Molecular Biomarkers of Malignant Transformation in Head and Neck Dysplasia
Abstract
Simple Summary
Abstract
1. Introduction
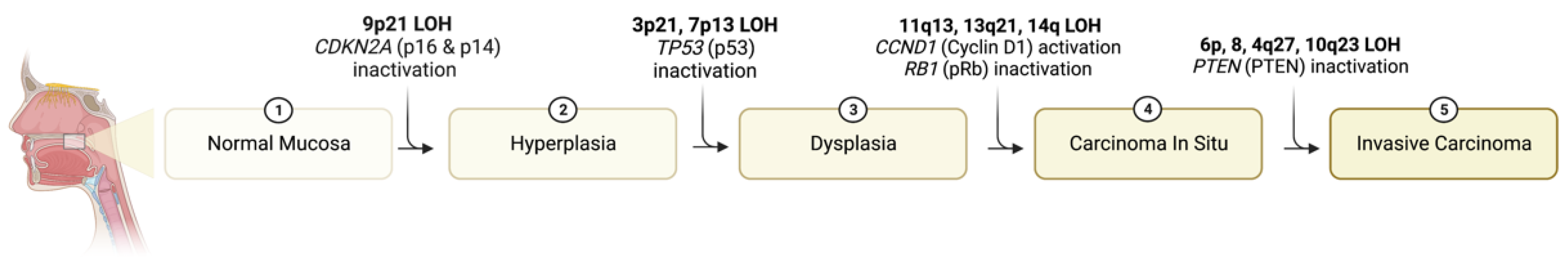
2. Genetic Mechanisms Underlying Progression of Dysplasia to Carcinoma
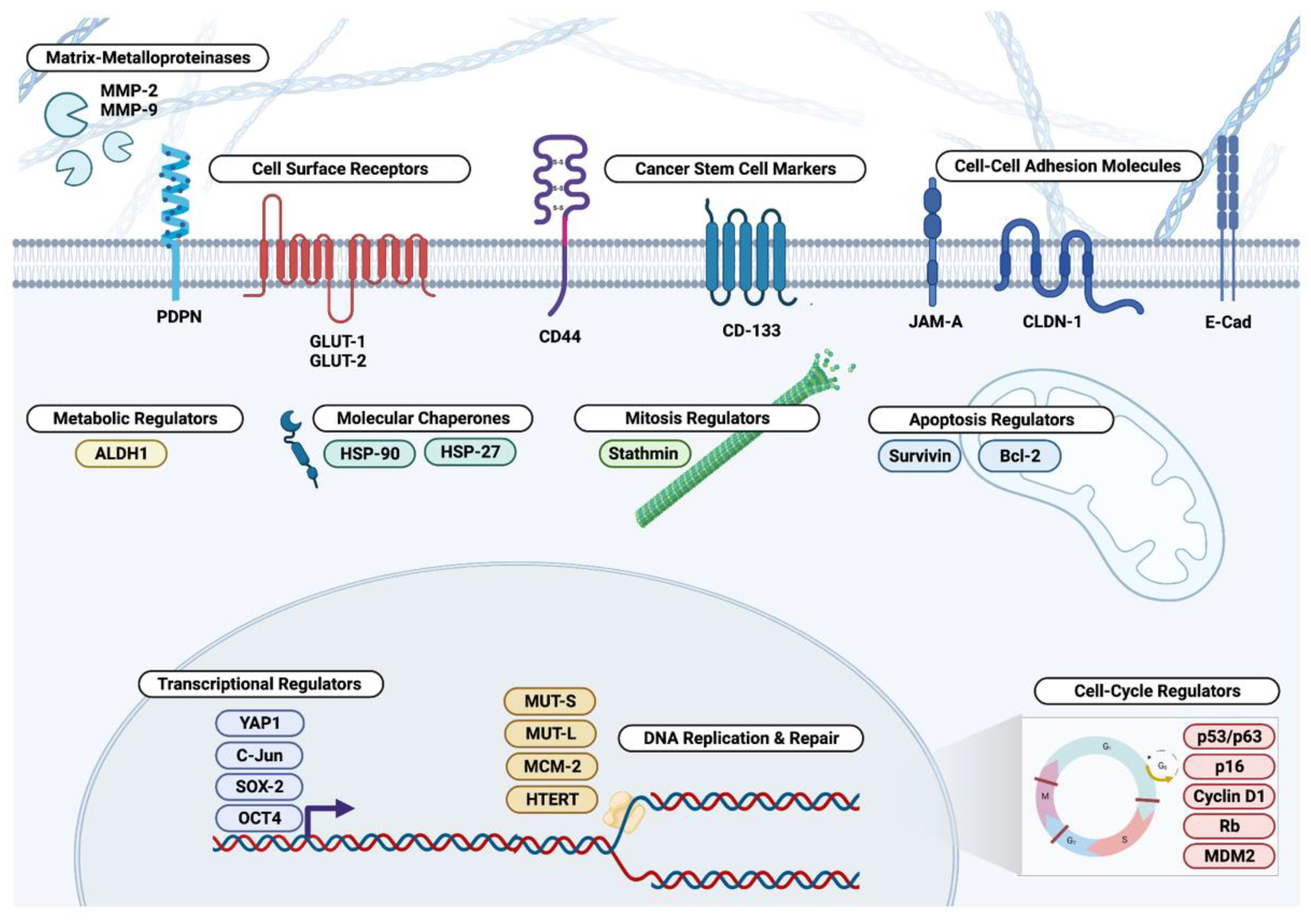
3. Extracellular and Cell Surface Biomarkers
3.1. Matrix Metalloproteinases
3.2. Podoplanin
3.3. Claudin, JAM-A, and E-Cadherin
3.4. CD44 and CD133
3.5. Glucose Transporters
4. Cytosolic Biomarkers
4.1. Aldehyde Dehydrogenases
4.2. Molecular Chaperones
4.3. Mitosis and Apoptosis Regulators
5. Nuclear Biomarkers
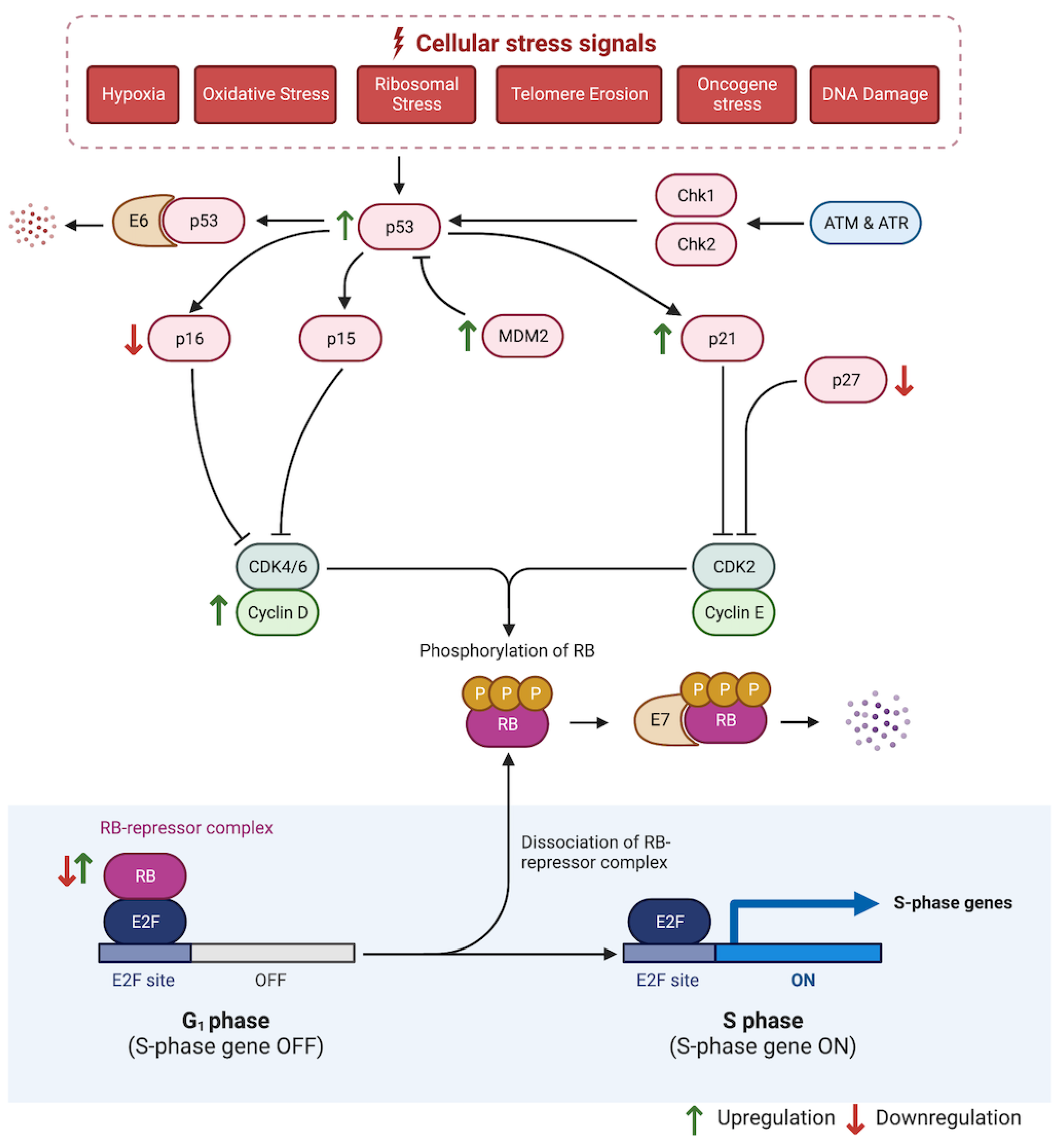
5.1. Cell Cycle Regulators
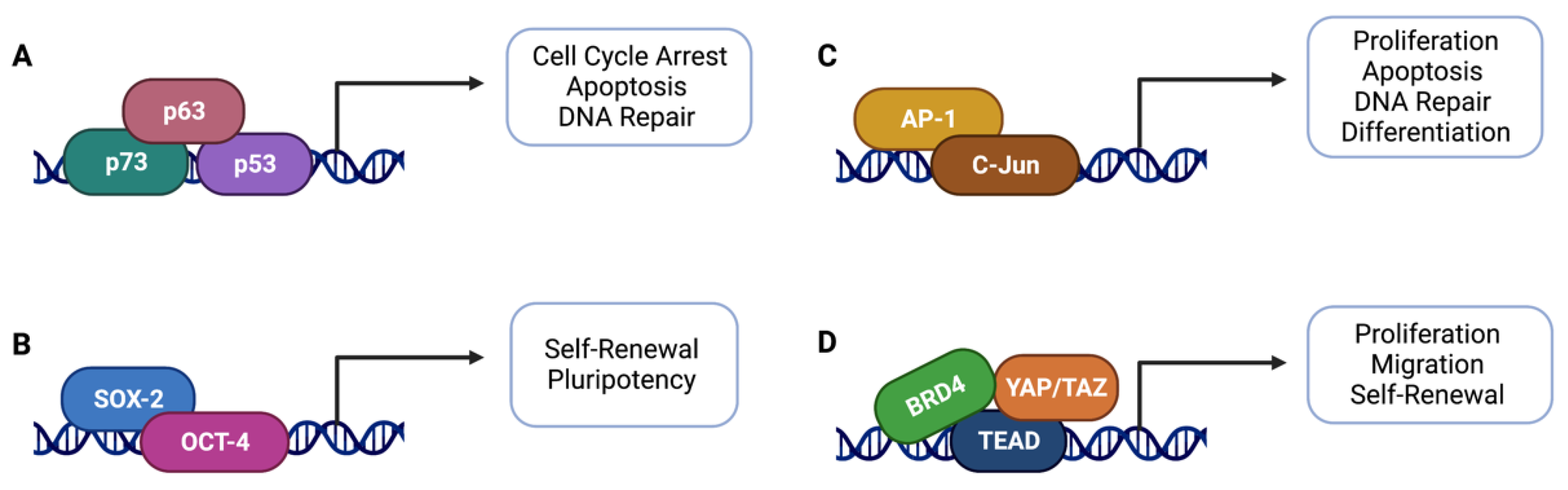
5.2. Transcriptional Regulators
5.3. DNA Replication and Repair Regulators
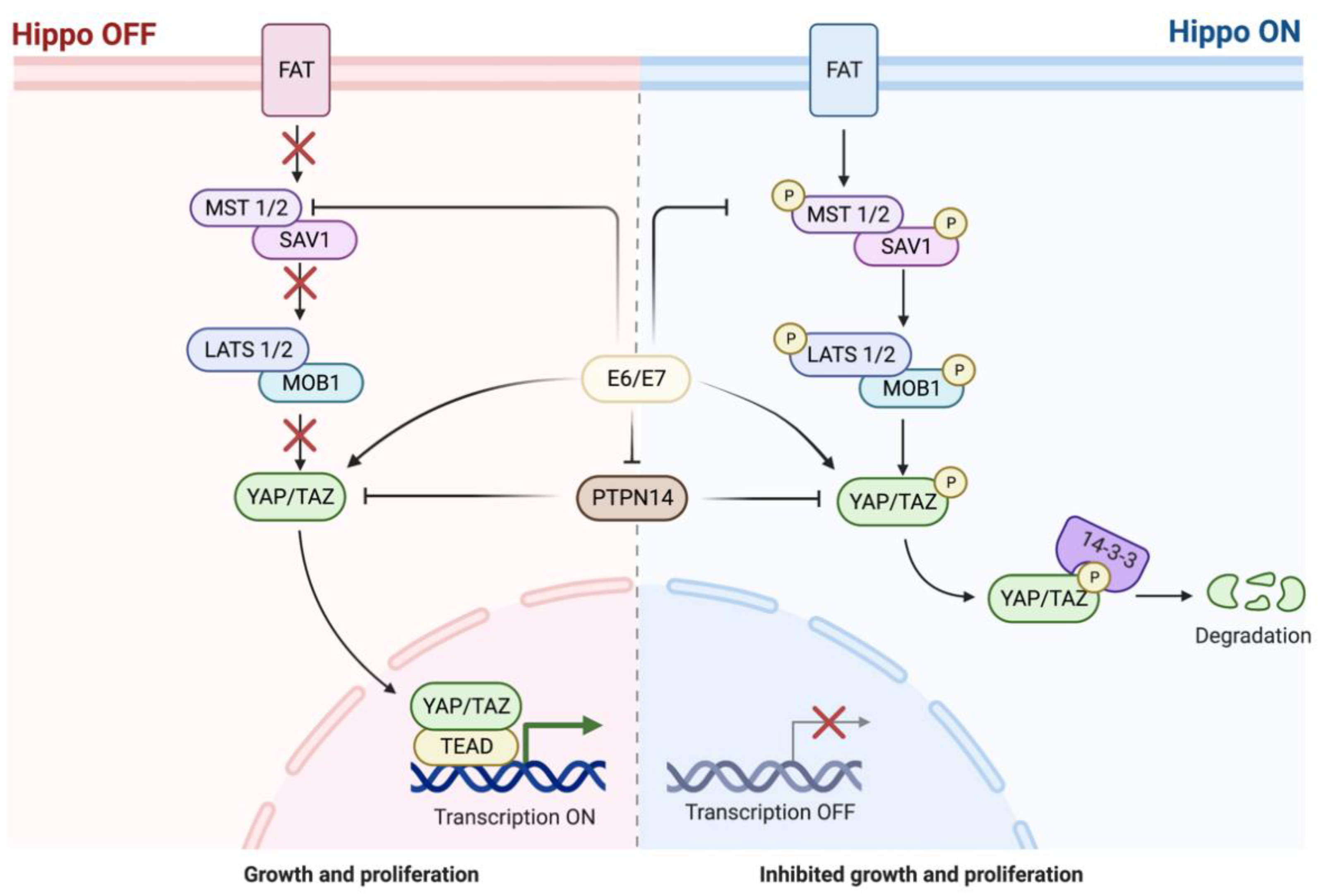
5.4. Hippo Pathway
6. Conclusions and Clinical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef]
- Marcu, L.G.; Yeoh, E. A review of risk factors and genetic alterations in head and neck carcinogenesis and implications for current and future approaches to treatment. J. Cancer Res. Clin. Oncol. 2009, 135, 1303–1314. [Google Scholar] [CrossRef]
- Stepnick, D.; Gilpin, D. Head and Neck Cancer: An Overview. Semin. Plast. Surg. 2010, 24, 107–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seiwert, T.Y.; Cohen, E.E.W. State-of-the-art management of locally advanced head and neck cancer. Br. J. Cancer 2005, 92, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Brenner, H. Changes in Survival in Head and Neck Cancers in the Late 20th and Early 21st Century: A Period Analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Galvis, M.; Loveless, R.; Kowalski, L.; Teng, Y. Impacts of Environmental Factors on Head and Neck Cancer Pathogenesis and Progression. Cells 2021, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D.; Grandis, J.R. The Molecular Pathogenesis of Head and Neck Cancer. Cancer Biol. Ther. 2010, 9, 1–7. [Google Scholar] [CrossRef]
- Helliwell, T. ‘Risky’ epithelium in the larynx—A practical diagnosis? Histopathology 1999, 34, 262–265. [Google Scholar] [CrossRef]
- Bosman, F.T. Dysplasia classification: Pathology in disgrace? J. Pathol. 2001, 194, 143–144. [Google Scholar] [CrossRef]
- Fleskens, S.; Slootweg, P. Grading systems in head and neck dysplasia: Their prognostic value, weaknesses and utility. Head Neck Oncol. 2009, 1, 11. [Google Scholar] [CrossRef]
- Kujan, O.; Oliver, R.J.; Khattab, A.; Roberts, S.A.; Thakker, N.; Sloan, P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006, 42, 987–993. [Google Scholar] [CrossRef]
- Garnis, C.; Chari, R.; Buys, T.P.H.; Zhang, L.; Ng, R.T.; Rosin, M.P.; Lam, W.L. Genomic imbalances in precancerous tissues signal oral cancer risk. Mol. Cancer 2009, 8, 50. [Google Scholar] [CrossRef]
- Chin, D.; Boyle, G.M.; Williams, R.M.; Ferguson, K.; Pandeya, N.; Pedley, J.; Campbell, C.M.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Novel markers for poor prognosis in head and neck cancer. Int. J. Cancer 2005, 113, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.; van der Riet, P.; Westra, W.; Nawroz, H.; Clayman, G.; Piantadosi, S.; Corio, R.; Lee, D.; Greenberg, B.; Koch, W.; et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996, 56, 2488–2492. [Google Scholar] [CrossRef]
- Haddad, R.I.; Shin, D.M. Recent Advances in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ordonez, B.; Beauchemin, M.; Jordan, R.C.K. Molecular biology of squamous cell carcinoma of the head and neck. J. Clin. Pathol. 2006, 59, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Loffek, S.; Schilling, O.; Franzke, C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef]
- Bosman, F.T. The borderline: Basement membranes and the transition from premalignant to malignant neoplasia. Microsc. Res. Tech. 1994, 28, 216–225. [Google Scholar] [CrossRef]
- Fan, H.-X.; Li, H.-X.; Chen, D.; Gao, Z.-X.; Zheng, J.-H. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: Relationships and prognostic implications. J. Exp. Clin. Cancer Res. 2012, 31, 90. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.; Mane, D.R. Correlation of matrix metalloproteinase-9 expression with morphometric analysis of mucosal vasculature in oral squamous cell carcinoma, oral epithelial dysplasia, and normal oral mucosa. Int. J. Health Sci. 2018, 12, 36–43. [Google Scholar]
