The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
- breast cancer cell context which includes differential expression of the ER and other as yet unidentified factors,
- breast cancer complexity associated with multiple classifications of tumors based on differences in their histopathology, gene expression, and other clinical parameters,
- ligand structure and the fact that selective AhR modulators (SAhRMs) exhibit tissue/cell-specific AhR agonist or antagonist activity,
- other mechanisms of action of the AhR which involve altered genomic and non-genomic (e.g., cell membrane) pathways that may be differentially be affected by AhR ligands some of which also activate more than one receptor. An example of dual receptor ligands are the polyaromatic hydrocarbons (PAHs) and other compounds which bind both the AhR and ER [36,37,38,39].
2. Selective AhR Modulators (SAhRMs)
3. AhR in Breast Cancer: Prognostic Significance
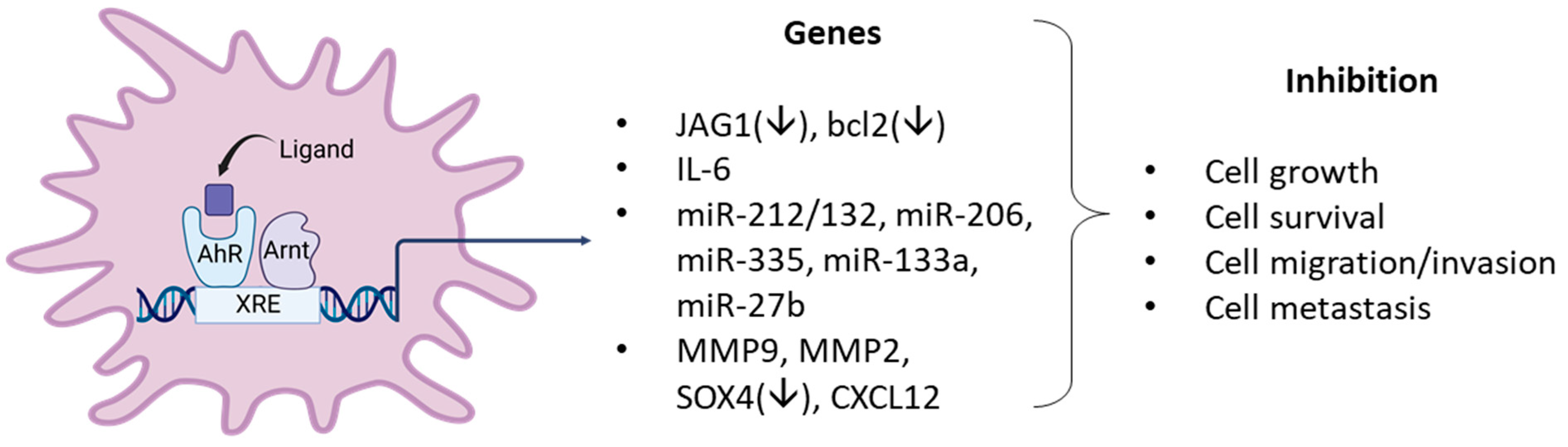
4. Role of the AhR and Its Ligands as Inhibitors Breast Cancer in Cellular and Rodent Models
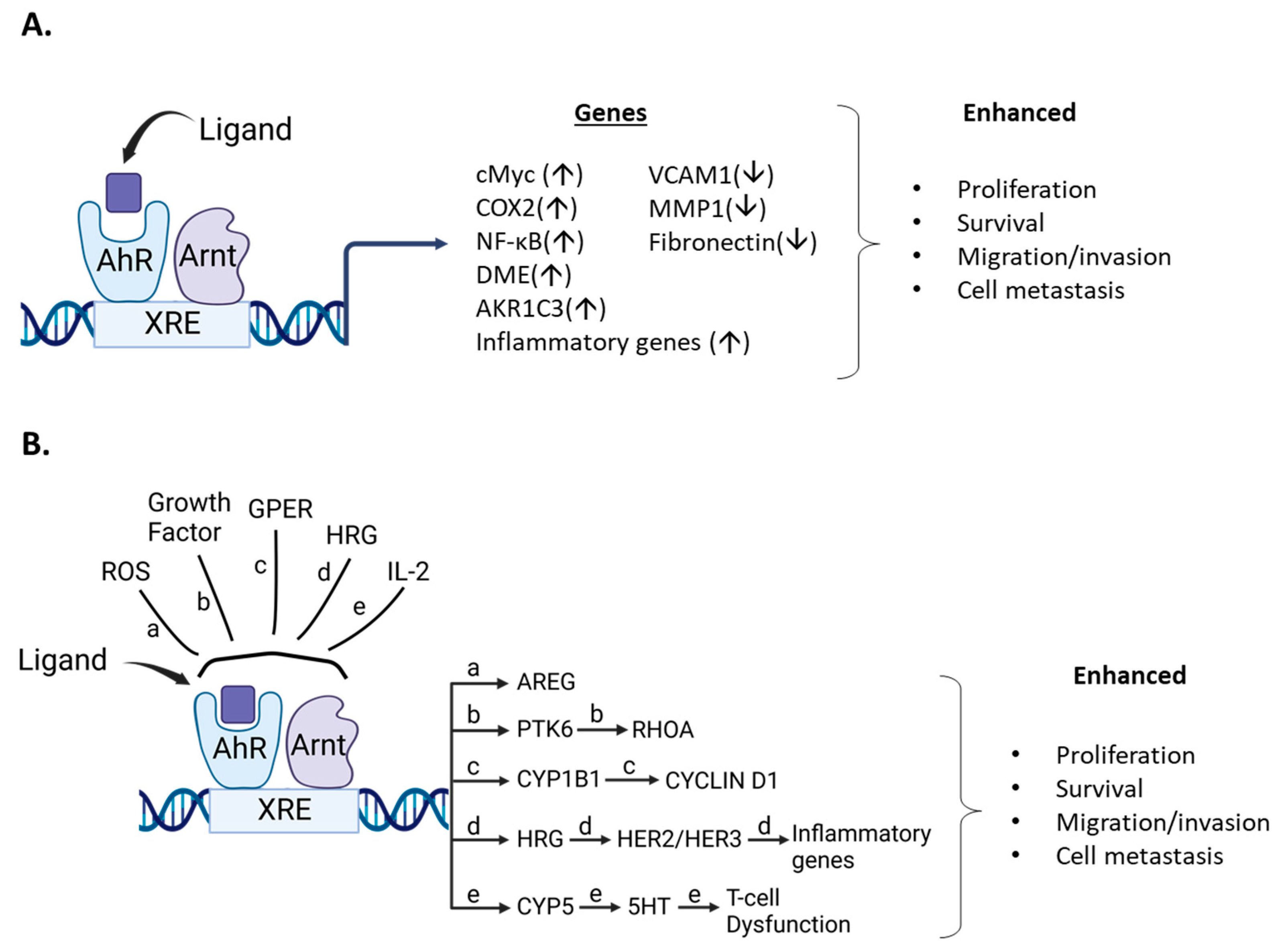
5. AhR and AhR Ligands Enhance Mammary Carcinogenesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [CrossRef]
- Poland, A.; Knutson, J.C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: Examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982, 22, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Avilla, M.N.; Malecki, K.M.C.; Hahn, M.E.; Wilson, R.H.; Bradfield, C.A. The Ah Receptor: Adaptive Metabolism, Ligand Diversity, and the Xenokine Model. Chem. Res. Toxicol. 2020, 33, 860–879. [Google Scholar] [CrossRef]
- Schmidt, J.V.; Bradfield, C.A. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996, 12, 55–89. [Google Scholar] [CrossRef]
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef]
- Whitlock, J.P., Jr.; Okino, S.T.; Dong, L.; Ko, H.P.; Clarke-Katzenberg, R.; Ma, Q.; Li, H. Cytochromes P450 5: Induction of cytochrome P4501A1: A model for analyzing mammalian gene transcription. FASEB J. 1996, 10, 809–818. [Google Scholar] [CrossRef]
- Whitlock Jr, J.P. Genetic and molecular aspects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin action. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 251–277. [Google Scholar] [CrossRef]
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340. [Google Scholar] [CrossRef]
- Evans, R.M. The nuclear receptor superfamily: A rosetta stone for physiology. Mol. Endocrinol. 2005, 19, 1429–1438. [Google Scholar] [CrossRef]
- Chambon, P. The nuclear receptor superfamily: A personal retrospect on the first two decades. Mol. Endocrinol. 2005, 19, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Santen, R.J. Celebrating 75 years of oestradiol. J. Mol. Endocrinol. 2015, 55, T1–T20. [Google Scholar] [CrossRef]
- Schmidt, J.V.; Su, G.H.; Reddy, J.K.; Simon, M.C.; Bradfield, C.A. Characterization of a murine AhR null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 1996, 93, 6731–6736. [Google Scholar] [CrossRef]
- Mimura, J.; Yamashita, K.; Nakamura, K.; Morita, M.; Takagi, T.N.; Nakao, K.; Ema, M.; Sogawa, K.; Yasuda, M.; Katsuki, M.; et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997, 2, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Fernandez-Salguero, P. The aryl hydrocarbon receptor: Studies using the AHR-null mice. Drug Metab Dispos. 1998, 26, 1194–1198. [Google Scholar]
- Safe, S.; Jin, U.H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef]
- Safe, S.; Han, H.; Goldsby, J.; Mohankumar, K.; Chapkin, R.S. Aryl Hydrocarbon Receptor (AhR) Ligands as Selective AhR Modulators: Genomic Studies. Curr. Opin. Toxicol. 2018, 11–12, 10–20. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Denison, M.S.; Seidel, S.D.; Rogers, W.J.; Ziccardi, M.H.; Winter, G.M.; Heath-Pagliuso, S. Natural and synthetic ligands for the Ah receptor. In Molecular Bioloy Approaches to Toxicology; Puga, A., Kendall, K.B., Eds.; Taylor and Francis: London, UK, 1998; pp. 3–33. [Google Scholar]
- Safe, S. Selective Ah receptor modulators (SAhRMs): Progress towards development of a new class of inhibitors of breast cancer growth. J. Women’s Cancer 2001, 3, 37–45. [Google Scholar]
- Wormke, M.; Stoner, M.; Saville, B.; Walker, K.; Abdelrahim, M.; Burghardt, R.; Safe, S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell Biol. 2003, 23, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Porter, W.; Santostefano, M.; Wang, X.; Safe, S. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol. Cell Biol. 1995, 15, 6710–6719. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Wormke, M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003, 16, 807–816. [Google Scholar] [CrossRef]
- Baker, J.R.; Sakoff, J.A.; McCluskey, A. The aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med. Res. Rev. 2020, 40, 972–1001. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-h.; Kohlhagen, G.; Liao, Z.-y.; Antony, S.; Sausville, E.; Pommier, Y. DNA-protein cross-links and replication-dependent histone H2AX phosphorylation induced by aminoflavone (NSC 686288), a novel anticancer agent active against human breast cancer cells. Cancer Res. 2005, 65, 5337–5343. [Google Scholar] [CrossRef]
- Terzuoli, E.; Puppo, M.; Rapisarda, A.; Uranchimeg, B.; Cao, L.; Burger, A.M.; Ziche, M.; Melillo, G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 2010, 70, 6837–6848. [Google Scholar] [CrossRef] [PubMed]
