IETA Ultrasonic Features Combined with GI-RADS Classification System and Tumor Biomarkers for Surveillance of Endometrial Carcinoma: An Innovative Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Inclusion Criteria for This Research Were as Shown Below
- (1)
- Tumor biomarkers HE4, CA125, CA15-3 and CA19-9 were detected in all cases within 1 month before the operation;
- (2)
- All cases underwent transrectal/transvaginal ultrasonography within 1 month before the operation;
- (3)
- The patients underwent human chorionic gonadotropin (HCG) testing to rule out pregnancy-related diseases;
- (4)
- Adenomyosis was not observed in all cases, and the abnormalities of the endometrium–myometrium junction caused by adenomyosis were excluded;
- (5)
- The enrolled patients underwent hysteroscopy, curettage or surgical resection, and the results were confirmed by pathological diagnosis or surgical records.
2.2. The Exclusion Criteria Were as Follows
- (1)
- All cases that did not meet the inclusion criteria;
- (2)
- The patients had previously undergone lower abdominal surgery, and the uterus had been excised;
- (3)
- Any patient who was allergic to ultrasound gel;
- (4)
- Patients who were not of legal age (under 18 years) were not included for medical ethical reasons;
- (5)
- The patients who have received preoperative hormone therapy, chemotherapy, radiation therapy or tumors in other organs;
- (6)
- The patients who have recently taken hormone drugs or pregnant or lactating women.
2.3. Instruments and Methods
2.4. The Theoretical Basis of IETA Ultrasonic Features GI-RADS Classification
2.5. The Benign IETA Ultrasonographic Signs (B-Signs) Were as Follows
- (1)
- Endometrial thickness: ≤4.0 mm (LR− < 0.1);
- (2)
- Uniform endometrial echogenicity: homogeneous hyperechoic, homogeneous hypoechoic, homogeneous isoechoic, three-layer pattern;
- (3)
- Non-uniform endometrial echogenicity: homogeneous with regular cysts;
- (4)
- Endometrial midline appearance: linear;
- (5)
- Endometrial–myometrial junction: regular;
- (6)
- “Bright edge”: yes;
- (7)
- Color score: 1~2 points;
- (8)
- Vascular pattern: no flow, single vessel (without branching), circular vessels.
2.6. The Malignant IETA Ultrasonographic Signs (M-Signs) Were as Follows
- (1)
- Endometrial thickness: premenopause ≥ 18.5 mm (LR+ > 10), postmenopause ≥ 15.5 mm (LR+ > 10);
- (2)
- Non-uniform endometrial echogenicity: heterogeneous with irregular cysts;
- (3)
- Endometrial midline appearance: not defined;
- (4)
- Endometrial–myometrial junction: interrupted, not defined;
- (5)
- Intracavitary fluid: ground glass, “mixed” echogenicity;
- (6)
- Color score: 3~4 points;
- (7)
- Vascular pattern: multiple vessels (focal origin), multiple vessels (multifocal origin).
2.7. The Undefined IETA Ultrasonographic Signs (U-Signs) Were as Follows
- (1)
- Non-uniform endometrial echogenicity: homogeneous with irregular cysts;
- (2)
- heterogeneous without cysts; heterogeneous with regular cysts;
- (3)
- Endometrial midline appearance: non-linear, irregular;
- (4)
- Endometrial–myometrial junction: irregular;
- (5)
- “Bright edge”: no;
- (6)
- Intracavitary fluid: no fluid; anechoic or of low-level echogenicity;
- (7)
- Vascular pattern: single vessel (with branching), scattered vessels.
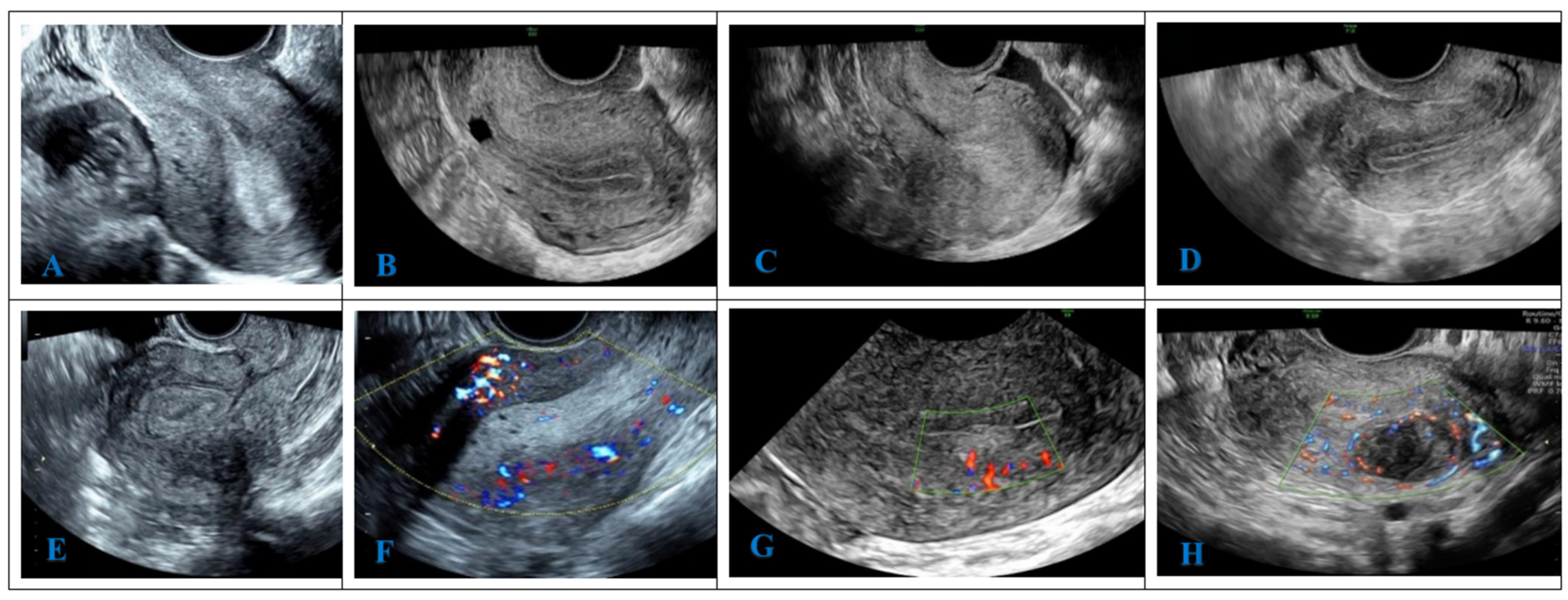
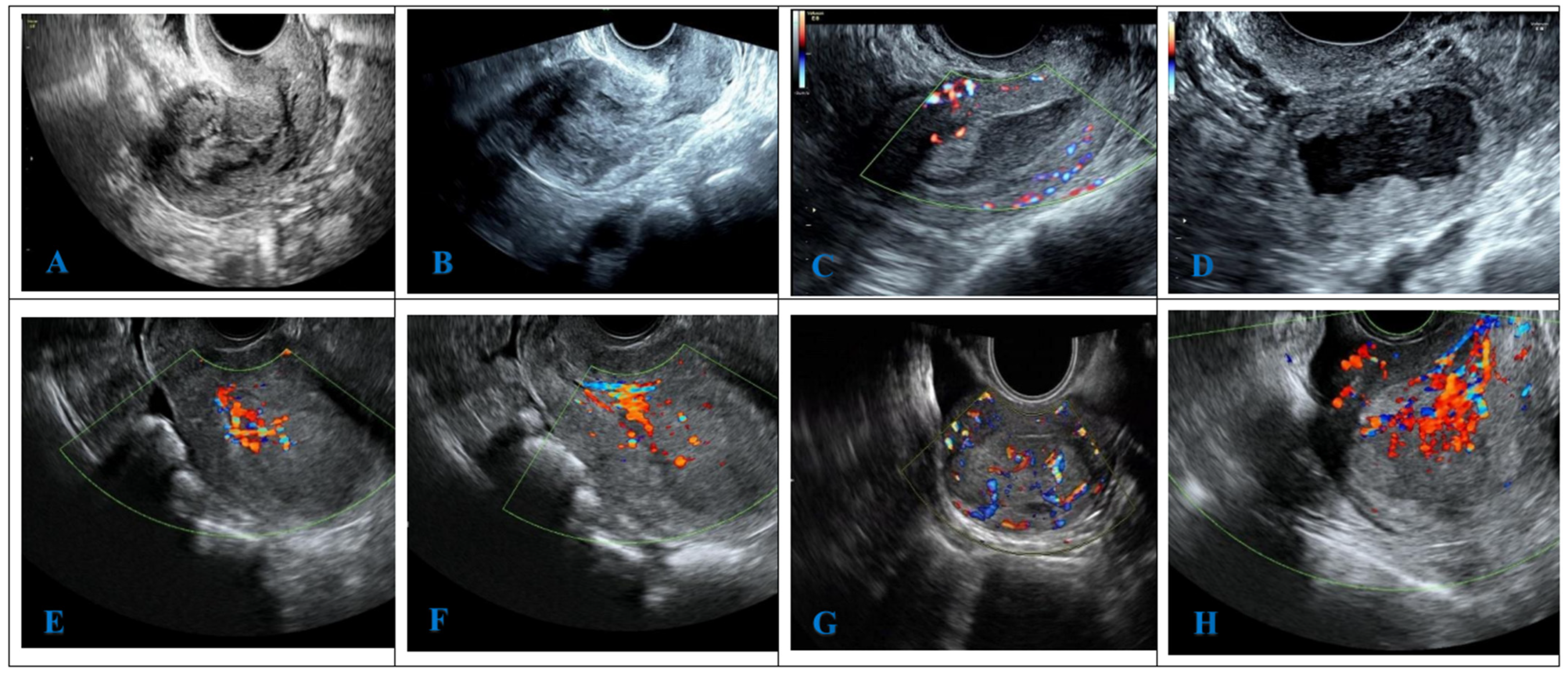
2.8. Ultrasonic Image Analysis
