Features on Endoscopy and MRI after Treatment with Contact X-ray Brachytherapy for Rectal Cancer: Explorative Results
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Contact X-ray Brachytherapy
2.2. Follow-Up
2.3. Evaluation of Endoscopy and MRI
2.4. Standard of Reference
2.5. Statistical Analysis

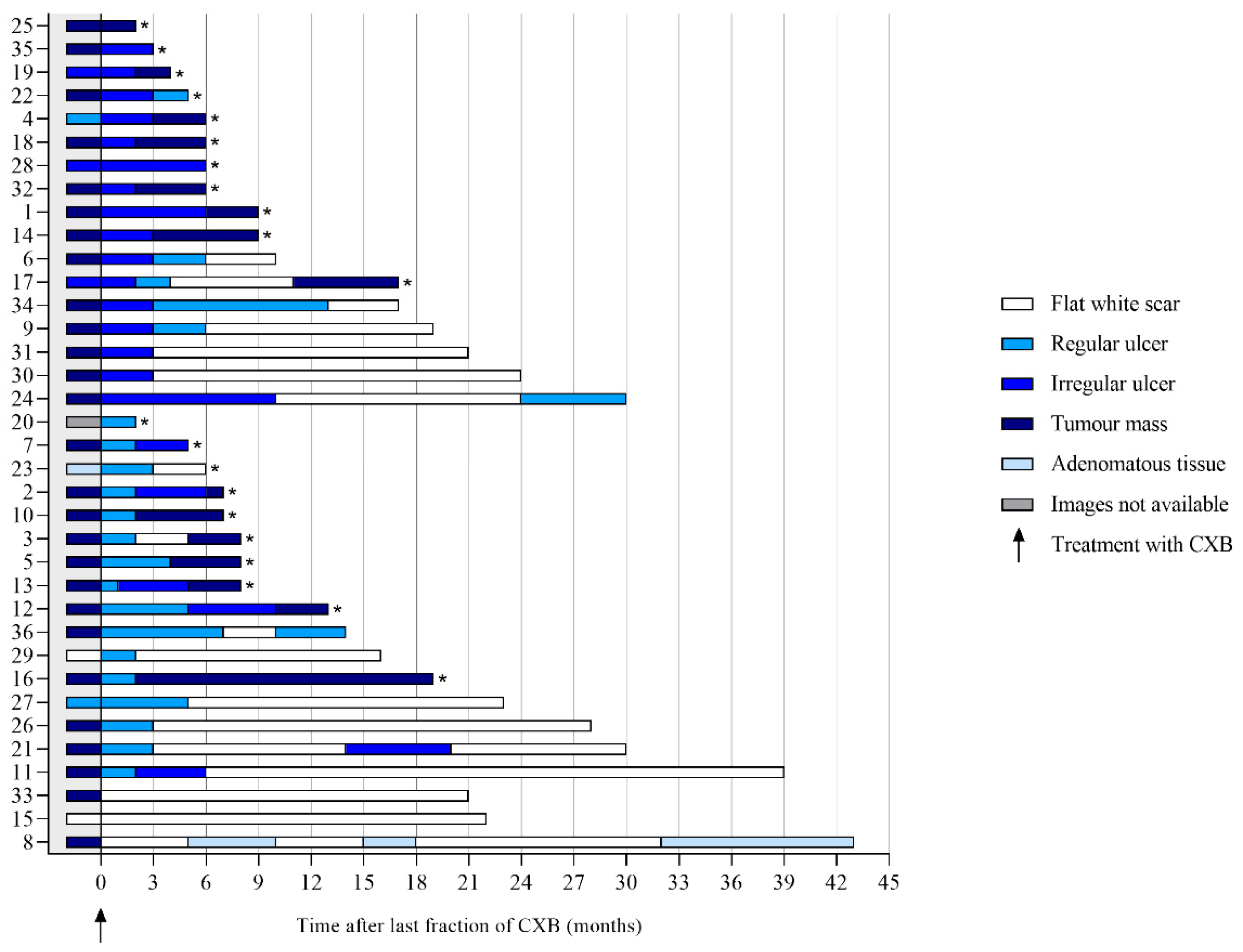
3. Results
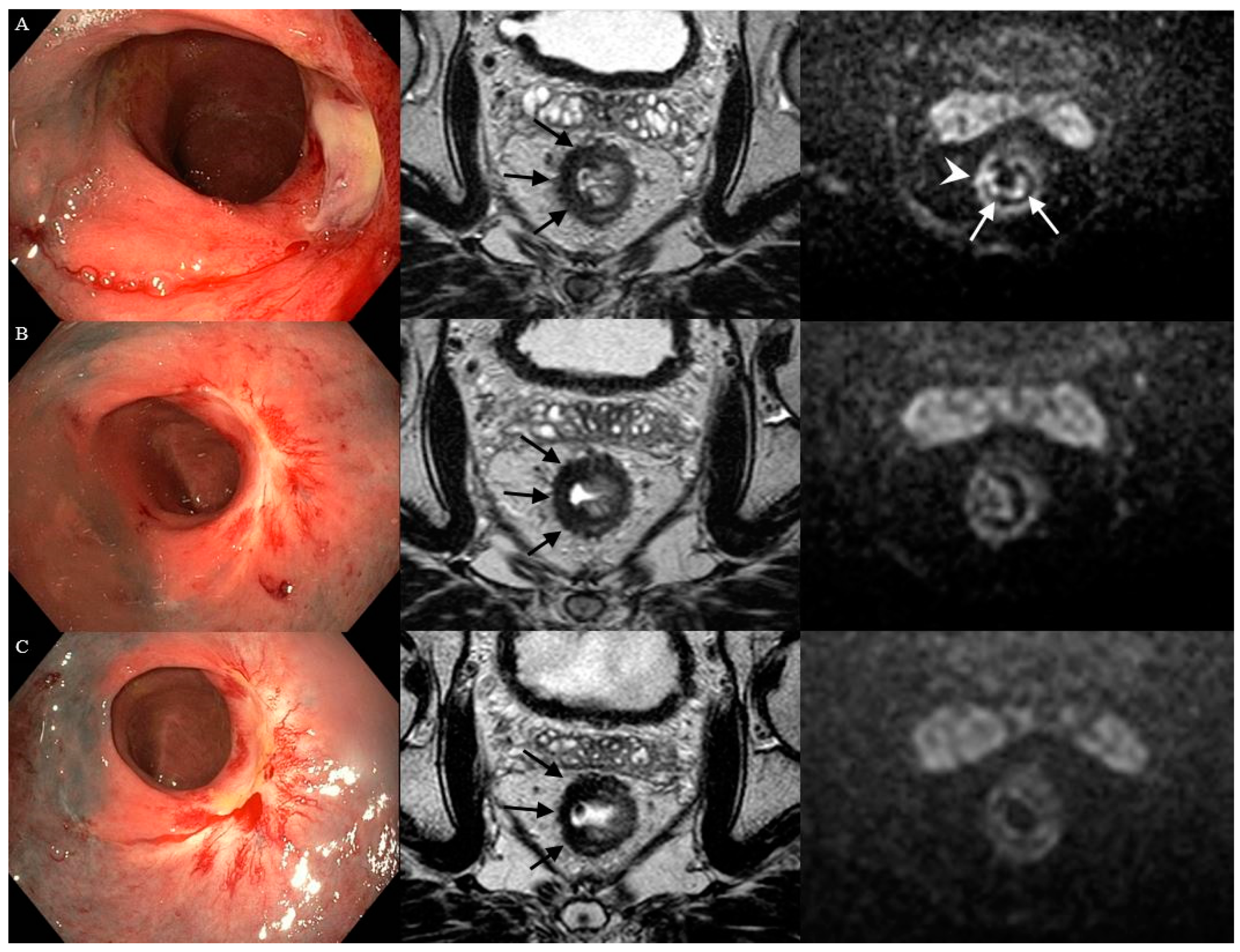
3.1. Features on Endoscopy
3.2. Features on T2W-MRI
3.3. Features on DWI
3.4. Diagnostic Performance of Endoscopy and MRI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717; discussion 717–718. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Beets-Tan, R.G.; Lambregts, D.M.; Lammering, G.; Nelemans, P.J.; Engelen, S.M.; van Dam, R.M.; Jansen, R.L.; Sosef, M.; Leijtens, J.W.; et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 2011, 29, 4633–4640. [Google Scholar] [CrossRef] [PubMed]
- Dhadda, A.S.; Martin, A.; Killeen, S.; Hunter, I.A. Organ Preservation Using Contact Radiotherapy for Early Rectal Cancer: Outcomes of Patients Treated at a Single Centre in the UK. Clin. Oncol. 2017, 29, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Sun Myint, A.; Smith, F.M.; Gollins, S.; Wong, H.; Rao, C.; Whitmarsh, K.; Sripadam, R.; Rooney, P.; Hershman, M.; Pritchard, D.M. Dose Escalation Using Contact X-ray Brachytherapy After External Beam Radiotherapy as Nonsurgical Treatment Option for Rectal Cancer: Outcomes From a Single-Center Experience. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 565–573. [Google Scholar] [CrossRef]
- Gérard, J.P.; Barbet, N.; Gal, J.; Dejean, C.; Evesque, L.; Doyen, J.; Coquard, R.; Gugenheim, J.; Benizri, E.; Schiappa, R.; et al. Planned organ preservation for early T2-3 rectal adenocarcinoma: A French, multicentre study. Eur. J. Cancer 2019, 108, 1–16. [Google Scholar] [CrossRef]
- Custers, P.A.; Geubels, B.M.; Huibregtse, I.L.; Peters, F.P.; Engelhardt, E.G.; Beets, G.L.; Marijnen, C.A.M.; van Leerdam, M.E.; van Triest, B. Contact X-ray Brachytherapy for Older or Inoperable Rectal Cancer Patients: Short-Term Oncological and Functional Follow-Up. Cancers 2021, 13, 6333. [Google Scholar] [CrossRef]
- Ortholan, C.; Romestaing, P.; Chapet, O.; Gerard, J.P. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e165–e171. [Google Scholar] [CrossRef]
- Appelt, A.L.; Ploen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef]
