Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
3. Results
3.1. Literature Search and Included Studies
3.2. Method of Obtaining the Cut-Off Value of NLR
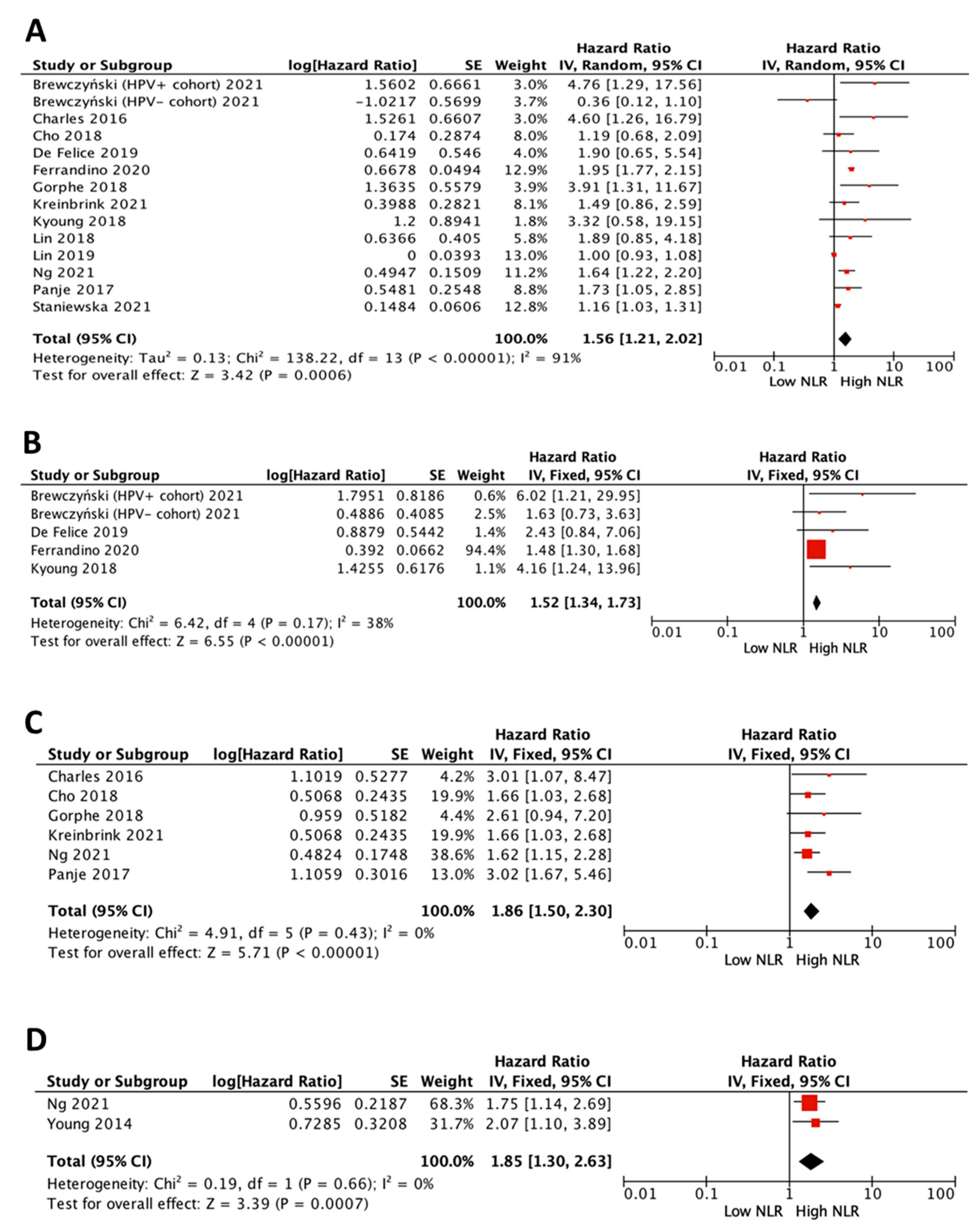
3.3. Impact of NLR on Survival in All Patients
3.4. Impact of NLR on Survival in HPV-Positive Patients
3.5. Impact of NLR on Survival in HPV-Negative Patients
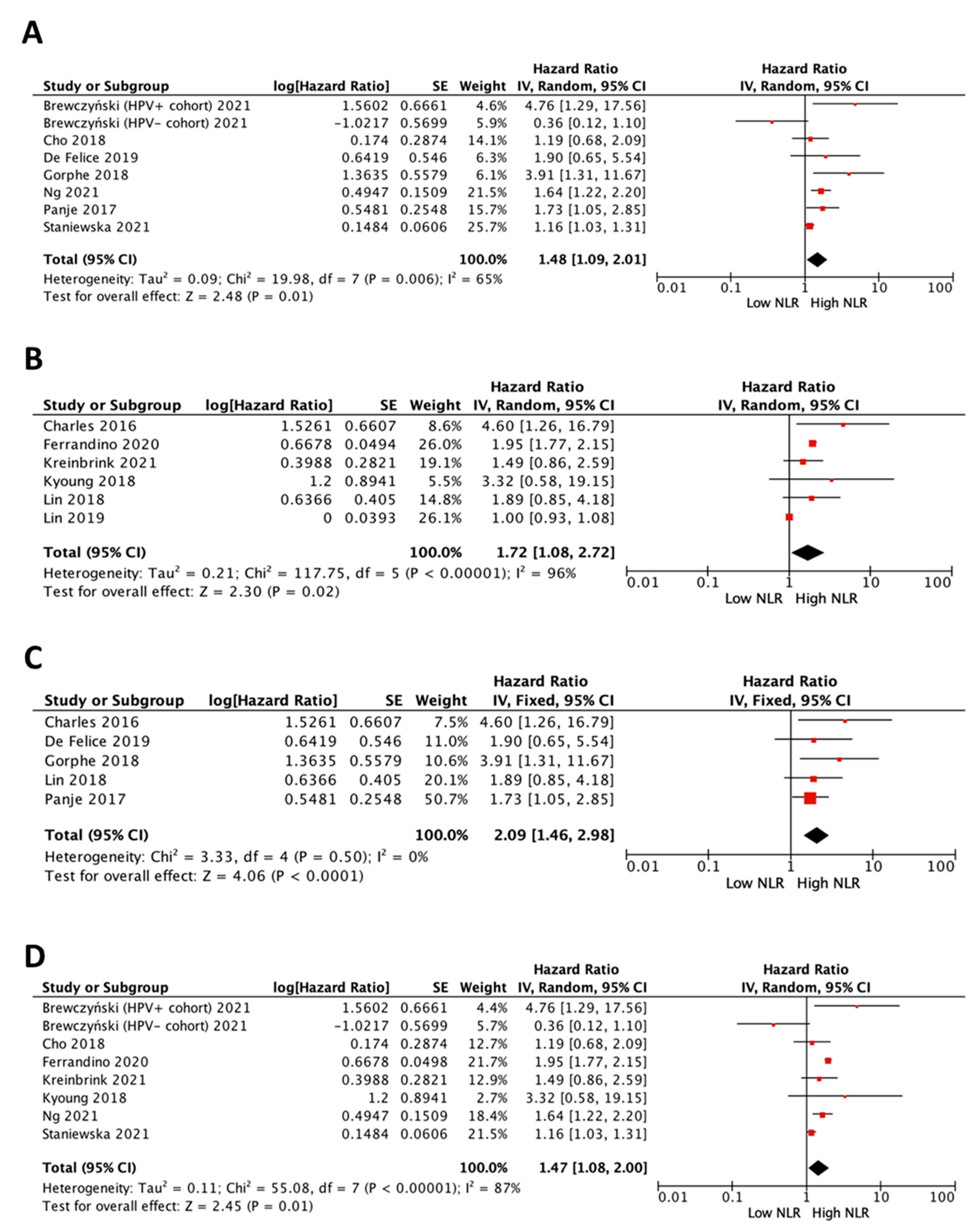
3.6. Impact of NLR on Survival in All Patients by Treatment
3.7. Impact of Cut-Off Value of NLR on Survival in All Patients
3.8. Published Status Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faraji, F.; Eisele, D.W.; Fakhry, C. Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2017, 2, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Nixon, I.J. Recent advances in the understanding and management of oropharyngeal cancer. F1000Research 2018, 7, F1000 Faculty Rev:1362. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- You, E.L.; Henry, M.; Zeitouni, A.G. Human papillomavirus-associated oropharyngeal cancer: Review of current evidence and management. Curr. Oncol. 2019, 26, 119–123. [Google Scholar] [CrossRef]
- Galon, J.; Pages, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.A.; Gajewski, T.F.; Ascierto, P.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, D.; Heng, Y.; Zhu, X.K.; Zhou, L.; Tao, L.; Lu, L.M. Prognostic Impact of Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma Patients. Laryngoscope 2021, 131, E1249–E1255. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 2017, 58, 1–13. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Q.; Guan, Z.; Diao, Q. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in patients with laryngeal cancer: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2021, 278, 417–425. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Marroquin-Muciño, M.; Perez-Medina, M.; Benito-Lopez, J.J.; Camarena, A.; Rumbo-Nava, U.; Lopez-Gonzalez, J.S. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front. Endocrinol. 2022, 13, 929572. [Google Scholar] [CrossRef]
- Kang, M.H.; Go, S.I.; Song, H.N.; Lee, A.; Kim, S.H.; Kang, J.H.; Jeong, B.K.; Kang, K.M.; Ling, H.; Lee, G.W. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br. J. Cancer 2014, 111, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.J.; Shen, S.L.; Li, S.Q.; Hua, Y.P.; Hu, W.J.; Liang, L.J.; Peng, B.G. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med. Oncol. 2013, 30, 721. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, R.M.; Roof, S.; Garneau, J.; Haidar, Y.; Bates, S.E.; Park, Y.A.; Bauml, J.M.; Genden, E.M.; Miles, B.; Sigel, K. Neutrophil-to-lymphocyte ratio as a prognostic indicator for overall and cancer-specific survival in squamous cell carcinoma of the head and neck. Head Neck 2020, 42, 2830–2840. [Google Scholar] [CrossRef] [PubMed]