2.9. The Serological Detection of Tumor Markers CA125, CA15-3, CA19-9 and HE4
2.10. The Comprehensive Evaluation
2.11. Statistical Analysis
3. Results
3.1. General Information
3.2. Univariate Analysis and Multivariate Logisitic Regression Analysis of IETA Ultrasonographic Features
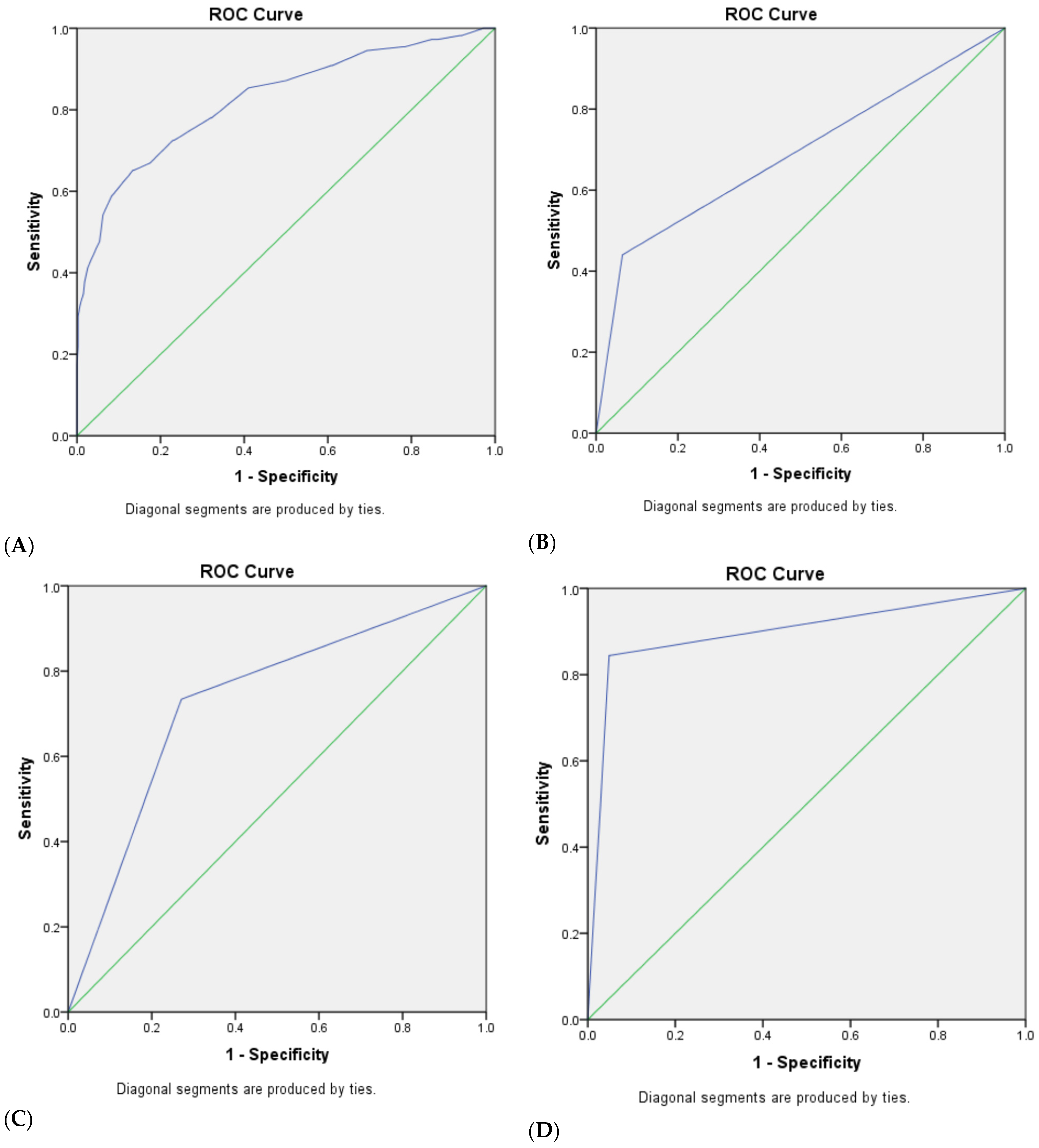
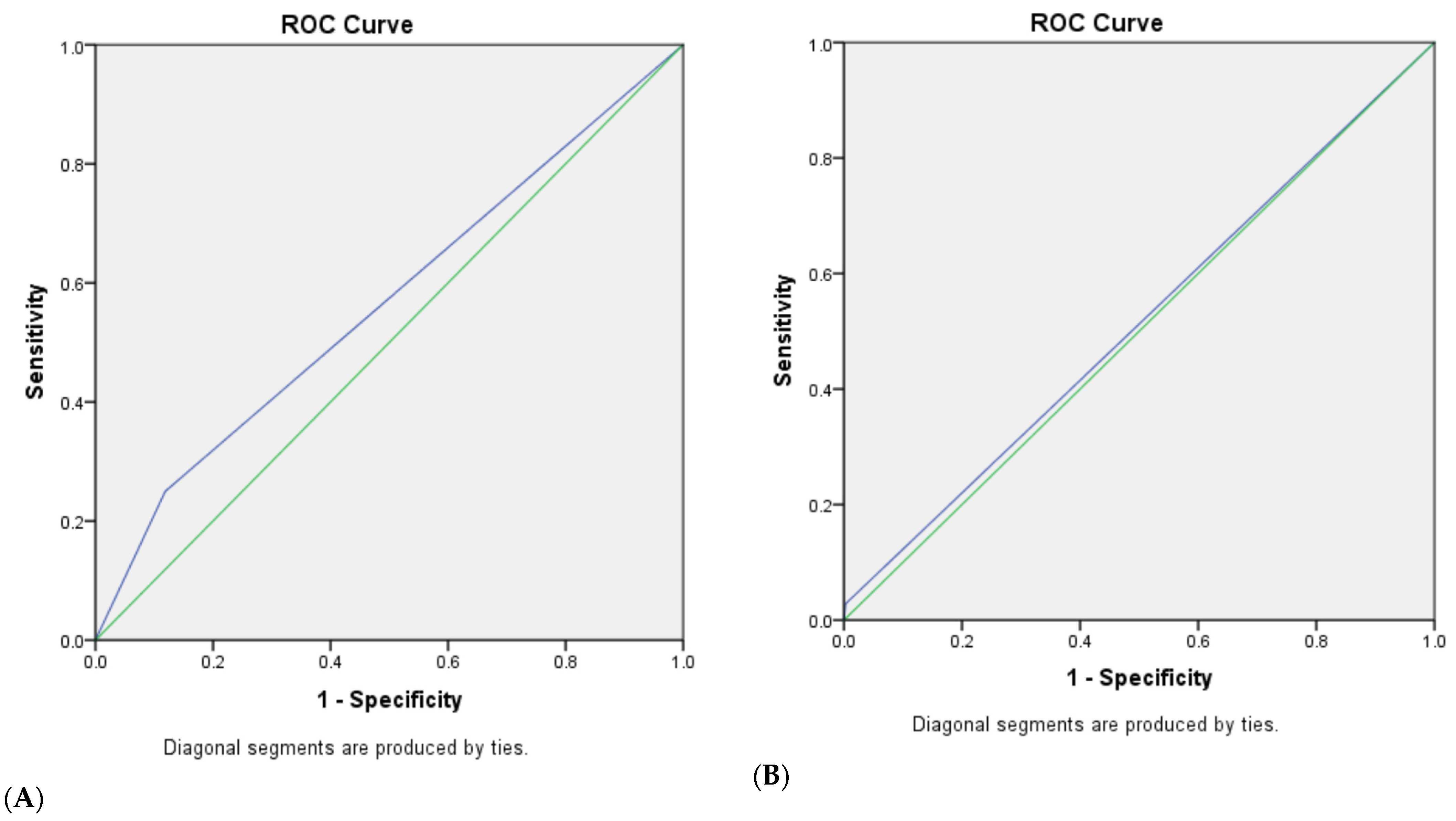
3.3. The Diagnostic Efficacy of Traditional and Modified Ultrasonic GI-RADS Classification in Predicting Benign and Malignant Uterine Cavity and Endometrial Lesions
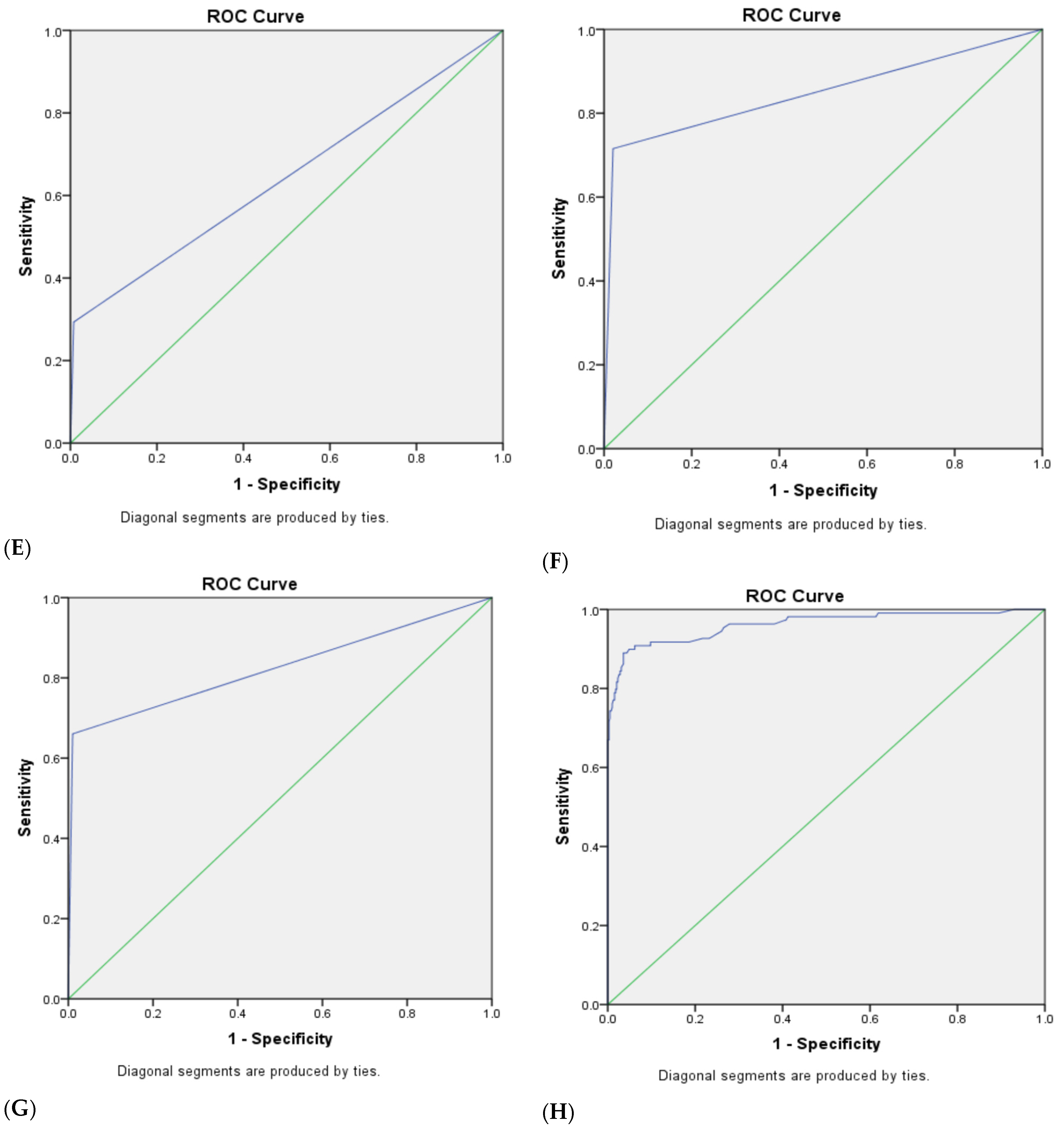
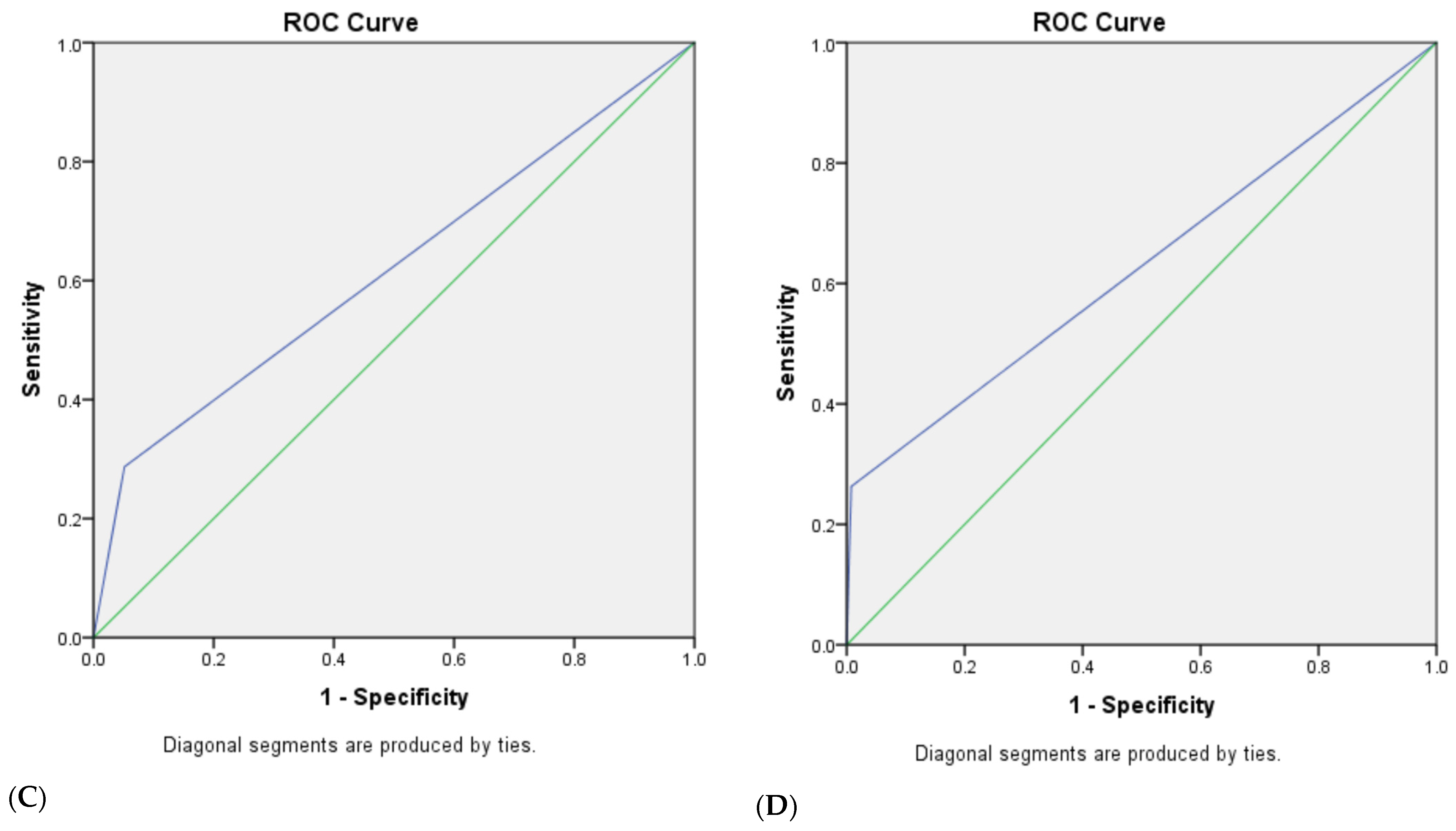
3.4. The Combined Diagnostic Efficacy of Ultrasonic GI-RADS Classification Combined with Serum Tumor Biomarker (CA125, CA15-3, CA19-9 and HE4) Results for Benign and Malignant Uterine Cavity and Endometrial Lesions
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haldorsen, I.S.; Salvesen, H.B. What Is the Best Preoperative Imaging for Endometrial Cancer? Curr. Oncol. Rep. 2016, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, G.; Hu, K.; Huang, K.; Zhang, M.; Zhou, J.; Teng, F.; Cao, J.; Dai, C.; Jia, X. A New Risk Index Combining d-Dimer, Fibrinogen, HE4, and CA199 Differentiates Suspecting Endometrial Cancer From Patients With Abnormal Vaginal Bleeding or Discharge. Technol. Cancer Res. Treat. 2020, 19, 1533033819901117. [Google Scholar] [CrossRef] [PubMed]
- Espiau Romera, A.; Cuesta Guardiola, T.; Benito Vielba, M.; De Bonrostro Torralba, C.; Coronado Martin, P.J.; Baquedano Mainar, L. HE4 tumor marker as a predictive factor for lymphatic metastasis in endometrial cancer. Int. J. Gynaecol. Obstet. 2020, 149, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, R.; Ryan, N.A.J.; Barr, C.E.; Derbyshire, A.E.; Wan, Y.L.; Maskell, Z.; Stocking, K.; Pemberton, P.W.; Bolton, J.; McVey, R.J.; et al. Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer. Cancers 2020, 12, 276. [Google Scholar] [CrossRef]
- Li, Y.; Cong, P.; Wang, P.; Peng, C.; Liu, M.; Sun, G. Risk factors for pelvic lymph node metastasis in endometrial cancer. Arch. Gynecol. Obstet. 2019, 300, 1007–1013. [Google Scholar] [CrossRef]
- Huang, G.Q.; Xi, Y.Y.; Zhang, C.J.; Jiang, X. Serum Human Epididymis Protein 4 Combined with Carbohydrate Antigen 125 for Endometrial Carcinoma Diagnosis: A Meta-Analysis and Systematic Review. Genet. Test Mol. Biomark. 2019, 23, 580–588. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef]
- Eriksson, L.S.; Lindqvist, P.G.; Floter Radestad, A.; Dueholm, M.; Fischerova, D.; Franchi, D.; Jokubkiene, L.; Leone, F.P.; Savelli, L.; Sladkevicius, P.; et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: Interobserver reproducibility among ultrasound experts and gynecologists. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 476–482. [Google Scholar] [CrossRef]
- Verbakel, J.Y.; Mascilini, F.; Wynants, L.; Fischerova, D.; Testa, A.C.; Franchi, D.; Fruhauf, F.; Cibula, D.; Lindqvist, P.G.; Fruscio, R.; et al. Validation of ultrasound strategies to assess tumor extension and to predict high-risk endometrial cancer in women from the prospective IETA (International Endometrial Tumor Analysis)-4 cohort. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 55, 115–124. [Google Scholar] [CrossRef]
- Epstein, E.; Fischerova, D.; Valentin, L.; Testa, A.C.; Franchi, D.; Sladkevicius, P.; Fruhauf, F.; Lindqvist, P.G.; Mascilini, F.; Fruscio, R.; et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: Prospective multicenter study. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 51, 818–828. [Google Scholar] [CrossRef]
