The Development of a Three-Dimensional Platform for Patient-Derived Ovarian Cancer Tissue Models: A Systematic Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Ovarian Cancer Tumour Characteristics
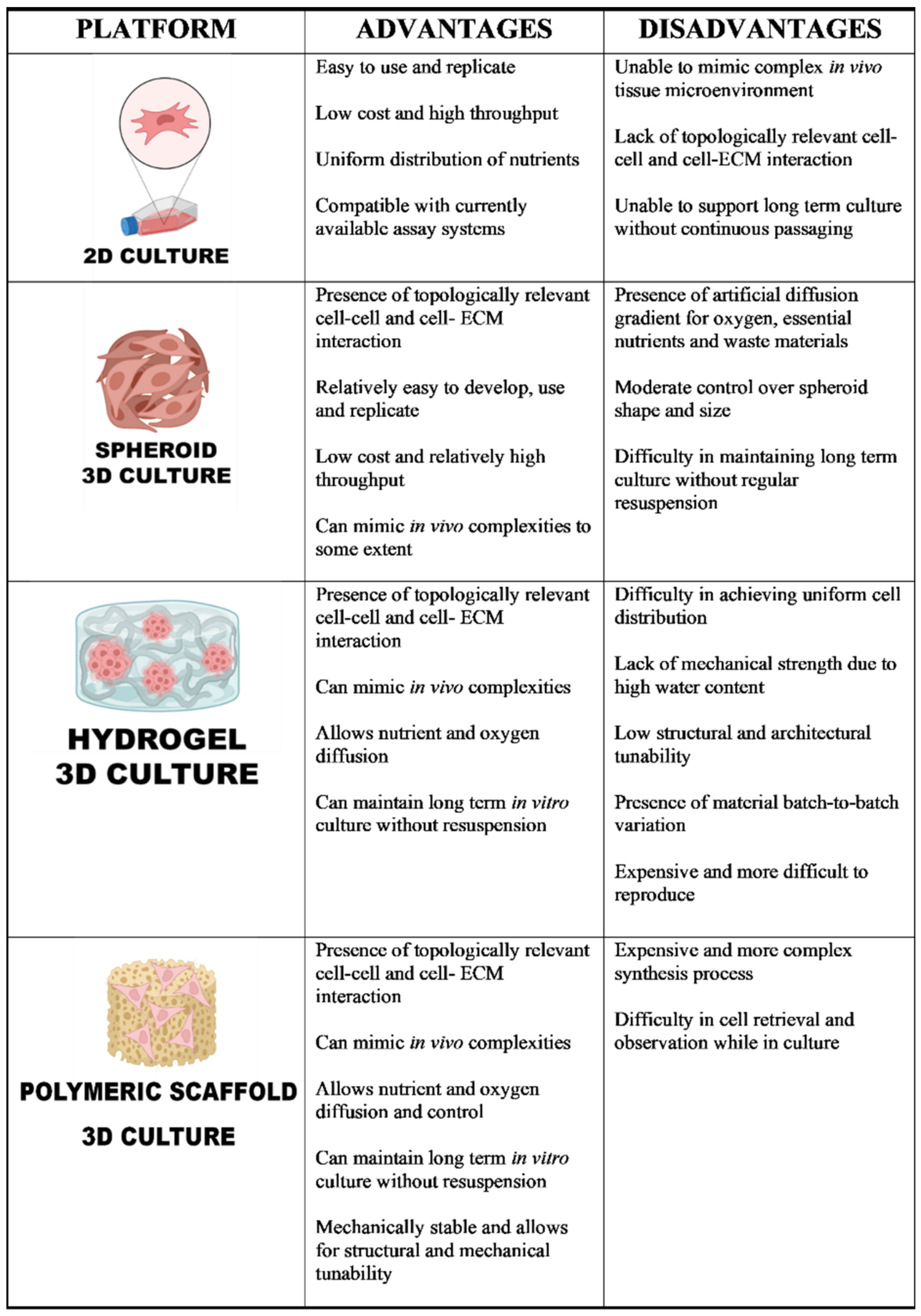
1.2. Current Models
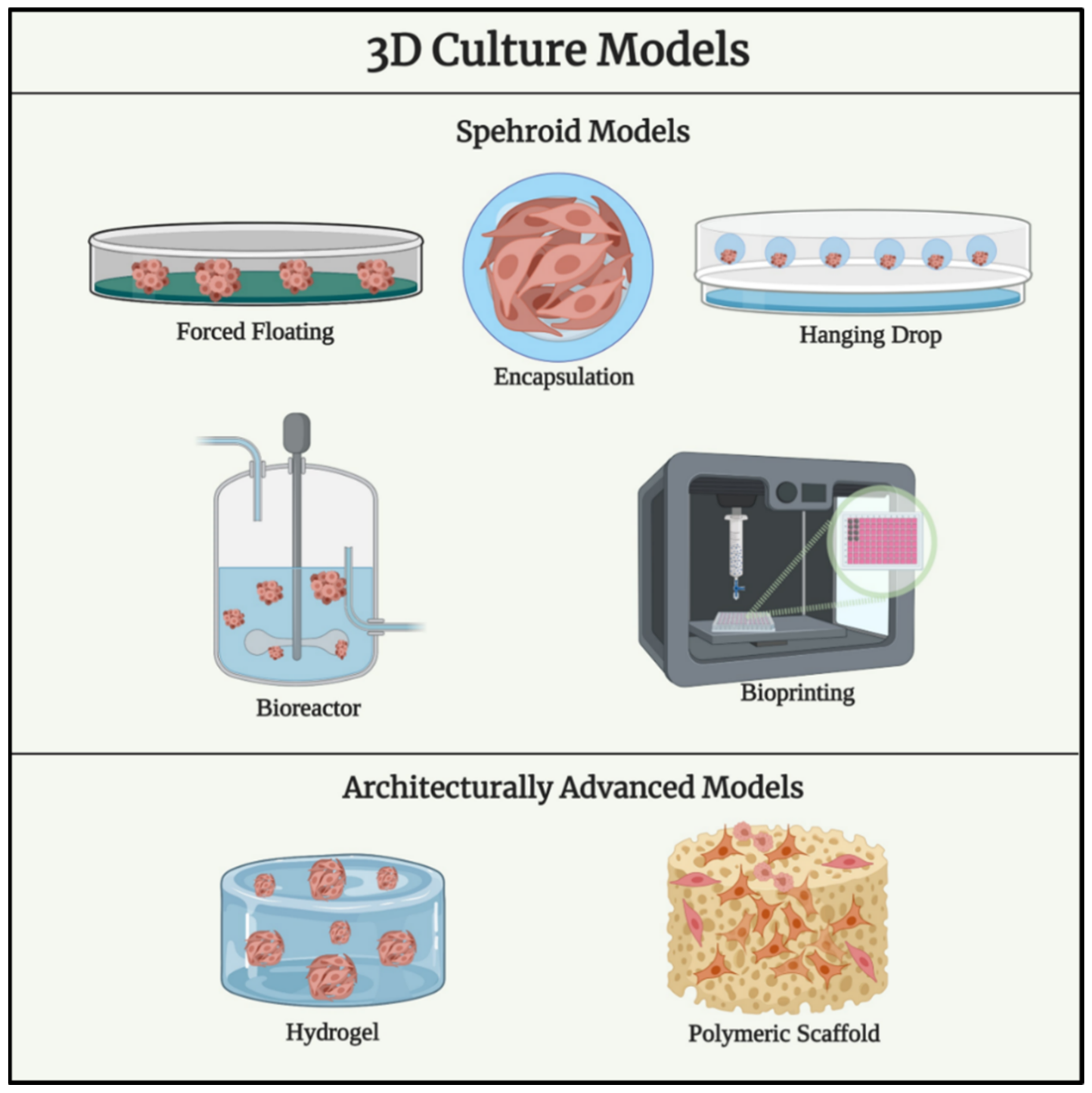
1.3. Spheroid Models for Ovarian Cancer
1.4. Ovarian Cancer Models Using Biomaterials with Advances Structural Complexity
1.5. Cell Sources in Available Ovarian Cancer In Vitro 3D Studies
2. Materials and Methods
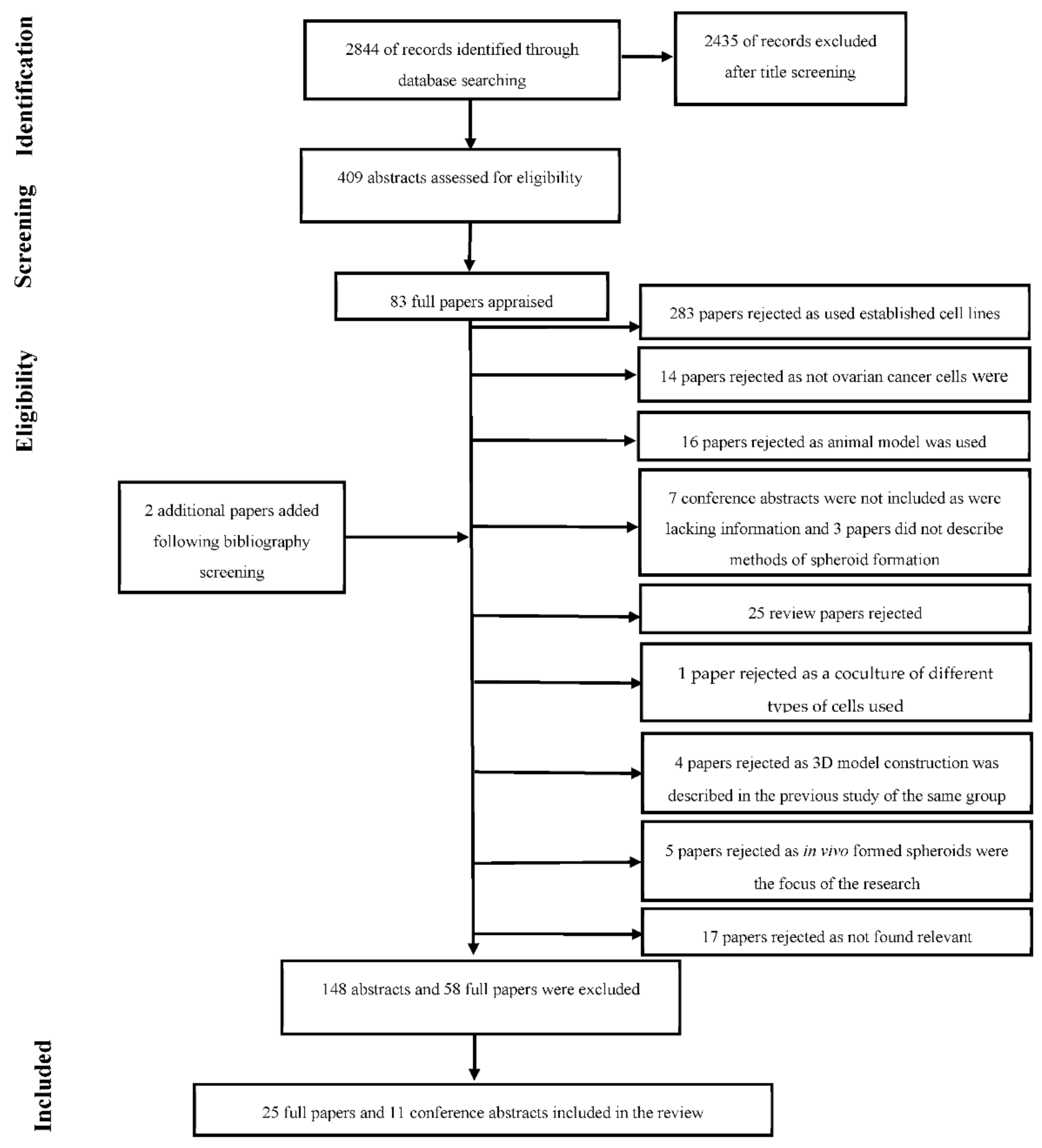
2.1. Search Strategy
2.2. Selection Process
2.3. Study Selection
- The type of cells used for the 3D model were exclusively patient derived OvCa cells or newly established cell lines derived directly from primary OvCa cells.
- Only multicellular tumour spheroid and tumour-derived spheroid models were reviewed in the current study.
- The main focus of the research was to build a three-dimensional model of OvCa cells regardless of method used to accomplish it.
2.4. Data Extraction
3. Results
- established OvCa cell lines were used for creating tumour spheroids opposed to patient derived samples;
- not OvCa cells used;
- animal models studied;
- only conference abstract was available which did not contain enough information for detailed analysis;
- review article;
- 3D model construction was described in the previous study of the same group, sensitivities were evaluated in the article;
- in vivo formed spheroids were the focus of the research;
- topic was found irrelevant.
4. Discussion
| Paper | Number of Patients | Type of Specimens Collected |
|---|---|---|
| Griffon et al. [6] | 18 | 8 solid tumours finely chopped and enzymatically disaggregated, 9 ascitic fluids and 1 pleural effusion |
| Zhang et al. [7] | 5 | Tumour specimens of stage III serous adenocarcinomas—minced and enzymatically disaggregated |
| Kryczek et al. [8] | 25 | Cells and tissues obtained from ascites and tumours of chemotherapy naïve patients with EOC |
| He et al. [12] | 6 | Tumour specimens from OvCa patients mechanically dissociated and enzymatically disaggregated within 30 min of surgery |
| Martinez- Serrano et al. [13] | 10 | Ovarian tumour mass from chemotherapy naïve patients with papillary serous EOC processed using enzymatic cell tissue dissociation |
| Rafehi et al. [14] | At least 4 independent patient samples and at least 3 experimental replicates | Ascites fluid obtained from OvCa patients at the time of debulking surgery or paracentesis |
| Raghavan et al. [15] | 3 | Primary patient ascites cells (centrifuged) from tumour bank with confirmed OvCa origin |
| Loessner et al. [16] | n/a | Primary OvCa cells isolated from patients with high grade serous OvCa |
| Shuford et al. [17] | 92 | Fresh tissue from either a primary debulking surgery (n = 76) or laparoscopic biopsy (n = 16) of chemotherapy naïve patients |
| Maru et al. [18] | 15 | Tissue fragments of approximately 500–1000 mm3 obtained from ovarian tumours immediately after tumour resection. Non-necrotic lesions with solid or papillary growth selected. Tissue fragments cut into 2–3 mm pieces and enzymatically disaggregated. |
| Nelson et al. [19] | 12 | Primary patient ascites cells (centrifuged) from tumour bank and solid tumour samples processed using a tumour dissociation kit |
| Park et al. [9] | 3 | Fresh tumours minced and dissociated with collagenase |
| Huang et al. [10] | 7 | Fresh tumours minced and dissociated with collagenase and hyaluronidase |
| Hedemann et al. [11] | n/a | Fresh tumour cleared, fragmentated and enzymatically disaggregated |
| Paper | Number of Patients | Type of Specimens Collected |
|---|---|---|
| Sonoda et al. [20] | n/a | OVMG-1 and OVMG-2 serous adenocarcinoma cell lines from surgical specimens |
| Zietarska et al. [21] | n/a | TOV-21G and TOV-112I cell lines from primary ovarian malignant tumours; OV-90 cell line from ovarian malignant ascites from chemotherapy naïve patients |
| Puiffe et al. [22] | OV-90 cell line derived from 1 patient; ascites of 54 EOC patients | OV-90 cell line derived from cellular fraction of ascites from a chemotherapy-naïve patient |
| Ouellet et al. [23] | 2 | TOV-1946 (scape method used) and TOV-2223G (collagenase method used) cell lines derived from solid tumours and OV-1946 cell lines—from a mass of cells from ascites (micro-dissection into small pieces) of chemotherapy naïve patients with grade 3 serous papillary cystadenoma at stage IIIC |
