Expression Pattern and Prognostic Value of CTLA-4, CD86, and Tumor-Infiltrating Lymphocytes in Rectal Cancer after Neoadjuvant Chemo(radio)therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
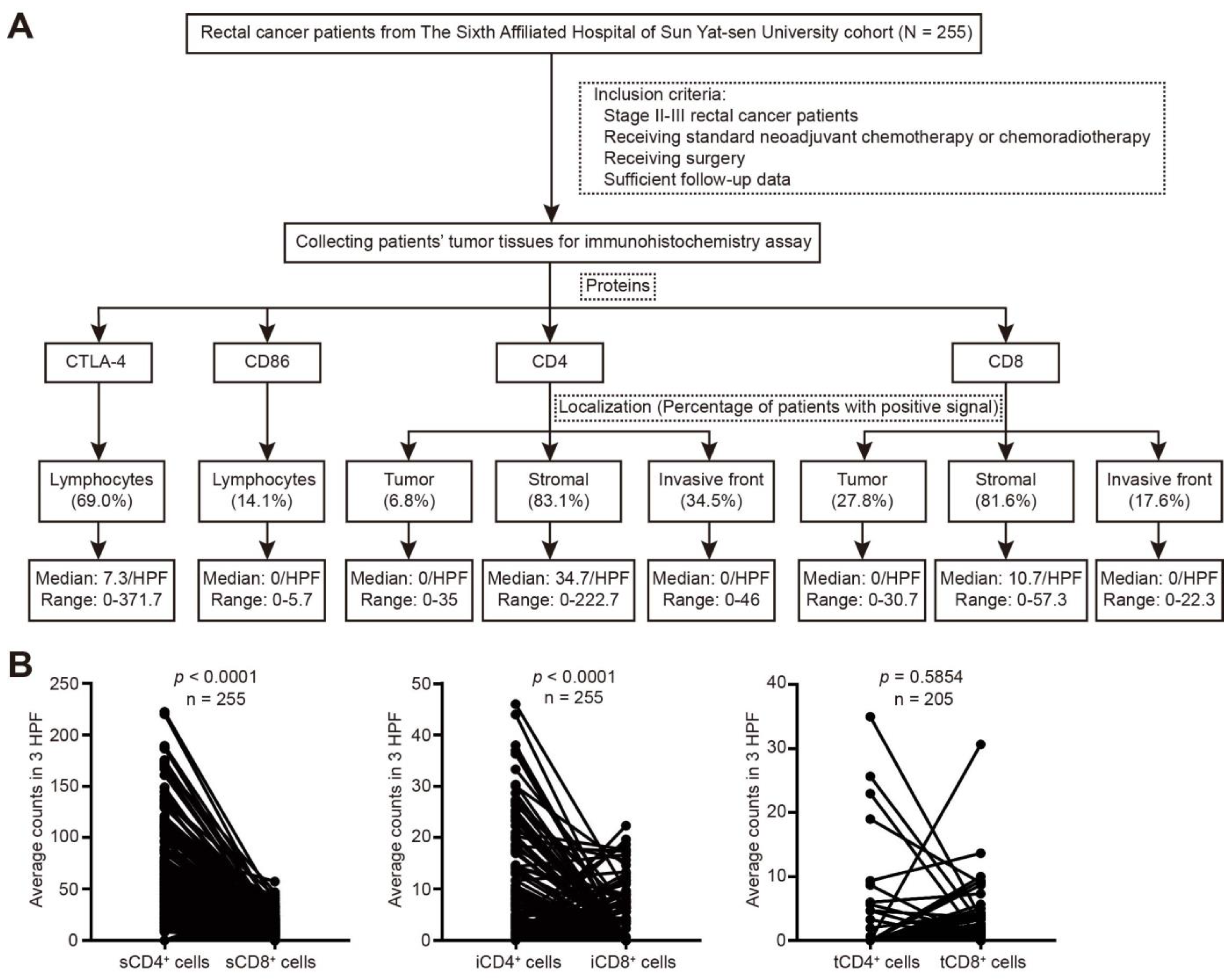
2.1. Patients and Treatment
2.2. Grading Standard and Evaluation Method for TRG
2.3. Immunohistochemistry (IHC)
2.4. Quantification of the Expression of Immune-Related Molecules
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
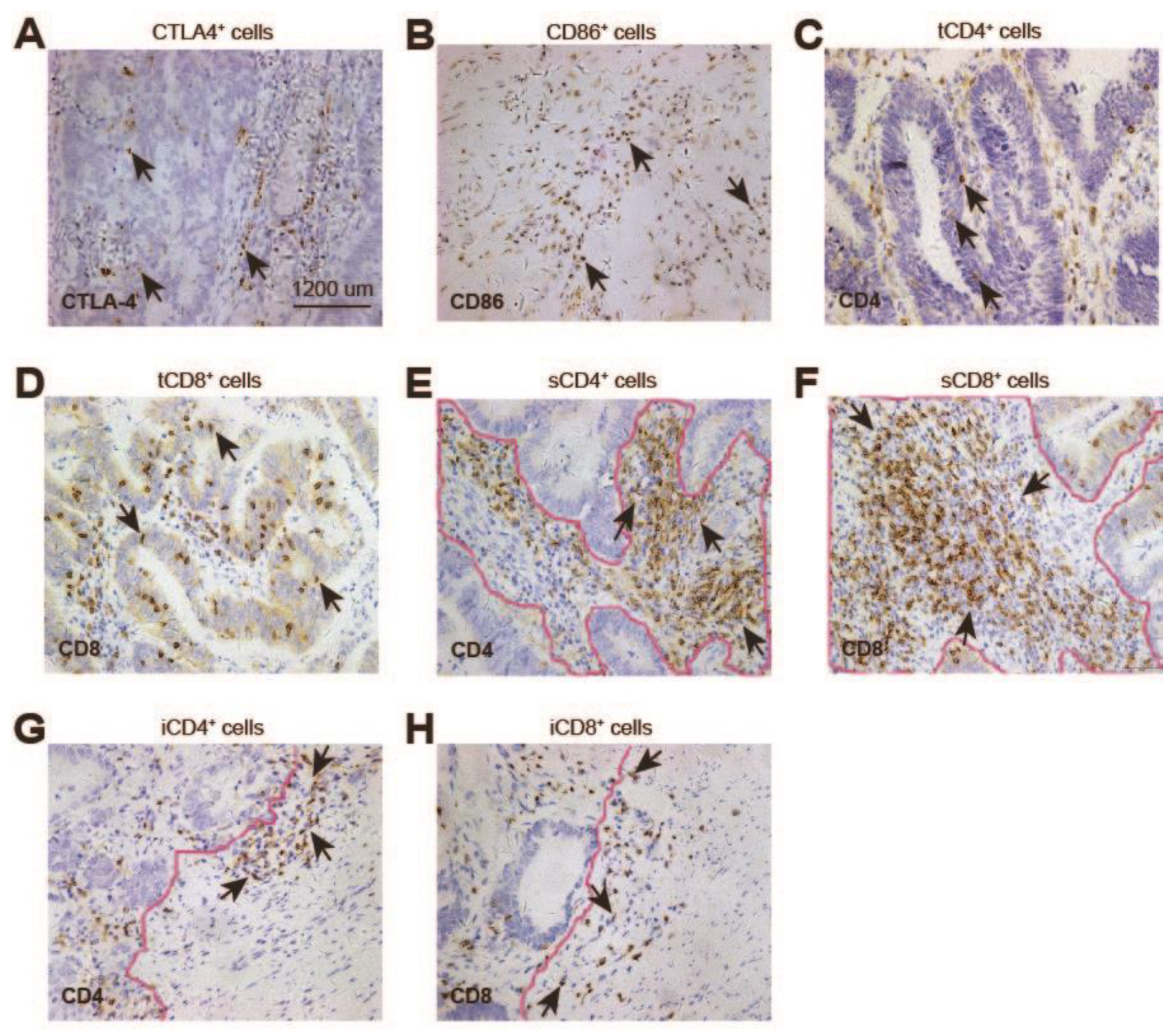
3.2. Expression of CTLA-4, CD86, CD4, and CD8 in Rectal Cancer after nCRT
3.3. Association between Expression of Immune-Related Molecules and Clinicopathologic Characteristics
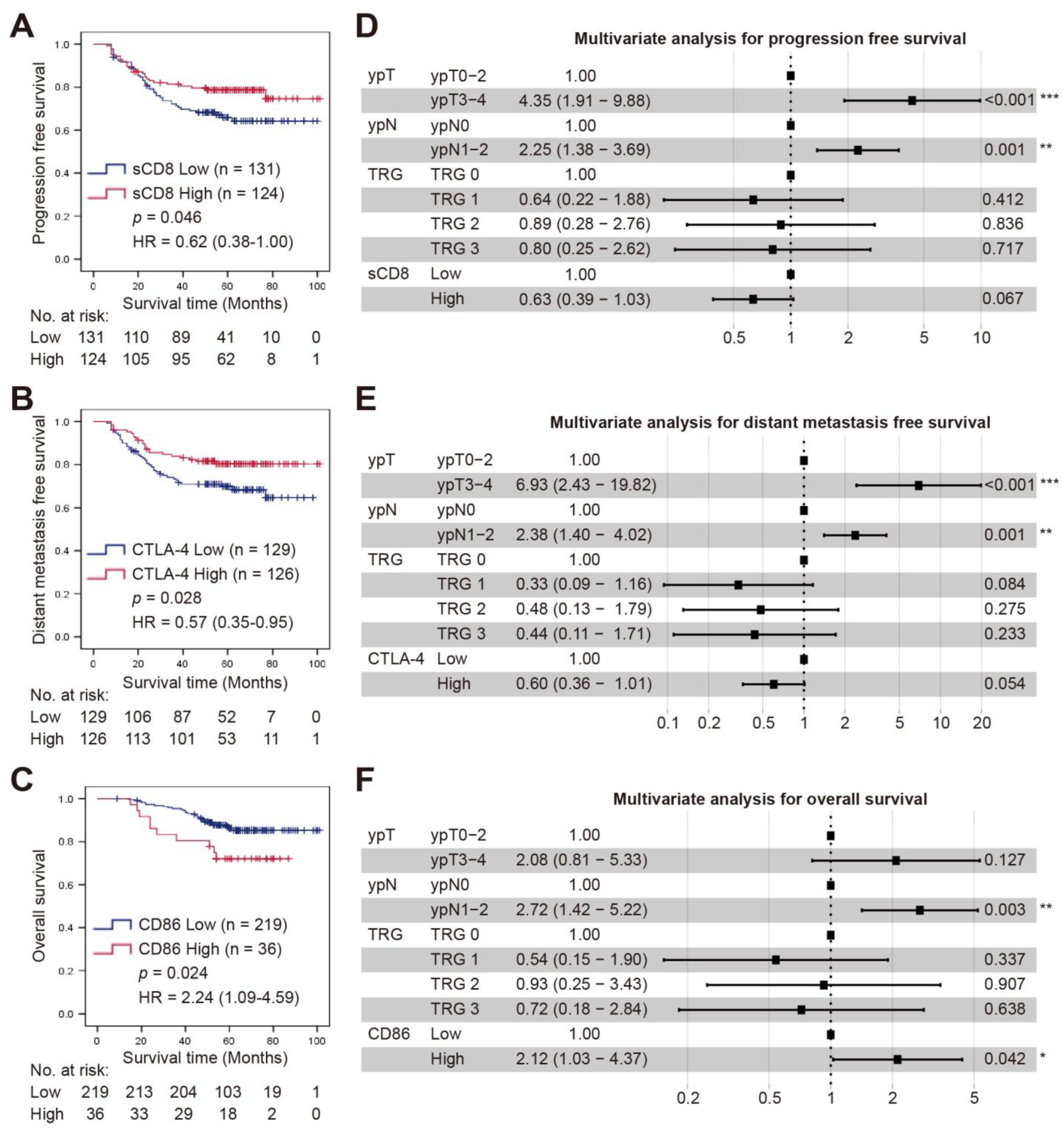
3.4. Survival Analysis
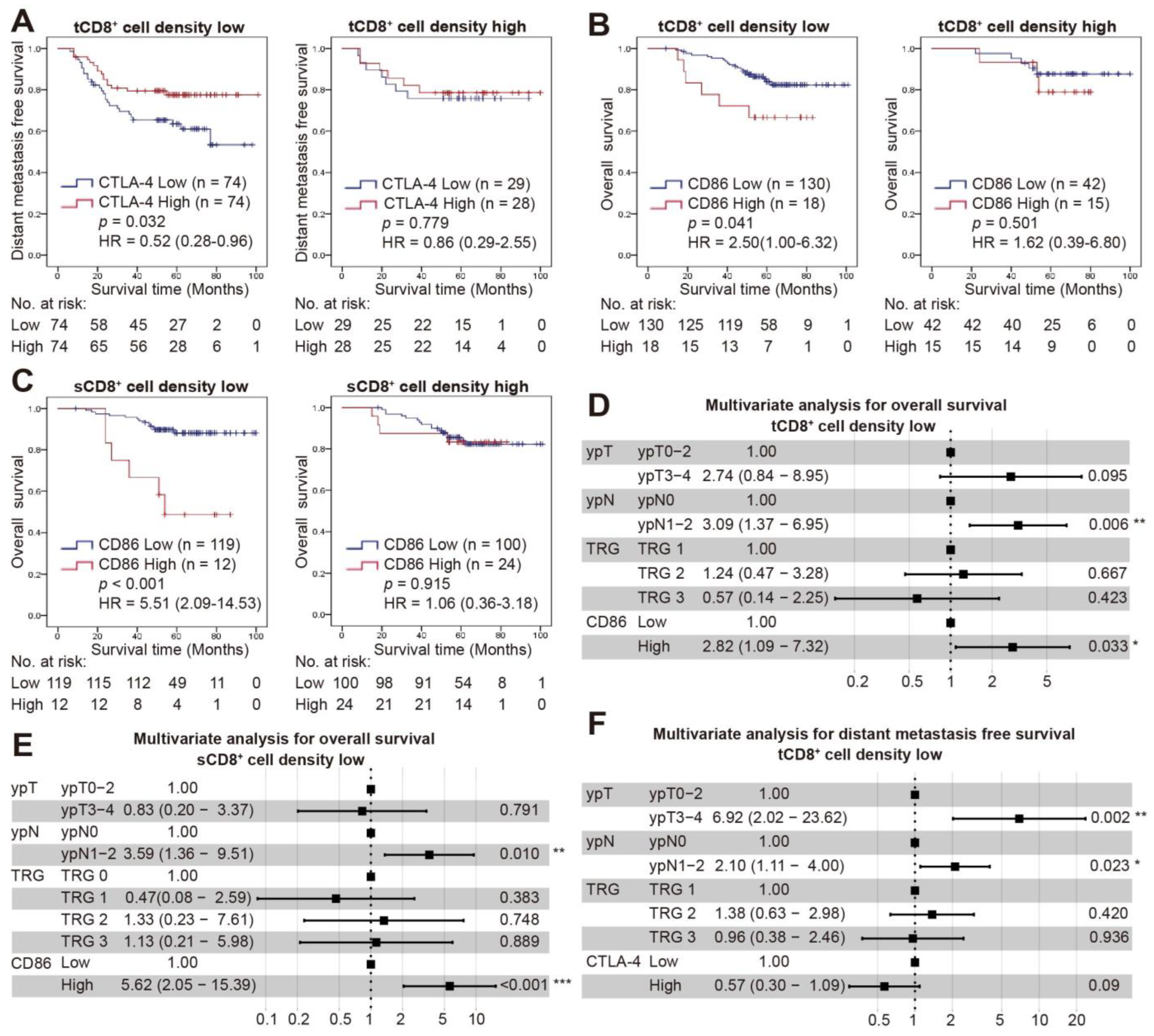
3.5. Combined Status of CD8+ Cell Density and CTLA-4/CD86 Expression and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar] [PubMed]
- Huyghe, N.; Baldin, P.; Van den Eynde, M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: What is the future beyond deficient mismatch-repair tumours? Gastroenterol. Rep. 2020, 8, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Hecht, M.; Büttner-Herold, M.; Erlenbach-Wünsch, K.; Haderlein, M.; Croner, R.; Grützmann, R.; Hartmann, A.; Fietkau, R.; Distel, L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer 2016, 65, 52–60. [Google Scholar] [CrossRef]
- Chiang, S.F.; Huang, C.Y.; Ke, T.W.; Chen, T.W.; Lan, Y.C.; You, Y.S.; Chen, W.T.; Chao, K.S.C. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol. Immunother. CII 2019, 68, 283–296. [Google Scholar] [CrossRef]