- Fraga, C.A.d.C.; Farias, L.C.; de Oliveira, M.V.M.; Domingos, P.L.B.; Pereira, C.S.; Silva, T.F.; Roy, A.; Gomez, R.S.; de Paula, A.M.B.; Guimarães, A.L.S. Increased VEGFR2 and MMP9 protein levels are associated with epithelial dysplasia grading. Pathol. Res. Pract. 2014, 210, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.C.K.; Macabeo-Ong, M.; Shiboski, C.H.; Dekker, N.; Ginzinger, D.G.; Wong, D.T.W.; Schmidt, B.L. Overexpression of Matrix Metalloproteinase-1 and -9 mRNA Is Associated with Progression of Oral Dysplasia to Cancer. Clin. Cancer Res. 2004, 10, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, W.; Zhang, Y.; Wang, X.; Liu, H.; Xu, S.; Zhao, Z.; Chen, D. Changes in the expression of Col IV, gelatinase and TIMP-1 in oral leukoplakia. Int. J. Clin. Exp. Pathol. 2017, 10, 8535–8543. [Google Scholar] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Mane, D.R.; Arora, M. Immunohistochemical expression of extracellular matrix metalloproteinase inducer (EMMPRIN) in normal oral mucosa, oral epithelial dysplasia and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2018, 22, 279–280. [Google Scholar] [CrossRef]
- Astarita, J.L.; Acton, S.E.; Turley, S.J. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3, 283. [Google Scholar] [CrossRef]
- Ugorski, M.; Dziegiel, P.; Suchanski, J. Podoplanin—A small glycoprotein with many faces. Am. J. Cancer Res. 2016, 6, 370–386. [Google Scholar] [PubMed]
- Yuan, P.; Temam, S.; El-Naggar, A.; Zhou, X.; Liu, D.D.; Lee, J.J.; Mao, L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 2006, 107, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; El-Naggar, A.K.; Papadimitrakopoulou, V.; Ren, H.; Fan, Y.-H.; Feng, L.; Lee, J.J.; Kim, E.; Hong, W.K.; Lippman, S.M.; et al. Podoplanin: A Novel Marker for Oral Cancer Risk in Patients with Oral Premalignancy. J. Clin. Oncol. 2008, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Chandrashekar, C. Evaluation of SOX2 and podoplanin expression in oral epithelial dysplasia and its correlation with malignant transformation. J. Investig. Clin. Dent. 2019, 10, e12450. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, B.; Nayak, R.; Kotrashetti, V.S. Immunohistochemical Expression of Podoplanin in Clinical Variants of Oral Leukoplakia and Its Correlation with Epithelial Dysplasia. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Grochau, K.J.; Safi, A.-F.; Drebber, U.; Grandoch, A.; Zöller, J.E.; Kreppel, M. Podoplanin expression in oral leukoplakia─a prospective study. J. Cranio-Maxillofac. Surg. 2019, 47, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Christofori, G. The potential role of podoplanin in tumour invasion. Br. J. Cancer 2007, 96, 1–5. [Google Scholar] [CrossRef]
- Wicki, A.; Lehembre, F.; Wick, N.; Hantusch, B.; Kerjaschki, D.; Christofori, G. Tumor invasion in the absence of epithelial-mesenchymal transition: Podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 2006, 9, 261–272. [Google Scholar] [CrossRef]
- Atsumi, N.; Ishii, G.; Kojima, M.; Sanada, M.; Fujii, S.; Ochiai, A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochem. Biophys. Res. Commun. 2008, 373, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, P.; Barhoi, D.; Giri, A.; Bhattacharjee, A.; Giri, S. Joint detection of claudin-1 and junctional adhesion molecule-A as a therapeutic target in oral epithelial dysplasia and oral squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 18117–18127. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Y.; Zhang, W.; Wei, F.; Yang, J.; Luo, X.; Zhou, T.; Hou, B.; Qian, S.; Deng, X.; et al. Junctional adhesion molecule-A, an epithelial–mesenchymal transition inducer, correlates with metastasis and poor prognosis in human nasopharyngeal cancer. Carcinogenesis 2015, 36, 41–48. [Google Scholar] [CrossRef]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.-R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Von Zeidler, S.V.; Botelho, T.d.S.; Mendonça, E.F.; Batista, A.C. E-cadherin as a potential biomarker of malignant transformation in oral leukoplakia: A retrospective cohort study. BMC Cancer 2014, 14, 972. [Google Scholar] [CrossRef]