| Grun et al. [24] | n/a | OV-TRL12B cell line established from cytobrushing of a squamous ovarian carcinoma |
| Létourneau et al. [25] | 3 | TOV cell lines (n = 4) derived from solid ovarian tumour (scape method) and OV cell lines (n = 5) established from the cellular fraction of ascites (centrifugation) |
| Liao et al. [26] | 30 | Primary EOC cell lines obtained from tumour specimens (finely minced) and ascitic fluid (centrifugation) obtained from patients undergoing tumour debulking surgery for EOC |
| Fleury et al. [27] | 6 | Solid ovarian tumour (TOV) derived cell lines (TOV2978G, TOV3041G, TOV3291G) (scrape method). The OV cell lines (OV866(2), OV4453, OV4485) established from the cellular fraction of ascites (centrifugation). |
| Noguchi et al. [28] | 1 | NCC-cOV1-C1 cell line derived from cellular fraction of ascites of a patient with clear cell carcinoma |
| Silva et al. [29] | 1 | IPO43 cell line established from the ascitic fluid of a patient with a diagnosis of high-grade serous carcinoma (HGSC) of the ovary, previously treated with chemotherapy |
| Parashar et al. [30] | 1 | Ovarian tumour samples minced and enzymatically disaggregated. Cells strained and centrifuged. |
| Hanging Drop | Forced Floating | Bioreactor | Others |
|---|---|---|---|
| Zietarska et al. (4 days) | Griffon et al. (4–5 days) | Grun et al. (3–4 weeks) | Loessner et al. (hydrogel system; 2 weeks) |
| Ouellet et al. (3 days) | Sonoda et al. (7 days) | Maru et al. (hydrogel-based sandwich method; 5 days) | |
| Létourneau et al. (4 days) | Puiffe et al. (4 days) | ||
| Fleury et al. (5–7 days) | Zhang et al. (11–14 days) | ||
| Raghavan et al. (7 days) | Kryczek et al. (1–6 weeks) | ||
| Liao et al. (3 weeks early culture- > dissociation and replating fornightly) | |||
| Rafehi et al. (72 h) | |||
| He et al. (14 days) | |||
| Martinez-Serrano et al. (average 28 days) | |||
| Shuford et al. (24–72 h) | |||
| Nelson et al. (2–4 days) | |||
| Vader et al. | |||
| Basten et al. | |||
| Mikkonen et al. | |||
| Nanki et al. | |||
| Park et al. (7 days) | |||
| Noguchi et al. (4 days) | |||
| Hedemann et al. (4 days) | |||
| Huang et al. | |||
| Silva et al. (72 h) | |||
| Parashar et al. (7 days) |
| Paper | Construct Development Method | Time of Incubation | Size of Spheroids | General Comment | ||
|---|---|---|---|---|---|---|
| Griffon et al. [6] | 6-well plates coated with 1 mL of 0.5% agarose (forced floating/aggregation) | 10 days in vitro | Mean of 198 (±7.7) µm | Radiosensitivities of spheroids obtained from human ovarian carcinoma cells tested. | ||
| Zhang et al. [7] | Ultra Low Attachment plates (forced floating/aggregation) | 11–14 days in vitro | Validation in vivo | 50–100 µm | Isolation and characterisation of highly tumorigenic subpopulation of cells (malignant progenitors) described. | |
| Kryczek et al. [8] | Ultralow attachment plates | (forced floating/aggregation) | Spheres counted for 1–6 weeks | Validation in vivo | >50 µm | Expression of multiple cancer stem cell markers in fresh OvCa and established primary OvCa cell lines investigated and the stem cell properties of potential OvCa stem cells in vitro and in vivo examined. |
| He et al. [12] | 96-well ultra-low attachment plates | (forced floating/aggregation) | 2 weeks in vitro | Validation in vivo | n/a | Subpopulation of stem cell-like cells that form spheroids and possess self-renewal capacity, strong tumour-initiating ability, and higher resistance to chemotherapy derived from high grade serous carcinoma studied. |
| Martinez-Serrano et al. [13] | Corning Ultra-Low attachment surface T25 flask | (forced floating/aggregation) | Median period of 28 days in vitro cultivation | n/a | Specificity of cell surface markers to discriminate the tumour initiating cells (isolated from EOC) from somatic stem cells (isolated from healthy women) investigated. | |
| Rafehi et al. [14] | Ultralow attachment plates | (forced floating/aggregation) | 3 days in vitro | n/a | Findings demonstrating that intact TGFβ signalling is required to control epithelial-mesenchymal transition in EOC ascites-derived cell spheroids, and it promotes the malignant characteristics of these structures. | |
| Raghavan et al. [15] | Spheroids formed by hanging drop method | 7 days in vitro | Validation in vivo | n/a | The responses to varying drug treatments were different in patient-derived samples and correlated with in vivo drug studies in xenografts. | |