- Huemer, F.; Klieser, E.; Neureiter, D.; Schlintl, V.; Rinnerthaler, G.; Pagès, F.; Kirilovsky, A.; El Sissy, C.; Iglseder, W.; Singhartinger, F.; et al. Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. J. Clin. Med. 2020, 9, 2775. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Akiyoshi, T.; Yamamoto, N.; Kawachi, H.; Ishikawa, Y.; Mori, S.; Oba, K.; Nagino, M.; Fukunaga, Y.; Ueno, M. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur. J. Cancer 2018, 91, 11–20. [Google Scholar] [CrossRef]
- Teng, F.; Meng, X.; Kong, L.; Mu, D.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl. Res. J. Lab. Clin. Med. 2015, 166, 721–732.e721. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Inselmann, S.; Saussele, S.; Dietz, C.T.; Mu Ller, M.C.; Eigendorff, E.; Brendel, C.A.; Metzelder, S.K.; Bru Mmendorf, T.H.; Waller, C.; et al. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia 2017, 31, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Pentcheva-Hoang, T.; Egen, J.G.; Wojnoonski, K.; Allison, J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 2004, 21, 401–413. [Google Scholar] [CrossRef]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef]
- Sansom, D.M.; Manzotti, C.N.; Zheng, Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003, 24, 314–319. [Google Scholar] [CrossRef]
- Wan, X.B.; Zhang, Q.; Chen, M.; Liu, Y.; Zheng, J.; Lan, P.; He, F. Prognostic Value of Interval Between the Initiation of Neoadjuvant Treatment to Surgery for Patients With Locally Advanced Rectal Cancer Following Neoadjuvant Chemotherapy, Radiotherapy and Definitive Surgery. Front. Oncol. 2020, 10, 1280. [Google Scholar] [CrossRef]
