Development and Validation of a Predictive Model for Toxicity of Neoadjuvant Chemoradiotherapy in Rectal Cancer in the CAO/ARO/AIO-04 Phase III Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
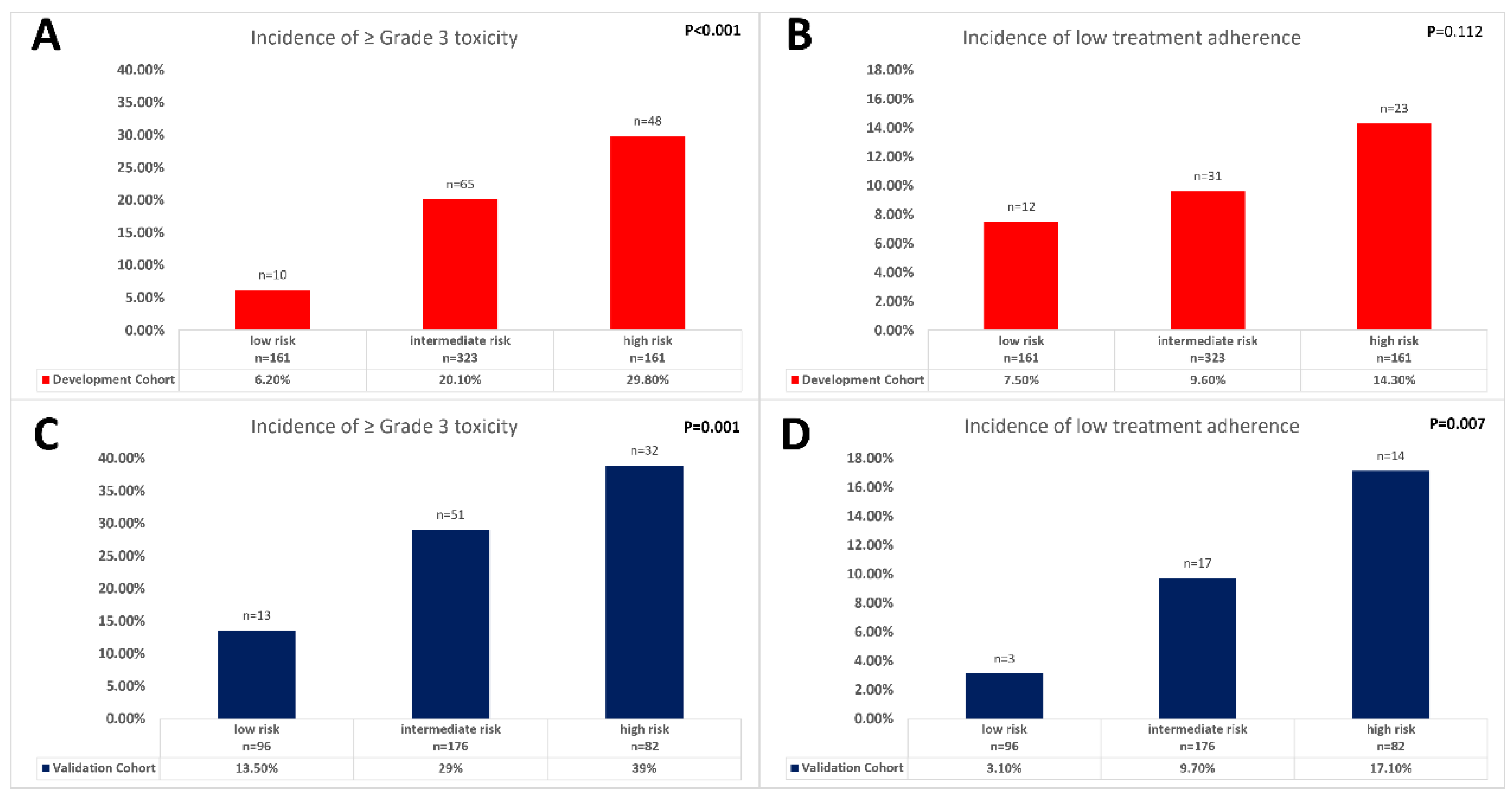
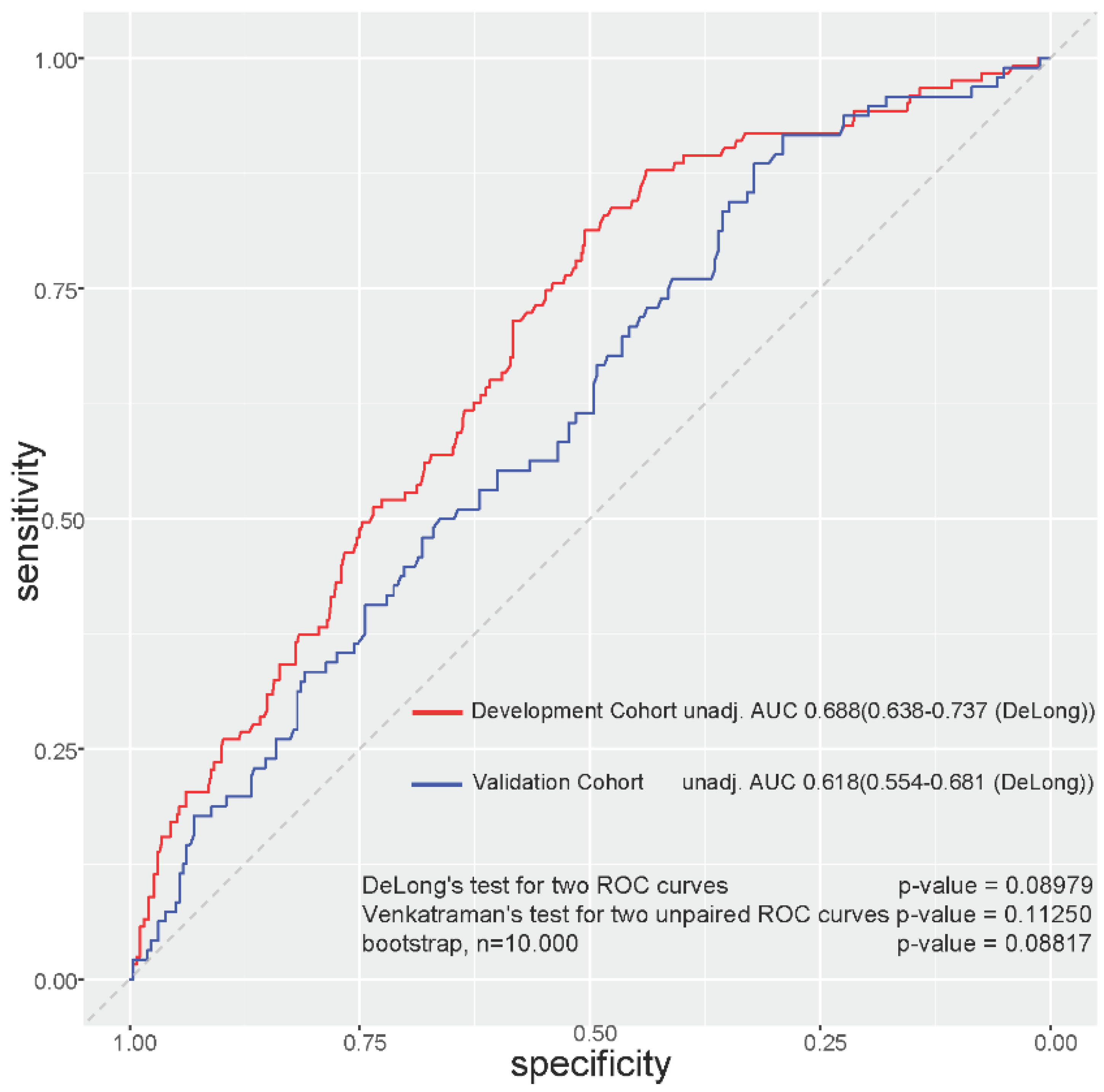
3.1. Development and Validation Cohort
3.2. Association between QLQ and Toxicity
3.3. Binary Logistic Regression Models for Clinical Characteristics, Blood Parameters, and QoL
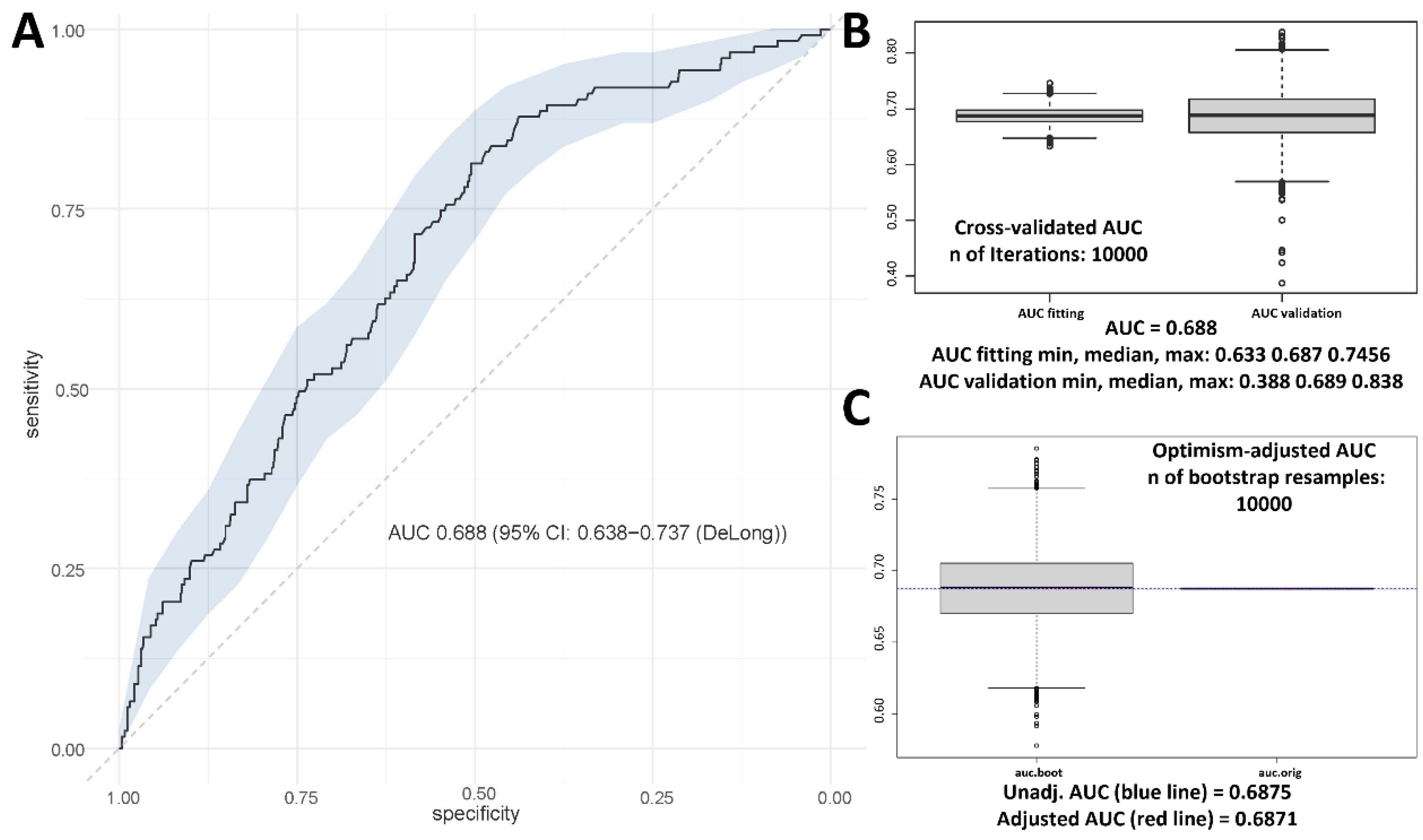
3.4. Best Subset Selection of the Predictive Model for Toxicity
3.5. Predictive Toxicity Model Using BMI, Gender, and Emotional Functioning
3.6. Development and Validation Cohort Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv263. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Allgauer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.M.; Jiang, J.; Li, N.; Liu, W.Y.; Chen, S.L.; Li, S.; Lu, N.N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef]
- Fernandez, L.M.; Sao Juliao, G.P.; Figueiredo, N.L.; Beets, G.L.; van der Valk, M.J.M.; Bahadoer, R.R.; Hilling, D.E.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Renehan, A.G.; et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: A retrospective, international, multicentre registry study. Lancet Oncol. 2021, 22, 43–50. [Google Scholar] [CrossRef]