| Loessner et al. [16] | Encapsulation based spheroid formation | 2 weeks in vitro | n/a | 3D culture showed cell proliferation profile and aggregation similar to in vivo. Expression of integrins, MMP enhanced in 3D culture in comparison to 2D. Spheroids showed higher chemoresistance in comparison to 2D for paclitaxel. | ||
| Shuford et al. [17] | 84-well spheroid microplates | (forced floating/aggregation) | 24 h in vitro | n/a | Analytical and prospective clinical validation of a new test that utilizes primary patient tissue in 3D cell culture to make patient specific response predictions prior to initiation of treatment in the clinic presented. | |
| Maru et al. [18] | Hydrogel based sandwich method | 5 days in vitro | n/a | 3D hydrogel-based model of patient samples was able to maintain original tumour characteristics. Spheroid based models are better for assessment of treatments in comparison to hydrogel-based 3D in vitro models. | ||
| Nelson et al. [19] | Matrigel in 24-well plate (forced floating/aggregation) | 2–4 days in vitro | n/a | Ex vivo cultures from patient biopsies used to provide models that support interrogation of chromosome instability mechanisms. | ||
| Park et al. [9] | Ultra-attachment 6-well culture plates | (forced floating/aggregation) | 7 days in vitro | n/a | mRNA expression of transcription factors and miRNA expression of spheroids derived from primary ovarian cancers to identify factors regulating ovarian cancer stem cells. | |
| Huang et al. [10] | 6-well ultra-low attachment plates. (forced floating/aggregation) | 7–10 days in vitro | Validation in vivo | >50 µm | Cell lines and primary tissue used to grow spheroids, which were tested against platinum-chemotherapy agents, correlated with in vivo drug studies in xenografts. | |
| Hedemann et al. [11] | Ultralow attachment plates | (forced floating/aggregation) | 4 days in vitro | ~150–300 µm | A combination of ADAM17 inhibitor with cisplatin tested in 2D and 3D culture of cells derived from cell lines and primary ovarian tumor- and ascites-derived cells. | |
| Paper | Construct Development Method | Time of Incubation | Size of Spheroids | General Comment | ||||
|---|---|---|---|---|---|---|---|---|
| Sonoda et al. [20] | 24-well culture plate coated with 1% agarose (forced floating/aggregation) | 7 days in vitro | Validation in vivo | n/a | Angiogenesis factors expression measured and compared in 2D, 3D and xenografts. | |||
| Zietarska et al. [21] | Spheroids formed by hanging drop method | 10 days in vitro (spheroids formed by day 4) | Validation in vivo | Maximum size of 500 µm | Molecular comparison of spheroid model versus 2D and xenograft model described. | |||
| Puiffe et al. [22] | Spheroids formed by modified hanging drop method | 4 days in vitro | Small in the absence And ~500 µm in the presence of ascites | Effect of the acellular fraction of ascites on OV-90 addressed. | ||||
| Ouellet et al. [23] | Spheroids formed by modified hanging drop method | 4 days in vitro | Validation in vivo | TOV-1946—aggregate | OV-1946—semi-compact | TOV-2223—none | New serous EOC cell lines from both solid tumours and ascites of the same patient were derived and characterised. | |
| Grun et al. [24] | Rotary Cell Culture System | 3–4 weeks in vitro (spheroids formed in 1 week) | Maximum diameter of 4 mm | 3D culture established, biological features (morphological characteristics, expression of tumour markers, proteomic profiles). compared between 2D, 3D and primary tumours. | ||||
| Létourneau et al. [25] | Spheroids formed by hanging drop method | 4 days in vitro | Validation in vivo | n/a | New OvCa cell lines described. | |||
| Liao et al. [26] | Ultralow attachment plates | (forced floating/aggregation) | Cultivation period n/a | Validation in vivo | n/a | To study EOC pathogenesis, EOC primary cells under stem cell selective conditions were cultured and generated anchorage-independent, self-renewing spheroids morphologically similar to spheroids isolated from patient ascites. | ||
| Fleury et al. [27] | Spheroids formed by hanging droplet method | 5–7 days in vitro | Validation in vivo | TOV2978G, OV4453—aggregate | OTOV3291G—semi compact | TOV3041G—compact | Six new EOC cell lines spontaneously derived from high grade serous tumours or ascites established and described. | |