- Vazquez-Manjarrez, S.E.; Rico-Rodriguez, O.C.; Guzman-Martinez, N.; Espinoza-Cruz, V.; Lara-Nunez, D. Imaging and diagnostic approach of the adnexal mass: What the oncologist should know. Chin. Clin. Oncol. 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kong, T.W.; Paek, J.; Chang, S.J.; Ryu, H.S. Predicting Model of Lymph Node Metastasis Using Preoperative Tumor Grade, Transvaginal Ultrasound, and Serum CA-125 Level in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 26, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, F.; Liu, J.; Zhang, S. Diagnostic performance of the Gynecology Imaging Reporting and Data System for malignant adnexal masses. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2017, 137, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Behnamfar, F.; Adibi, A.; Khadra, H.; Moradi, M. Diagnostic accuracy of gynecology imaging reporting and data system in evaluation of adnexal lesions. J. Res. Med. Sci. 2019, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.A.; Metwally, M.I.; Gamil, S.A.; Khater, H.M.; Aly, S.A.; El Sammak, A.A.; Zaitoun, M.M.A.; Khattab, E.M.; Azmy, T.M.; Alayouty, N.A.; et al. Comparison of O-RADS, GI-RADS, and IOTA simple rules regarding malignancy rate, validity, and reliability for diagnosis of adnexal masses. Eur. Radiol. 2021, 31, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.A.; Refaat, R.; Ibrahim, S.A.; Madkour, N.M.; Awad, A.M.; Mohamed, E.M.; El Sammak, A.A.; Zaitoun, M.M.A.; Dawoud, H.A.; Khamis, M.E.M.; et al. Gynecology Imaging Reporting and Data System (GI-RADS): Diagnostic performance and inter-reviewer agreement. Eur. Radiol. 2019, 29, 5981–5990. [Google Scholar] [CrossRef]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef]
- Leone, F.P.; Timmerman, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: A consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef]
- Kabil Kucur, S.; Temizkan, O.; Atis, A.; Gozukara, I.; Uludag, E.U.; Agar, S.; Davas, I. Role of endometrial power Doppler ultrasound using the international endometrial tumor analysis group classification in predicting intrauterine pathology. Arch. Gynecol. Obstet. 2013, 288, 649–654. [Google Scholar] [CrossRef]
- Dueholm, M.; Moller, C.; Rydbjerg, S.; Hansen, E.S.; Ortoft, G. An ultrasound algorithm for identification of endometrial cancer. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2014, 43, 557–568. [Google Scholar] [CrossRef]
- Sladkevicius, P.; Valentin, L. Prospective validation of two mathematical models to calculate the risk of endometrial malignancy in patients with postmenopausal bleeding and sonographic endometrial thickness ≥4.5 mm. Eur. J. Cancer 2016, 59, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, J.L.; Pascual, M.A.; Ajossa, S.; de Lorenzo, C.; Piras, A.; Hereter, L.; Juez, L.; Fabbri, P.; Graupera, B.; Guerriero, S. Reproducibility of the International Endometrial Analysis Group Color Score for Assigning the Amount of Flow Within the Endometrium Using Stored 3-Dimensional Volumes. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2017, 36, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.; Hjorth, I.M.D.; Dahl, K.; Hansen, E.S.; Ortoft, G. Ultrasound Scoring of Endometrial Pattern for Fast-track Identification or Exclusion of Endometrial Cancer in Women with Postmenopausal Bleeding. J. Minim. Invasive Gynecol. 