Rectal Sparing Approaches after Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-Analysis Comparing Local Excision and Watch and Wait
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Registration
2.2. Literature Search and Review, and Studies Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Statistical Methods
2.5. Quality Evaluation of Evidence
3. Results
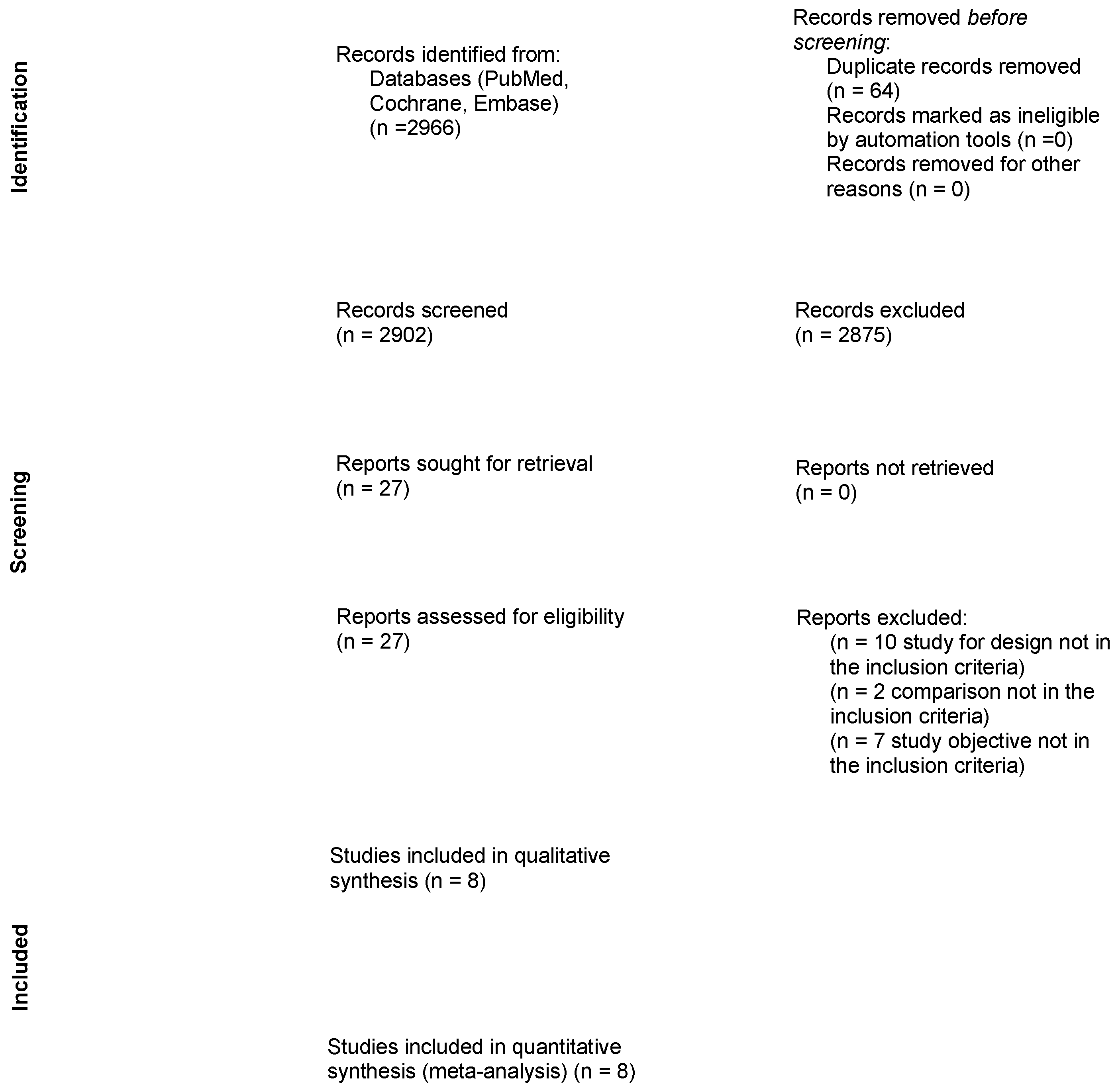
3.1. Study Selection
3.2. Study Design, Definition of Clinical Response, and Aspects of Staging and Treatment
| Author | Year | Design of the Study | NAT | Assessment of Clinical Response | Definition of Complete Clinical Response | Indication for Completion Surgery Following LE | |
|---|---|---|---|---|---|---|---|
| Timing (Weeks) | Exams | ||||||
| Vaccaro | 2016 | Retrospective, monocenter | LCCRT | 8–12 | Rectoscopy, CEA, MRI, and CT scan. | Flat mucosa or white scar, teleangectasia. | Not reported |
| Creavin | 2016 | Retrospective, monocenter | LCCRT | 6–8 | DRE, sigmoidoscopy, CEA, MRI, and CT scan. | Visible scar only or residual scar/ulcer <3 cm | ypT ≥ 2 Positive margins |
| Asoglu | 2019 | Retrospective, monocenter | TNT | 4 | Sigmoidoscopy, MRI, PET-CT | Flat mucosa or white scar, teleangectasia. MRI and PET-CT complete response, MRI TRG 1–2, absence of FDG uptake | Not reported |