| Noguchi et al. [28] | 96-well culture plates (forced floating/aggregation) | 4 days in vitro | n/a | NCC-cOV1-C1 cell line established and characterised. Anticancer drug screening conducted. | ||||
| Silva et al. [29] | Stirred-tank culture system placed on a magnetic stirrer | 3 days of in vitro | n/a | IPO43 cell line established and characterised. | ||||
| Parashar et al. [30] | 24-well, growth factor reduced Matrigel-coated non-adherent plates (forced floating/aggregation) | 7 days in vitro | ~30–100 µm | MCW-OV-SL-3 endometrioid subtype of ovarian cancer cell line established, chemoresistance mechanisms studied. | ||||
| Abstract | Year | Number of Patients, Specimen and Method | General Comment |
|---|---|---|---|
| Sun et al. [31] | 2012 | Fresh specimens of OvCa minced, enzymatically digested, rinsed, incubated in DMEM (monolayer cultures) Mammosphere media (spheroids). Validation in vivo. | Spheroids are enriched for expression of markers including CD133, CD44, NANOG and OCT4, suggesting that spheroid formation enhances stem cell-like markers. Increased expression of miR-26b in spheroids compared to monolayer culture. |
| Shuford et al. [32] | 2014 | OvCa samples—standard mincing & digestion. | Ex vivo 3D (EV3D™) culture and testing of primary human OvCa was described. Carboplatin & taxane based combination therapy was used in most cases. |
| Ishiguro et al. [33] | 2014 | OvCa cells from surgical specimen. Validation in vivo. | Differentiation of spheroid cells associated with the downregulation of the stem cell-specific regulators Nanog, Sox2, and ALDH1A1 and the up-regulation of cytokeratin and it is associated with increased paclitaxel resistance. The changes are reversible. |
| Desrochers et al. [34] | 2015 | OvCa samples (newly diagnosed, treatment naïve and relapsed) standard mincing & digestion. 3D spheroids were developed and 3D perfused Ovarian Microtumours were cultured using the 3DKUBETM. | Carboplatin, gemcitabine, erlotinib and afatanib responses tested. |
| Vader et al. [35] Basten et al. [36] Dijkmans et al. [37] | 2017 2018 2018 | 3D cultures embedded in a protein-rich hydrogel (384 well plates) are generated from tumour biopsies (endometrial, cervical, and OvCa patients–fresh and cryopreserved material). | 3D cultures exposed to standard-of-care therapies, targeted therapies and drug combinations. |
| Mikkonen et al. [38] | 2018 | Processed fresh cancer tissue (ovarian)—cells cultivated in Matrigel or in cellulose-based hydrogel, GrowDex. | Genetic profiling and image-based phenotyping, phenomics done. Drug responses (52 agents) tested in 2D and 3D, significant differences in sensitivity to several drugs observed. |
| Nanki et al. [39] | 2018 | Intraoperative ascites and tissue samples from primary ovarian, peritoneal, and fallopian tube cancer patients. 3D culture obtained using 96-well plates—14 days. | Spheroids-like structures were formed in 30% (1/3) of ascites samples and 50% (4/8) of tissue samples. The tumorigenicity and invasiveness of the cells were demonstrated using new 3D model cultured in vitro by NanoCulture Plate LH96. |
| Tanaka et al. [40] | 2018 | 13 primary ovarian tumour surgical samples (8—OvCa, 2—borderline, 3—benign) and 1 malignant effusion (ascitic and pleural) of OvCa patient. Matrigel-based organoid culture, or spheroid culture. | Long-term 3D cultures established from 4 samples. Drug responses tested for 2 cultures (cisplatin and paclitaxel). |
| Ito et al. [41] | 2018 | OvCa cells from patient tumours (61 cancer tissue-originated spheroid (CTOS) method). | Sensitivity assay for paclitaxel and carboplatin conducted and compared to clinical outcome. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OvCa | ovarian cancer |
| EOC | epithelial ovarian cancer |
| 3D | three dimensional |
| 2D | two dimensional |
| TME | tumour microenvironment |
| ECM | extracellular matrix |
| PEG | poly-ethylene glycol |
| EMT | epithelial to mesenchymal transition |
| MMP | matrix metalloproteinases |
| CTOS | cancer tissue originated spheroids |
| TGFβ | transforming growth factor β |
| VEGF | vascular endothelial growth factor |
Appendix A
References
- Cancer Research, UK. Ovarian Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer (accessed on 27 January 2020).
- Torre, L.A.; Trabert, B.; Desantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®): Health Professional Version; National Cancer Instutute: Bethesda, MD, USA, 2002.