- Adams, C.L.; Chen, Y.-T.; Smith, S.J.; Nelson, W.J. Mechanisms of Epithelial Cell–Cell Adhesion and Cell Compaction Revealed by High-resolution Tracking of E-Cadherin–Green Fluorescent Protein. J. Cell Biol. 1998, 142, 1105–1119. [Google Scholar] [CrossRef]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef]
- Sathish, I.I.; Asokan, K.; Krithika, C.L.; Ramanathan, A. Expression of E- Cadherin and Levels of Dysplasia in Oral Leukoplakia—A Prospective Cohort Study. Asian Pac. J. Cancer Prev. 2020, 21, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Santos García, A.; Abad Hernández, M.M.; Fonseca Sánchez, E.; Gonzalez, R.J.; Galindo Villardón, P.; Cruz Hernández, J.J.; Bullon-Sopelana, A. E-cadherin, laminin and collagen IV expression in the evolution from dysplasia to oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E100–E105. [Google Scholar] [PubMed]
- Zhang, W.; Alt-Holland, A.; Margulis, A.; Shamis, Y.; Fusenig, N.E.; Rodeck, U.; Garlick, J.A. E-cadherin loss promotes the initiation of squamous cell carcinoma invasion through modulation of integrin-mediated adhesion. J. Cell Sci. 2006, 119, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sneath, R.J.; Mangham, D.C. The normal structure and function of CD44 and its role in neoplasia. J. Clin. Pathol. Mol. Pathol. 1998, 51, 191–200. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Saghravanian, N.; Mirhashemi, M.; Ghazi, N.; Taghipour, A.; Mohajertehran, F. Evaluation of CD24 and CD44 as cancer stem cell markers in squamous cell carcinoma and epithelial dysplasia of the oral cavity by q-RT-PCR. Dent. Res. J. 2020, 17, 208. [Google Scholar] [CrossRef]
- Ghazi, N.; Saghravanian, N.; Ghazi, A.; Shakeri, M.T.; Khajehbahrami, H. CD44 Expression in Dysplastic and Non-Dysplastic Oral Lichen Planus. Int. J. Cancer Manag. 2020, 13, e98061. [Google Scholar] [CrossRef]
- Godge, P.; Poonja, L.; Py, G.; Ls, P. Quantitative assessment of expression of cell adhesion molecule (CD44) splice variants: CD44 standard (CD44s) and v5, v6 isoforms in oral leukoplakias: An immunohistochemical study. Indian J. Dent. Res. 2011, 22, 493. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.A.; Bravo, M.; Ruiz-Ávila, I.; Esteban, F.; Bascones-Martínez, A.; González-Moles, S. Adhesion molecule CD44 expression in non-tumour epithelium adjacent to tongue cancer. Oral Oncol. 2004, 40, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hirvikoski, P.; Tammi, R.; Kumpulainen, E.; Virtaniemi, J.; Parkkinen, J.J.; Tammi, M.; Johansson, R.; Ågren, U.; Karhunen, J.; Kosma, V.-M. Irregular expression of hyaluronan and its CD44 receptor is associated with metastatic phenotype in laryngeal squamous cell carcinoma. Virchows Arch. 1999, 434, 37–44. [Google Scholar] [CrossRef]
- Soukka, T.; Salmi, M.; Joensuu, H.; Häkkinen, L.; Sointu, P.; Koulu, L.; Kalimo, K.; Klemi, P.; Grenman, R.; Jalkanen, S. Regulation of CD44v6-containing isoforms during proliferation of normal and malignant epithelial cells. Cancer Res. 1997, 57, 2281–2289. [Google Scholar]
- Mesrati, M.H.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Glumac, P.M.; Lebeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef]
- Ravindran, G.; Devaraj, H. Aberrant expression of CD133 and musashi-1 in preneoplastic and neoplastic human oral squamous epithelium and their correlation with clinicopathological factors. Head Neck 2012, 34, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Jiajia, Q.; Yan, S.; Changqing, Y.; Wenjing, J.; Han, Z.; Yuanpan, C.; Qiuyan, L. Clinical significance of CD44 and CD133 expression in oral potentially malignant disorder and oral squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi 2017, 35, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, J.; Ma, L.; Liu, W.; Zhou, Z. CD133 expression in oral lichen planus correlated with the risk for progression to oral squamous cell carcinoma. Ann. Diagn. Pathol. 2013, 17, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, L.; Shen, X.-M.; Shi, L.-J.; Zhang, C.-P.; Xu, L.-Q.; Zhou, Z.-T. Expression patterns of cancer stem cell markers ALDH1 and CD133 correlate with a high risk of malignant transformation of oral leukoplakia. Int. J. Cancer 2012, 132, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.C.M.; Bezerra, T.M.M.; de Barros Silva, P.G.; Cavalcante, R.B.; Costa, F.W.G.; Alves, A.P.N.N.; Chaves, F.N.; Pereira, K.M.A. CD133 Role in Oral Carcinogenesis. Asian Pac. J. Cancer Prev. 2020, 21, 2501–2506. [Google Scholar] [CrossRef]