2019, 26, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.; Hjorth, I.M.D.; Dahl, K.; Pedersen, L.K.; Ortoft, G. Identification of endometrial cancers and atypical hyperplasia: Development and validation of a simplified system for ultrasound scoring of endometrial pattern. Maturitas 2019, 123, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Votino, A.; Van den Bosch, T.; Installe, A.J.; Van Schoubroeck, D.; Kaijser, J.; Kacem, Y.; De Moor, B.; Van Pachterbeke, C.; Timmerman, D. Optimizing the ultrasound visualization of the endometrial-myometrial junction (EMJ). Facts Views Vis. Obgyn. 2015, 7, 60–63. [Google Scholar] [PubMed]
- Sladkevicius, P.; Opolskiene, G.; Valentin, L. Prospective temporal validation of mathematical models to calculate risk of endometrial malignancy in patients with postmenopausal bleeding. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sladkevicius, P.; Installe, A.; Van Den Bosch, T.; Timmerman, D.; Benacerraf, B.; Jokubkiene, L.; Di Legge, A.; Votino, A.; Zannoni, L.; De Moor, B.; et al. International Endometrial Tumor Analysis (IETA) terminology in women with postmenopausal bleeding and sonographic endometrial thickness ≥4.5 mm: Agreement and reliability study. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 51, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Verbakel, J.Y.; Valentin, L.; Wynants, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladevicius, P.; Alcazar, J.L.; Votino, A.; et al. Typical ultrasound features of various endometrial pathology described using the International Endometrial Tumor Analysis (IETA) terminology in women with abnormal uterine bleeding. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 57, 164–172. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, L.; Zhu, Y.; Huang, Y.; Yuan, K.; Liu, W.; Li, S.; Guo, X.; Hao, Y. Combination IETA Ultrasonographic Characteristics Simple Scoring Method With Tumor Biomarkers Effectively Improves the Differentiation Ability of Benign and Malignant Lesions in Endometrium and Uterine Cavity. Front. Oncol. 2021, 11, 605847. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tokinaga-Uchiyama, A.; Mizushima, T.; Maruyama, Y.; Mogami, T.; Shikata, N.; Ikeda, A.; Yamamoto, H.; Miyagi, E. Normalization of abnormal plasma amino acid profile-based indexes in patients with gynecological malignant tumors after curative treatment. BMC Cancer 2018, 18, 973. [Google Scholar] [CrossRef]
- Kozakiewicz, B.; Chadzynska, M.; Dmoch-Gajzlerska, E.; Stefaniak, M. Monitoring the treatment outcome in endometrial cancer patients by CEA and TATI. Tumour. Biol. 2016, 37, 9367–9374. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, L. The incidence and predictors of gynecologic malignancies among postmenopausal patients with endometrial fluid collection. Arch. Gynecol. Obstet. 2019, 299, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Qu, W.; Wang, J.; Jiang, S.W. Comparison of serum human epididymis protein 4 and CA125 on endometrial cancer detection: A meta-analysis. Clin. Chim. Acta 2019, 488, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, C.; Madhav, M.R.; Sabarimurugan, S.; Lakhotiya, K.; Pandey, V.; Priyadharshini, T.; Baxi, S.; Gothandam, K.M.; Jayaraj, R. Diagnostic and prognostic role of HE4 expression in multiple carcinomas: A protocol for systematic review and meta-analysis. Medicine 2019, 98, e15336. [Google Scholar] [CrossRef] [PubMed]