- Callero, M.A.; Suarez, G.V.; Luzzani, G.; Itkin, B.; Nguyen, B.; Loaiza-Perez, A.I. Aryl hydrocarbon receptor activation by aminoflavone: New molecular target for renal cancer treatment. Int. J. Oncol. 2012, 41, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kuffel, M.J.; Schroeder, J.C.; Pobst, L.J.; Naylor, S.; Reid, J.M.; Kaufmann, S.H.; Ames, M.M. Activation of the antitumor agent aminoflavone (NSC 686288) is mediated by induction of tumor cell cytochrome P450 1A1/1A2. Mol. Pharmacol. 2002, 62, 143–153. [Google Scholar] [CrossRef]
- Guarnieri, T. Aryl Hydrocarbon Receptor Connects Inflammation to Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5264. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Liu, D.; Farago, M.; Seldin, D.C.; Belguise, K.; Sonenshein, G.E.; Sherr, D.H. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol. Chem. 2006, 387, 1175–1187. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Murray, T.J.; Mitchell, G.C.; Hesterman, E.V.; Karchner, S.I.; Merson, R.R.; Hahn, M.E.; Sherr, D.H. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene 2005, 24, 7869–7881. [Google Scholar] [CrossRef]
- Narasimhan, S.; Stanford Zulick, E.; Novikov, O.; Parks, A.J.; Schlezinger, J.J.; Wang, Z.; Laroche, F.; Feng, H.; Mulas, F.; Monti, S.; et al. Towards Resolving the Pro- and Anti-Tumor Effects of the Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2018, 19, 1388. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Kolluri, S.K.; Jin, U.H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Karki, K.; Cheng, Y.; Michelhaugh, S.K.; Mittal, S.; Safe, S. The aryl hydrocarbon receptor is a tumor suppressor–like gene in glioblastoma. J. Biol. Chem. 2019, 294, 11342–11353. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Ariazi, E.; Kim, K.; Khan, S.; Barhoumi, R.; Burghardt, R.; Liu, S.; Hill, D.; Finnell, R.; Wlodarczyk, B. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor α. Cancer Res. 2006, 66, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Abdelrahim, M.; Khan, S.; Ariazi, E.; Jordan, V.C.; Safe, S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells. Biol. Chem. 2006, 387, 1209–1213. [Google Scholar] [CrossRef]
- Shipley, J.M.; Waxman, D.J. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methycholanthrene. Toxicol. Appl. Pharmacol. 2006, 213, 87–97. [Google Scholar] [CrossRef]
- Swedenborg, E.; Rüegg, J.; Hillenweck, A.; Rehnmark, S.; Faulds, M.H.; Zalko, D.; Pongratz, I.; Pettersson, K. 3-Methylcholanthrene displays dual effects on estrogen receptor (ER) α and ERβ signaling in a cell-type specific fashion. Mol. Pharmacol. 2008, 73, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Rannug, A.; Rannug, U.; Rosenkranz, H.S.; Winqvist, L.; Westerholm, R.; Agurell, E.; Grafstrom, A.K. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 1987, 262, 15422–15427. [Google Scholar] [CrossRef]
- Song, J.; Clagett-Dame, M.; Peterson, R.E.; Hahn, M.E.; Westler, W.M.; Sicinski, R.R.; DeLuca, H.F. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc. Natl. Acad. Sci. USA 2002, 99, 14694–14699. [Google Scholar] [CrossRef] [PubMed]
- Chiaro, C.R.; Morales, J.L.; Prabhu, K.S.; Perdew, G.H. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry 2008, 47, 8445–8455. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Toshimitsu, T.; Matsuoka, S.; Maruyama, A.; Oh-Oka, K.; Takamura, T.; Nakamura, Y.; Ishimaru, K.; Fujii-Kuriyama, Y.; Ikegami, S.; et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 2014, 92, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Mexia, N.; Gaitanis, G.; Velegraki, A.; Soshilov, A.; Denison, M.S.; Magiatis, P. Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch. Biochem. Biophys. 2015, 571, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, M.; Safe, S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994, 82, 43–47. [Google Scholar] [CrossRef]
- Ramamoorthy, K.; Gupta, M.S.; Sun, G.; McDougal, A.; Safe, S. 3,3’,4,4’-Tetrachlorobiphenyl exhibits antiestrogenic and antitumorigenic activity in the rodent uterus and mammary and in human breast cancer cells. Carcinogenesis 1999, 20, 115–123. [Google Scholar] [CrossRef][Green Version]
- Chen, I.; McDougal, A.; Wang, F.; Safe, S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 1998, 19, 1631–1639. [Google Scholar] [CrossRef]
- McDougal, A.; Wilson, C.; Safe, S. Inhibition of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997, 120, 53–63. [Google Scholar] [CrossRef]
- McDougal, A.; Sethi Gupta, M.; Ramamoorthy, K.; Sun, G.; Safe, S.H. Inhibition of carcinogen-induced rat mammary tumor growth and other estrogen-dependent responses by symmetrical dihalo-substituted analogs of diindolylmethane. Cancer Lett. 2000, 151, 169–179. [Google Scholar] [CrossRef]
- McDougal, A.; Wormke, M.; Calvin, J.; Safe, S. Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective aryl hydrocarbon receptor modulator. Cancer Res. 2001, 61, 3902–3907. [Google Scholar]
- Huggins, C.B. Selective induction of hormone-dependent mammary adenocarcinoma in the rat. J. Lab. Clin. Med. 1987, 109, 262–266. [Google Scholar] [PubMed]