- Botha, H.; Farah, C.; Koo, K.; Cirillo, N.; McCullough, M.; Paolini, R.; Celentano, A. The Role of Glucose Transporters in Oral Squamous Cell Carcinoma. Biomolecules 2021, 11, 1070. [Google Scholar] [CrossRef]
- Angadi, V.C.; Angadi, P.V. GLUT-1 immunoexpression in oral epithelial dysplasia, oral squamous cell carcinoma, and verrucous carcinoma. J. Oral Sci. 2015, 57, 115–122. [Google Scholar] [CrossRef]
- Feitosa, S.G.; Viana, K.F.; Luna, E.C.M.; Costa, F.W.G.; Cavalcante, R.B.; Chaves, F.N.; Chaves, H.V.; Pereira, K.M.A. Immunohistochemical Evaluation of GLUT-3 and GLUT-4 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 1779–1783. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chi, T.; Chang, W.-M.; Su, C.-Y.; Lin, Y.-F.; Chen, C.-L.; Chen, M.-H.; Chang, P.M.-H.; Wu, A.T.H.; Hsiao, M. Glucose transporter 4 promotes head and neck squamous cell carcinoma metastasis through the TRIM24-DDX58 axis. J. Hematol. Oncol. 2017, 10, 11. [Google Scholar] [CrossRef]
- Pereira, K.M.A.; Feitosa, S.G.; Lima, A.T.T.; Luna, E.C.M.; Cavalcante, R.B.; de Lima, K.C.; Chaves, F.N.; Costa, F.W.G. Immunohistochemical Evaluation of Glucose Transporter Type 1 in Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2016, 17, 147–151. [Google Scholar] [CrossRef]
- Reisser, C.; Eichhorn, K.; Herold-Mende, C.; Born, A.I.; Bannasch, P. Expression of facilitative glucose transport proteins during development of squamous cell carcinomas of the head and neck. Int. J. Cancer. 1999, 80, 194–198. [Google Scholar] [CrossRef]
- Brooks Robey, R.; Weisz, J.; Kuemmerle, N.B.; Salzberg, A.C.; Berg, A.; Brown, D.G.; Kubik, L.; Palorini, R.; Al-Mulla, F.; Al-Temaimi, R.; et al. Metabolic reprogramming and dysregulated metabolism: Cause, consequence and/or enabler of environmental carcinogenesis? Carcinogenesis 2015, 36, S203–S231. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Raju, K.L.; Augustine, D.; Patil, S. Prognostic Significance of ALDH1, Bmi1, and OCT4 Expression in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Cancer Control 2020, 27, 1073274820904959. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, Y.-W.; Hsu, H.-S.; Tseng, L.-M.; Huang, P.-I.; Lu, K.-H.; Chen, D.-T.; Tai, L.-K.; Yung, M.-C.; Chang, S.-C.; et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009, 385, 307–313. [Google Scholar] [CrossRef]
- Dhumal, S.N.; Choudhari, S.K.; Patankar, S.; Ghule, S.S.; Jadhav, Y.B.; Masne, S. Cancer Stem Cell Markers, CD44 and ALDH1, for Assessment of Cancer Risk in OPMDs and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Head Neck Pathol. 2021, 16, 453–465. [Google Scholar] [CrossRef]
- Dubey, A.; Prajapati, K.S.; Swamy, M.; Pachauri, V. Heat shock proteins: A therapeutic target worth to consider. Vet. World 2015, 8, 46–51. [Google Scholar] [CrossRef]
- Hendrick, J.P.; Hartl, F.-U. Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 1993, 62, 349–384. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Majumdar, S.; Kotina, S.; Uppala, D.; Balla, H. Expression of Heat Shock Protein 70 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma: An Immunohistochemical Study. Contemp. Clin. Dent. 2019, 10, 185–190. [Google Scholar] [CrossRef]
- Patil, P.; Nandimath, K.; Prabhu, S.; Naikmasur, V.G. Heat shock protein (HSP70) as a marker of epithelial dysplasia in oral dysplastic lesions: A clinicopathological study. J. Oral Maxillofac. Pathol. 2015, 19, 53–57. [Google Scholar] [CrossRef]
- Bar, J.K.; Cierpikowski, P.; Lis-Nawara, A.; Duc, P.; Hałoń, A.; Radwan-Oczko, M. Comparison of p53, HSP90, E-cadherin and HPV in oral lichen planus and oral squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2021, 41, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Sobel, A. Stathmin: A relay phosphoprotein for multiple signal transduction? Trends Biochem. Sci. 1991, 16, 301–305. [Google Scholar] [CrossRef]