- So, Y.K.; Lee, G.; Oh, D.; Byeon, S.; Park, W.; Chung, M.K. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients with Human Papillomavirus-Positive Oropharyngeal Cancer. Otolaryngol. Head Neck Surg. 2018, 159, 303–309. [Google Scholar] [CrossRef]
- De Felice, F.; Tombolini, M.; Abate, G.; Salerno, F.; Bulzonetti, N.; Tombolini, V.; Musio, D. Prognostic Significance of the Neutrophil/Lymphocyte Ratio in Patients with Non-Human Papilloma Virus-Related Oropharyngeal Cancer: A Retrospective Cohort Study. Oncology 2019, 96, 8–13. [Google Scholar] [CrossRef]
- Lin, A.J.; Gang, M.; Rao, Y.J.; Campian, J.; Daly, M.; Gay, H.; Oppelt, P.; Jackson, R.S.; Rich, J.; Paniello, R.; et al. Association of Posttreatment Lymphopenia and Elevated Neutrophil-to-Lymphocyte Ratio With Poor Clinical Outcomes in Patients With Human Papillomavirus-Negative Oropharyngeal Cancers. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 413–421. [Google Scholar] [CrossRef]
- Kreinbrink, P.J.; Li, J.; Parajuli, S.; Wise-Draper, T.M.; Choi, D.L.; Tang, A.L.; Takiar, V. Pre-treatment absolute lymphocyte count predicts for improved survival in human papillomavirus (HPV)-driven oropharyngeal squamous cell carcinoma. Oral Oncol. 2021, 116, 105245. [Google Scholar] [CrossRef]
- Lin, A.J.; Rao, Y.J.; Chin, R.I.; Campian, J.; Mullen, D.; Thotala, D.; Daly, M.; Gay, H.; Oppelt, P.; Hallahan, D.; et al. Post-operative radiation effects on lymphopenia, neutrophil to lymphocyte ratio, and clinical outcomes in palatine tonsil cancers. Oral Oncol. 2018, 86, 1–7. [Google Scholar] [CrossRef]
- Cho, J.K.; Kim, M.W.; Choi, I.S.; Moon, U.Y.; Kim, M.J.; Sohn, I.; Kim, S.; Jeong, H.S. Optimal cutoff of pretreatment neutrophil-to-lymphocyte ratio in head and neck cancer patients: A meta-analysis and validation study. BMC Cancer 2018, 18, 969. [Google Scholar] [CrossRef]
- Brewczyński, A.; Jabłońska, B.; Mazurek, A.M.; Mrochem-Kwarciak, J.; Mrowiec, S.; Śnietura, M.; Kentnowski, M.; Kołosza, Z.; Składowski, K.; Rutkowski, T. Comparison of Selected Immune and Hematological Parameters and Their Impact on Survival in Patients with HPV-Related and HPV-Unrelated Oropharyngeal Cancer. Cancers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Staniewska, E.; Tomasik, B.; Tarnawski, R.; Łaszczych, M.; Miszczyk, M. The prognostic value of red cell distribution width (RDW), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in radiotherapy for oropharyngeal cancer. Rep. Pract. Oncol. Radiother. 