- Hu, W.; Sorrentino, C.; Denison, M.S.; Kolaja, K.; Fielden, M.R. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 2007, 71, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Lee, S.O.; Safe, S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J. Pharm. Exp. 2012, 343, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Lee, S.O.; Pfent, C.; Safe, S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer 2014, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Kim, S.B.; Safe, S. Omeprazole Inhibits Pancreatic Cancer Cell Invasion through a Nongenomic Aryl Hydrocarbon Receptor Pathway. Chem. Res. Toxicol. 2015, 28, 907–918. [Google Scholar] [CrossRef]
- Saito, R.; Miki, Y.; Hata, S.; Takagi, K.; Iida, S.; Oba, Y.; Ono, K.; Ishida, T.; Suzuki, T.; Ohuchi, N. Aryl hydrocarbon receptor in breast cancer—A newly defined prognostic marker. Horm. Cancer 2014, 5, 11–21. [Google Scholar] [CrossRef]
- Vacher, S.; Castagnet, P.; Chemlali, W.; Lallemand, F.; Meseure, D.; Pocard, M.; Bieche, I.; Perrot-Applanat, M. High AHR expression in breast tumors correlates with expression of genes from several signaling pathways namely inflammation and endogenous tryptophan metabolism. PLoS ONE 2018, 13, e0190619. [Google Scholar] [CrossRef]
- Tryggvadottir, H.; Sandén, E.; Björner, S.; Bressan, A.; Ygland Rödström, M.; Khazaei, S.; Edwards, D.P.; Nodin, B.; Jirström, K.; Isaksson, K. The Prognostic Impact of Intratumoral Aryl Hydrocarbon Receptor in Primary Breast Cancer Depends on the Type of Endocrine Therapy: A Population-Based Cohort Study. Front. Oncol. 2021, 11, 1671. [Google Scholar] [CrossRef]
- Jeschke, U.; Zhang, X.; Kuhn, C.; Jalaguier, S.; Colinge, J.; Pfender, K.; Mayr, D.; Ditsch, N.; Harbeck, N.; Mahner, S. The prognostic impact of the aryl hydrocarbon receptor (AhR) in primary breast cancer depends on the lymph node status. Int. J. Mol. Sci. 2019, 20, 1016. [Google Scholar] [CrossRef]
- Li, Y.; Qin, H.Z.; Song, Q.; Wu, X.D.; Zhu, J.H. Lack of association between the aryl hydrocarbon receptor rs2066853 polymorphism and breast cancer: A meta-analysis on Ahr polymorphism and breast cancer. Genet. Mol. Res. 2015, 14, 16162–16168. [Google Scholar] [CrossRef]
- Marshall, N.B.; Kerkvliet, N.I. Dioxin and immune regulation: Emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 2010, 1183, 25–37. [Google Scholar] [CrossRef]
- Gutierrez-Vazquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharm. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.C.; Hudgins, S.; Lewis, A.; Schorr, K.; Sommer, R.; Peterson, R.E.; Flaws, J.A.; Furth, P.A. In utero and lactational treatment with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin impairs mammary gland differentiation but does not block the response to exogenous estrogen in the postpubertal female rat. Toxicol. Sci. 2001, 62, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lew, B.J.; Collins, L.L.; O’Reilly, M.A.; Lawrence, B.P. Activation of the aryl hydrocarbon receptor during different critical windows in pregnancy alters mammary epithelial cell proliferation and differentiation. Toxicol. Sci. 2009, 111, 151–162. [Google Scholar] [CrossRef]
- Vorderstrasse, B.A.; Fenton, S.E.; Bohn, A.A.; Cundiff, J.A.; Lawrence, B.P. A novel effect of dioxin: Exposure during pregnancy severely impairs mammary gland differentiation. Toxicol. Sci. 2004, 78, 248–257. [Google Scholar] [CrossRef]
- Kociba, R.J.; Keyes, D.G.; Beyer, J.E.; Carreon, R.M.; Wade, C.E.; Dittenber, D.A.; Kalnins, R.P.; Frauson, L.E.; Park, C.L.; Barnard, S.D.; et al. Results of a 2-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in rats. Toxicol. Appl. Pharmacol. 1978, 46, 279–303. [Google Scholar] [CrossRef]
- Walker, N.J.; Wyde, M.E.; Fischer, L.J.; Nyska, A.; Bucher, J.R. Comparison of chronic toxicity and carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in 2-year bioassays in female Sprague-Dawley rats. Mol. Nutr. Food Res. 2006, 50, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Rowell, C.; Wang, J.; Lamartiniere, C.A. Prenatal TCDD exposure predisposes for mammary cancer in rats. Reprod. Toxicol. 2007, 23, 391–396. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Gavin, H.M.; Arlt, V.M.; Lawrence, B.P.; Fenton, S.E.; Medina, D.; Vorderstrasse, B.A. Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int. J. Cancer 2011, 128, 1509–1523. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, P.; Liu, X.; Li, X.; Walker, K.; Kotha, L.; Rowlands, C.; Safe, S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr. Relat. Cancer 2009, 16, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Spink, B.C.; Bennett, J.A.; Lostritto, N.; Cole, J.R.; Spink, D.C. Expression of the aryl hydrocarbon receptor is not required for the proliferation, migration, invasion, or estrogen-dependent tumorigenesis of MCF-7 breast cancer cells. Mol. Carcinog. 2013, 52, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Hiromori, Y.; Aoki, A.; Kimura, T.; Fujii-Kuriyama, Y.; Nagase, H.; Nakanishi, T. Possible aryl hydrocarbon receptor-independent pathway of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced antiproliferative response in human breast cancer cells. Toxicol. Lett. 2012, 211, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, E.-Y.; Huang, W.-Y.; Chang, Y.-C.; Tsai, M.-H.; Chuang, E.Y.; Kuok, Q.-Y.; Bai, S.-T.; Chao, L.-Y.; Sher, Y.-P.; Lai, L.-C. Aryl hydrocarbon receptor activates NDRG1 transcription under hypoxia in breast cancer cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Luecke-Johansson, S.; Gralla, M.; Rundqvist, H.; Ho, J.C.; Johnson, R.S.; Gradin, K.; Poellinger, L. A Molecular Mechanism To Switch the Aryl Hydrocarbon Receptor from a Transcription Factor to an E3 Ubiquitin Ligase. Mol. Cell Biol. 2017, 37, e00630-16. [Google Scholar] [CrossRef] [PubMed]