- Trakarnsanga, A.; Gönen, M.; Shia, J.; Nash, G.M.; Temple, L.K.; Guillem, J.G.; Paty, P.B.; Goodman, K.A.; Wu, A.; Gollub, M.; et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J. Natl. Cancer Inst. 2014, 106, dju248. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef] [PubMed]
- Sędek, Ł.; Theunissen, P.; Sobral da Costa, E.; van der Sluijs-Gelling, A.; Mejstrikova, E.; Gaipa, G.; Sonsala, A.; Twardoch, M.; Oliveira, E.; Novakova, M.; et al. Differential expression of CD73, CD86 and CD304 in normal vs. leukemic B-cell precursors and their utility as stable minimal residual disease markers in childhood B-cell precursor acute lymphoblastic leukemia. J. Immunol. Methods 2019, 475, 112429. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Kabani, K.; Favaloro, J.; Yang, S.; Ho, P.J.; Gibson, J.; Fromm, P.; Suen, H.; Woodland, N.; Nassif, N.; et al. CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood 2012, 120, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, J.P.; Haruki, K.; Lau, M.C.; Väyrynen, S.A.; Zhong, R.; Dias Costa, A.; Borowsky, J.; Zhao, M.; Fujiyoshi, K.; Arima, K.; et al. The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol. Res. 2021, 9, 8–19. [Google Scholar] [CrossRef]
- Chang, C.S.; Chang, J.H.; Hsu, N.C.; Lin, H.Y.; Chung, C.Y. Expression of CD80 and CD86 costimulatory molecules are potential markers for better survival in nasopharyngeal carcinoma. BMC Cancer 2007, 7, 88. [Google Scholar] [CrossRef]