- Stiekema, A.; Lok, C.; Korse, C.M.; van Driel, W.J.; van der Noort, V.; Kenter, G.G.; Van de Vijver, K.K. Serum HE4 is correlated to prognostic factors and survival in patients with endometrial cancer. Virchows Arch. 2017, 470, 655–664. [Google Scholar] [CrossRef]
- Fanfani, F.; Restaino, S.; Cicogna, S.; Petrillo, M.; Montico, M.; Perrone, E.; Radillo, O.; De Leo, R.; Ceccarello, M.; Scambia, G.; et al. Preoperative Serum Human Epididymis Protein 4 Levels in Early Stage Endometrial Cancer: A Prospective Study. Int. J. Gynecol. Cancer 2017, 27, 1200–1205. [Google Scholar] [CrossRef]
- Dong, C.; Liu, P.; Li, C. Value of HE4 Combined with Cancer Antigen 125 in the Diagnosis of Endometrial Cancer. Pak. J. Med. Sci. 2017, 33, 1013–1017. [Google Scholar] [CrossRef]
- Reijnen, C.; Visser, N.C.; Kasius, J.C.; Boll, D.; Geomini, P.M.; Ngo, H.; Van Hamont, D.; Pijlman, B.M.; Vos, M.C.; Bulten, J.; et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: A multicenter prospective cohort study. J. Gynecol. Oncol. 2019, 30, e70. [Google Scholar] [CrossRef]
- Unsal, M.; Kimyon Comert, G.; Karalok, A.; Basaran, D.; Turkmen, O.; Boyraz, G.; Tasci, T.; Koc, S.; Boran, N.; Tulunay, G.; et al. The preoperative serum CA125 can predict the lymph node metastasis in endometrioid-type endometrial cancer. Ginekol. Pol. 2018, 89, 599–606. [Google Scholar] [CrossRef]
- Hao, Y.; Ren, G.; Yang, W.; Zheng, W.; Wu, Y.; Li, W.; Li, X.; Li, Y.; Guo, X. Combination diagnosis with elastography strain ratio and molecular markers effectively improves the diagnosis rate of small breast cancer and lymph node metastasis. Quant. Imaging Med. Surg. 2020, 10, 678–691. [Google Scholar] [CrossRef]
- Nithin, K.U.; Sridhar, M.G.; Srilatha, K.; Habebullah, S. CA 125 is a better marker to differentiate endometrial cancer and abnormal uterine bleeding. Afr. Health Sci. 2018, 18, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, X.; Ma, B.; Zhu, L.; Liu, L. Relationship between ultrasound elastography and myofibroblast distribution in breast cancer and its clinical significance. Sci. Rep. 2016, 6, 19584. [Google Scholar] [CrossRef] [PubMed]
- Koneczny, J.; Czekierdowski, A.; Florczak, M.; Poziemski, P.; Stachowicz, N.; Borowski, D. The use of sonographic subjective tumor assessment, IOTA logistic regression model 1, IOTA Simple Rules and GI-RADS system in the preoperative prediction of malignancy in women with adnexal masses. Ginekol. Pol. 2017, 88, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zou, X.; Xu, H.; Zhang, T.; Zhao, Y.; Gao, L.; Duan, W.; Ma, X.; Zhang, L. The diagnostic performance of the Gynecologic Imaging Reporting and Data System (GI-RADS) in adnexal masses. Ann. Transl. Med. 2021, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Amor, F.; Vaccaro, H.; Alcazar, J.L.; Leon, M.; Craig, J.M.; Martinez, J. Gynecologic imaging reporting and data system: A new proposal for classifying adnexal masses on the basis of sonographic findings. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2009, 28, 285–291. [Google Scholar] [CrossRef]
- Migda, M.; Bartosz, M.; Migda, M.S.; Kierszk, M.; Katarzyna, G.; Malenczyk, M. Diagnostic value of the gynecology imaging reporting and data system (GI-RADS) with the ovarian malignancy marker CA-125 in preoperative adnexal tumor assessment. J. Ovarian Res. 2018, 11, 92. [Google Scholar] [CrossRef]