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef]
- Lopez-Campos, F.; Martin-Martin, M.; Fornell-Perez, R.; Garcia-Perez, J.C.; Die-Trill, J.; Fuentes-Mateos, R.; Lopez-Duran, S.; Dominguez-Rullan, J.; Ferreiro, R.; Riquelme-Oliveira, A.; et al. Watch and wait approach in rectal cancer: Current controversies and future directions. World J. Gastroenterol. 2020, 26, 4218–4239. [Google Scholar] [CrossRef]
- Bosset, J.F.; Calais, G.; Daban, A.; Berger, C.; Radosevic-Jelic, L.; Maingon, P.; Bardet, E.; Pierart, M.; Briffaux, A.; Group, E.R. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: Assessment of acute toxicity and treatment compliance. Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur. J. Cancer 2004, 40, 219–224. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Meadows, H.M.; Lopes, A.; Muirhead, R.; Sebag-Montefiore, D.; Adams, R.; ACTII Study Group. Impact of compliance to chemoradiation on long-term outcomes in squamous cell carcinoma of the anus: Results of a post hoc analysis from the randomised phase III ACT II trial. Ann. Oncol. 2020, 31, 1376–1385. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Tu, H.A.; Tourangeau, A.; Howell, D.; Fitch, M.; Springall, E.; Alibhai, S.M.H. Factors influencing adherence to cancer treatment in older adults with cancer: A systematic review. Ann. Oncol. 2014, 25, 564–577. [Google Scholar] [CrossRef]