- Goode, G.; Pratap, S.; Eltom, S.E. Depletion of the aryl hydrocarbon receptor in MDA-MB-231 human breast cancer cells altered the expression of genes in key regulatory pathways of cancer. PLoS ONE 2014, 9, e100103. [Google Scholar] [CrossRef]
- Hall, J.M.; Barhoover, M.A.; Kazmin, D.; McDonnell, D.P.; Greenlee, W.F.; Thomas, R.S. Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol. Endocrinol. 2010, 24, 359–369. [Google Scholar] [CrossRef]
- Goode, G.D.; Ballard, B.R.; Manning, H.C.; Freeman, M.L.; Kang, Y.; Eltom, S.E. Knockdown of aberrantly upregulated aryl hydrocarbon receptor reduces tumor growth and metastasis of MDA-MB-231 human breast cancer cell line. Int. J. Cancer. 2013, 133, 2769–2780. [Google Scholar] [CrossRef]
- Li, B.B.; Scott, E.Y.; Olafsen, N.E.; Matthews, J.; Wheeler, A.R. Analysis of the effects of aryl hydrocarbon receptor expression on cancer cell invasion via three-dimensional microfluidic invasion assays. Lab A Chip 2022, 22, 313–325. [Google Scholar] [CrossRef]
- Barhoover, M.A.; Hall, J.M.; Greenlee, W.F.; Thomas, R.S. Aryl hydrocarbon receptor regulates cell cycle progression in human breast cancer cells via a functional interaction with cyclin-dependent kinase 4. Mol. Pharmacol. 2010, 77, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Porter, W.; Burghardt, R.; Safe, S.H. Mechanism of inhibition of MDA-MB-468 breast cancer cell growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Carcinogenesis 1997, 18, 925–933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vogel, C.; Abel, J. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on growth factor expression in the human breast cancer cell line MCF-7. Arch. Toxicol. 1995, 69, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.L.; Yoon, D.; Choi, H.H.; Wang, F.; Taylor, R.T.; Chen, N.; Zhang, R.; Hankinson, O. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol. Sci. Off. J. Soc. Toxicol. 2007, 98, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol. Cancer 2015, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kim, K.; Jin, U.H.; Pfent, C.; Cao, H.; Amendt, B.; Liu, X.; Wilson-Robles, H.; Safe, S. Aryl hydrocarbon receptor agonists induce microRNA-335 expression and inhibit lung metastasis of estrogen receptor negative breast cancer cells. Mol. Cancer 2012, 11, 108–118. [Google Scholar] [CrossRef]
- Gierthy, J.F.; Bennett, J.A.; Bradley, L.M.; Cutler, D.S. Correlation of in vitro and in vivo growth suppression of MCF-7 human breast cancer by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res. 1993, 53, 3149–3153. [Google Scholar]
- Wang, T.; Wyrick, K.L.; Meadows, G.G.; Wills, T.B.; Vorderstrasse, B.A. Activation of the aryl hydrocarbon receptor by TCDD inhibits mammary tumor metastasis in a syngeneic mouse model of breast cancer. Toxicol. Sci. 2011, 124, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Perez, A.I.; Kenney, S.; Boswell, J.; Hollingshead, M.; Alley, M.C.; Hose, C.; Ciolino, H.P.; Yeh, G.C.; Trepel, J.B.; Vistica, D.T.; et al. Aryl hydrocarbon receptor activation of an antitumor aminoflavone: Basis of selective toxicity for MCF-7 breast tumor cells. Mol. Cancer 2004, 3, 715–725. [Google Scholar] [CrossRef]
- Callero, M.A.; Loaiza-Pérez, A.I. The role of aryl hydrocarbon receptor and crosstalk with estrogen receptor in response of breast cancer cells to the novel antitumor agents benzothiazoles and aminoflavone. Int. J. Breast Cancer 2011, 2011, 923250. [Google Scholar] [CrossRef][Green Version]
- McLean, L.; Soto, U.; Agama, K.; Francis, J.; Jimenez, R.; Pommier, Y.; Sowers, L.; Brantley, E. Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. Int. J. Cancer 2008, 122, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.S.; Mavingire, N.; Khan, S.; Rowland, L.K.; Wooten, J.V.; Opoku-Agyeman, A.; Guevara, A.; Soto, U.; Cavalli, F.; Loaiza-Pérez, A.I. AhR ligand aminoflavone suppresses α6-integrin–Src–Akt signaling to attenuate tamoxifen resistance in breast cancer cells. J. Cell. Physiol. 2019, 234, 108–121. [Google Scholar] [CrossRef]
- Brantley, E.; Callero, M.A.; Berardi, D.E.; Campbell, P.; Rowland, L.; Zylstra, D.; Amis, L.; Yee, M.; Simian, M.; Todaro, L. AhR ligand Aminoflavone inhibits α6-integrin expression and breast cancer sphere-initiating capacity. Cancer Lett. 2016, 376, 53–61. [Google Scholar] [CrossRef]
- Callero, M.A.; Rodriguez, C.E.; Sólimo, A.; Bal de Kier Joffé, E.; Loaiza Perez, A.I. The immune system as a new possible cell target for AFP 464 in a spontaneous mammary cancer mouse model. J. Cell. Biochem. 2017, 118, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Perez, A.I.; Trapani, V.; Hose, C.; Singh, S.S.; Trepel, J.B.; Stevens, M.F.; Bradshaw, T.D.; Sausville, E.A. Aryl hydrocarbon receptor mediates sensitivity of MCF-7 breast cancer cells to antitumor agent 2-(4-amino-3-methylphenyl) benzothiazole. Mol. Pharmacol. 2002, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.-S.; Kashiyama, E.; Bradshaw, T.D.; Stinson, S.F.; Brantley, E.; Sausville, E.A.; Stevens, M.F. Role of CYP1A1 in modulation of antitumor properties of the novel agent 2-(4-amino-3-methylphenyl) benzothiazole (DF 203, NSC 674495) in human breast cancer cells. Cancer Res. 2000, 60, 5196–5203. [Google Scholar]