| Park | 2019 | Retrospective, monocenter | LCCRT | 4–6 | DRE, sigmoidoscopy, CT, MRI | Not defined | ypT ≥ 2 ypT1, with SM ≥ 2, LNI, PNI, tumor budding, positive margins |
| Yeom | 2019 | Retrospective, monocenter | LCCRT | 5–6 | MRI | No residual tumor or residual fibrosis; no suspicious metastatic lymph nodes | Not reported |
| Al-Najami | 2021 | Retrospective, monocenter | LCRRT | 16 | DRE, sigmoidoscopy, MRI | Flat mucosa or white scar, MRI TRG1 | Not reported |
| Lee | 2021 | Retrospective, multicenter | LCCRT | 4–12 | DRE, sigmoidoscopy, CEA, MRI, and CT scan. | Scar or small ulcer, ycT0N0 | Not reported |
| Wang | 2022 | Phase II, monocenter | TNT | 16–20 | DRE, sigmoidoscopy, CEA, MRI, and CT scan. | Flat, white scar, no ulcer, no nodularity. DRE: normal. MRI: T2 dark signal, no visible lymphnodes, DWI no visible signal, lack/low signal ADC | Poor differentiation, LNI, PNI, positive margins |
3.3. Post-Treatment Complications and Short-Terms Outcomes
3.4. Local Regrowth, Local and Distant Recurrences, Rectum-Preservation Rate
| Author | Year | Patients | Completion TME in LE | Follow-Up | Local Regrowth | Local Recurrence | Distant Recurrence | Organ Preservation | Disease-Free Survival (DFS) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | LE | Required/Performed | WW | LE | WW | LE | WW | LE | WW | LE | ||||