- Sato, T.; Takagi, K.; Higuchi, M.; Abe, H.; Kojimahara, M.; Sagawa, M.; Tanaki, M.; Miki, Y.; Suzuki, T.; Hojo, H. Immunolocalization of CD80 and CD86 in Non-Small Cell Lung Carcinoma: CD80 as a Potent Prognostic Factor. Acta Histochem. Cytochem. 2022, 55, 25–35. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Sun, Y.; Dou, X.; Hu, X.; Zou, W.; Sun, Y.; Hu, Q.; Yue, J. Neoadjuvant Chemoradiotherapy Changes the Landscape of Soluble Immune Checkpoint Molecules in Patients With Locally Advanced Rectal Cancer. Front. Oncol. 2022, 12, 756811. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Kelly, R.J.; Zaidi, A.H.; Smith, M.A.; Omstead, A.N.; Kosovec, J.E.; Matsui, D.; Martin, S.A.; DiCarlo, C.; Werts, E.D.; Silverman, J.F.; et al. The Dynamic and Transient Immune Microenvironment in Locally Advanced Esophageal Adenocarcinoma Post Chemoradiation. Ann. Surg. 2018, 268, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- van den Ende, T.; van den Boorn, H.G.; Hoonhout, N.M.; van Etten-Jamaludin, F.S.; Meijer, S.L.; Derks, S.; de Gruijl, T.D.; Bijlsma, M.F.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Priming the tumor immune microenvironment with chemo(radio)therapy: A systematic review across tumor types. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, X.; Chen, H.; Hu, Y.; Zhang, H.; Wu, L.; Yang, Y.; Mao, B.; Wang, H. The mutational pattern of homologous recombination-related (HRR) genes in Chinese colon cancer and its relevance to immunotherapy responses. Aging 2020, 13, 2365–2378. [Google Scholar] [CrossRef]