- McLean, L.S.; Watkins, C.N.; Campbell, P.; Zylstra, D.; Rowland, L.; Amis, L.H.; Scott, L.; Babb, C.E.; Livingston, W.J.; Darwanto, A. Aryl hydrocarbon receptor ligand 5F 203 induces oxidative stress that triggers DNA damage in human breast cancer cells. Chem. Res. Toxicol. 2015, 28, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.L.; Citossi, F.; Singh, R.; Kaur, B.; Gaskell, M.; Farmer, P.B.; Monks, A.; Hose, C.; Stevens, M.F.; Leong, C.-O. Antitumour benzothiazoles. Part 32: DNA adducts and double strand breaks correlate with activity; synthesis of 5F203 hydrogels for local delivery. Bioorganic Med. Chem. 2015, 23, 6891–6899. [Google Scholar] [CrossRef]
- Trapani, V.; Patel, V.; Leong, C.; Ciolino, H.; Yeh, G.; Hose, C.; Trepel, J.; Stevens, M.; Sausville, E.; Loaiza-Perez, A. DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. Br. J. Cancer 2003, 88, 599–605. [Google Scholar] [CrossRef]
- Bradshaw, T.D.; Bibby, M.C.; Double, J.A.; Fichtner, I.; Cooper, P.A.; Alley, M.C.; Donohue, S.; Stinson, S.F.; Tomaszewjski, J.E.; Sausville, E.A. Preclinical evaluation of amino acid prodrugs of novel antitumor 2-(4-amino-3-methylphenyl) benzothiazoles. Mol. Cancer Ther. 2002, 1, 239–246. [Google Scholar] [PubMed]
- Brantley, E.; Antony, S.; Kohlhagen, G.; Meng, L.; Agama, K.; Stinson, S.F.; Sausville, E.A.; Pommier, Y. Anti-tumor drug candidate 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole induces single-strand breaks and DNA-protein cross-links in sensitive MCF-7 breast cancer cells. Cancer Chemother. Pharmacol. 2006, 58, 62–72. [Google Scholar] [CrossRef]
- Leong, C.O.; Gaskell, M.; Martin, E.A.; Heydon, R.T.; Farmer, P.B.; Bibby, M.C.; Cooper, P.A.; Double, J.A.; Bradshaw, T.D.; Stevens, M.F. Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. Br. J. Cancer 2003, 88, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; De Iuliis, G.N.; McCluskey, A.; Sakoff, J.A. A novel naphthalimide that selectively targets breast cancer via the arylhydrocarbon receptor pathway. Sci. Rep. 2020, 10, 13978. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R.; Pollard, B.L.; Lin, A.J.S.; Gilbert, J.; Paula, S.; Zhu, X.; Sakoff, J.A.; McCluskey, A. Modelling and Phenotypic Screening of NAP-6 and 10-Cl-BBQ, AhR Ligands Displaying Selective Breast Cancer Cytotoxicity in Vitro. ChemMedChem 2021, 16, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhong, H.; Li, N.; Yu, N.; Wang, Y.; Chen, L.; Sun, J. Discovery of Novel Anti-Breast-Cancer Inhibitors by Synergistically Antagonizing Microtubule Polymerization and Aryl Hydrocarbon Receptor Expression. J. Med. Chem. 2021, 64, 12964–12977. [Google Scholar] [CrossRef]
- Gilbert, J.; De Iuliis, G.N.; Tarleton, M.; McCluskey, A.; Sakoff, J.A. (Z)-2-(3,4-Dichlorophenyl)-3-(1H-Pyrrol-2-yl)Acrylonitrile Exhibits Selective Antitumor Activity in Breast Cancer Cell Lines via the Aryl Hydrocarbon Receptor Pathway. Mol. Pharmacol. 2018, 93, 168–177. [Google Scholar] [CrossRef]
- Baker, J.R.; Russell, C.C.; Gilbert, J.; McCluskey, A.; Sakoff, J.A. Amino alcohol acrylonitriles as broad spectrum and tumour selective cytotoxic agents. RSC Med. Chem. 2021, 12, 929–942. [Google Scholar] [CrossRef]
- Stanton, D.T.; Baker, J.R.; McCluskey, A.; Paula, S. Development and interpretation of a QSAR model for in vitro breast cancer (MCF-7) cytotoxicity of 2-phenylacrylonitriles. J. Comput. Aided. Mol. Des. 2021, 35, 613–628. [Google Scholar] [CrossRef]
- Zulauf, K.E.; Kirby, J.E. Discovery of small-molecule inhibitors of multidrug-resistance plasmid maintenance using a high-throughput screening approach. Proc. Natl. Acad. Sci. USA 2020, 117, 29839–29850. [Google Scholar] [CrossRef]
- O’Donnell, E.F., 3rd; Jang, H.S.; Liefwalker, D.F.; Kerkvliet, N.I.; Kolluri, S.K. Discovery and Mechanistic Characterization of a Select Modulator of AhR-regulated Transcription (SMAhRT) with Anti-cancer Effects. Apoptosis 2021, 26, 307–322. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, X.; Chen, H.; Huang, H.; An, X.; Sun, M.; Yao, Q.; Kim, K.; Zhang, H.; Chu, M.; et al. Carbidopa suppresses estrogen receptor-positive breast cancer via AhR-mediated proteasomal degradation of ERalpha. Investig. New Drugs, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Subramaniam, V.; Abdalla, S.; Jothy, S.; Prud’homme, G.J. Tranilast inhibits the growth and metastasis of mammary carcinoma. Anticancer Drugs 2009, 20, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.; Chakrabarti, R.; Prud’homme, G.J.; Jothy, S. Tranilast inhibits cell proliferation and migration and promotes apoptosis in murine breast cancer. Anticancer Drugs 2010, 21, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.; Ace, O.; Prud’homme, G.J.; Jothy, S. Tranilast treatment decreases cell growth, migration and inhibits colony formation of human breast cancer cells. Exp. Mol. Pathol. 2011, 90, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, C.X.; Bu, Y.; Bottum, K.M.; Tischkau, S.A. Beta-naphthoflavone (DB06732) mediates estrogen receptor-positive breast cancer cell cycle arrest through AhR-dependent regulation of PI3K/AKT and MAPK/ERK signaling. Carcinogenesis 2014, 35, 703–713. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.F.; Koch, D.C.; Bisson, W.H.; Jang, H.S.; Kolluri, S.K. The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell Death Dis. 2014, 5, e1038. [Google Scholar] [CrossRef]