| Vaccaro | 2016 | 23 | 7 | 0/0 | 46 | 4 (18.5) | 0 (0) | 1 (4.3) | 1 (14.3) | 21 (91) | 7 (100) | 3-year 94.1% | ||
| Creavin | 2016 | 10 | 50 | 15/15 | 42 | 29 | 1 (10) | 3 (6) | 1 (10) | 5 (10) | 9 (90) | 33 (66) | 80% | 80% |
| Asoglu | 2019 | 39 | 6 | 0/0 | 22 | 6 (15.4) | 1 (16.6) | 2 (5.1) | 1 (16.6) | 36 (92.3) | 5 (83.3) | |||
| Park | 2019 | 32 | 42 | 11/0 | 73 | 65 | 10 (31.3) | 2 (4.8) | 1 (3.1) | 4 (9.5) | 27 (84.3) | 42 (100) | 5-year RFS 69.8% | 5-year RFS 84.6% |
| Yeom | 2019 | 15 | 25 | NR | 20 | 30 | 4 (26.7) | 4 (16) | 5 (33.3) | 6 (24) | 14 (93.3) | 23 (92.0) | 3-year DFS 72.9% | 3-year DFS 55.5% |
| Al-Najami | 2021 | 42 | 22 | 16/3 | 53 | 11 (26) | 10 (45) | 4 (18.2) | 38 (95) | 16 (73) | 3-year 66.2% | |||
| Lee | 2021 | 14 | 30 | 64.7 | 3 (21.4) | 4 (13.3) | 0 (0) | 2 (6.7) | 12 (85.7) | 27 (90) | 5-year DFS 76.4% | |||
| Wang | 2022 | 38 | 6 | 0/0 | 39.5 | 7 (18.4) | 0 (0) | 2 (5.3) | 1 (16.7) | 33 (86.8) | 6 (100) | |||
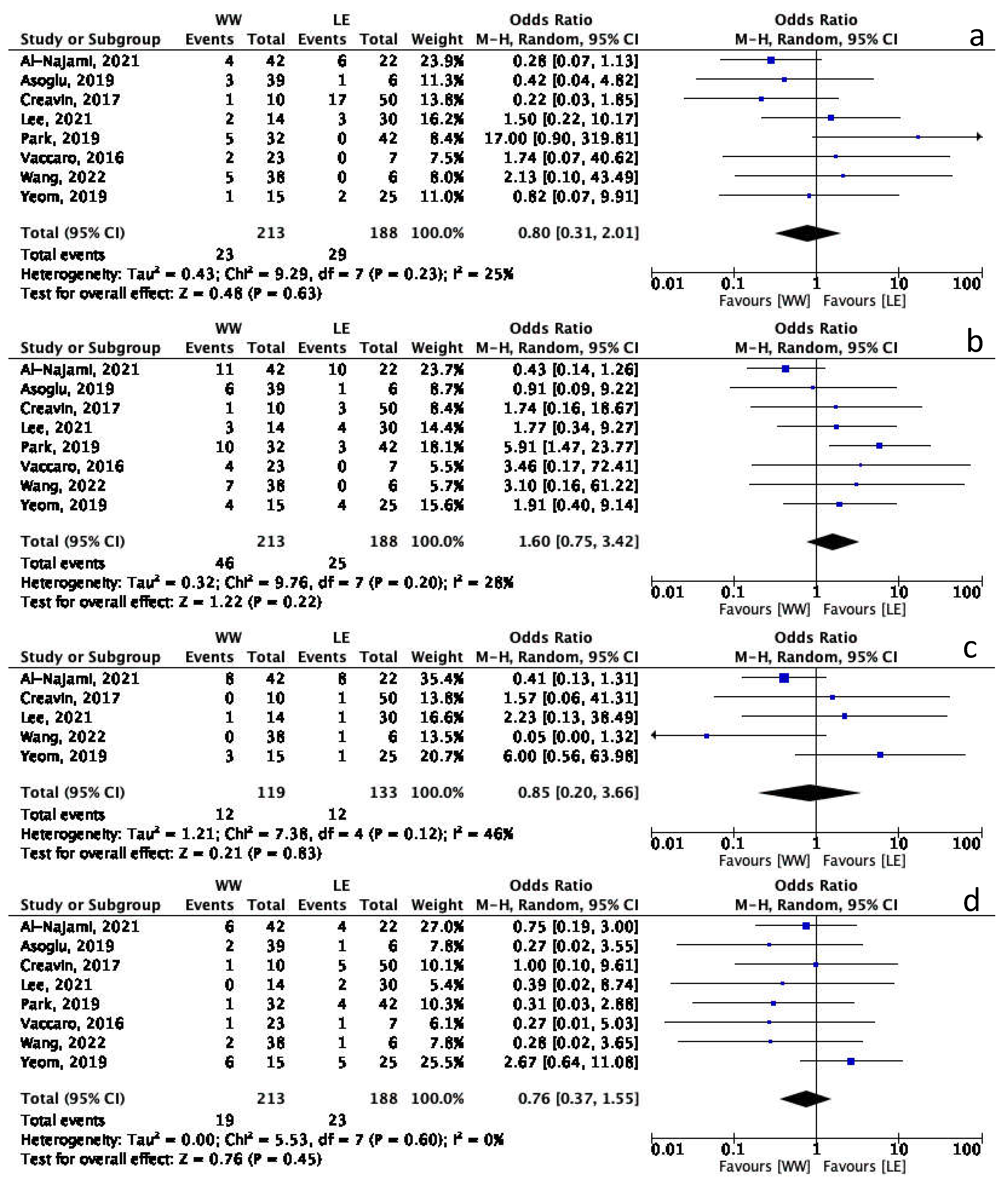
3.5. Watch and Wait vs. Local Excision: Long-Term Outcomes Meta-Analysis
3.6. Overall Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network. Rectal Cancer (Version 1.2022). 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 1 July 2022).