2021, 26, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Young, C.A.; Murray, L.J.; Karakaya, E.; Thygesen, H.H.; Sen, M.; Prestwich, R.J. The Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Oropharyngeal Carcinoma Treated with Chemoradiotherapy. Clin. Med. Insights Oncol. 2014, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Charles, K.A.; Harris, B.D.; Haddad, C.R.; Clarke, S.J.; Guminski, A.; Stevens, M.; Dodds, T.; Gill, A.J.; Back, M.; Veivers, D.; et al. Systemic inflammation is an independent predictive marker of clinical outcomes in mucosal squamous cell carcinoma of the head and neck in oropharyngeal and non-oropharyngeal patients. BMC Cancer 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Panje, C.; Riesterer, O.; Glanzmann, C.; Studer, G. Neutrophil-lymphocyte ratio complements volumetric staging as prognostic factor in patients treated with definitive radiotherapy for oropharyngeal cancer. BMC Cancer 2017, 17, 643. [Google Scholar] [CrossRef]
- Gorphe, P.; Chekkoury Idrissi, Y.; Tao, Y.; Schernberg, A.; Ou, D.; Temam, S.; Casiraghi, O.; Blanchard, P.; Mirghani, H. Anemia and neutrophil-to-lymphocyte ratio are prognostic in p16-positive oropharyngeal carcinoma treated with concurrent chemoradiation. Papillomavirus Res. 2018, 5, 32–37. [Google Scholar] [CrossRef]
- Ng, S.P.; Bahig, H.; Jethanandani, A.; Sturgis, E.M.; Johnson, F.M.; Elgohari, B.; Gunn, G.B.; Ferrarotto, R.; Phan, J.; Rosenthal, D.I.; et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br. J. Cancer 2021, 124, 628–633. [Google Scholar] [CrossRef]
- Valero, C.; Pardo, L.; López, M.; García, J.; Camacho, M.; Quer, M.; León, X. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck 2017, 39, 219–226. [Google Scholar] [CrossRef]
- Rassouli, A.; Saliba, J.; Castano, R.; Hier, M.; Zeitouni, A.G. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015, 37, 103–110. [Google Scholar] [CrossRef]
- Rachidi, S.; Wallace, K.; Wrangle, J.M.; Day, T.A.; Alberg, A.J.; Li, Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck 2016, 38 (Suppl. 1), E1068–E1074. [Google Scholar] [CrossRef]
- Selzer, E.; Grah, A.; Heiduschka, G.; Kornek, G.; Thurnher, D. Primary radiotherapy or postoperative radiotherapy in patients with head and neck cancer: Comparative analysis of inflammation-based prognostic scoring systems. Strahlenther. Onkol. 2015, 191, 486–494. [Google Scholar] [CrossRef]
- Kano, S.; Homma, A.; Hatakeyama, H.; Mizumachi, T.; Sakashita, T.; Kakizaki, T.; Fukuda, S. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck 2017, 39, 247–253. [Google Scholar] [CrossRef]