- Schuurhuizen, C.; Marino, P.; Braamse, A.M.J.; Buffart, L.M.; Joly, F.; Fizazi, K.; Habibian, M.; Boher, J.M.; Soulie, M.; Oudard, S.; et al. Impact of Patient- and Clinician-Reported Cumulative Toxicity on Quality of Life in Patients With Metastatic Castration-Naive Prostate Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 1481–1488. [Google Scholar] [CrossRef]
- Gomez, D.; Calderon, C.; Carmona-Bayonas, A.; Cacho Lavin, D.; Munoz, M.M.; Martinez Cabanez, R.; Jimenez-Fonseca, P. Impact of adjuvant therapy toxicity on quality of life and emotional symptoms in patients with colon cancer: A latent class analysis. Clin. Transl. Oncol. 2021, 23, 657–662. [Google Scholar] [CrossRef]
- Gani, C.; Gani, N.; Zschaeck, S.; Eberle, F.; Schaeffeler, N.; Hehr, T.; Berger, B.; Fischer, S.G.; Classen, J.; Zipfel, S.; et al. Organ Preservation in Rectal Cancer: The Patients’ Perspective. Front. Oncol. 2019, 9, 318. [Google Scholar] [CrossRef]
- Wolff, H.A.; Conradi, L.C.; Schirmer, M.; Beissbarth, T.; Sprenger, T.; Rave-Frank, M.; Hennies, S.; Hess, C.F.; Becker, H.; Christiansen, H.; et al. Gender-specific acute organ toxicity during intensified preoperative radiochemotherapy for rectal cancer. Oncologist 2011, 16, 621–631. [Google Scholar] [CrossRef]