- Pucciarelli, S.; Giandomenico, F.; De Paoli, A.; Gavaruzzi, T.; Lotto, L.; Mantello, G.; Barba, C.; Zotti, P.; Flora, S.; Del Bianco, P. Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br. J. Surg. 2017, 104, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717; discussion 717–718. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, S.; De Paoli, A.; Guerrieri, M.; La Torre, G.; Maretto, I.; De Marchi, F.; Mantello, G.; Gambacorta, M.A.; Canzonieri, V.; Nitti, D.; et al. Local excision after preoperative chemoradiotherapy for rectal cancer: Results of a multicenter phase II clinical trial. Dis. Colon Rectum 2013, 56, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, L.; Bao, Q.R.; Spolverato, G.; Capelli, G.; Del Bianco, P.; Albertoni, L.; De Paoli, A.; Guerrieri, M.; Mantello, G.; Gambacorta, M.A.; et al. Long-Term Outcomes of Local Excision Following Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Ann. Surg. Oncol. 2020, 28, 2801–2808. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Austin, T.M.; Richter, R.R.; Sebelski, C.A. Introduction to the GRADE approach for guideline development: Considerations for physical therapist practice. Phys. Ther. 2014, 94, 1652–1659. [Google Scholar] [CrossRef]
- Sammour, T.; Price, B.A.; Krause, K.J.; Chang, G.J. Nonoperative Management or ‘Watch and Wait’ for Rectal Cancer with Complete Clinical Response After Neoadjuvant Chemoradiotherapy: A Critical Appraisal. Ann. Surg. Oncol. 2017, 24, 1904–1915. [Google Scholar] [CrossRef]
- Al-Najami, I.; Jones, H.J.; Dickson, E.A.; Muirhead, R.; Deding, U.; James, D.R.; Cunningham, C. Rectal cancer: Watch-and-wait and continuing the rectal-preserving strategy with local excision for incomplete response or limited regrowth. Surg. Oncol. 2021, 37, 101574. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Wynn, G.; Marks, J.; Kessler, H.; Gama-Rodrigues, J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 2010, 53, 1692–1698. [Google Scholar] [CrossRef]
- Vaccaro, C.A.; Yazyi, F.J.; Ojra Quintana, G.; Santino, J.P.; Sardi, M.E.; Beder, D.; Tognelli, J.; Bonadeo, F.; Lastiri, J.M.; Rossi, G.L. Locally advanced rectal cancer: Preliminary results of rectal preservation after neoadjuvant chemoradiotherapy. Cir. Esp. 2016, 94, 274–279. [Google Scholar] [CrossRef]
- Creavin, B.; Ryan, E.; Martin, S.T.; Hanly, A.; O’Connell, P.R.; Sheahan, K.; Winter, D.C. Organ preservation with local excision or active surveillance following chemoradiotherapy for rectal cancer. Br. J. Cancer 2017, 116, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.S.; Lee, S.Y.; Kim, C.H.; Kim, Y.J.; Nam, T.K.; Kim, H.R. Non-operative treatment outcome for rectal cancer patient with clinical complete response after neoadjuvant chemoradiotherapy. Asian J. Surg. 2019, 42, 823–831. [Google Scholar] [CrossRef]