- Matsutani, S.; Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Nakao, S.; Hirakawa, K.; Ohira, M. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018, 109, 966–979. [Google Scholar] [CrossRef]
- Richards, C.H.; Roxburgh, C.S.; Powell, A.G.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. The clinical utility of the local inflammatory response in colorectal cancer. Eur. J. Cancer 2014, 50, 309–319. [Google Scholar] [CrossRef]
- Salvi, S.; Fontana, V.; Boccardo, S.; Merlo, D.F.; Margallo, E.; Laurent, S.; Morabito, A.; Rijavec, E.; Dal Bello, M.G.; Mora, M.; et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol. Immunother. CII 2012, 61, 1463–1472. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, K.; Chie, E.K.; Kim, S.; Lee, K.B.; Jang, J.Y.; Kim, S.W.; Oh, D.Y.; Bang, Y.J. Clinical Implications of Cytotoxic T Lymphocyte Antigen-4 Expression on Tumor Cells and Tumor-Infiltrating Lymphocytes in Extrahepatic Bile Duct Cancer Patients Undergoing Surgery Plus Adjuvant Chemoradiotherapy. Target. Oncol. 2017, 12, 211–218. [Google Scholar] [CrossRef]
- Winograd, R.; Byrne, K.T.; Evans, R.A.; Odorizzi, P.M.; Meyer, A.R.; Bajor, D.L.; Clendenin, C.; Stanger, B.Z.; Furth, E.E.; Wherry, E.J.; et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol. Res. 2015, 3, 399–411. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Patients |
|---|---|
| Gender | |
| Male | 178 (69.8) |
| Female | 77 (30.2) |
| Age | |
| ≤55 | 128 (50.2) |
| >55 | 127 (49.8) |
| cT stage | |
| cT3 | 203 (79.6) |
| cT4 | 52 (20.4) |
| cN stage | |
| cN0 | 57 (22.4) |