- Moon, H.; Roh, J.L.; Lee, S.W.; Kim, S.B.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother. Oncol. 2016, 118, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Waldron, J.N.; Milosevic, M.; Shen, X.; Ringash, J.; Su, J.; Tong, L.; Perez-Ordonez, B.; Weinreb, I.; Bayley, A.J.; et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer 2015, 121, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bojaxhiu, B.; Templeton, A.J.; Elicin, O.; Shelan, M.; Zaugg, K.; Walser, M.; Giger, R.; Aebersold, D.M.; Dal Pra, A. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat. Oncol. 2018, 13, 216. [Google Scholar] [CrossRef]
- Valero, C.; Pardo, L.; Sansa, A.; Garcia Lorenzo, J.; López, M.; Quer, M.; León, X. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck 2020, 42, 336–343. [Google Scholar] [CrossRef]
- Li, L.; Liu, G.; Lu, H.; Jin, K.; Zhai, X.; Zhou, M.; Duan, Y.; Yue, K.; Wu, Y.; Wang, X. Pre-treatment circulating neutrophil count is an independent prognostic factor in oropharyngeal cancer. Ann. Transl. Med. 2020, 8, 1135. [Google Scholar] [CrossRef]
- Ye, J.; Liao, B.; Jiang, X.; Dong, Z.; Hu, S.; Liu, Y.; Xiao, M. Prognosis Value of Platelet Counts, Albumin and Neutrophil-Lymphocyte Ratio of Locoregional Recurrence in Patients with Operable Head and Neck Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Sumner, W.A.; Stokes, W.A.; Oweida, A.; Berggren, K.L.; McDermott, J.D.; Raben, D.; Abbott, D.; Jones, B.; Gan, G.; Karam, S.D. Survival impact of pre-treatment neutrophils on oropharyngeal and laryngeal cancer patients undergoing definitive radiotherapy. J. Transl. Med. 2017, 15, 168. [Google Scholar] [CrossRef]
- Mascarella, M.A.; Mannard, E.; Silva, S.D.; Zeitouni, A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck 2018, 40, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck 2018, 40, 647–655. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Zhou, L.; Dai, Y.; Hu, G. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2019, 41, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, H.; Yan, A.; Wang, H.; Li, X.; Liu, J.; Li, W. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: A meta-analysis. BMC Cancer 2018, 18, 383. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Zanoni, D.K.; McGill, M.R.; Ganly, I.; Morris, L.G.T.; Quer, M.; Shah, J.P.; Wong, R.J.; León, X.; Patel, S.G. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer 2020, 126, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Kotha, N.V.; Voora, R.S.; Qian, A.S.; Kumar, A.; Qiao, E.M.; Stewart, T.F.; Rose, B.S.; Orosco, R.K. Prognostic Utility of Pretreatment Neutrophil-Lymphocyte Ratio in Advanced Larynx Cancer. Biomark. Insights 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, J.; Qiu, Z.; Guo, J.; Chang, W. Prognostic role of neutrophil-to-lymphocyte ratio to laryngeal squamous cell carcinoma: A meta-analysis. Braz. J. Otorhinolaryngol. 