- Moschetta, M.; Boussios, S.; Rassy, E.; Samartzis, E.P.; Funingana, G.; Uccello, M. Neoadjuvant treatment for newly diagnosed advanced ovarian cancer: Where do we stand and where are we going? Ann. Transl. Med. 2020, 8, 1710. [Google Scholar] [CrossRef] [PubMed]
- Lal-Nag, M.; McGee, L.; Titus, S.A.; Brimacombe, K.; Michael, S.; Sittampalam, G.; Ferrer, M. Exploring Drug Dosing Regimens In Vitro Using Real-Time 3D Spheroid Tumor Growth Assays. SLAS Discov. 2017, 22, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Griffon, G.; Marchal, C.; Merlin, J.L.; Marchal, S.; Parache, R.M.; Bey, P. Radiosensitivity of multicellular tumour spheroids obtained from human ovarian cancers. Eur. J. Cancer 1995, 31A, 85–91. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.M.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Kryczek, I.; Liu, S.; Roh, M.; Vatan, L.; Szeliga, W.; Wei, S.; Banerjee, M.; Mao, Y.; Kotarski, J.; Wicha, M.S.; et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int. J. Cancer 2012, 130, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hwang, S.; Jeong, J.Y.; Jung, S.G.; Choi, M.C.; Joo, W.D.; Song, S.H.; Lee, C.; An, H.J. Integrative analysis of transcription factors and microRNAs in ovarian cancer cell spheroids. J. Ovarian Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Huang, Z.; Kondoh, E.; Visco, Z.R.; Baba, T.; Matsumura, N.; Dolan, E.; Whitaker, R.S.; Konishi, I.; Fujii, S.; Berchuck, A.; et al. Targeting dormant ovarian cancer cells in vitro and in an in vivo mouse model of platinum resistance. Mol. Cancer Ther. 2021, 20, 85–95. [Google Scholar] [CrossRef]
- Hedemann, N.; Herz, A.; Schiepanski, J.H.; Dittrich, J.; Sebens, S.; Dempfle, A.; Feuerborn, J.; Rogmans, C.; Tribian, N.; Flörkemeier, I.; et al. Adam17 inhibition increases the impact of cisplatin treatment in ovarian cancer spheroids. Cancers 2021, 13, 2039. [Google Scholar] [CrossRef]
- He, Q.Z.; Luo, X.Z.; Wang, K.; Zhou, Q.; Ao, H.; Yang, Y.; Li, S.X.; Li, Y.; Zhu, H.T.; Duan, T. Isolation and characterization of cancer stem cells from high-grade serous ovarian carcinomas. Cell. Physiol. Biochem. 2014, 33, 173–184. [Google Scholar] [CrossRef]
- Martínez-Serrano, M.J.; Caballero-Baños, M.; Vilella, R.; Vidal, L.; Pahisa, J.; Martínez-Roman, S. Is sphere assay useful for the identification of cancer initiating cells of the ovary? Int. J. Gynecol. Cancer 2015, 25, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, S.; Valdes, Y.R.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; Dimattia, G.E.; Shepherd, T.G. TGFβ signaling regulates Epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids. Endocr. Relat. Cancer 2016, 23, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Mehta, P.; Ward, M.R.; Bregenzer, M.E.; Fleck, E.M.A.; Tan, L.; McLean, K.; Buckanovich, R.J.; Mehta, G. Personalized medicine–based approach to model patterns of chemoresistance and tumor recurrence using ovarian cancer stem cell spheroids. Clin. Cancer Res. 2017, 23, 6934–6945. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Rockstroh, A.; Shokoohmand, A.; Holzapfel, B.M.; Wagner, F.; Baldwin, J.; Boxberg, M.; Schmalfeldt, B.; Lengyel, E.; Clements, J.A.; et al. Biomaterials A 3D tumor microenvironment regulates cell proliferation, peritoneal growth and expression patterns. Biomaterials 2019, 190–191, 63–75. [Google Scholar] [CrossRef]
- Shuford, S.; Wilhelm, C.; Rayner, M.; Elrod, A.; Millard, M.; Mattingly, C.; Lotstein, A.; Smith, A.M.; Guo, Q.J.; O’Donnell, L.; et al. Prospective Validation of an Ex Vivo, Patient-Derived 3D Spheroid Model for Response Predictions in Newly Diagnosed Ovarian Cancer. Sci. Rep. 2019, 9, 11153. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Itami, M.; Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol. 2019, 154, 189–198. [Google Scholar] [CrossRef]
- Nelson, L.; Tighe, A.; Golder, A.; Littler, S.; Bakker, B.; Moralli, D.; Murtuza Baker, S.; Donaldson, I.J.; Spierings, D.C.J.; Wardenaar, R.; et al. A living biobank of ovarian cancer ex vivo models reveals profound mitotic heterogeneity. Nat. Commun. 2020, 11, 822. [Google Scholar] [CrossRef]
- Sonoda, T.; Kobayashi, H.; Kaku, T.; Hirakawa, T.; Nakano, H. Expression of angiogenesis factors in monolayer culture, multicellular spheroid and in vivo transplanted tumor by human ovarian cancer cell lines. Cancer Lett. 2003, 196, 229–237. [Google Scholar] [CrossRef]
- Zietarska, M.; Maugard, C.M.; Filali-Mouhim, A.; Alam-Fahmy, M.; Tonin, P.N.; Provencher, D.M.; Mes-Masson, A.M. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol. Carcinog. 2007, 46, 872–885. [Google Scholar] [CrossRef]
- Puiffe, M.L.; Le Page, C.; Filali-Mouhim, A.; Zietarska, M.; Ouellet, V.; Tonin, P.N.; Chevrette, M.; Provencher, D.M.; Mes-Masson, A.M. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007, 9, 820–829. [Google Scholar] [CrossRef]
- Ouellet, V.; Zietarska, M.; Portelance, L.; Lafontaine, J.; Madore, J.; Puiffe, M.L.; Arcand, S.L.; Shen, Z.; Hébert, J.; Tonin, P.N.; et al. Characterization of three new serous epithelial ovarian cancer cell lines. BMC Cancer 2008, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Grun, B.; Benjamin, E.; Sinclair, J.; Timms, J.F.; Jacobs, I.J.; Gayther, S.A.; Dafou, D. Three-dimensional in vitro cell biology models of ovarian and endometrial cancer. Cell Prolif. 2009, 42, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Létourneau, I.J.; Quinn, M.C.J.; Wang, L.L.; Portelance, L.; Caceres, K.Y.; Cyr, L.; Delvoye, N.; Meunier, L.; de Ladurantaye, M.; Shen, Z.; et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer 2012, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- Fleury, H.; Communal, L.; Carmona, E.; Portelance, L.; Arcand, S.L.; Rahimi, K.; Tonin, P.N.; Provencher, D.; Mes-Masson, A.M. Novel high-grade serous epithelial ovarian cancer cell lines that reflect the molecular diversity of both the sporadic and hereditary disease. Genes Cancer 2015, 6, 378–398. [Google Scholar] [CrossRef]
- Noguchi, R.; Yoshimatsu, Y.; Sei, A.; Yoshida, H.; Katou, T.; Kondo, T. Drug screening and kinase activity profiling of a novel patient-derived cell line of clear cell ovarian carcinoma. J. Electrophor. 2021, 65, 23–31. [Google Scholar] [CrossRef]
- Silva, F.; Coelho, F.; Peixoto, A.; Pinto, P.; Martins, C.; Frombach, A.S.; Santo, V.E.; Brito, C.; Guimarães, A.; Félix, A. Establishment and characterization of a novel ovarian high-grade serous carcinoma cell line—IPO43. Cancer Cell Int. 2022, 22, 175. [Google Scholar] [CrossRef]
- Parashar, D.; Geethadevi, A.; Mittal, S.; McAlarnen, L.A.; George, J.; Kadamberi, I.P.; Gupta, P.; Uyar, D.S.; Hopp, E.E.; Drendel, H.; et al. Correction to: Parashar et al. Patient-Derived Ovarian Cancer Spheroids Rely on PI3K-AKT Signaling Addiction for Cancer Stemness and Chemoresistance. Cancers 2022, 14, 958. [Google Scholar] [CrossRef]
- Sun, G.; Meng, F.; Zhong, M.; Yu, Y.; Shan, W.; Anderson, M.; Brewer, M.A. Abstract 1406: A new ovarian cancer metastasis model using multicellular spheroids generated from human ovarian cancers tissues. Cancer Res. 2012, 72, 1406. [Google Scholar] [CrossRef]