- DuSell, C.D.; Nelson, E.R.; Wittmann, B.M.; Fretz, J.A.; Kazmin, D.; Thomas, R.S.; Pike, J.W.; McDonnell, D.P. Regulation of aryl hydrocarbon receptor function by selective estrogen receptor modulators. Mol. Endocrinol. 2010, 24, 33–46. [Google Scholar] [CrossRef]
- Kim, E.J.; Shin, M.; Park, H.; Hong, J.E.; Shin, H.K.; Kim, J.; Kwon, D.Y.; Park, J.H. Oral administration of 3,3’-diindolylmethane inhibits lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. J. Nutr. 2009, 139, 2373–2379. [Google Scholar] [CrossRef]
- Chang, X.; Tou, J.C.; Hong, C.; Kim, H.A.; Riby, J.E.; Firestone, G.L.; Bjeldanes, L.F. 3,3’-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 2005, 26, 771–778. [Google Scholar] [CrossRef]
- Hsu, E.L.; Chen, N.; Westbrook, A.; Wang, F.; Zhang, R.; Taylor, R.T.; Hankinson, O. CXCR4 and CXCL12 down-regulation: A novel mechanism for the chemoprotection of 3,3’-diindolylmethane for breast and ovarian cancers. Cancer Lett. 2008, 265, 113–123. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Bargil, S.; Bar, Y.; Ozlavo, R.; Tuval, S.; Rapson, Y.; Pomerantz, A.; Zoref, D.; Sharon, E.; Caspi, O.; et al. 3,3-Diindolylmethane (DIM): A nutritional intervention and its impact on breast density in healthy BRCA carriers. A prospective clinical trial. Carcinogenesis 2020, 41, 1395–1401. [Google Scholar] [CrossRef]
- Bjeldanes, L.F.; Kim, J.Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, E.P.; Heukers, R.; Manson, M.M. EGFR and Src are involved in indole-3-carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis 2007, 28, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.N.; Jun, W.; Choue, R.; Lee, J. I3C and ICZ inhibit migration by suppressing the EMT process and FAK expression in breast cancer cells. Mol. Med. Rep. 2013, 7, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hargraves, K.G.; He, L.; Firestone, G.L. Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol. Carcinog. 2016, 55, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, Y.L.; Chen, L.; Vaziri, N.D.; Zhao, Y.Y. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit. Rev. Toxicol. 2019, 49, 445–460. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Jorge Rodriguez, M.E.; Veitia, M.S.; Giner, R.M. Plant Occurring Flavonoids as Modulators of the Aryl Hydrocarbon Receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef]
- Tiong, C.T.; Chen, C.; Zhang, S.J.; Li, J.; Soshilov, A.; Denison, M.S.; Lee, L.S.; Tam, V.H.; Wong, S.P.; Xu, H.E.; et al. A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ERalpha protein. Carcinogenesis 2012, 33, 1089–1097. [Google Scholar] [CrossRef]
- Hanieh, H.; Mohafez, O.; Hairul-Islam, V.I.; Alzahrani, A.; Bani Ismail, M.; Thirugnanasambantham, K. Novel Aryl Hydrocarbon Receptor Agonist Suppresses Migration and Invasion of Breast Cancer Cells. PLoS ONE 2016, 11, e0167650. [Google Scholar] [CrossRef]
- Pham, T.H.; Lecomte, S.; Le Guevel, R.; Lardenois, A.; Evrard, B.; Chalmel, F.; Ferriere, F.; Balaguer, P.; Efstathiou, T.; Pakdel, F. Characterization of Glyceollins as Novel Aryl Hydrocarbon Receptor Ligands and Their Role in Cell Migration. Int. J. Mol. Sci. 2020, 21, 1368. [Google Scholar] [CrossRef]
- Yamashita, N.; Taga, C.; Ozawa, M.; Kanno, Y.; Sanada, N.; Kizu, R. Camalexin, an indole phytoalexin, inhibits cell proliferation, migration, and mammosphere formation in breast cancer cells via the aryl hydrocarbon receptor. J. Nat. Med. 2022, 76, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jiang, C.; Fu, C.; Chang, P.; Yang, B.; Wu, J.; Zhao, X.; Ma, S. Antiproliferative effect of 2-Hydroxy-6-tridecylbenzoic acid from ginkgo biloba sarcotestas through the aryl hydrocarbon receptor pathway in triple-negative breast cancer cells. Nat. Prod. Res. 2020, 34, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H.; Ibrahim, H.M.; Mohammed, M.; Alwassil, O.I.; Abukhalil, M.H.; Farhan, M. Activation of aryl hydrocarbon receptor signaling by gallic acid suppresses progression of human breast cancer in vitro and in vivo. Phytomedicine 2022, 96, 153817. [Google Scholar] [CrossRef] [PubMed]
- Piwarski, S.A.; Thompson, C.; Chaudhry, A.R.; Denvir, J.; Primerano, D.A.; Fan, J.; Salisbury, T.B. The putative endogenous AHR ligand ITE reduces JAG1 and associated NOTCH1 signaling in triple negative breast cancer cells. Biochem. Pharmacol. 2020, 174, 113845. [Google Scholar] [CrossRef] [PubMed]
- Mobini, K.; Tamaddon, G.; Fardid, R.; Keshavarzi, M.; Mohammadi-Bardbori, A. Aryl hydrocarbon-estrogen alpha receptor-dependent expression of miR-206, miR-27b, and miR-133a suppress cell proliferation and migration in MCF-7 cells. J. Biochem. Mol. Toxicol. 2019, 33, e22304. [Google Scholar] [CrossRef] [PubMed]
- Sari, Z.; Miko, E.; Kovacs, T.; Boratko, A.; Ujlaki, G.; Janko, L.; Kiss, B.; Uray, K.; Bai, P. Indoxylsulfate, a Metabolite of the Microbiome, Has Cytostatic Effects in Breast Cancer via Activation of AHR and PXR Receptors and Induction of Oxidative Stress. Cancers 2020, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Sari, Z.; Miko, E.; Kovacs, T.; Janko, L.; Csonka, T.; Lente, G.; Sebo, E.; Toth, J.; Toth, D.; Arkosy, P.; et al. Indolepropionic Acid, a Metabolite of the Microbiome, Has Cytostatic Properties in Breast Cancer by Activating AHR and PXR Receptors and Inducing Oxidative Stress. Cancers 2020, 12, 2411. [Google Scholar] [CrossRef]