- Wolff, H.A.; Conradi, L.C.; Beissbarth, T.; Leha, A.; Hohenberger, W.; Merkel, S.; Fietkau, R.; Raab, H.R.; Tschmelitsch, J.; Hess, C.F.; et al. Gender affects acute organ toxicity during radiochemotherapy for rectal cancer: Long-term results of the German CAO/ARO/AIO-94 phase III trial. Radiother Oncol. 2013, 108, 48–54. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Benson, A.B., 3rd; Macdonald, J.S.; Fuchs, C.S. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003, 98, 484–495. [Google Scholar] [CrossRef]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.D.; Ghadimi, M.; Minsky, B.D.; Fleischmann, M.; Fokas, E.; Rodel, C. Impact of body-mass index on treatment and outcome in locally advanced rectal cancer: A secondary, post-hoc analysis of the CAO/ARO/AIO-04 randomized phase III trial. Radiother Oncol. 2021, 164, 223–231. [Google Scholar] [CrossRef]
- Marijnen, C.A. Organ preservation in rectal cancer: Have all questions been answered? Lancet Oncol. 2015, 16, e13–e22. [Google Scholar] [CrossRef]
- Rodel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- EORTC. EORTC QLQ-C30 Scoring Manual. Available online: https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf (accessed on 9 September 2022).