- Shuford, S.; Widener, R.; Cheluvaraju, C.; Desrochers, T.; Mattingly, C.; Puls, L.; Gevaert, M.; Orr, D.; Crosswell, H.E. Abstract LB-36: Chemotherapy testing of primary human ovarian cancers in an ex vivo 3D culture platform: A novel method of phenotypic profiling for clinical trial selection and personalized medicine. Cancer Res. 2014, 74, LB-36. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Nakagama, H.; Okamoto, K.; Tanaka, K.; Enomoto, T. Abstract 3056: Human ovarian cancer stem cells: In vitro cultivation and characterization. Cancer Res. 2014, 74, 3056. [Google Scholar] [CrossRef]
- Desrochers, T.; Shuford, S.; Mattingly, C.; Holmes, L.; Gevaert, M.; Elder, J.; Orr, D.; Corless, C.; Puls, L.; Crosswell, H.E. Abstract LB-282: Ex vivo 3d drug response profiling of primary human ovarian cancer differentiates treatment-naive and relapsed patients and molecular subtypes. Cancer Res. 2015, 75, LB-282. [Google Scholar] [CrossRef]
- Vader, W.; Price, L.; Herpers, B.; Basten, S. 3D cultured tumour from patients to predict treatment response. Ann. Oncol. 2017, 28, V451. [Google Scholar] [CrossRef]
- Basten, S.; Herpers, B.; Yan, K.; Vader, W.; Price, L. Abstract LB-A09: Predicting PARPi sensitivity in patient derived ex vivo 3D tumor cultures. Mol. Cancer Ther. 2018, 17, LB-A09. [Google Scholar] [CrossRef]
- Dijkmans, T.; Basten, S.; Herpers, B.; Yan, K.; Giesemann, T.; Schueler, J.; Vader, W.; Price, L. Abstract 4644: Patient-derived 3D tumor cultures for clinical diagnostics and pre-clinical drug development. Cancer Res. 2018, 78, 4644. [Google Scholar] [CrossRef]
- Mikkonen, P.; Turunen, L.; Paasonen, L.; Potdar, S.; Paavolainen, L.; Murumägi, A.; Kallioniemi, O.; Pietiäinen, V.M. Abstract 5029: Precision cancer medicine based on 3D drug profiling of patient-derived cancer cell spheroid models. Cancer Res. 2018, 78, 5029. [Google Scholar] [CrossRef]
- Nanki, Y.; Hirasawa, A.; Nomura, H.; Okubo, A.; Itoh, M.; Akahane, T.; Chiyoda, T.; Kataoka, F.; Tominaga, E.; Aoki, D. Abstract A61: Ascites-derived and tissue-derived ovarian cancer cell primary 3D cultures aimed for personalized medicine. Clin. Cancer Res. 2018, 24, A61. [Google Scholar] [CrossRef]
- Tanaka, N.; Suzuka, K.; Maru, Y.; Hippo, Y.; Itami, M. Development of three dimensional culture method for ovarian cancer toward clinical application. Int. J. Gynecol. Cancer 2018, 28, 209. [Google Scholar] [CrossRef]
- Ito, Y.; Endo, H.; Kondo, J.; Matsuzaki, S.; Ueda, Y.; Kimura, T.; Yoshino, K. Ex vivo chemosensitivity assay using patient-derived spheroids of epithelial ovarian cancer. Cancer Sci. 2018, 109, 603. [Google Scholar] [CrossRef]
- Lengyel, E.; Burdette, J.E.; Kenny, H.A.; Matei, D.; Pilrose, J.; Haluska, P.; Hales, D.B.; Stack, M.S. Epithelial Ovarian Cancer Experimental Models. Oncogene 2014, 33, 3619–3633. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N.; Birrer, M.J.; Sood, A.K. Early events in the pathogenesis of epithelial ovarian cancer. J. Clin. Oncol. 2008, 26, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Pearce, O.M.T.; Delaine-Smith, R.; Maniati, E.; Nichols, S.; Wang, J.; Böhm, S.; Rajeeve, V.; Ullah, D.; Chakravarty, P.; Jones, R.R.; et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers Europe PMC Funders Group. Cancer Discov. 2018, 8, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.N.; Bregenzer, M.E.; Mehta, P.; Snyder, C.S.; Repetto, T.; Yang-Hartwich, Y.; Mehta, G. Personalized models of heterogeneous 3D epithelial tumor microenvironments: Ovarian cancer as a model. Acta Biomater. 2021, 132, 401–420. [Google Scholar] [CrossRef]
- Worzfeld, T.; von Strandmann, E.P.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Müller, R. The unique molecular and cellular microenvironment of Ovarian cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef]
- Kenny, H.A.; Dogan, S.; Zillhardt, M.; Anirban Mitra, S.D.Y.; Krausz, T.; Lengyel, E. Organotypic Models of Metastasis: A 3 Dimensional Culture Mimicking the Human Peritoneum and Omentum for the Study of the Early Steps of Ovarian Cancer Metastasis; Springer: New York, NY, USA, 2013; pp. 3–6. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chiang, C.Y.; Daifotis, H.A.; Nieman, K.M.; Fahrmann, J.F.; Lastra, R.R.; Romero, I.L.; Fiehn, O.; Lengyel, E. Adipocyte-induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res. 2020, 80, 1748–1761. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Furuya, M. Ovarian cancer stroma: Pathophysiology and the roles in cancer development. Cancers 2012, 4, 701–724. [Google Scholar] [CrossRef]
- Cai, J.; Tang, H.; Xu, L.; Wang, X.; Yang, C.; Ruan, S.; Guo, J.; Hu, S.; Wang, Z. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis 2012, 33, 20–29. [Google Scholar] [CrossRef]
- Yeung, T.L.; Leung, C.S.; Yip, K.P.; Yeung, C.L.A.; Wong, S.T.C.; Mok, S.C. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: Cell and molecular processes in cancer metastasis. Am. J. Physiol.—Cell Physiol. 2015, 309, C444–C456. [Google Scholar] [CrossRef]
- Nwani, N.G.; Sima, L.E.; Nieves-Neira, W.; Matei, D. Targeting the microenvironment in high grade serous ovarian cancer. Cancers 2018, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.J.; Al-Attar, A.; Rolland, P.; Scott, I.V.; Deen, S.; Liu, D.T.Y.; Spendlove, I.; Durrant, L.G. Vascular endothelial growth factor expression in ovarian cancer: A model for targeted use of novel therapies? Clin. Cancer Res. 2008, 14, 3030–3035. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, H.C.; Kohn, E.C.; Steinberg, S.M.; Rothenberg, M.L.; Merino, M.J. Tumor angiogenesis in advanced stage ovarian carcinoma. Am. J. Pathol. 1995, 147, 33–41. [Google Scholar]
- Gasparini, G.; Bonoldi, E.; Viale, G.; Verderio, P.; Boracchi, P.; Panizzoni, G.A.; Radaelli, U.; Di Bacco, A.; Guglielmi, R.B.; Bevilacqua, P. Prognostic and predictive value of tumour angiogenesis in ovarian carcinomas. Int. J. Cancer 1996, 69, 205–211. [Google Scholar] [CrossRef]
- Orre, M.; Lotfi-Miri, M.; Mamers, P.; Rogers, P.A.W. Increased microvessel density in mucinous compared with malignant serous and benign tumours of the ovary. Br. J. Cancer 1998, 77, 2204–2209. [Google Scholar] [CrossRef]
- Boussios, S.; Karathanasi, A.; Cooke, D.; Neille, C.; Sadauskaite, A.; Moschetta, M.; Zakynthinakis-Kyriakou, N.; Pavlidis, N. PARP inhibitors in ovarian cancer: The route to “ITHacA". Diagnostics 2019, 9, 55. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Lima, D.; Reis, R.L.; Oliveira, J.M.; Correlo, V.M. Anti-Cancer Drug Validation: The Contribution of Tissue Engineered Models. Stem Cell Rev. Rep. 2017, 13, 347–363. [Google Scholar] [CrossRef]
- Totti, S.; Vernardis, S.I.; Meira, L.; Pérez-Mancera, P.A.; Costello, E.; Greenhalf, W.; Palmer, D.; Neoptolemos, J.; Mantalaris, A.; Velliou, E.G. Designing a bio-inspired biomimetic in vitro system for the optimization of ex vivo studies of pancreatic cancer. Drug Discov. Today 2017, 22, 690–701. [Google Scholar] [CrossRef]
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239. [Google Scholar] [CrossRef]
- Velliou, E.G.; Dos Santos, S.B.; Papathanasiou, M.M.; Fuentes-Gari, M.; Misener, R.; Panoskaltsis, N.; Pistikopoulos, E.N.; Mantalaris, A. Towards unravelling the kinetics of an acute myeloid leukaemia model system under oxidative and starvation stress: A comparison between two- and three-dimensional cultures. Bioprocess Biosyst. Eng. 2015, 38, 1589–1600. [Google Scholar] [CrossRef]