2022, 88, 717–724. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Andrukhov, O.; Wang, T.; Song, S.; Yan, C.; Zhang, F. Meta-analysis of the prognostic value of the neutrophil-to-lymphocyte ratio in oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 353–358. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Ren, F.; Guo, R.; Zhang, P. Prognostic and clinicopathological significance of neutrophil-to-lymphocyte ratio in patients with oral cancer. Biosci. Rep. 2018, 38, BSR20181550. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, J.; Tang, D.; Zhou, L.; Chou, L.; Chou, K.Y.; Tao, L.; Lu, L.M. Neutrophil infiltration mediated by CXCL5 accumulation in the laryngeal squamous cell carcinoma microenvironment: A mechanism by which tumour cells escape immune surveillance. Clin. Immunol. 2017, 175, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Matlung, S.E.; Wilhelmina van Kempen, P.M.; Bovenschen, N.; van Baarle, D.; Willems, S.M. Differences in T-cell infiltrates and survival between HPV+ and HPV- oropharyngeal squamous cell carcinoma. Future Sci. OA 2016, 2, FSO88. [Google Scholar] [CrossRef]


| References | Year | Country | Sample Size | Clinical Stage | Median Age | Median Follow-Up | HPV Status |
|---|---|---|---|---|---|---|---|
| Ferrandino et al. [13] | 2020 | United States | 5169 | I, II, III, IV | 64 | 60 | All patients |
| So et al. [14] | 2018 | Republic of Korea | 104 | I, II, III, IV | 57 | 60 | Positive |
| De Felice et al. [15] | 2019 | Italy | 57 | I, II, III, IV | NA | 60 | Negative |
| Lin et al. [16] | 2019 | United States | 108 | I, II, III, IV | 56 | 37 | Negative |
| Kreinbrink et al. [17] | 2021 | United States | 201 | I, II, III, IV | 58 | 40 | All patients |
| Lin et al. [18] | 2018 | United States | 99 | I, II, III, IV | 54 | 69.6 | All patients |
| Cho et al. [19] | 2018 | Republic of Korea | 56 | I, II, III, IV | 60 | 39 | All patients |
| Brewczyński et al. [20] | 2021 | Poland | 127 | I, II, III, IV | 61 | 74.58 | Positive and negative |
| Staniewska et al. [21] | 2021 | Poland | 208 | I, II, III, IV | 59 | NA | All patients |
| Young et al. [22] | 2014 | United Kingdom | 249 | I, II, III, IV | 55 | 46 | All patients |
| Charles et al. [23] | 2016 | Australia | 67 | I, II, III, IV | 63 | 29 | All patients |
| Panje et al. [24] | 2017 | Switzerland | 187 | I, II, III, IV | 62 | 61.2 | All patients |