- Lycke, M.; Kristjansdottir, B.; Sundfeldt, K. A multicenter clinical trial validating the performance of HE4, CA125, risk of ovarian malignancy algorithm and risk of malignancy index. Gynecol. Oncol. 2018, 151, 159–165. [Google Scholar] [CrossRef]
- Bian, J.; Sun, X.; Li, B.; Ming, L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients With Endometrial Cancer. Technol. Cancer Res. Treat. 2017, 16, 435–439. [Google Scholar] [CrossRef]
| Ultrasound Characteristics | Histopathology | ||||
|---|---|---|---|---|---|
| Benign Lesions | Malignant Lesions | χ2 | p Value | ||
| Endometrial thickness (mm) | 9.91 ± 4.13 | 18.84± 9.21 | 0.000 * | ||
| Premenopause (mean ± SD) | 10.01 ± 4.04 | 18.02 ± 9.23 | 0.000 * | ||
| Postmenopause (mean ± SD) | 8.89 ± 4.81 | 19.52 ± 9.21 | 0.000 * | ||
| Uniform endometrial echogenicity | 28/388 (7.2%) | 1/109 (0.9%) | 6.217 | 0.010 * | |
| Homogeneous hyperechoic | 17/388 (4.4%) | 1/109 (0.9%) | |||
| Homogeneous hypoechoic | 0 | 0 | |||
| Homogeneous isoechoic | 2/388 (0.5%) | 0 | |||
| Three-layer pattern | 9/388 (2.3%) | 0 | |||
| Non-uniform endometrial echogenicity | 360/388 (92.8%) | 108/109 (99.1%) | |||
| Homogeneous with regular cysts | 19/388 (4.9%) | 0/109 (0%) | 5.550 | 0.019 * | |
| Homogeneous with irregular cysts | 2/388 (0.5%) | 0/109 (0%) | 0.564 | 1.000 | |
| Heterogeneous without cysts | 305/388 (78.6%) | 61/109 (55.9%) | 22.480 | 0.000 * | |
| Heterogeneous with regular cysts | 9/388 (2.3%) | 3/109 (2.8%) | 0.068 | 0.795 | |
| Heterogeneous with irregular cysts | 25/388 (6.4%) | 44/109 (40.4%) | 81.908 | 0.000 * | |
| Endometrial midline appearance | |||||
| Linear | 124/388 (31.9%) | 3/109 (2.7%) | 38.885 | 0.000 * | |
| Non-linear | 68/388 (17.5%) | 4/109 (3.7%) | 13.188 | 0.000 * | |
| Irregular | 91/388 (23.5%) | 22/109 (20.2%) | 0.518 | 0.472 | |
| Not defined | 105/388 (27.1%) | 80/109 (73.4%) | 78.174 | 0.000 * | |
| Endometrial–myometrial junction | |||||
| Regular | 368/388 (94.8%) | 17/109 (15.6%) | 306.143 | 0.000 * | |
| Irregular | 1/388 (0.3%) | 0/109 (0%) | 0.282 | 1.000 | |
| Interrupted | 14/388 (3.6%) | 66/109 (60.6%) | 208.036 | 0.000 * | |
| Not defined | 5/388 (1.3%) | 26/109 (23.9%) | 74.083 | 0.000 * | |
| “Bright edge” | 53.137 | 0.000 * | |||
| Yes | 146/388 (37.6%) | 2/109 (1.8%) | |||
| No | 242/388 (62.4%) | 107/109 (98.2%) | |||
| Intracavitary fluid | |||||
| No fluid | 359/388 (92.5%) | 70/109 (64.2%) | 57.729 | 0.000 * | |
| Anechoic or of low-level echogenicity | 26/388 (6.7%) | 9/109(8.3%) | 0.315 | 0.575 | |
| Ground glass | 2/388 (0.5%) | 8/109 (7.3%) | 20.098 | 0.000 * | |
| “Mixed” echogenicity | 1/388 (0.3%) | 22/109 (20.2%) | 76.549 | 0.000 * | |
| Color score | |||||
| 1 point | 116/388 (29.9%) | 1/109 (0.9%) | 39.703 | 0.000 * | |
| 2 points | 264/388 (68.0%) | 31/109 (28.4%) | 55.316 | 0.000 * | |
| 3 points | 8/388 (2.1%) | 45/109 (41.3%) | 138.130 | 0.000 * | |
| 4 points | 0/388 (0%) | 32/109 (29.4%) | 121.747 | 0.000 * | |
| Vascular pattern | |||||
| No flow | 116/388 (29.9%) | 1/109 (0.9%) | 39.703 | 0.000 * | |
| Single vessel (Without branching) | 144/388 (37.1%) | 1/109 (0.9%) | 53.954 | 0.000 * | |
| Single vessel (With branching) | 14/388 (3.6%) | 8/109 (7.3%) | 2.800 | 0.112 | |
| Scattered vessels | 86/388 (22.2%) | 27/109 (24.8%) | 0.329 | 0.566 | |