- Law, C.C.; Tak Lam, W.W.; Fu, Y.T.; Wong, K.H.; Sprangers, M.A.; Fielding, R. Validation of the Chinese version of the EORTC colorectal cancer-specific quality-of-life questionnaire module (QLQ-CR38). J. Pain Symptom Manag. 2008, 35, 203–213. [Google Scholar] [CrossRef]
- Magnuson, A.; Sedrak, M.S.; Gross, C.P.; Tew, W.P.; Klepin, H.D.; Wildes, T.M.; Muss, H.B.; Dotan, E.; Freedman, R.A.; O’Connor, T.; et al. Development and Validation of a Risk Tool for Predicting Severe Toxicity in Older Adults Receiving Chemotherapy for Early-Stage Breast Cancer. J. Clin. Oncol. 2021, 39, 608–618. [Google Scholar] [CrossRef]
- Hurria, A.; Mohile, S.; Gajra, A.; Klepin, H.; Muss, H.; Chapman, A.; Feng, T.; Smith, D.; Sun, C.L.; De Glas, N.; et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J. Clin. Oncol. 2016, 34, 2366–2371. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Palta, M.; Willett, C.G.; Czito, B.G. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer 2017, 123, 1497–1506. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs. Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.C.; Csajka, C.; Dotto, G.P.; Wagner, A.D. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. J Clin. Oncol. 2018, 36, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, E.M.; Krumholz, H.A.; Krumholz, H.M. Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries. PLoS Med. 2016, 13, e1001998. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, D.L.P.; Partridge, M.; Hawkins, M.A. Systematic review and meta-analysis of small bowel dose-volume and acute toxicity in conventionally-fractionated rectal cancer radiotherapy. Radiother. Oncol. 2019, 138, 38–44. [Google Scholar] [CrossRef]
- Tavoli, A.; Tavoli, Z.; Montazeri, A. The Relationship Between Emotional Functioning of the EORTC QLQ-C30 and A Measure of Anxiety and Depression (HADS) in Cancer Patients. Int. J. Cancer Manag. 2019, 12, e94568. [Google Scholar] [CrossRef]
- Reisinger, M.W.; Moss, M.; Clark, B.J. Is lack of social support associated with a delay in seeking medical care? A cross-sectional study of Minnesota and Tennessee residents using data from the Behavioral Risk Factor Surveillance System. BMJ Open 2018, 8, e018139. [Google Scholar] [CrossRef]
- Amonoo, H.L.; El-Jawahri, A.; Deary, E.C.; Traeger, L.N.; Cutler, C.S.; Antin, J.A.; Huffman, J.C.; Lee, S.J. Yin and Yang of Psychological Health in the Cancer Experience: Does Positive Psychology Have a Role? J. Clin. Oncol. 2022, 40, 2402–2407. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; Palmer, M.J.; Brundage, M.; Calvert, M.; Stockler, M.R.; King, M.T. Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: A systematic review. BMJ Open 2016, 6, e010938. [Google Scholar] [CrossRef]
- Gilbert, A.; Ziegler, L.; Martland, M.; Davidson, S.; Efficace, F.; Sebag-Montefiore, D.; Velikova, G. Systematic Review of Radiation Therapy Toxicity Reporting in Randomized Controlled Trials of Rectal Cancer: A Comparison of Patient-Reported Outcomes and Clinician Toxicity Reporting. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 555–567. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- EMA. EMA Recommendations on DPD Testing Prior to Treatment with Fluorouracil, Capecitabine, Tegafur and Flucytosine. Available online: https://www.ema.europa.eu/en/documents/referral/fluorouracil-fluorouracil-related-substances-article-31-referral-ema-recommendations-dpd-testing_en.pdf (accessed on 5 June 2022).