- Kim, D.W.; Gazourian, L.; Quadri, S.A.; Romieu-Mourez, R.; Sherr, D.H.; Sonenshein, G.E. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 2000, 19, 5498–5506. [Google Scholar] [CrossRef] [PubMed]
- Novikov, O.; Wang, Z.; Stanford, E.A.; Parks, A.J.; Ramirez-Cardenas, A.; Landesman, E.; Laklouk, I.; Sarita-Reyes, C.; Gusenleitner, D.; Li, A.; et al. An Aryl Hydrocarbon Receptor-Mediated Amplification Loop That Enforces Cell Migration in ER-/PR-/Her2- Human Breast Cancer Cells. Mol. Pharmacol. 2016, 90, 674–688. [Google Scholar] [CrossRef]
- Parks, A.J.; Pollastri, M.P.; Hahn, M.E.; Stanford, E.A.; Novikov, O.; Franks, D.G.; Haigh, S.E.; Narasimhan, S.; Ashton, T.D.; Hopper, T.G.; et al. In silico identification of an aryl hydrocarbon receptor antagonist with biological activity in vitro and in vivo. Mol. Pharmacol. 2014, 86, 593–608. [Google Scholar] [CrossRef]
- Murray, T.J.; Yang, X.; Sherr, D.H. Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res. BCR 2006, 8, R17. [Google Scholar] [CrossRef] [PubMed]
- Stanford, E.A.; Wang, Z.; Novikov, O.; Mulas, F.; Landesman-Bollag, E.; Monti, S.; Smith, B.W.; Seldin, D.C.; Murphy, G.J.; Sherr, D.H. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Degner, S.C.; Papoutsis, A.J.; Selmin, O.; Romagnolo, D.F. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3’-diindolylmethane in breast cancer cells. J. Nutr. 2009, 139, 26–32. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, N.C.; Rogers, T.J.; Gordon, M.A.; Greene, L.I.; Cochrane, D.R.; Spoelstra, N.S.; Nemkov, T.G.; D’Alessandro, A.; Hansen, K.C.; Richer, J.K. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015, 75, 4651–4664. [Google Scholar] [CrossRef]
- Shadboorestan, A.; Tarfiei, G.A.; Montazeri, H.; Sepand, M.R.; Zangooei, M.; Khedri, A.; Ostad, S.N.; Ghahremani, M.H. Invasion and migration of MDA-MB-231 cells are inhibited by block of AhR and NFAT: Role of AhR/NFAT1/β4 integrin signaling. J. Appl. Toxicol. 2019, 39, 375–384. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Tsai, C.F.; Hsu, C.Y.; Kuo, P.L.; Lee, J.N.; Chai, C.Y.; Wang, S.C.; Tsai, E.M. Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. FASEB J. 2012, 26, 778–787. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Hsu, C.Y.; Yang, P.J.; Chiu, C.C.; Liang, S.S.; Ou-Yang, F.; Kan, J.Y.; Hou, M.F.; Wang, T.N.; Tsai, E.M. DEHP mediates drug resistance by directly targeting AhR in human breast cancer. Biomed. Pharm. 2022, 145, 112400. [Google Scholar] [CrossRef]
- Shan, A.; Leng, L.; Li, J.; Luo, X.M.; Fan, Y.J.; Yang, Q.; Xie, Q.H.; Chen, Y.S.; Ni, C.S.; Guo, L.M.; et al. TCDD-induced antagonism of MEHP-mediated migration and invasion partly involves aryl hydrocarbon receptor in MCF7 breast cancer cells. J. Hazard Mater 2020, 398, 122869. [Google Scholar] [CrossRef]
- Bekki, K.; Vogel, H.; Li, W.; Ito, T.; Sweeney, C.; Haarmann-Stemmann, T.; Matsumura, F.; Vogel, C.F. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic. Biochem. Physiol. 2015, 120, 5–13. [Google Scholar] [CrossRef]
- Yamashita, N.; Kanno, Y.; Saito, N.; Terai, K.; Sanada, N.; Kizu, R.; Hiruta, N.; Park, Y.; Bujo, H.; Nemoto, K. Aryl hydrocarbon receptor counteracts pharmacological efficacy of doxorubicin via enhanced AKR1C3 expression in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2019, 516, 693–698. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Lazennec, G.; Kado, S.Y.; Dahlem, C.; He, Y.; Castaneda, A.; Ishihara, Y.; Vogeley, C.; Rossi, A.; Haarmann-Stemmann, T.; et al. Targeting the Aryl Hydrocarbon Receptor Signaling Pathway in Breast Cancer Development. Front. Immunol. 2021, 12, 625346. [Google Scholar] [CrossRef] [PubMed]
- Kubli, S.P.; Bassi, C.; Roux, C.; Wakeham, A.; Gobl, C.; Zhou, W.; Jafari, S.M.; Snow, B.; Jones, L.; Palomero, L.; et al. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.R.; Kerkvliet, C.P.; Krutilina, R.I.; Playa, H.C.; Parke, D.N.; Thomas, W.A.; Smeester, B.A.; Moriarity, B.S.; Seagroves, T.N.; Lange, C.A. Breast Tumor Kinase (Brk/PTK6) Mediates Advanced Cancer Phenotypes via SH2-Domain Dependent Activation of RhoA and Aryl Hydrocarbon Receptor (AhR) Signaling. Mol. Cancer Res. MCR 2021, 19, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Lappano, R.; Bruno, L.; Rizzuti, B.; Grande, F.; Guzzi, R.; Briguori, S.; Miglietta, A.M.; Nakajima, M.; Di Martino, M.T.; et al. AHR and GPER mediate the stimulatory effects induced by 3-methylcholanthrene in breast cancer cells and cancer-associated fibroblasts (CAFs). J. Exp. Clin. Cancer Res. 2019, 38, 335. [Google Scholar] [CrossRef]
- Hollingshead, B.D.; Beischlag, T.V.; Dinatale, B.C.; Ramadoss, P.; Perdew, G.H. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008, 68, 3609–3617. [Google Scholar] [CrossRef]
- Yamashita, N.; Saito, N.; Zhao, S.; Terai, K.; Hiruta, N.; Park, Y.; Bujo, H.; Nemoto, K.; Kanno, Y. Heregulin-induced cell migration is promoted by aryl hydrocarbon receptor in HER2-overexpressing breast cancer cells. Exp. Cell Res. 2018, 366, 34–40. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, N.; Zhou, L.; Wang, J.; Zhou, Y.; Zhang, T.; Fang, Y.; Deng, J.; Gao, Y.; Liang, X.; et al. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021, 22, 358–369. [Google Scholar] [CrossRef]

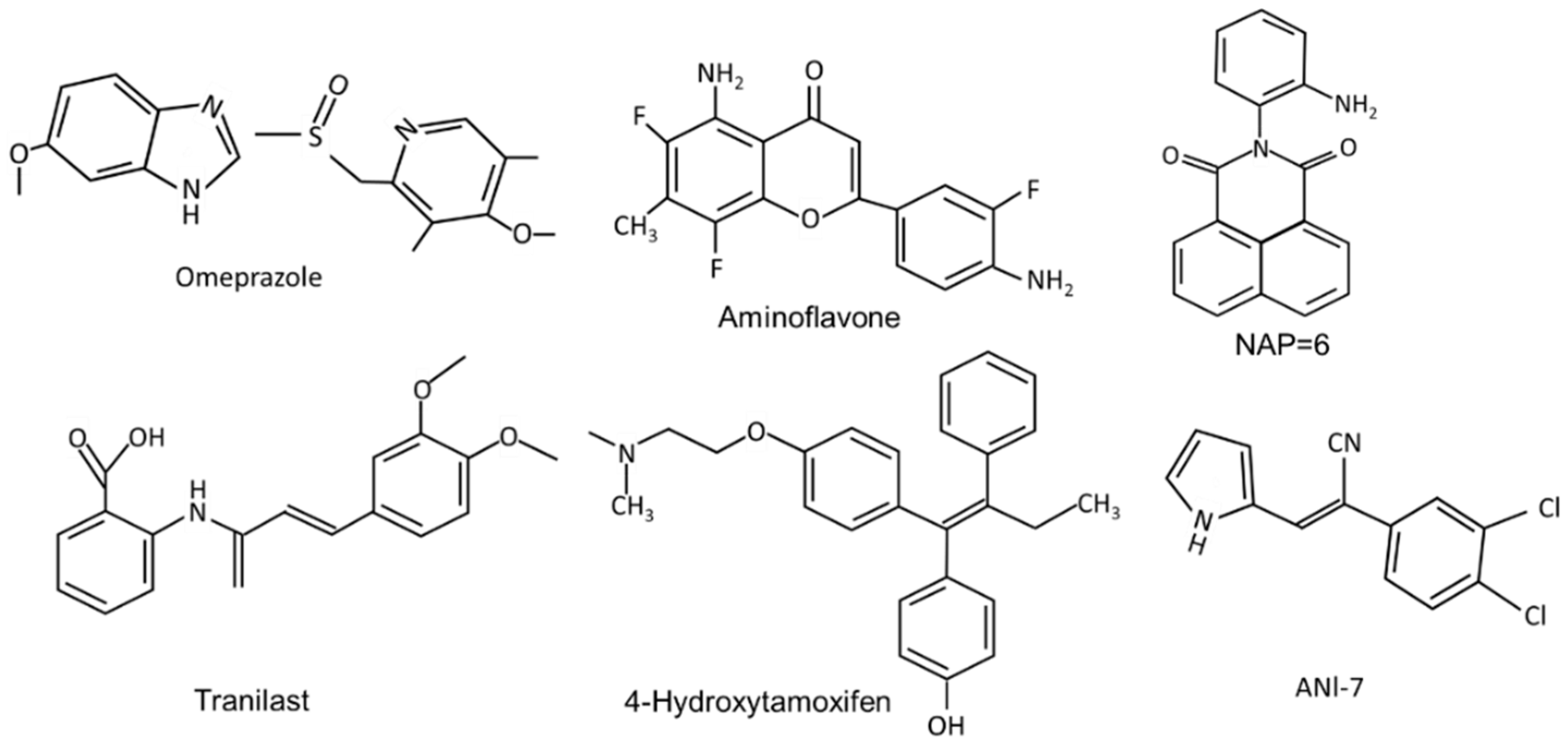
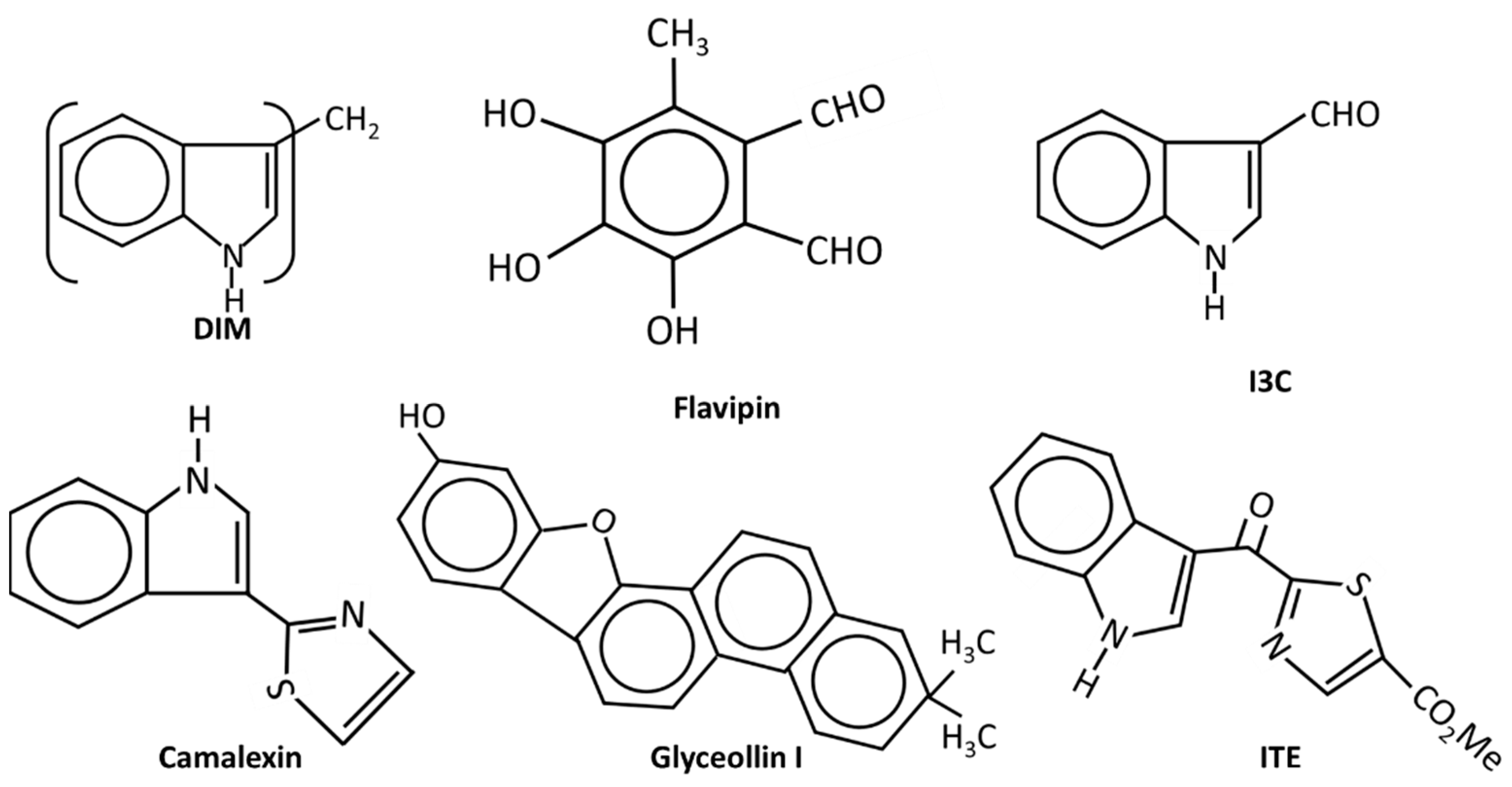
| Compounds | Responses | Cell | KO | In Vivo | Reference |
|---|---|---|---|---|---|
| TCDD | multiple | MDA-MB-231 MDA-MB-436 MDA-MB-157 MDA-MB-435 and BT474 | ✓ | [73] | |