| cN1-2 | 198 (77.6) |
| Therapy method | |
| nCRT | 149 (58.4) |
| nCT | 106 (41.6) |
| ypT | |
| ypT0-2 | 124 (48.6) |
| ypT3-4 | 131 (51.4) |
| ypN | |
| ypN0 | 201 (78.8) |
| ypN1- | 54 (21.2) |
| Tumor Regression Grade | |
| TRG 0 | 50 (19.6) |
| TRG 1 | 81 (31.8) |
| TRG 2 | 82 (32.2) |
| TRG 3 | 42 (16.5) |
| Variables | Therapy Method | p | Tumor Regression Grade | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| nCT | nCRT | TRG 0 | TRG 1 | TRG 2 | TRG 3 | ||||
| CTLA-4+ cells | Low | 54 (50.9) | 75 (50.3) | 0.924 | 26 (52.0) | 45 (55.6) | 42 (51.2) | 16 (38.1) | 0.324 |
| High | 52 (49.1) | 74 (49.7) | 24 (48.0) | 36 (44.4) | 40 (48.8) | 26 (61.9) | |||
| CD86+ cells | Low | 85 (80.2) | 134 (89.9) | 0.028 * | 47 (94.0) | 72 (88.9) | 67 (81.7) | 33 (78.6) | 0.096 |
| High | 21 (19.8) | 15 (10.1) | 3 (6.0) | 9 (11.1) | 15 (18.3) | 15 (21.4) | |||
| tCD4+ cells | Low | 85 (88.5) | 106 (97.2) | 0.014 * | 0 | 79 (97.5) | 77 (93.9) | 35 (83.3) | 0.012 * |
| High | 11 (11.5) | 3 (2.8) | 0 | 2 (2.5) | 5 (6.1) | 7 (16.7) | |||
| NA | 10 | 40 | 50 | 0 | 0 | 0 | |||
| sCD4+ cells | Low | 41 (38.7) | 85 (57.0) | 0.004 * | 31 (62.0) | 45 (55.6) | 37 (45.1) | 13 (31.0) | 0.013 * |
| High | 65 (61.3) | 64 (43.0) | 19 (38.0) | 36 (44.4) | 45 (54.9) | 29 (69.0) | |||
| iCD4+ cells | Low | 52 (49.1) | 115 (77.2) | 0.000 * | 44 (88.0) | 61 (75.3) | 44 (53.7) | 18 (42.9) | 0.000 * |
| High | 54 (50.9) | 34 (22.8) | 6 (12.0) | 20 (24.7) | 38 (46.3) | 24 (57.1) | |||
| tCD8+ cells | Low | 58 (60.4) | 90 (82.6) | 0.000 * | 0 | 64 (79.0) | 58 (70.7) | 26 (61.9) | 0.124 |