| Circular vessels | 24/388 (6.2%) | 0/109 (0%) | 7.084 | 0.008 * | |
| Multiple vessels (focal origin) | 1/388 (0.3%) | 35/109 (32.1%) | 128.497 | 0.000 * | |
| Multiple vessels (multifocal origin) | 3/388 (0.8%) | 37/109 (33.9%) | 126.525 | 0.000 * | |
| Ultrasound Characteristics | B | S.E. | Wald | p Value | Exp(B) | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Endometrial thickness | 0.246 | 0.026 | 89.186 | 0.000 * | 1.279 | 1.215 | 1.346 | |
| Premenopause | 0.252 | 0.036 | 48.868 | 0.000 * | 1.287 | 1.199 | 1.381 | |
| Postmenopause | 0.235 | 0.052 | 20.452 | 0.000 * | 1.266 | 1.143 | 1.401 | |
| Non-uniform endometrial echogenicity | ||||||||
| Heterogeneous with irregular cysts | 2.436 | 0.283 | 74.184 | 0.000 * | 11.426 | 6.564 | 19.889 | |
| Endometrial midline appearance | ||||||||
| Not defined | 2.006 | 0.245 | 67.037 | 0.000 * | 7.435 | 4.600 | 12.019 | |
| Endometrial–myometrial junction | ||||||||
| Interrupted or Not defined | 4.655 | 0.354 | 173.299 | 0.000 * | 105.102 | 52.556 | 210.184 | |
| “Bright edge” | ||||||||
| No | 3.474 | 0.721 | 23.199 | 0.000 * | 32.277 | 7.850 | 132.707 | |
| Intracavitary fluid | ||||||||
| Ground glass or “Mixed” echogenicity | 3.977 | 0.617 | 41.595 | 0.000 * | 53.333 | 15.928 | 178.579 | |
| Color score | 3~4 points | 4.783 | 0.416 | 132.484 | 0.000 * | 119.516 | 52.927 | 269.881 |
| Vascular pattern | Multiple vessels (focal origin) or Multiple vessels (multifocal origin) | 5.230 | 0.542 | 93.193 | 0.000 * | 186.811 | 64.601 | 540.209 |
| Ultrasound Characteristics | Benign Signs | Malignant Signs | Undefined Signs |
|---|---|---|---|
| Endometrial thickness | ≤4.0 mm (LR− < 0.1) | Premenopause ≥ 18.5 mm (LR+ > 10), Postmenopause ≥ 15.5 mm (LR+ > 10) | |
| Uniform endometrial echogenicity | Homogeneous hyperechoic; Homogeneous hypoechoic; Homogeneous isoechoic; Three-layer pattern | ||
| Non-uniform endometrial echogenicity | Homogeneous with regular cysts | Heterogeneous with irregular cysts | Homogeneous with irregular cysts; Heterogeneous without cysts; Heterogeneous with regular cysts |
| Endometrial midline appearance | Linear | Not defined | Non-linear Irregular |
| Endometrial–myometrial junction | Regular | Interrupted; Not defined | Irregular |
| “Bright edge” | Yes | No | |
| Intracavitary fluid | Ground glass; “Mixed” echogenicity | No fluid Anechoic or of low-level echogenicity; | |
| Color score | 1~2 points | 3~4 points | |
| Vascular pattern | No flow; Single vessel (Without branching); Circular vessels | Multiple vessels (focal origin); Multiple vessels (multifocal origin) | Single vessel (With branching); Scattered vessels; |
| Classification | U-T-GI-RADS | Standard of Classification | U-M-GI-RADS | Standard of Classification |
|---|---|---|---|---|
| 1 | Definite benign | No lesions | Definite benign | No lesions |
| 2 | Most likely benign | It fits the benign description, not one of the malignant ones | Most likely benign | It fits the benign description, not one of the malignant ones |
| 3 | Probably benign | There are undefined signs, but not malignant ones | Probably benign | There are undefined signs, but not malignant ones |
| 4 | Probably malignant | Probably malignant | ||
| 4a | Contains 1 malignant sign | 4a | Contains 1 malignant sign | |
| 4b | Contains 2 malignant signs | 4b | Contains 2 malignant signs | |
| 4c | Contains 3 malignant signs | |||