- Raghavan, S.; Ward, M.R.; Rowley, K.R.; Wold, R.M.; Buckanovich, R.J.; Mehta, G.; Arbor, A.; Arbor, A.; Arbor, A.; Arbor, A. Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol. Oncol. 2016, 138, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, X.; Long, C.; Xu, H.; Cheng, X.; Chang, J.; Zhang, C.; Zhang, C.; Wang, X. Collagen-based three-dimensional culture microenvironment promotes epithelial to mesenchymal transition and drug resistance of human ovarian cancer: In vitro. RSC Adv. 2018, 8, 8910–8919. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yin, F.; Wu, H.; Hu, X.; Zheng, L.; Zhao, J. In vitro ovarian cancer model based on three-dimensional agarose hydrogel. J. Tissue Eng. 2014, 5, 2041731413520438. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, X. A 3D model of ovarian cancer cell lines on peptide nanofiber scaffold to explore the cell-scaffold interaction and chemotherapeutic resistance of anticancer drugs. Int. J. Nanomed. 2011, 6, 303–310. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef]
- Lal-Nag, M.; McGee, L.; Guha, R.; Lengyel, E.; Kenny, H.A.; Ferrer, M. A high throughput screening model of the tumor microenvironment for ovarian cancer cell growth. SLAS Discov. 2017, 22, 494–506. [Google Scholar] [CrossRef]
- Heredia-Soto, V.; Redondo, A.; Berjón, A.; Martín, M.M.; Díaz, E.; Crespo, R.; Hernández, A.; Yébenes, L.; Gallego, A.; Feliu, J.; et al. High-throughput 3-dimensional culture of epithelial ovarian cancer cells as preclinical model of disease. Oncotarget 2018, 9, 21893–21903. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Chen, D.; Yang, J.; Yang, C.; Zhang, Y.; Zhang, H.; Dou, J. Evaluation of characteristics of CD44+CD117+ ovarian cancer stem cells in three dimensional basement membrane extract scaffold versus two dimensional monocultures. BMC Cell Biol. 2013, 14, 7. [Google Scholar] [CrossRef]
- Myungjin Lee, J.; Mhawech-Fauceglia, P.; Lee, N.; Cristina Parsanian, L.; Gail Lin, Y.; Andrew Gayther, S.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hoffman, R.M. Human ovarian carcinoma metastatic models constructed in nude mice by orthoptic transplantation of histologically-intact patient specimens. Anticancer Res. 1993, 57, 283–286. [Google Scholar]
- Ricci, F.; Bizzaro, F.; Cesca, M.; Guffanti, F.; Ganzinelli, M.; Decio, A.; Ghilardi, C.; Perego, P.; Fruscio, R.; Buda, A.; et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014, 74, 6980–6990. [Google Scholar] [CrossRef] [PubMed]
- Heo, E.J.; Cho, Y.J.; Cho, W.C.; Hong, J.E.; Jeon, H.K.; Oh, D.Y.; Choi, Y.L.; Song, S.Y.; Choi, J.J.; Bae, D.S.; et al. Patient-derived xenograft models of epithelial ovarian cancer for preclinical studies. Cancer Res. Treat. 2017, 49, 915–926. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.X.; Shi, W.; Ma, A.H.; De Vere White, R.W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- Magnottia, E.; Marascoa, W.A. The latest animal models of ovarian cancer for novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 249–257. [Google Scholar] [CrossRef]
- Totti, S.; Allenby, M.C.; Dos Santos, S.B.; Mantalaris, A.; Velliou, E.G. A 3D bioinspired highly porous polymeric scaffolding system for: In vitro simulation of pancreatic ductal adenocarcinoma. RSC Adv. 2018, 8, 20928–20940. [Google Scholar] [CrossRef]
- De Jaeghere, E.; De Vlieghere, E.; Van Hoorick, J.; Van Vlierberghe, S.; Wagemans, G.; Pieters, L.; Melsens, E.; Praet, M.; Van Dorpe, J.; Boone, M.N.; et al. Heterocellular 3D scaffolds as biomimetic to recapitulate the tumor microenvironment of peritoneal metastases in vitro and in vivo. Biomaterials 2018, 158, 95–105. [Google Scholar] [CrossRef]
- Brooks, E.A.; Gencoglu, M.F.; Corbett, D.C.; Stevens, K.R.; Peyton, S.R. An omentum-inspired 3D PEG hydrogel for identifying ECM-drivers of drug resistant ovarian cancer. APL Bioeng. 2019, 3, 026106. [Google Scholar] [CrossRef]
- Johnson, P.A.; Giles, J.R. The hen as a model of ovarian cancer. Nat. Rev. Cancer 2013, 13, 432–436. [Google Scholar] [CrossRef]
- Bolland, D.E.; McLean, K. Preclinical models in ovarian cancer. In Animal Models in Cancer Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–57. ISBN 9780128147054. [Google Scholar]
- Boettcher, A.N.; Kiupel, M.; Adur, M.K.; Cocco, E.; Santin, A.D.; Bellone, S.; Charley, S.E.; Blanco-Fernandez, B.; Risinger, J.I.; Ross, J.W.; et al. Human ovarian cancer tumor formation in severe combined immunodeficient (SCID) pigs. Front. Oncol. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Krausz, T.; Yamada, S.D.; Lengyel, E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int. J. Cancer. 2007, 121, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Masiello, T.; Dhall, A.; Hemachandra, L.; Tokranova, N.; Melendez, J.; Castracane, J. A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress. Cells 2018, 7, 277. [Google Scholar] [CrossRef]
- Totti, S.; Ng, K.W.; Dale, L.; Lian, G.; Chen, T.; Velliou, E.G. A novel versatile animal-free 3D tool for rapid low-cost assessment of immunodiagnostic microneedles. Sens. Actuators B Chem. 2019, 296, 126652. [Google Scholar] [CrossRef]
- Fuentes-Garí, M.; Velliou, E.; Misener, R.; Pefani, E.; Rende, M.; Panoskaltsis, N.; Mantalaris, A.; Pistikopoulos, E.N. A systematic framework for the design, simulation and optimization of personalized healthcare: Making and healing blood. Comput. Chem. Eng. 2015, 81, 80–93. [Google Scholar] [CrossRef]
- Velliou, E.; Fuentes-Garí, M.; Misener, R.; Pefani, E.; Rende, M.; Panoskaltsis, N.; Mantalaris, A.; Pistikopoulos, E.N. A framework for the design, modeling and optimization of biomedical systems. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 34, pp. 225–236. [Google Scholar]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Watters, K.M.; Bajwa, P.; Kenny, H.A. Organotypic 3D models of the ovarian cancer tumor microenvironment. Cancers 2018, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005, 65, 3025–3029. [Google Scholar] [CrossRef]
- Desoize, B.; Jardillier, J.C. Multicellular resistance: A paradigm for clinical resistance? Crit. Rev. Oncol. Hematol. 2000, 36, 193–207. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kurokawa, T.; Nishikawa, Y.; Orisa, M.; Kleinman, H.K.; Kotsuji, F. Laminin-1-derived scrambled peptide AG73T disaggregates laminin-1-induced ovarian cancer cell spheroids and improves the efficacy of cisplatin. Int. J. Oncol. 2008, 32, 673–681. [Google Scholar] [CrossRef][Green Version]
- Wan, X.; Ball, S.; Willenbrock, F.; Yeh, S.; Vlahov, N.; Koennig, D.; Green, M.; Brown, G.; Jeyaretna, S.; Li, Z.; et al. Perfused Three-dimensional Organotypic Culture of Human Cancer Cells for Therapeutic Evaluation. Sci. Rep. 2017, 7, 9408. [Google Scholar] [CrossRef] [PubMed]
- Jong, B.K.; Stein, R.; O’Hare, M.J. Three-dimensional in vitro tissue culture models of breast cancer—A review. Breast Cancer Res. Treat. 2004, 85, 281–291. [Google Scholar] [CrossRef]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roman, N.; Stevenson, K.; Gilmour, L.; Hamilton, G.; Chalmers, A.J. A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses. Neuro. Oncol. 2017, 19, 229–241. [Google Scholar] [CrossRef]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 877. [Google Scholar] [CrossRef]