- Kang, W.; Tong, J.H.M.; Chan, A.; Lung, R.W.M.; Chau, S.L.; Wong, Q.W.L.; Wong, N.; Yu, J.; Cheng, A.; To, K.F. Stathmin1 plays oncogenic role and is a target of MicroRNA-223 in gastric cancer. PLoS ONE 2012, 7, e33919. [Google Scholar] [CrossRef] [PubMed]
- Curmi, P.A.; Noguès, C.; Lachkar, S.; Carelle, N.; Gonthier, M.P.; Sobel, A.; Lidereau, R.; Bièche, I. Overexpression of stathmin in breast carcinomas points out to highly proliferative tumours. Br. J. Cancer 2000, 82, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Huang, W.-G.; Chen, Z.-C.; Peng, F.; Zhang, P.-F.; Li, M.-Y.; Li, F.; Li, J.-L.; Li, C.; Yi, H.; et al. Identification of Novel Nasopharyngeal Carcinoma Biomarkers by Laser Capture Microdissection and Proteomic Analysis. Clin. Cancer Res. 2008, 14, 435–445. [Google Scholar] [CrossRef]
- Kouzu, Y.; Uzawa, K.; Koike, H.; Saito, K.; Nakashima, D.; Higo, M.; Endo, Y.; Kasamatsu, A.; Shiiba, M.; Bukawa, H.; et al. Overexpression of stathmin in oral squamous-cell carcinoma: Correlation with tumour progression and poor prognosis. Br. J. Cancer 2006, 94, 717–723. [Google Scholar] [CrossRef][Green Version]
- Vadla, P.; Deepthi, G.; Arun Kumar, C.; Bashamalla, R.; Syeda, N.; Naramala, S. Immunohistochemical expression of stathmin in oral dysplasia: An original study with an insight of its action on microtubules. J. Oral Maxillofac. Pathol. 2021, 25, 247–252. [Google Scholar] [CrossRef]
- Vadla, P.; Yeluri, S.; Deepthi, G.; Guttikonda, V.R.; Taneeru, S.; Naramala, S. Stathmin! An immunohistochemical analysis of the novel marker in Oral Squamous Cell Carcinoma and Oral Leukoplakia. Asian Pac. J. Cancer Prev. 2020, 21, 3317–3323. [Google Scholar] [CrossRef]
- Pallavi, N.; Nalabolu, G.R.K.; Hiremath, S.K.S. Bcl-2 and c-Myc expression in oral dysplasia and oral squamous cell carcinoma: An immunohistochemical study to assess tumor progression. J. Oral Maxillofac. Pathol. 2018, 22, 325. [Google Scholar] [CrossRef]
- Altieri, D.C. Survivin in apoptosis control and cell cycle regulation in cancer. Prog. Cell Cycle Res. 2003, 5, 447–452. [Google Scholar]
- Chipuk, J.E.; Green, D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008, 18, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef] [PubMed]
- Juneja, S.; Chaitanya, N.B.; Agarwal, M. Immunohistochemical expression of Bcl-2 in oral epithelial dysplasia and oral squamous cell carcinoma. Indian J. Cancer 2015, 52, 505. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Petronacci, C.M.; de Mendoza, I.L.-I.; Suarez-Peñaranda, J.M.; Padin-Iruegas, E.; Blanco-Carrion, A.; Lorenzo-Pouso, A.I.; Ortega, K.L.; Pérez-Sayáns, M. Immunohistochemical Characterization of Bcl-2 in Oral Potentially Malignant Disorders. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 706–712. [Google Scholar] [CrossRef]
- Tanaka, C.; Uzawa, K.; Shibahara, T.; Yokoe, H.; Noma, H.; Tanzawa, H. Expression of an Inhibitor of Apoptosis, Survivin, in Oral Carcinogenesis. J. Dent. Res. 2003, 82, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Angelin, D.; Nair, B.J. Comparative evaluation of survivin expression in leukoplakia, lichen planus, and oral squamous cell carcinoma: An immunohistochemical study. J. Cancer Res. Ther. 2020, 16, 569. [Google Scholar] [CrossRef]
- Muzio, L.L.; Pannone, G.; Leonardi, R.; Staibano, S.; Mignogna, M.; de Rosa, G.; Kudo, Y.; Takata, T.; Altieri, D. Survivin, a Potential Early Predictor of Tumor Progression in the Oral Mucosa. J. Dent. Res. 2003, 82, 923–928. [Google Scholar] [CrossRef]
- Pietsch, E.C.; Sykes, S.M.; McMahon, S.B.; E Murphy, M. The p53 family and programmed cell death. Oncogene 2008, 27, 6507–6521. [Google Scholar] [CrossRef]
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Gordon, E.M.; Ravicz, J.R.; Liu, S.; Chawla, S.P.; Hall, F.L. Cell cycle checkpoint control: The cyclin G1/Mdm2/p53 axis emerges as a strategic target for broad-spectrum cancer gene therapy—A review of molecular mechanisms for oncologists. Mol. Clin. Oncol. 2018, 9, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; Levine, A.J. The p53 pathway: Positive and negative feedback loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Qin, J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-p53 pathway revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar] [CrossRef]