| High | 38 (39.6) | 19 (17.4) | 0 | 17 (21.0) | 24 (29.3) | 16 (38.1) | |||
| NA | 10 | 40 | 50 | 0 | 0 | 0 | |||
| sCD8+ cells | Low | 51 (48.1) | 80 (53.7) | 0.380 | 33 (66.0) | 40 (49.4) | 35 (42.7) | 23 (54.8) | 0.069 |
| High | 55 (51.9) | 69 (46.3) | 17 (34.0) | 41 (50.6) | 47 (57.3) | 19 (45.2) | |||
| iCD8+ cells | Low | 79 (74.5) | 131 (87.9) | 0.006 * | 50 (100.0) | 67 (82.7) | 61 (74.4) | 32 (76.2) | 0.002 * |
| High | 27 (25.5) | 18 (12.1) | 0 (0.0) | 14 (17.3) | 21 (25.6) | 10 (23.8) | |||
| TRG | TRG 0 | 10 (9.4) | 40 (26.8) | 0.000 * | - | - | - | - | - |
| TRG 1 | 24 (22.6) | 57 (38.3) | - | - | - | - | |||
| TRG 2 | 35 (33.0) | 47 (31.5) | - | - | - | - | |||
| TRG 3 | 37 (34.9) | 5 (3.4) | - | - | - | - | |||
| Variables | CTLA-4 Expression | p | CD86 Expression | p | |||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| tCD4+ cells | Low | 98 (95.1) | 93 (91.2) | 0.260 | 162 (94.2) | 29 (87.9) | 0.188 |
| High | 5 (4.9) | 9 (8.8) | 10 (5.8) | 4 (12.1) | |||
| sCD4+ cells | Low | 66 (51.2) | 60 (47.6) | 0.571 | 109 (49.8) | 17 (47.2) | 0.777 |
| High | 63 (48.8) | 66 (52.4) | 110 (50.2) | 19 (52.8) | |||
| iCD4+ cells | Low | 90 (69.8) | 77 (61.1) | 0.146 | 146 (66.7) | 21 (58.3) | 0.330 |
| High | 39 (30.2) | 49 (38.9) | 73 (33.3) | 15 (41.7) | |||
| tCD8+ cells | Low | 84 (81.6) | 64 (62.7) | 0.003 * | 130 (75.6) | 18 (54.5) | 0.013 * |
| High | 19 (18.4) | 38 (37.3) | 42 (24.4) | 15 (45.5) | |||
| sCD8+ cells | Low | 68 (52.7) | 63 (50.0) | 0.665 | 119 (54.3) | 12 (33.3) | 0.019 * |