| 2,3,7,8-TCDF | multiple | MDA-MB-231 MDA-MB-436 MDA-MB-157 MDA-MB-435 and BT474 | ✓ | [73] | |
| 2,3,4,7,8-PeCDD | multiple | MDA-MB-231 MDA-MB-436 MDA-MB-157 MDA-MB-435 and BT474 | ✓ | [73] | |
| 1,2,3,7,8-PeCDD | multiple | MDA-MB-231 MDA-MB-436 MDA-MB-157 MDA-MB-435 and BT474 | ✓ | [73] | |
| 3,3′4,4′,5-PeCB | multiple | MDA-MB-231 MDA-MB-436 MDA-MB-157 MDA-MB-435 and BT474 | ✓ | [73] | |
| TCDD | cell cycle prog. | MCF-7 | – | – | [82] |
| TCDD | gr. | MDA-MB-468 | – | ✓ | [83] |
| TCDD | gr. | MCF-7 | – | – | [84] |
| TCDD | CXCR4/CXCL12 | Multiple | [85] | ||
| TCDD | Inv | MDA-MB-231, SKBR3 | ✓ | – | [79] |
| TCDD | Inv | MDA-MB-231, SKBR3 | ✓ | – | [79] |
| TCDD | Inv | MDA-MB-231, T47D | ✓ | – | [86] |
| TCDD | Inv | MDA-MB-231 and BT474 | ✓ | – | [87] |
| TCDD | gr | MCF-7 | – | ✓ | [88] |
| TCDD | met | 4T-1 | – | ✓ | [89] |
| Compounds | Responses | Cells | KO | In Vivo | Reference |
|---|---|---|---|---|---|
| MCDF | gr | MDA-MB-453, MDA-MB-436, HCC-38, MDA-MB-435, BT-474, MDA-MB-157 | ✓ | [73] | |
| MCDF | inv | MDA-MB-231 and BT474 | ✓ | ✓ | [87] |
| MCDF | gr | MDA-MB-468 | – | ✓ | [83] |
| Aminoflavone | gr, DNA damage cytotoxicity, ROS apoptosis | MCF-7, MDA-MB-231, T47D. ZR-75, MDA-MB-468 | – | ✓ | [24,25,26,90,91,92,93,94,95] |
| Aminobenzothiazoles (DF-203 and SF-203) | gr, ROS, DNA damage | MCF-7, MDA-MB-468, CRL2335, MDA-MB-435 | – | ✓ | [96,97,98,99,100,101,102,103] |
| Naphthylamide der-invatives (NAP6) | gr | MCF-7, MDA-MB-231, BT26, BT474, MDA-MB-468 | – | ✓ | [104,105] |
| #12 (quinazoline derivative) | gr, apoptosis, MMP, ROS | MCF-7 | – | ✓ | [106] |
| ANI -7 (acrylo-nitriles) | gr | MCF-7, T47D, ZR-75 SKBR3, MDA-MB-468, BT20, and BT474 | ✓ | – | [107,108,109] |
| Aminoglycoside CG3-15943 | MDA-MB-468 | ✓ | – | [110,111] | |
| Flutamide | migr. | MDA-MB-468 | – | – | [54] |
| Leflunomide | migr. | MDA-MB-468 | – | – | [54] |
| Nimodipine | migr. | MDA-MB-468 | – | – | [54] |
| Omeprazole | migr. | MDA-MB-468 | – | – | [54] |
| Sulindac | migr. | MDA-MB-468 | – | – | [54] |
| Sulindac | migr | MDA-MB-468 | – | – | [54] |
| Tranilast | inv, migr, gr, met | MDA-MB-468, 4T1 | – | ✓ | [54,113,114,115] |
| β-Naphthoflavone | gr | MCF-7, MDA-MB-231 | ✓ | – | [116] |
| Raloxifene | Apoptosis | MDA-MB-231 | ✓ | – | [117] |
| Carbidopa | multiple | MCF-7, MDA-MB-231 | ✓ | [112] | |
| 4-Hydroxytamoxifen | diff | MCF-7 | – | – | [118] |
| Compounds | Responses | Cells | KO | In vivo | Reference |
|---|---|---|---|---|---|
| DIM | gr, invasion, met | MDA-MB-231, MCF-7, 4T-1 | ✓ | ✓ | [78,86,119,120,121,122] |
| I3C | gr, migr. apoptosis | MIF-7, MDA-MB-231, MDA-MB-468, T47D | – | – | [123,124,125,126] |
| ICZ | migr | MCF-7, MDA-MB-231, | – | – | [125] |
| Luteolin | inv, gr, met | MDA-MB-231 | – | – | [129] |
| Icaritin MIR-212/132 | gr | MCF-7 | ✓ | ✓ | [130] |
| Flavipin | gr, inv, migr | MDA-MB-231, T47D | ✓ | ✓ | [131] |
| Glyceollins CI and II | migr | MDA-MB-231 | – | – | [132] |
| Camalexin | gr, migr (mammosphere) | MCF-7, T47D | – | ✓ | [133] |
| 2-Hydroxy-6-tridecylbenzone acia | gr | MDA-MB-231 | – | – | [134] |
| Gallic acid | apoptosis, migr, inv, gr | T47D, MDA-MB-231 | [135] | ||
| ITE | MCF7, MDA-MB-231. MDA-MB-157 | ✓ | – | [136] | |
| FICZ | gr, migr | MCF-7 | ✓ | – | [137] |
| Indoxylsulfate | ROS, met, migr | 4T1 | ✓ | – | [138] |
| Indolepropionic acid | gr, ROS, met | 4T1, SKBR3 | ✓ | – | [139] |
| Compound | Responses/Pathway | Cells | KO | In Vivo | Reference |
|---|---|---|---|---|---|
| TCDD | gr, Myc/Rel-AhR | Hs5787 | – | – | [30,140] |
| FICZ, BaP, TCDD, XA, Kyn | migr/AhR-TDO-Kyn | SUM149, Hs578T | ✓ | ✓ | [141] |
| CB7993113 DMBA | migr, inv, tox | BP1, Hs5787 | ✓ | – | [142] |
| FICZ, BaP, TCDD | migr/AhR-SOX2 | Hs578T, MCF-7,SUM149 | ✓ | – | [144] |
| Galangin, NF, MC | gr/genes | Hs5787 | ✓ | – | [143] |
| DIM, TCDD | colony form, migr | BP1, Hs578T, SUM149, MDA-MB-231 | ✓ | – | [31,145] |
| Kyn | colonies, inv met/AhR-TDO-KYN, NFkB | BT59, SUM159, MDA-MB-231 | ✓ | – | [146] |
| BaP | inv, gr, migr | MDA-MB-231 | ✓ | – | [147] |
| Phthalates | migr, inv/HDAC6 | MCF-7, MDA-MB-231 | ✓ | – | [148,149,150] |
| TCDD, Kyn | surv, infl/COX2, NFkB | MDA-MB-231, SKBR3, others | ✓ | – | [151] |
| MC | cytotox/AKR1C3 | MDA-MB-231 | ✓ | – | [152] |
| TCDD | apoptosis, gr, AhRR | MDA-MB-231, MCF-7, others | ✓ | – | [153] |
| CH223191 | Proangiogenic/AhR-AREG-ROS | multiple | ✓ | ✓ | [154] |
| CH223191 | met, migr, motility | MDA-MB-231, Hs578T Others | ✓ | ✓ | [155] |
| MC | gr/AhR-GPER | SKBR3 | ✓ | – | [156] |
| TCDD, BaP | infl, IL6 | MCF-7 | ✓ | – | [157] |
| MC | migr/HRG-AhR | MCF-7 | ✓ | – | [158] |
| 5-Hydroxtryptophan | IL-2-CD8 +T cell exhaustion | 4T1 | ✓ | ✓ | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safe, S.; Zhang, L. The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer. Cancers 2022, 14, 5574. https://doi.org/10.3390/cancers14225574
Safe S, Zhang L. The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer. Cancers. 2022; 14(22):5574. https://doi.org/10.3390/cancers14225574
Chicago/Turabian StyleSafe, Stephen, and Lei Zhang. 2022. "The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer" Cancers 14, no. 22: 5574. https://doi.org/10.3390/cancers14225574
APA StyleSafe, S., & Zhang, L. (2022). The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer. Cancers, 14(22), 5574. https://doi.org/10.3390/cancers14225574