- Dale, R.G. The radiobiology of Papillon-type treatments. Clin. Oncol. 2007, 19, 649–654. [Google Scholar] [CrossRef]
- Maas, M.; Lambregts, D.M.; Nelemans, P.J.; Heijnen, L.A.; Martens, M.H.; Leijtens, J.W.; Sosef, M.; Hulsewe, K.W.; Hoff, C.; Breukink, S.O.; et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann. Surg. Oncol. 2015, 22, 3873–3880. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Wynn, G.; Marks, J.; Kessler, H.; Gama-Rodrigues, J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 2010, 53, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.J.; Delli Pizzi, A.; Lahaye, M.J.; van Griethuysen, J.J.M.; Maas, M.; Beets, G.L.; Bakers, F.C.H.; Beets-Tan, R.G.H. A Pattern-Based Approach Combining Tumor Morphology on MRI With Distinct Signal Patterns on Diffusion-Weighted Imaging to Assess Response of Rectal Tumors After Chemoradiotherapy. Dis. Colon Rectum 2018, 61, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.J.; Boellaard, T.N.; Beets-Tan, R.G.H. Response evaluation after neoadjuvant treatment for rectal cancer using modern MR imaging: A pictorial review. Insights Into Imaging 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- van der Sande, M.E.; Maas, M.; Melenhorst, J.; Breukink, S.O.; van Leerdam, M.E.; Beets, G.L. Predictive Value of Endoscopic Features for a Complete Response After Chemoradiotherapy for Rectal Cancer. Ann. Surg. 2019, 274, e541–e547. [Google Scholar] [CrossRef]
- Dunstan, M.J.D.; Rockall, T.A.; Potter, K.; Stewart, A.J. Radiological and clinical findings following rectal contact X-ray brachytherapy (Papillon technique)-how to assess response. J. Contemp. Brachytherapy 2018, 10, 179–189. [Google Scholar] [CrossRef]
- Haak, H.E.; Maas, M.; Lahaye, M.J.; Boellaard, T.N.; Delli Pizzi, A.; Mihl, C.; van der Zee, D.; Fabris, C.; van der Sande, M.E.; Melenhorst, J.; et al. Selection of Patients for Organ Preservation After Chemoradiotherapy: MRI Identifies Poor Responders Who Can Go Straight to Surgery. Ann. Surg. Oncol. 2020, 27, 2732–2739. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef]
- Santiago, I.; Barata, M.; Figueiredo, N.; Pares, O.; Henriques, V.; Galzerano, A.; Carvalho, C.; Matos, C.; Heald, R.J. The split scar sign as an indicator of sustained complete response after neoadjuvant therapy in rectal cancer. Eur. Radiol. 2020, 30, 224–238. [Google Scholar] [CrossRef]