| Characteristics | No. | Low-Grade Toxicity n = 676 | High-Grade Toxicity n = 155 | p-Value |
|---|---|---|---|---|
| Percentage | 81.3% | 18.7% | ||
| Treatment Arm | ||||
| 5-FU arm | 420 | 347 (82.6%) | 73 (17.4%) | |
| 5-FU/Ox arm | 408 | 326 (79.9%) | 82 (20.1%) | 0.316 * |
| Age | ||||
| continuous | 831 | 676 (81.3%) | 155 (18.7%) | 0.749 ** |
| (median age) | 63.50 years | 62.70 years | ||
| Gender | ||||
| Male | 590 | 501 (84.9%) | 89 (15.1%) | |
| Female | 241 | 175 (72.6%) | 66 (27.4%) | <0.001 * |
| ECOG | ||||
| Grade 0 | 617 | 508 (82.3%) | 109 (17.7%) | |
| Grade 1 + 2 | 202 | 159 (78.7%) | 43 (21.3%) | 0.251 * |
| BMI | ||||
| continuous | 827 | 672 (81.3%) | 155 (18.7%) | 0.001 ** |
| (median BMI) | 26.75 kg/m2 | 25.3 kg/m2 | ||
| cT-category | ||||
| cT2 | 41 | 29 (70.7%) | 12 (29.3%) | |
| cT3 | 736 | 605 (82.2%) | 131 (17.8%) | |
| cT4 | 49 | 37 (75.5%) | 12 (24.5%) | 0.107 * |
| cN-category | ||||
| cN0 | 219 | 177 (80.8%) | 42 (19.2%) | |
| cN1/cN2 | 593 | 482 (81.3%) | 111 (18.7%) | 0.882 * |
| Grading | ||||
| G1 | 49 | 43 (87.8%) | 6 (12.2%) | |
| G2 | 658 | 531 (80.7%) | 127 (19.3%) | |
| G3 | 70 | 62 (88.6%) | 8 (11.4%) | 0.145 * |
| Tumor Localization | ||||
| Low | 308 | 247 (80.2%) | 61 (19.8%) | |
| Middle/High | 511 | 418 (81.8%) | 93 (18.2%) | 0.569 * |
| Parameter | OR (95% CI) | Beta Coefficient | Response | Formula | Value |
|---|---|---|---|---|---|
| Gender | 1.922 (1.259–2.932) | 0.653 | Male Female | 44 points 88 points | |
| BMI (continuous) | 0.933 (0.888–0.980) | −0.070 | kg/m2 | ________ kg/m2 × 5 | -_______points |
| Emotional functioning | 0.985 (0.977–0.992) | −0.015 | |||
| Calculation as follows: | |||||
| Did you feel tense? | |||||
| Not at All | A Little | Quite a Bit | Very Much | ||
| 1 point | 2 points | 3 points | 4 points | ||
| Did you worry? | |||||
| Not at All | A Little | Quite a Bit | Very Much | ||
| 1 point | 2 points | 3 points | 4 points | ||
| Did you feel irritable? | |||||
| Not at All | A Little | Quite a Bit | Very Much | ||
| 1 point | 2 points | 3 points | 4 points | ||
| Did you feel depressed? | |||||
| Not at All | A Little | Quite a Bit | Very Much | ||
| 1 point | 2 points | 3 points | 4 points | ||
| Formula to calculate points (Please insert the sum of the points according to your answers in the gray field of the formula) : | -______points | ||||
| Sum | ______points | ||||
| Risk Prediction | low toxicity risk | intermediate toxicity risk | high toxicity risk | ||
| <−176.333 points | −176.333 to −118.083 points | >−118.083 points | |||
| Characteristics | No. | Development Cohort n = 831 | Validation Cohort n = 405 | p-Value |
|---|---|---|---|---|
| Percentage | 67.2% | 32.8% | ||
| Treatment Arm | ||||
| 5-FU arm | 625 | 420 (50.7%) | 205 (50.7%) | |
| 5-FU/Ox arm | 607 | 408 (49.3%) | 199 (49.3%) | 0.995 * |
| Age | ||||
| continuous | 1236 | 831 (67.2%) | 405 (32.8%) | 0.788 ** |
| ≤67.89 years | 824 | 560 (32.0%) | 264 (34.2%) | |
| >67.89 years | 412 | 271 (68.0%) | 141 (65.8%) | 0.441 * |