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 4. [Google Scholar] [CrossRef]
- Moraya, A.I.; Ali, J.L.; Samadder, P.; Liang, L.; Morrison, L.C.; Werbowetski-Ogilvie, T.E.; Ogunsina, M.; Schweizer, F.; Arthur, G.; Nachtigal, M.W. Novel glycolipid agents for killing cisplatin-resistant human epithelial ovarian cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 67. [Google Scholar] [CrossRef]
- Burdett, E.; Kasper, F.K.; Mikos, A.G.; Ludwig, J.A. Engineering Tumors: A Tissue Engineering Perspective in Cancer Biology. Tissue Eng. Part B Rev. 2010, 16, 351–359. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Rizzi, S.C.; Stok, K.S.; Fuehrmann, T.; Hollier, B.; Magdolen, V.; Hutmacher, D.W.; Clements, J.A. A bioengineered 3D ovarian cancer model for the assessment ofpeptidase-mediated enhancement of spheroid growth andintraperitoneal spread. Biomaterials 2013, 34, 7389–7400. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hu, X.; Huang, Y.; Xu, G.; Yang, J.; Li, L. In vivo bioengineered ovarian tumors based on collagen, matrigel, alginate and agarose hydrogels: A comparative study. Biomed. Mater. 2015, 10, 15016. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E.; Melchels, F.P.W.; Holzapfel, B.M.; Meckel, T.; Hutmacher, D.W.; Loessner, D. Gelatine methacrylamide-based hydrogels: An alternative three-dimensional cancer cell culture system. Acta Biomater. 2014, 10, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Sodek, K.L.; Brown, T.J.; Ringuette, M.J. Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration. BMC Cancer 2008, 8, 223. [Google Scholar] [CrossRef]
- Zhou, N.; Hu, K.; Guo, Z.; Zhang, Q.; Chen, J.; Zhang, T.; Gu, N. Thermo-Sensitive PLGA-PEG-PLGA Tri-Block Copolymer Hydrogel as Three-Dimensional Cell Culture Matrix for Ovarian Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 5252–5255. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Chen, J.; Fang, K.; Dou, J.; Gu, N. The controllable preparation of porous PLGA microspheres by the oil/water emulsion method and its application in 3D culture of ovarian cancer cells. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 452, 115–124. [Google Scholar] [CrossRef]
- Sun, B.; Taha, M.S.; Ramsey, B.; Torregrosa-Allen, S.; Elzey, B.D.; Yeo, Y. Intraperitoneal chemotherapy of ovarian cancer by hydrogel depot of paclitaxel nanocrystals. J. Control. Release 2016, 235, 91–98. [Google Scholar] [CrossRef]
- McKenzie, A.J.; Hicks, S.R.; Svec, K.V.; Naughton, H.; Edmunds, Z.L.; Howe, A.K. The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Sci. Rep. 2018, 8, 7228. [Google Scholar] [CrossRef]
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, 8998. [Google Scholar] [CrossRef] [PubMed]
- Girard, Y.K.; Wang, C.; Ravi, S.; Howell, M.C.; Mallela, J.; Alibrahim, M.; Green, R.; Hellermann, G.; Mohapatra, S.S.; Mohapatra, S. A 3D Fibrous Scaffold Inducing Tumoroids: A Platform for Anticancer Drug Development. PLoS ONE 2013, 8, 75345. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Desai, U.; Andey, T.; Sams, A.; Singh, M. AlgiMatrixTM Based 3D Cell Culture System as an In-Vitro Tumor Model for Anticancer Studies. PLoS ONE 2013, 8, 53708. [Google Scholar] [CrossRef] [PubMed]
- Matta-Domjan, B.; King, A.; Totti, S.; Matta, C.; Dover, G.; Martinez, P.; Zakhidov, A.; La Ragione, R.; Macedo, H.; Jurewicz, I.; et al. Biophysical interactions between pancreatic cancer cells and pristine carbon nanotube substrates: Potential application for pancreatic cancer tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Fu, Z.; Yin, H. A bioengineered metastatic pancreatic tumor model for mechanistic investigation of chemotherapeutic drugs. J. Biotechnol. 2013, 166, 166–173. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, X.; Zhang, X.; Han, H.; Han, B.; Xu, J.; Tang, K.; Fu, Z.; Yin, H. A tissue-engineered subcutaneous pancreatic cancer model for antitumor drug evaluation. Int. J. Nanomed. 2013, 8, 1167–1176. [Google Scholar] [CrossRef]
- Ricci, C.; Mota, C.; Moscato, S.; D’Alessandro, D.; Ugel, S.; Sartoris, S.; Bronte, V.; Boggi, U.; Campani, D.; Funel, N.; et al. Interfacing polymeric scaffolds with primary pancreatic ductal adenocarcinoma cells to develop 3D cancer models. Biomatter 2014, 4, e955386. [Google Scholar] [CrossRef]
- Gupta, P.; Totti, S.; Pérez-Mancera, P.A.; Dyke, E.; Nisbet, A.; Schettino, G.; Webb, R.; Velliou, E.G. Chemoradiotherapy screening in a novel biomimetic polymer based pancreatic cancer model. RSC Adv. 2019, 9, 41649–41663. [Google Scholar] [CrossRef]
- Gupta, P.; Perez-Mancera, P.; Kocher, H.; Nisbet, A.; Schettino, G.; Velliou, E. A novel scaffold based hybrid multicellular model for pancreatic ductal adenocarcinoma—Towards a better mimicry of the in vivo tumour microenvironment. Front. Bioeng. Biotechnol. 2020, 8, 290. [Google Scholar] [CrossRef]
- Rijal, G.; Bathula, C.; Li, W. Application of Synthetic Polymeric Scaffolds in Breast Cancer 3D Tissue Cultures and Animal Tumor Models. Int. J. Biomater. 2017, 2017, 8074890. [Google Scholar] [CrossRef]
- Liu, Z.; Vunjak-Novakovic, G. Modeling tumor microenvironments using custom-designed biomaterial scaffolds. Curr. Opin. Chem. Eng. 2016, 11, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 1000097. [Google Scholar] [CrossRef] [PubMed]
| Key Element/Characteristic of Ovarian Tumour Model | In Vivo Function/Repercussion |
|---|---|
| Complex microenvironment (cellular and architectural) [42,43,44,45] | • Reflects tumour histology • Tumour growth • Resistance to chemotherapeutic agents |
| Mesothelial cells [43,47] | • Attachment and invasion of cancer cells |
| Fibronectin/Integrins [43] | • Spheroidal structure growth |
| Fibroblasts [52] | • Tumour growth, adhesion and invasiveness |
| Adipocytes [49,50,51] | • Tumour growth and metastasis promotion |
| Extracellular matrix and stroma [51] | • Tumour growth, adhesion |
| Extracellular microvesicles [47] | • Invasion and methastasis • Drug-resistance |
| Angiogenesis (PARP/VEGFR3)/Neovascularisation [54,55,59] | • Ability to grow over a certain size • Invasion and metastasis • Drug-resistance |
| Ability to self-organise in 3D structures [43] | • Invasion and metastasis • Drug-resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevinyan, L.; Gupta, P.; Velliou, E.; Madhuri, T.K. The Development of a Three-Dimensional Platform for Patient-Derived Ovarian Cancer Tissue Models: A Systematic Literature Review. Cancers 2022, 14, 5628. https://doi.org/10.3390/cancers14225628
Sevinyan L, Gupta P, Velliou E, Madhuri TK. The Development of a Three-Dimensional Platform for Patient-Derived Ovarian Cancer Tissue Models: A Systematic Literature Review. Cancers. 2022; 14(22):5628. https://doi.org/10.3390/cancers14225628
Chicago/Turabian StyleSevinyan, Lusine, Priyanka Gupta, Eirini Velliou, and Thumuluru Kavitha Madhuri. 2022. "The Development of a Three-Dimensional Platform for Patient-Derived Ovarian Cancer Tissue Models: A Systematic Literature Review" Cancers 14, no. 22: 5628. https://doi.org/10.3390/cancers14225628
APA StyleSevinyan, L., Gupta, P., Velliou, E., & Madhuri, T. K. (2022). The Development of a Three-Dimensional Platform for Patient-Derived Ovarian Cancer Tissue Models: A Systematic Literature Review. Cancers, 14(22), 5628. https://doi.org/10.3390/cancers14225628