- Rijkmans, E.C.; van Triest, B.; Nout, R.A.; Kerkhof, E.M.; Buijsen, J.; Rozema, T.; Franssen, J.H.; Velema, L.A.; Laman, M.S.; Cats, A.; et al. Evaluation of clinical and endoscopic toxicity after external beam radiotherapy and endorectal brachytherapy in elderly patients with rectal cancer treated in the HERBERT study. Radiother. Oncol. 2018, 126, 417–423. [Google Scholar] [CrossRef]
- Hupkens, B.J.P.; Maas, M.; Martens, M.H.; Deserno, W.; Leijtens, J.W.A.; Nelemans, P.J.; Bakers, F.C.H.; Lambregts, D.M.J.; Beets, G.L.; Beets-Tan, R.G.H. MRI surveillance for the detection of local recurrence in rectal cancer after transanal endoscopic microsurgery. Eur. Radiol. 2017, 27, 4960–4969. [Google Scholar] [CrossRef]
- Gerard, J.P.; Myint, A.S.; Barbet, N.; Dejean, C.; Thamphya, B.; Gal, J.; Montagne, L.; Vuong, T. Targeted Radiotherapy Using Contact X-ray Brachytherapy 50 kV. Cancers 2022, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.-P.; Barbet, N.N.; Pacé-Loscos, T.; Magné, N.; Serrand, J.; Mineur, L.; Deberne, M.; Zilli, T.; Dhadda, A.S.; Myint, A.S. Contact x-ray brachytherapy (Papillon) in addition to chemoradiotherapy to improve organ preservation in early cT2-T3 rectal adenocarcinoma: The 3-year results of OPERA randomized trial (NCT02505750). J. Clin. Oncol. 2022, 40, 3512. [Google Scholar] [CrossRef]
- Marcu, L.G. The first Rs of radiotherapy: Or standing on the shoulders of giants. Australas. Phys. Eng. Sci. Med. 2015, 38, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, D.A.; Geijsen, D.E.; van Leersum, N.J.; Punt, C.J.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J.; Dutch Surgical Colorectal, A. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. J. Br. Surg. 2013, 100, 933–939. [Google Scholar] [CrossRef]
- Petrelli, F.; Sgroi, G.; Sarti, E.; Barni, S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann. Surg. 2016, 263, 458–464. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Sao Juliao, G.P.; Fernandez, L.M.; Vailati, B.B.; Andrade, A.; Araujo, S.E.A.; Gama-Rodrigues, J.; Perez, R.O. Achieving a Complete Clinical Response After Neoadjuvant Chemoradiation That Does Not Require Surgical Resection: It May Take Longer Than You Think! Dis. Colon Rectum 2019, 62, 802–808. [Google Scholar] [CrossRef]