| 5 | Most likely malignant | Contains more than or equal to 3 malignant signs | Most likely malignant | Contains more than or equal to 4 malignant signs |
| 6 | Pathology confirmed | Pathology confirmed |
| Parameter | Benign Lesions | Malignant Lesions | p Value |
|---|---|---|---|
| Cases number (n) | 388 | 109 | |
| Premenopause (%) | 352/388 (90.7%) | 49/109 (45.0%) | 0.000 * |
| Postmenopause (%) | 36/388 (9.3%) | 60/109 (55.0%) | 0.000 * |
| Age (years, mean ± SD) | 38.37 ± 9.27 | 53.00 ± 11.15 | 0.000 * |
| BMI (kg/m2) | 22.65 ± 3.27 | 24.62 ± 3.94 | 0.000 * |
| Gravidity (mean ± SD) | 2.43 ± 1.89 | 2.59 ± 1.57 | 0.419 |
| Parity (mean ± SD) | 1.36 ± 1.06 | 1.83 ± 1.18 | 0.000 * |
| Abortion (mean ± SD) | 1.06 ± 1.38 | 0.74 ± 1.02 | 0.029 * |
| Clinical symptoms | |||
| Irregular menstruation (%) | 212/388 (54.6%) | 54/109 (49.5%) | 0.346 |
| Irregular bleeding of the vagina (%) | 90/388 (23.2%) | 88/109 (80.7%) | 0.000 * |
| Leucorrhea with blood or contact bleeding (%) | 11/388 (2.8%) | 10/109 (9.2%) | 0.012 * |
| Hypogastralgia (%) | 33/388 (8.5%) | 8/109 (7.3%) | 0.696 |
| No symptom (%) | 111/388 (28.6%) | 7/109 (6.4%) | 0.000 * |
| Methods | Sensitivity (%) | Specificity (%) | Positive Predictive Value (PPV, %) | Negative Predictive Value (NPV, %) | Diagnostic Accuracy Rate |
|---|---|---|---|---|---|
| U-T-GI-RADS | |||||
| 4a | 97.2 | 65.2 | 44.0 | 98.8 | 72.2 |
| 4b | 88.1 | 92.0 | 75.6 | 96.5 | 91.2 |
| 5 | 75.2 | 98.5 | 93.2 | 93.4 | 93.4 |
| U-T-GI-RADS combined tumor biomarkers | |||||
| 4a | 89.9 | 85.6 | 63.6 | 96.8 | 86.5 |
| 4b | 81.7 | 95.9 | 84.8 | 94.9 | 92.8 |
| 5 | 48.6 | 99.0 | 93.0 | 87.3 | 87.9 |
| U-M-GI-RADS | |||||
| 4a | 97.2 | 65.2 | 44.0 | 98.8 | 72.2 |
| 4b | 88.1 | 92.3 | 76.2 | 96.5 | 91.3 |
| 4c | 75.2 | 98.7 | 94.3 | 93.4 | 93.6 |
| 5 | 66.1 | 99.7 | 98.6 | 91.3 | 92.4 |
| U-M-GI-RADS combined tumor biomarkers | |||||
| 4a | 89.9 | 85.6 | 63.6 | 96.8 | 86.5 |
| 4b | 81.7 | 95.9 | 84.8 | 94.9 | 92.8 |
| 4c | 71.6 | 98.7 | 94.0 | 92.5 | 92.8 |
| 5 | 45.0 | 100.0 | 100.0 | 86.6 | 87.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Wang, H.; Liu, L.; Zhao, L.; Chen, J.; Tian, H.; Gao, L.; Wu, B.; Zhang, J.; Guo, X.; et al. IETA Ultrasonic Features Combined with GI-RADS Classification System and Tumor Biomarkers for Surveillance of Endometrial Carcinoma: An Innovative Study. Cancers 2022, 14, 5631. https://doi.org/10.3390/cancers14225631
Lin D, Wang H, Liu L, Zhao L, Chen J, Tian H, Gao L, Wu B, Zhang J, Guo X, et al. IETA Ultrasonic Features Combined with GI-RADS Classification System and Tumor Biomarkers for Surveillance of Endometrial Carcinoma: An Innovative Study. Cancers. 2022; 14(22):5631. https://doi.org/10.3390/cancers14225631
Chicago/Turabian StyleLin, Dongmei, Hui Wang, Lu Liu, Liang Zhao, Jing Chen, Hongyan Tian, Lei Gao, Beibei Wu, Jing Zhang, Xia Guo, and et al. 2022. "IETA Ultrasonic Features Combined with GI-RADS Classification System and Tumor Biomarkers for Surveillance of Endometrial Carcinoma: An Innovative Study" Cancers 14, no. 22: 5631. https://doi.org/10.3390/cancers14225631
APA StyleLin, D., Wang, H., Liu, L., Zhao, L., Chen, J., Tian, H., Gao, L., Wu, B., Zhang, J., Guo, X., & Hao, Y. (2022). IETA Ultrasonic Features Combined with GI-RADS Classification System and Tumor Biomarkers for Surveillance of Endometrial Carcinoma: An Innovative Study. Cancers, 14(22), 5631. https://doi.org/10.3390/cancers14225631