| Gorphe et al. [25] | 2018 | France | 167 | I, II, III | 59 | 32 | Positive |
| Ng et al. [26] | 2021 | United States | 848 | II, III, IV | 57 | 59 | All patients |
| References | Treatment Method | Covariant | Cut-Off Value of NLR | Method of Obtaining the Cut-Off Value | Survival Analysis | Analysis Method | NOS |
| Ferrandino et al. [13] | S, R, C, CR, S + CR, S + R | Top tertile NLR | 3.4 (OS) 3.7 (DFS) | Tertile | OS, DFS | M | 9 |
| So et al. [14] | S, R, CR, S + CR, S + R | High NLR | 2.42 | ROC | OS, DFS | M | 8 |
| De Felice et al. [15] | R, CR | High NLR | 4.7 | ROC | OS, DFS | M | 9 |
| Lin et al. [16] | S + R, R, CR | High NLR | NA | Median | OS | U | 8 |
| Kreinbrink et al. [17] | S + R, R, CR | High NLR | 3.0 | Median | OS, RFS | U | 8 |
| Lin et al. [18] | S + R, S + CR | High NLR | 3.8 | ROC | OS | U | 9 |
| Cho et al. [19] | R, CR | High NLR | 2.7 | ROC | OS, RFS | M/U | 9 |
| Brewczyński et al. [20] | R, CR | High NLR | 2.13 (OS) 2.29 (DFS) | ROC | OS, DFS | M/U | 9 |
| Staniewska et al. [21] | R, CR | High NLR | 2.009 | ROC | OS | M | 7 |
| Young et al. [22] | CR | High NLR | 5 | NA | LRC | M | 7 |
| Charles et al. [23] | S + R, S + CR, R, CR | High NLR | 5 | Review of literature | OS, RFS | M | 8 |
| Panje et al. [24] | R, CR | Top quartile NLR | 4.68 | Quartile | OS, RFS | M | 9 |
| Gorphe et al. [25] | CR | High NLR | 5 | Review of literature | OS, RFS | M | 7 |
| Ng et al. [26] | R, CR | High NLR | 3.0 | Median | OS, RFS, LRC | M | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, J.P.; Sánchez-Canteli, M.; Triantafyllou, A.; de Bree, R.; Mäkitie, A.A.; Franchi, A.; Hellquist, H.; Saba, N.F.; Stenman, G.; Takes, R.P.; et al. Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 802. https://doi.org/10.3390/cancers15030802
Rodrigo JP, Sánchez-Canteli M, Triantafyllou A, de Bree R, Mäkitie AA, Franchi A, Hellquist H, Saba NF, Stenman G, Takes RP, et al. Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(3):802. https://doi.org/10.3390/cancers15030802
Chicago/Turabian StyleRodrigo, Juan P., Mario Sánchez-Canteli, Asterios Triantafyllou, Remco de Bree, Antti A. Mäkitie, Alessandro Franchi, Henrik Hellquist, Nabil F. Saba, Göran Stenman, Robert P. Takes, and et al. 2023. "Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis" Cancers 15, no. 3: 802. https://doi.org/10.3390/cancers15030802
APA StyleRodrigo, J. P., Sánchez-Canteli, M., Triantafyllou, A., de Bree, R., Mäkitie, A. A., Franchi, A., Hellquist, H., Saba, N. F., Stenman, G., Takes, R. P., Valero, C., Zidar, N., & Ferlito, A. (2023). Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 15(3), 802. https://doi.org/10.3390/cancers15030802