- Lee, J.K.; Cho, J.R.; Song, K.S.; Oh, J.H.; Jeong, S.Y.; Kim, M.J.; Lee, J.; Kim, M.H.; Oh, H.K.; Kim, D.W.; et al. Oncologic comparison between nonradical management and total mesorectal excision in good responders after chemoradiotherapy in patients with mid-to-low rectal cancer. Ann. Surg. Treat. Res. 2021, 101, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Asoglu, O.; Tokmak, H.; Bakir, B.; Demir, G.; Ozyar, E.; Atalar, B.; Goksel, S.; Koza, B.; Guven Mert, A.; Demir, A.; et al. The impact of total neo-adjuvant treatment on nonoperative management in patients with locally advanced rectal cancer: The evaluation of 66 cases. Eur. J. Surg. Oncol. 2020, 46, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Chow, O.S.; Gollub, M.J.; Nash, G.M.; Temple, L.K.; Weiser, M.R.; Guillem, J.G.; Paty, P.B.; Avila, K.; Garcia-Aguilar, J. Organ Preservation in Rectal Adenocarcinoma: A phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015, 15, 767. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.Y.; Zhao, Y.M.; Li, S.J.; Li, Z.W.; Sun, Y.S.; Wang, W.H.; Wu, A.W. Intentional Watch & Wait or Organ Preservation Surgery Following Neoadjuvant Chemoradiotherapy Plus Consolidation CAPEOX for MRI-defined Low-risk Rectal Cancer: Findings from a Prospective Phase 2 Trial (PKUCH-R01 Trial, NCT02860234). Ann. Surg. 2022. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, J.L.; Yoon, Y.S.; Kim, C.W.; Lim, S.B.; Yu, C.S.; Kim, J.C. Oncologic Outcomes of Organ Preserving Approaches in Patients With Rectal Cancer Treated With Preoperative Chemoradiotherapy. Ann. Coloproctol. 2019, 35, 65–71. [Google Scholar] [CrossRef]
- Rullier, E.; Rouanet, P.; Tuech, J.J.; Valverde, A.; Lelong, B.; Rivoire, M.; Faucheron, J.L.; Jafari, M.; Portier, G.; Meunier, B.; et al. Organ preservation for rectal cancer (GRECCAR 2): A prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017, 390, 469–479. [Google Scholar] [CrossRef]
- Levic Souzani, K.; Bulut, O.; Kuhlmann, T.P.; Gögenur, I.; Bisgaard, T. Completion total mesorectal excision following transanal endoscopic microsurgery does not compromise outcomes in patients with rectal cancer. Surg. Endosc. 2022, 36, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Clermonts, S.; Köeter, T.; Pottel, H.; Stassen, L.P.S.; Wasowicz, D.K.; Zimmerman, D.D.E. Outcomes of completion total mesorectal excision are not compromised by prior transanal minimally invasive surgery. Color. Dis. 2020, 22, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Aref, A.; Alame, A.M.; Drelichman, E.R.; Hawasli, A. Should Local Excision After Neoadjuvant Therapy Be Included in the National Guidelines for the Treatment of Locally Advanced Rectal Cancer? Dis. Colon Rectum 2022, 65, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Dattani, M.; Heald, R.J.; Goussous, G.; Broadhurst, J.; Sao Juliao, G.P.; Habr-Gama, A.; Perez, R.O.; Moran, B.J. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann. Surg. 2018, 268, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Capelli, G.; De Simone, I.; Spolverato, G.; Cinquini, M.; Moschetti, I.; Lonardi, S.; Masi, G.; Carlomagno, C.; Corsi, D.; Luppi, G.; et al. Non-Operative Management Versus Total Mesorectal Excision for Locally Advanced Rectal Cancer with Clinical Complete Response After Neoadjuvant Chemoradiotherapy: A GRADE Approach by the Rectal Cancer Guidelines Writing Group of the Italian Association of Medical Oncology (AIOM). J. Gastrointest. Surg. 2020, 24, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Strombom, P.; Chow, O.S.; Roxburgh, C.S.; Lynn, P.; Eaton, A.; Widmar, M.; Ganesh, K.; Yaeger, R.; Cercek, A.; et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019, 5, e185896. [Google Scholar] [CrossRef] [PubMed]