| Gender | ||||
| Male | 831 | 590 (71.0%) | 284 (70.1%) | |
| Female | 405 | 241 (29.0%) | 121 (29.9%) | 0.751 * |
| ECOG | ||||
| Grade 0 | 819 | 617 (75.3%) | 341 (84.6%) | |
| Grade 1 + 2 | 403 | 202 (24.7%) | 62 (15.4%) | <0.001 * |
| BMI | ||||
| continuous | 1232 | 827 (67.1%) | 405 (32.9%) | 0.220 ** |
| <20 kg/m2 | 52 | 37 (4.5%) | 15 (3.7%) | |
| 20–24.9 kg/m2 | 369 | 253 (30.6%) | 116 (28.6%) | |
| 25–26.9 kg/m2 | 234 | 159 (19.2%) | 75 (18.5%) | |
| 27–29.9 kg/m2 | 307 | 206 (24.9%) | 101 (24.9%) | |
| ≥30 kg/m2 | 270 | 172 (20.8%) | 88 (24.2%) | 0.693 * |
| cT-category | ||||
| cT2 | 54 | 41 (5.0%) | 13 (3.2%) | |
| cT3 | 1086 | 736 (89.1%) | 350 (86.4%) | |
| cT4 | 91 | 49 (5.9%) | 42 (10.4%) | 0.009 * |
| cN-category | ||||
| cN0 | 305 | 219 (27.0%) | 86 (21.7%) | |
| cN1/cN2 | 903 | 593 (73.0%) | 310 (78.3%) | 0.049 * |
| Grading | ||||
| G1 | 64 | 49 (6.3%) | 15 (3.9%) | |
| G2 | 998 | 658 (84.7%) | 340 (88.5%) | |
| G3 | 99 | 70 (9.0%) | 29 (7.6%) | 0.152 * |
| Tumor Localization | ||||
| Low | 465 | 308 (37.6%) | 157 (39.0%) | |
| Middle/High | 757 | 511 (62.4%) | 246 (61.0%) | 0.647 * |
| Overall neoadjuvant treatment toxicity | ||||
| Grade 0 + 1 + 2 | 971 | 676 (81.3%) | 295 (72.8%) | |
| Grade 3 + 4 | 265 | 155 (18.7%) | 110 (27.2%) | 0.001 * |
| Neoadjuvant Treatment Adherence | ||||
| complete/nearly complete | 1097 | 733 (88.2%) | 364 (89.9%) | |
| incomplete | 139 | 88 (11.8%) | 41 (10.1%) | 0.383 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diefenhardt, M.; Martin, D.; Ludmir, E.B.; Fleischmann, M.; Hofheinz, R.-D.; Ghadimi, M.; Kosmala, R.; Polat, B.; Friede, T.; Minsky, B.D.; et al. Development and Validation of a Predictive Model for Toxicity of Neoadjuvant Chemoradiotherapy in Rectal Cancer in the CAO/ARO/AIO-04 Phase III Trial. Cancers 2022, 14, 4425. https://doi.org/10.3390/cancers14184425
Diefenhardt M, Martin D, Ludmir EB, Fleischmann M, Hofheinz R-D, Ghadimi M, Kosmala R, Polat B, Friede T, Minsky BD, et al. Development and Validation of a Predictive Model for Toxicity of Neoadjuvant Chemoradiotherapy in Rectal Cancer in the CAO/ARO/AIO-04 Phase III Trial. Cancers. 2022; 14(18):4425. https://doi.org/10.3390/cancers14184425
Chicago/Turabian StyleDiefenhardt, Markus, Daniel Martin, Ethan B. Ludmir, Maximilian Fleischmann, Ralf-Dieter Hofheinz, Michael Ghadimi, Rebekka Kosmala, Bülent Polat, Tim Friede, Bruce D. Minsky, and et al. 2022. "Development and Validation of a Predictive Model for Toxicity of Neoadjuvant Chemoradiotherapy in Rectal Cancer in the CAO/ARO/AIO-04 Phase III Trial" Cancers 14, no. 18: 4425. https://doi.org/10.3390/cancers14184425
APA StyleDiefenhardt, M., Martin, D., Ludmir, E. B., Fleischmann, M., Hofheinz, R.-D., Ghadimi, M., Kosmala, R., Polat, B., Friede, T., Minsky, B. D., Rödel, C., & Fokas, E. (2022). Development and Validation of a Predictive Model for Toxicity of Neoadjuvant Chemoradiotherapy in Rectal Cancer in the CAO/ARO/AIO-04 Phase III Trial. Cancers, 14(18), 4425. https://doi.org/10.3390/cancers14184425