| High | 61 (47.3) | 63 (50.0) | 100 (45.7) | 24 (66.7) | |||
| iCD8+ cells | Low | 108 (83.7) | 102 (81.0) | 0.562 | 184 (84.0) | 26 (72.2) | 0.085 |
| High | 21 (16.3) | 24 (19.0) | 35 (16.0) | 10 (27.8) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.-K.; Wang, C.; Feng, L.-L.; Bai, S.-M.; Feng, W.-X.; Ouyang, N.-T.; Chu, Z.-H.; Fan, X.-J.; Qin, Q.-Y. Expression Pattern and Prognostic Value of CTLA-4, CD86, and Tumor-Infiltrating Lymphocytes in Rectal Cancer after Neoadjuvant Chemo(radio)therapy. Cancers 2022, 14, 5573. https://doi.org/10.3390/cancers14225573
Yin X-K, Wang C, Feng L-L, Bai S-M, Feng W-X, Ouyang N-T, Chu Z-H, Fan X-J, Qin Q-Y. Expression Pattern and Prognostic Value of CTLA-4, CD86, and Tumor-Infiltrating Lymphocytes in Rectal Cancer after Neoadjuvant Chemo(radio)therapy. Cancers. 2022; 14(22):5573. https://doi.org/10.3390/cancers14225573
Chicago/Turabian StyleYin, Xin-Ke, Chao Wang, Li-Li Feng, Shao-Mei Bai, Wei-Xing Feng, Neng-Tai Ouyang, Zhong-Hua Chu, Xin-Juan Fan, and Qi-Yuan Qin. 2022. "Expression Pattern and Prognostic Value of CTLA-4, CD86, and Tumor-Infiltrating Lymphocytes in Rectal Cancer after Neoadjuvant Chemo(radio)therapy" Cancers 14, no. 22: 5573. https://doi.org/10.3390/cancers14225573
APA StyleYin, X.-K., Wang, C., Feng, L.-L., Bai, S.-M., Feng, W.-X., Ouyang, N.-T., Chu, Z.-H., Fan, X.-J., & Qin, Q.-Y. (2022). Expression Pattern and Prognostic Value of CTLA-4, CD86, and Tumor-Infiltrating Lymphocytes in Rectal Cancer after Neoadjuvant Chemo(radio)therapy. Cancers, 14(22), 5573. https://doi.org/10.3390/cancers14225573