- Rullier, E.; Vendrely, V.; Asselineau, J.; Rouanet, P.; Tuech, J.J.; Valverde, A.; de Chaisemartin, C.; Rivoire, M.; Trilling, B.; Jafari, M.; et al. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol. Hepatol. 2020, 5, 465–474. [Google Scholar] [CrossRef]
- Marchegiani, F.; Palatucci, V.; Capelli, G.; Guerrieri, M.; Belluco, C.; Rega, D.; Morpurgo, E.; Coco, C.; Restivo, A.; De Franciscis, S.; et al. Rectal Sparing Approach After Neoadjuvant Therapy in Patients with Rectal Cancer: The Preliminary Results of the ReSARCh Trial. Ann. Surg. Oncol. 2022, 29, 1880–1889. [Google Scholar] [CrossRef]
- Felder, S.I.; Patil, S.; Kennedy, E.; Garcia-Aguilar, J. Endoscopic Feature and Response Reproducibility in Tumor Assessment after Neoadjuvant Therapy for Rectal Adenocarcinoma. Ann. Surg. Oncol. 2021, 28, 5205–5223. [Google Scholar] [CrossRef]
- Fokas, E.; Appelt, A.; Glynne-Jones, R.; Beets, G.; Perez, R.; Garcia-Aguilar, J.; Rullier, E.; Joshua Smith, J.; Marijnen, C.; Peters, F.P.; et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat. Rev. Clin. Oncol. 2021, 18, 805–816. [Google Scholar] [CrossRef]
- Kong, J.C.; Soucisse, M.; Michael, M.; Tie, J.; Ngan, S.Y.; Leong, T.; McCormick, J.; Warrier, S.K.; Heriot, A.G. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann. Surg. Oncol. 2021, 28, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Barina, A.; De Paoli, A.; Delrio, P.; Guerrieri, M.; Muratore, A.; Bianco, F.; Vespa, D.; Asteria, C.; Morpurgo, E.; Restivo, A.; et al. Rectal sparing approach after preoperative radio- and/or chemotherapy (RESARCH) in patients with rectal cancer: A multicentre observational study. Tech. Coloproctol. 2017, 21, 633–640. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Q.R.; Ferrari, S.; Capelli, G.; Ruffolo, C.; Scarpa, M.; Agnes, A.; Chiloiro, G.; Palazzari, E.; Urso, E.D.L.; Pucciarelli, S.; et al. Rectal Sparing Approaches after Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-Analysis Comparing Local Excision and Watch and Wait. Cancers 2023, 15, 465. https://doi.org/10.3390/cancers15020465
Bao QR, Ferrari S, Capelli G, Ruffolo C, Scarpa M, Agnes A, Chiloiro G, Palazzari E, Urso EDL, Pucciarelli S, et al. Rectal Sparing Approaches after Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-Analysis Comparing Local Excision and Watch and Wait. Cancers. 2023; 15(2):465. https://doi.org/10.3390/cancers15020465
Chicago/Turabian StyleBao, Quoc Riccardo, Stefania Ferrari, Giulia Capelli, Cesare Ruffolo, Marco Scarpa, Amedea Agnes, Giuditta Chiloiro, Elisa Palazzari, Emanuele Damiano Luca Urso, Salvatore Pucciarelli, and et al. 2023. "Rectal Sparing Approaches after Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-Analysis Comparing Local Excision and Watch and Wait" Cancers 15, no. 2: 465. https://doi.org/10.3390/cancers15020465
APA StyleBao, Q. R., Ferrari, S., Capelli, G., Ruffolo, C., Scarpa, M., Agnes, A., Chiloiro, G., Palazzari, E., Urso, E. D. L., Pucciarelli, S., & Spolverato, G. (2023). Rectal Sparing Approaches after Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-Analysis Comparing Local Excision and Watch and Wait. Cancers, 15(2), 465. https://doi.org/10.3390/cancers15020465