- Boaz, K.; Pandya, J.A.; Natarajan, S.; Manaktala, N.; Nandita, K.; Lewis, A.J. A correlation of immunohistochemical expression of TP53 and CDKN1A in oral epithelial dysplasia and oral squamous cell carcinoma. J. Cancer Res. Ther. 2018, 14, 666–670. [Google Scholar] [CrossRef]
- Abrahao, A.; Bonelli, B.V.; Nunes, F.D.; Dias, E.P.; Cabral, M.G. Immunohistochemical expression of p53, p16 and hTERT in oral squamous cell carcinoma and potentially malignant disorders. Braz. Oral Res. 2011, 25, 34–41. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; García-Pedrero, J.M.; Suárez, C.; Takes, R.P.; Thompson, L.; Slootweg, P.J.; Woolgar, J.A.; Westra, W.H.; Brakenhoff, R.H.; Rinaldo, A.; et al. Biomarkers predicting malignant progression of laryngeal epithelial precursor lesions: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 1073–1083. [Google Scholar] [CrossRef]
- Kawano, S.; Matsubara, R.; Kiyosue, T.; Goto, Y.; Hirano, M.; Jinno, T.; Toyoshima, T.; Kitamura, R.; Oobu, K.; Nakamura, S. Increased ΔNp63 expression is predictive of malignant transformation in oral epithelial dysplasia and poor prognosis in oral squamous cell carcinoma. Int. J. Oncol. 2011, 39, 1391–1399. [Google Scholar] [CrossRef]
- Ono, S.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H. Immunohistochemistry of YAP and dNp63 and survival analysis of patients bearing precancerous lesion and oral squamous cell carcinoma. Int. J. Med. Sci. 2019, 16, 766–773. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Ms, S.-S.H.; Lin, L.-M. p73 expression for human buccal epithelial dysplasia and squamous cell carcinoma: Does it correlate with nodal status of carcinoma and is there a relationship with malignant change of epithelial dysplasia? Head Neck 2004, 26, 945–952. [Google Scholar] [CrossRef]
- Bascones-Martínez, A.; López-Durán, M.; Cano-Sánchez, J.; Sánchez-Verde, L.; Díez-Rodríguez, A.; Aguirre-Echebarría, P.; Álvarez-Fernández, E.; González-Moles, M.A.; Bascones-Ilundain, J.; Muzio, L.L.; et al. Differences in the expression of five senescence markers in oral cancer, oral leukoplakia and control samples in humans. Oncol. Lett. 2012, 3, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.T.; Budnick, S.D.; Logani, S. Immunohistochemical detection of p16INK4a in dysplastic lesions of the oral cavity. Mod. Pathol. 2006, 19, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Kaur, J.; Kumar, A.; Chakravarti, N.; Mathur, M.; Bahadur, S.; Shukla, N.K.; Deo, S.V.; Ralhan, R. Alterations of Rb Pathway Components Are Frequent Events in Patients with Oral Epithelial Dysplasia and Predict Clinical Outcome in Patients with Squamous Cell Carcinoma. Oncology 2005, 68, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Garces de los Fayos Alonso, I.; Liang, H.-C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef]
- Meng, Q.; Xia, Y. c-Jun, at the crossroad of the signaling network. Protein Cell 2011, 2, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Turatti, E.; Neves, A.d.C.; de Magalhães, M.H.C.G.; de Sousa, S.O.M. Assessment of c-Jun, c-Fos and cyclin D1 in premalignant and malignant oral lesions. J. Oral Sci. 2005, 47, 71–76. [Google Scholar] [CrossRef][Green Version]
- Shraddha, K.; Niranjan, K.; Hallikeri, K. Immunolocalization of c-Jun in normal mucosa, oral submucous fibrosis, epithelial dysplasia, and oral squamous cell carcinoma: A comparative study. J. Cancer Res. Ther. 2018, 14, 1180–1183. [Google Scholar] [CrossRef]
- Lima, J.S.; Correa, L.; Klingbeil, M.F.G.; de Sousa, S.C.O.M. C-Jun, pc-Jun, and p27 are differently expressed in oral leukoplakias in smokers and never-smokers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 73–80. [Google Scholar] [CrossRef]
- Rizzino, A. Concise Review: The Sox2-Oct4 Connection: Critical Players in a Much Larger Interdependent Network Integrated at Multiple Levels. Stem Cells 2013, 31, 1033–1039. [Google Scholar] [CrossRef]
- Ghazi, N.; Aali, N.; Shahrokhi, V.-R.; Mohajertehran, F.; Saghravanian, N. Relative Expression of SOX2 and OCT4 in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia. Rep. Biochem. Mol. Biol. 2020, 9, 171–179. [Google Scholar] [CrossRef]
