |  |  |  |
| Li [31] |  |  |  |  |  |  |  |
| Lopci [30] |  |  |  |  |  |  |  |
| Zhang L [29] |  |  |  |  |  |  |  |
| Wang [28] |  |  |  |  |  |  |  |
| Liu [27] |  |  |  |  |  |  |  |
| Chinnappan [26] |  |  |  |  |  |  |  |
| Jiao [25] |  |  |  |  |  |  |  |
| Jain [24] |  |  |  |  |  |  |  |
| Emmett [23] |  |  |  |  |  |  |  |
| Nuo [22] |  |  |  |  |  |  |  |
 Low risk;
Low risk;  high risk;
high risk;  unclear risk.
unclear risk.| Trial Identifier Number | Phase | Status | Radiotracer | AIM | Country |
|---|---|---|---|---|---|
| NCT05154162 | III | Recruiting | Not specified | To evaluate PSMA PET/CT additive value for sPCa diagnosis in men with negative/equivocal MRI | Australia |
| NCT02282137 | II | Recruiting | [68Ga]PSMA-11 | Sensitivity of PSMA PET/CT for the detection of tumor location | USA |
| NCT04179968 | NA | Recruiting | [68Ga]PSMA-11 | To assess the ability of PSMA PET/CT to increase diagnostic accuracy in localizing primary and metastatic lesions in patients with suspected prostate cancer and elevated PI-RADS scores and PSA | USA |
| NCT05002465 | NA | Available | [68Ga]PSMA-11 | To provide PSMA PET/CT for clinical use in the diagnosis, staging, and restaging | USA |
| NCT05297162 | NA | Recruiting | [68Ga]PSMA-11 | To compare PSMA PET/TRUS vs. mpMRI/TRUS fusion prostate biopsy in men with a high suspicion of PCa after at least one negative biopsy | Italy |
| NCT05160597 | I | Recruiting | [68Ga]PSMA-11 | To identify prostate cancer in men with a prior negative or inconclusive prostate biopsy | USA |
| NCT05137561 | NA | Recruiting | [68Ga]PSMA | To compare the diagnostic yield of robotic-arm-assisted PSMA PET/CT–guided prostate biopsy and MRI-directed TRUS-guided prostate biopsy in patients with PI-RADS grading of 4/5 | India |
| NCT04867603 | III | Recruiting | [68Ga]PSMA | Diagnostic performance of digital PET/CT using 68Ga PSMA for the characterization of prostate lesions | USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracciolo, M.; Castello, A.; Urso, L.; Borgia, F.; Ortolan, N.; Uccelli, L.; Cittanti, C.; Castellani, M.; Bartolomei, M.; Lazzeri, M.; et al. The Role of [68Ga]PSMA PET/CT for Clinical Suspicion of Prostate Cancer in Patients with or without Previous Negative Biopsy: A Systematic Review. Cancers 2022, 14, 5036. https://doi.org/10.3390/cancers14205036
Caracciolo M, Castello A, Urso L, Borgia F, Ortolan N, Uccelli L, Cittanti C, Castellani M, Bartolomei M, Lazzeri M, et al. The Role of [68Ga]PSMA PET/CT for Clinical Suspicion of Prostate Cancer in Patients with or without Previous Negative Biopsy: A Systematic Review. Cancers. 2022; 14(20):5036. https://doi.org/10.3390/cancers14205036
Chicago/Turabian StyleCaracciolo, Matteo, Angelo Castello, Luca Urso, Francesca Borgia, Naima Ortolan, Licia Uccelli, Corrado Cittanti, Massimo Castellani, Mirco Bartolomei, Massimo Lazzeri, and et al. 2022. "The Role of [68Ga]PSMA PET/CT for Clinical Suspicion of Prostate Cancer in Patients with or without Previous Negative Biopsy: A Systematic Review" Cancers 14, no. 20: 5036. https://doi.org/10.3390/cancers14205036
APA StyleCaracciolo, M., Castello, A., Urso, L., Borgia, F., Ortolan, N., Uccelli, L., Cittanti, C., Castellani, M., Bartolomei, M., Lazzeri, M., & Lopci, E. (2022). The Role of [68Ga]PSMA PET/CT for Clinical Suspicion of Prostate Cancer in Patients with or without Previous Negative Biopsy: A Systematic Review. Cancers, 14(20), 5036. https://doi.org/10.3390/cancers14205036