- Stewart, A.J.; Van Limbergen, E.J.; Gerard, J.P.; Appelt, A.L.; Verhaegen, F.; Berbee, M.; Vuong, T.; Brooker, C.; Rockall, T.; Sun Myint, A. GEC ESTRO ACROP consensus recommendations for contact brachytherapy for rectal cancer. Clin. Transl. Radiat. Oncol. 2022, 33, 15–22. [Google Scholar] [CrossRef]



| Characteristics | Total Cohort (n = 36) | Patients with a cCR (n = 15) | Patients with Residual Tumor (n = 21) | |||
|---|---|---|---|---|---|---|
| Median age (years) (range) | 66 | 38–86 | 67 | 54–86 | 66 | 38–79 |
| Sex (n, %) | ||||||
| Male | 21 | 58 | 9 | 60 | 12 | 57 |
| Female | 15 | 42 | 6 | 40 | 9 | 43 |
| Clinical tumor stage (n, %) | ||||||
| cT2 | 9 | 25 | 6 | 40 | 3 | 14 |
| cT3 | 26 | 72 | 8 | 53 | 18 | 86 |
| cT4 | 1 | 3 | 1 | 7 | 0 | 0 |
| Clinical nodal stage (n, %) | ||||||
| cN0 | 20 | 56 | 9 | 60 | 11 | 52 |
| cN1 | 12 | 33 | 6 | 40 | 6 | 29 |
| cN2 | 4 | 11 | 0 | 0 | 4 | 19 |
| Neoadjuvant radiotherapy (n, %) | ||||||
| Short-course radiotherapy | 7 | 19 | 7 | 47 | 0 | 0 |
| (Chemo)radiotherapy | 29 | 81 | 8 | 53 | 21 | 100 |
| Clinical nodal stage prior to CXB (n, %) | ||||||
| ycN0 | 34 | 94 | 14 | 93 | 20 | 95 |
| ycN1 | 2 | 6 | 1 | 7 | 1 | 4 |
| Clinical distant metastasis prior to CXB (n, %) | ||||||
| ycM0 | 33 | 92 | 14 | 93 | 19 | 90 |
| ycM1 | 3 | 8 | 1 | 7 | 2 | 10 |
| Median size tumor prior to CXB on MRI (cm) (range) | 2.2 | 0.8–4.2 | 1.9 | 1.0–3.0 | 2.0 | 0.8–4.2 |
| Median size tumor prior to CXB on endoscopy (cm) (range) | 2.0 | 1.0–4.0 | 2.0 | 1.0–2.5 | 2.0 | 1.0–4.0 |
| Median interval neoadjuvant radiotherapy and CXB (months) (range) | 3 | 2–38 | 2 | 2–21 | 4 | 2–38 |
| Patients treated with TME surgery following CXB (n, %) | 19 | 53 | 1 | 7 | 18 | 86 |
| Characteristics | Total Cohort (n = 19) | Patients with a cCR (n = 1) | Patients with Residual Tumor (n = 18) | |||
|---|---|---|---|---|---|---|
| Median interval end CXB and TME surgery (months) (range) | 10 | 5–24 | 24 | 9 | 5–20 | |
| Pathological tumor stage of patients treated with TME surgery following CXB (n, %) | ||||||
| ypT0 | 1 | 5 | 1 | 100 | 0 | 0 |
| ypT1 | 1 | 5 | 0 | 0 | 1 | 6 |
| ypT2 | 6 | 32 | 0 | 0 | 6 | 33 |
| ypT3 | 10 | 53 | 0 | 0 | 10 | 56 |
| ypT4 | 1 | 5 | 0 | 0 | 1 | 6 |
| Pathological nodal stage of patients treated with TME surgery following CXB (n, %) | ||||||
| ypN0 | 16 | 84 | 0 | 0 | 16 | 89 |
| ypN1 | 3 | 16 | 1 | 100 | 2 | 11 |
| Tumor Response | Diagnostic Performance % | ||||||
| Features at 3 Months | Residual Tumor | cCR | Sensitivity | Specificity | PPV | NPV | Accuracy |