- Hussenet, T.; Du Manoir, S. SOX2 in squamous cell carcinoma: Amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle 2010, 9, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-L.; Hu, F.-W.; Lee, S.-S.; Yu, C.-H.; Yu, C.-C.; Chang, Y.-C. Oct4 Mediates Tumor Initiating Properties in Oral Squamous Cell Carcinomas through the Regulation of Epithelial-Mesenchymal Transition. PLoS ONE 2014, 9, e87207. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, B.N.; Sanjai, K.; Kumaraswamy, J.; Papaiah, L.; Pandey, B.; Jyothi, B.M. Expression of human telomerase reverse transcriptase protein in oral epithelial dysplasia and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2016, 20, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Tye, B.K. MCM Proteins in DNA Replication. Annu. Rev. Biochem. 1999, 68, 649–686. [Google Scholar] [CrossRef]
- Zakaria, S.H.; Farag, H.A.; Khater, D.S. Immunohistochemical Expression of MCM-2 in Oral Epithelial Dysplasias. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Jessri, M.; Dalley, A.; Farah, C. MutSα and MutLα immunoexpression analysis in diagnostic grading of oral epithelial dysplasia and squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 74–82. [Google Scholar] [CrossRef]
- Awasthi, P.; Foiani, M.; Kumar, A. ATM and ATR signaling at a glance. J. Cell Sci. 2015, 128, 4255–4262. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, W.; Shi, L.; Xiao, X.; Wu, W.; Wu, L.; Zhou, Z. Expression of DNA doublestrand repair proteins in oral leukoplakia and the risk of malignant transformation. Oncol. Lett. 2018, 15, 9827–9835. [Google Scholar] [CrossRef]
- Ho, M.W.; Ryan, M.P.; Gupta, J.; Triantafyllou, A.; Risk, J.M.; Shaw, R.J.; Wilson, J.B. Loss of FANCD2 and related proteins may predict malignant transformation in oral epithelial dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 377–387. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Pastushenko, I.; Mauri, F.; Song, Y.; de Cock, F.; Meeusen, B.; Swedlund, B.; Impens, F.; van Haver, D.; Opitz, M.; Thery, M.; et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 2021, 589, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Golczer, G.; Ghose, S.; Lin, B.; Langenbucher, A.; Webb, J.; Bhanot, H.; Abt, N.B.; Lin, D.; Varvares, M.; et al. YAP1 maintains active chromatin state in head and neck squamous cell carcinomas that promotes tumorigenesis through cooperation with BRD4. Cell Rep. 2022, 39, 110970. [Google Scholar] [CrossRef] [PubMed]
- Hatterschide, J.; Castagnino, P.; Kim, H.W.; Sperry, S.M.; Montone, K.T.; Basu, D.; A White, E. YAP1 activation by human papillomavirus E7 promotes basal cell identity in squamous epithelia. eLife 2022, 11, e75466. [Google Scholar] [CrossRef]
- Olmedo-Nieva, L.; Muñoz-Bello, J.O.; Manzo-Merino, J.; Lizano, M. New insights in Hippo signalling alteration in human papillomavirus-related cancers. Cell. Signal. 2020, 76, 109815. [Google Scholar] [CrossRef]
- Omori, H.; Nishio, M.; Masuda, M.; Miyachi, Y.; Ueda, F.; Nakano, T.; Sato, K.; Mimori, K.; Taguchi, K.; Hikasa, H.; et al. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci. Adv. 2020, 6, eaay3324. [Google Scholar] [CrossRef] [PubMed]
- Schaaij-Visser, T.B.; Brakenhoff, R.H.; Leemans, C.R.; Heck, A.J.; Slijper, M. Protein biomarker discovery for head and neck cancer. J. Proteom. 2010, 73, 1790–1803. [Google Scholar] [CrossRef]
- Hunter, K.D.; Parkinson, E.K.; Harrison, P.R. Profiling early head and neck cancer. Nat. Rev. Cancer 2005, 5, 127–135. [Google Scholar] [CrossRef]
- Ranganathan, K.; Kavitha, L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27. [Google Scholar] [CrossRef]
- Mahmood, H.; Bradburn, M.; Rajpoot, N.; Islam, N.M.; Kujan, O.; Khurram, S.A. Prediction of malignant transformation and recurrence of oral epithelial dysplasia using architectural and cytological feature specific prognostic models. Mod. Pathol. 2022, 35, 1151–1159. [Google Scholar] [CrossRef]
- Cilona, M.; Locatello, L.G.; Novelli, L.; Gallo, O. The Mismatch Repair System (MMR) in Head and Neck Carcinogenesis and Its Role in Modulating the Response to Immunotherapy: A Critical Review. Cancers 2020, 12, 3006. [Google Scholar] [CrossRef]
- Ameri, A.; Mortazavi, N.; Ahmadi, H.K.; Novin, K. ERCC1 Expression Can Predict Response to Platinum-Based Induction Chemotherapy in Head and Neck Cancer Cases. Asian Pac. J. Cancer Prev. 2016, 17, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Eze, N.; Lo, Y.-C.; Burtness, B. Biomarker driven treatment of head and neck squamous cell cancer. Cancers Head Neck 2017, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Rieke, D.T.; Klinghammer, K.; Keilholz, U. Targeted Therapy of Head and Neck Cancer. Oncol. Res. Treat. 2016, 39, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, A.; Brakenhoff, R. Targeted Treatment of Head and Neck (Pre)Cancer: Preclinical Target Identification and Development of Novel Therapeutic Applications. Cancers 2021, 13, 2774. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Epstein, J.B.; Marur, S.; Gillespie, M.B.; Feldman, L.; Tsai, H.-L.; Zhang, Z.; Wang, H.; Sciubba, J.; Ferris, R.L.; et al. Cetuximab activity in dysplastic lesions of the upper aerodigestive tract. Oral Oncol. 2016, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- William, W.N., Jr.; Papadimitrakopoulou, V.; Lee, J.J.; Mao, L.; Cohen, E.E.W.; Lin, H.Y.; Gillenwater, A.M.; Martin, J.W.; Lingen, M.W.; Boyle, J.O.; et al. Erlotinib and the Risk of Oral Cancer: The Erlotinib Prevention of Oral Cancer (EPOC) Randomized Clinical Trial. JAMA Oncol. 2016, 2, 209–216. [Google Scholar] [CrossRef]
- Mishra, A.; Verma, M. Cancer Biomarkers: Are We Ready for the Prime Time? Cancers 2010, 2, 190–208. [Google Scholar] [CrossRef]
- Negm, R.S.; Verma, M.; Srivastava, S. The promise of biomarkers in cancer screening and detection. Trends Mol. Med. 2002, 8, 288–293. [Google Scholar] [CrossRef]
| Biomarker Category | Member (s) | Physiologic Function | Role in Tumorigenesis |
|---|---|---|---|
| Extracellular and Cell-Surface | |||
| Extracellular Degradation | MMP-2, MMP-9 | Type IV Collagenase |
|
| Cell Motility | Podoplanin | Transmembrane Glycoprotein |
|
| Cell-Cell Adhesion | Claudin | Tight Junction Protein |
|
| JAM-A | Tight Junction Protein |
| |
| E-Cadherin | Adherens Junction Protein |
| |
| Solute Transport | GLUT-1, GLUT-4 | Glucose Transporter |
|
| Cancer Stem Cell Markers | CD44 | Transmembrane Glycoprotein |
|
| CD133 | Transmembrane Glycoprotein |
| |
| Cytosolic Markers | |||
| Metabolic Regulators | ALDH-1 | Phase-I Oxidase |
|
| Molecular Chaperones | HSP70, HSP90 | Heat Shock Proteins |
|
| Mitosis Regulators | Stathmin | Cytoskeleton Phosphoprotein |
|
| Apoptosis Regulators | Bcl-2 | Inhibitor of Apoptosis |
|
| Survivin | Inhibitor of Apoptosis |
| |
| Nuclear Markers | |||
| Cell Cycle Regulators | p53 p63 p16 Cyclin D1 pRb MDM2 | Tumor Suppressor Tumor Suppressor Tumor Suppressor Inhibitor of pRb Tumor Suppressor E3 Ubiquitin Ligase |
|
| Transcriptional Regulators | C-Jun | AP-1 Transcription Factor |
|
| SOX-2/OCT-4 | Reprogramming Transcription Factors |
| |
| YAP/TAZ | Hippo Pathway Mediator |
| |
| DNA Replication and Repair Regulators | hTERT | Telomerase Protein |
|
| MCM2 | Replication Initiation Factor |
| |
| MutSα MutLα | DNA Mismatch Repair Protein |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranganath, K.; Feng, A.L.; Franco, R.A.; Varvares, M.A.; Faquin, W.C.; Naunheim, M.R.; Saladi, S.V. Molecular Biomarkers of Malignant Transformation in Head and Neck Dysplasia. Cancers 2022, 14, 5581. https://doi.org/10.3390/cancers14225581
Ranganath K, Feng AL, Franco RA, Varvares MA, Faquin WC, Naunheim MR, Saladi SV. Molecular Biomarkers of Malignant Transformation in Head and Neck Dysplasia. Cancers. 2022; 14(22):5581. https://doi.org/10.3390/cancers14225581
Chicago/Turabian StyleRanganath, Kushi, Allen L. Feng, Ramon A. Franco, Mark A. Varvares, William C. Faquin, Matthew R. Naunheim, and Srinivas Vinod Saladi. 2022. "Molecular Biomarkers of Malignant Transformation in Head and Neck Dysplasia" Cancers 14, no. 22: 5581. https://doi.org/10.3390/cancers14225581
APA StyleRanganath, K., Feng, A. L., Franco, R. A., Varvares, M. A., Faquin, W. C., Naunheim, M. R., & Saladi, S. V. (2022). Molecular Biomarkers of Malignant Transformation in Head and Neck Dysplasia. Cancers, 14(22), 5581. https://doi.org/10.3390/cancers14225581