| Tumor mass on endoscopy | |||||||
| + | 3 (TP) | 0 (FP) | 14 | 100 | 100 | 45 | 50 |
| - | 18 (FN) | 15 (TN) | |||||
| Focal tumor signal on T2W-MRI | |||||||
| + | 0 (TP) | 0 (FP) | 0 | 0 | 0 | 0 | 0 |
| − | 0 (FN) | 0 (TN) | |||||
| Mass-like high signal on DWI | |||||||
| + | 5 (TP) | 1 (FP) | 24 | 93 | 83 | 47 | 53 |
| − | 16 (FN) | 14 (TN) | |||||
| Tumor Response | Diagnostic Performance % | ||||||
| Features at 6 Months | Residual Tumor | cCR | Sensitivity | Specificity | PPV | NPV | Accuracy |
| Tumor mass on endoscopy | |||||||
| + | 8 (TP) | 0 (FP) | 47 | 100 | 100 | 63 | 72 |
| − | 9 (FN) | 15 (TN) | |||||
| Focal tumor signal on T2W-MRI | |||||||
| + | 5 (TP) | 0 (FP) | 26 | 100 | 100 | 48 | 56 |
| − | 14 (FN) | 13 (TN) | |||||
| Mass-like high signal on DWI | |||||||
| + | 11 (TP) | 0 (FP) | 58 | 100 | 100 | 62 | 75 |
| − | 8 (FN) | 13 (TN) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custers, P.A.; Maas, M.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Beets, G.L.; Peters, F.P.; Marijnen, C.A.M.; van Leerdam, M.E.; Huibregtse, I.L.; van Triest, B. Features on Endoscopy and MRI after Treatment with Contact X-ray Brachytherapy for Rectal Cancer: Explorative Results. Cancers 2022, 14, 5565. https://doi.org/10.3390/cancers14225565
Custers PA, Maas M, Lambregts DMJ, Beets-Tan RGH, Beets GL, Peters FP, Marijnen CAM, van Leerdam ME, Huibregtse IL, van Triest B. Features on Endoscopy and MRI after Treatment with Contact X-ray Brachytherapy for Rectal Cancer: Explorative Results. Cancers. 2022; 14(22):5565. https://doi.org/10.3390/cancers14225565
Chicago/Turabian StyleCusters, Petra A., Monique Maas, Doenja M. J. Lambregts, Regina G. H. Beets-Tan, Geerard L. Beets, Femke P. Peters, Corrie A. M. Marijnen, Monique E. van Leerdam, Inge L. Huibregtse, and Baukelien van Triest. 2022. "Features on Endoscopy and MRI after Treatment with Contact X-ray Brachytherapy for Rectal Cancer: Explorative Results" Cancers 14, no. 22: 5565. https://doi.org/10.3390/cancers14225565
APA StyleCusters, P. A., Maas, M., Lambregts, D. M. J., Beets-Tan, R. G. H., Beets, G. L., Peters, F. P., Marijnen, C. A. M., van Leerdam, M. E., Huibregtse, I. L., & van Triest, B. (2022). Features on Endoscopy and MRI after Treatment with Contact X-ray Brachytherapy for Rectal Cancer: Explorative Results. Cancers, 14(22), 5565. https://doi.org/10.3390/cancers14225565







