Simultaneous Onset of Haematological Malignancy and COVID: An Epicovideha Survey
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Patients
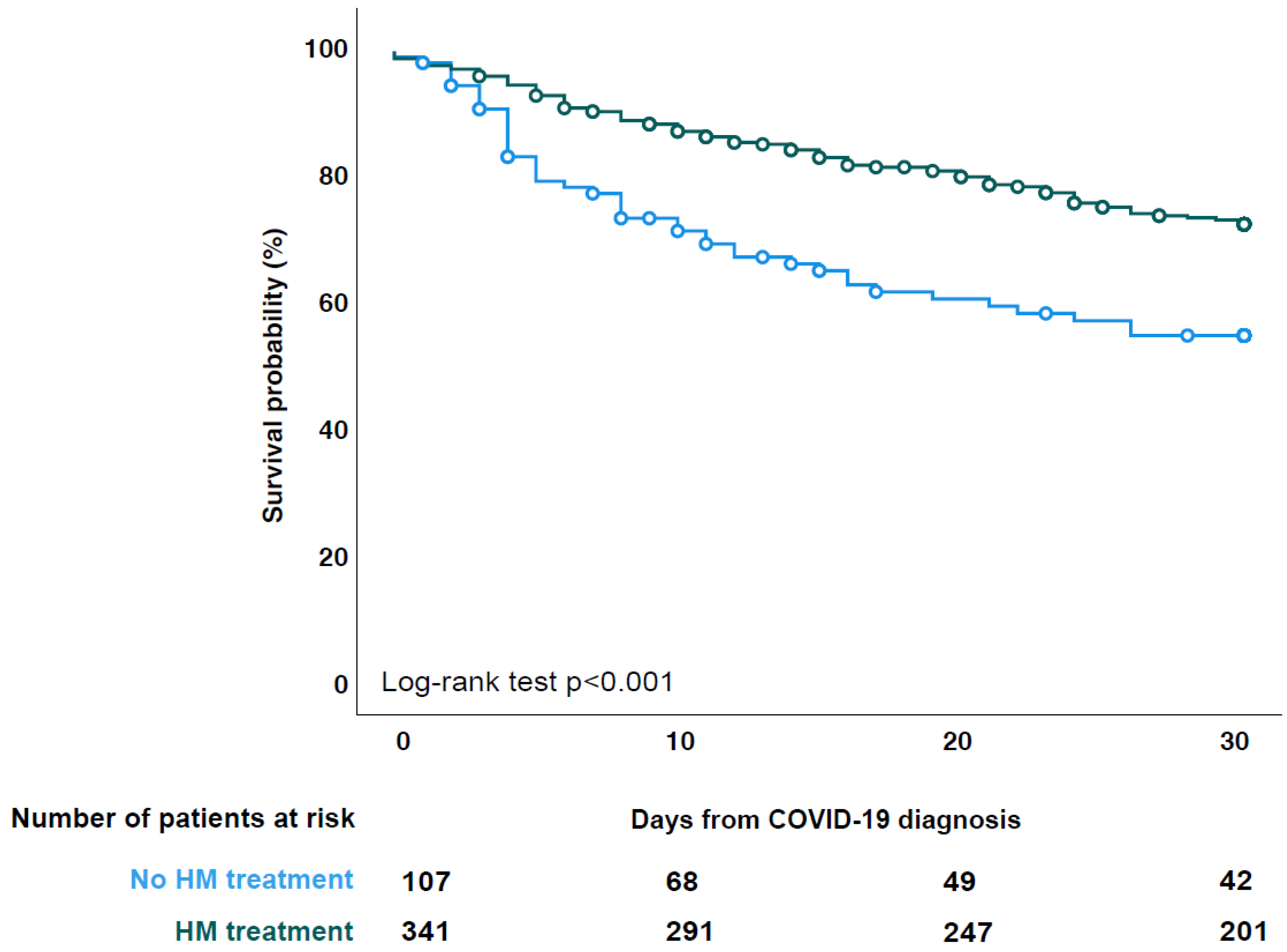
3.2. HM Treatment
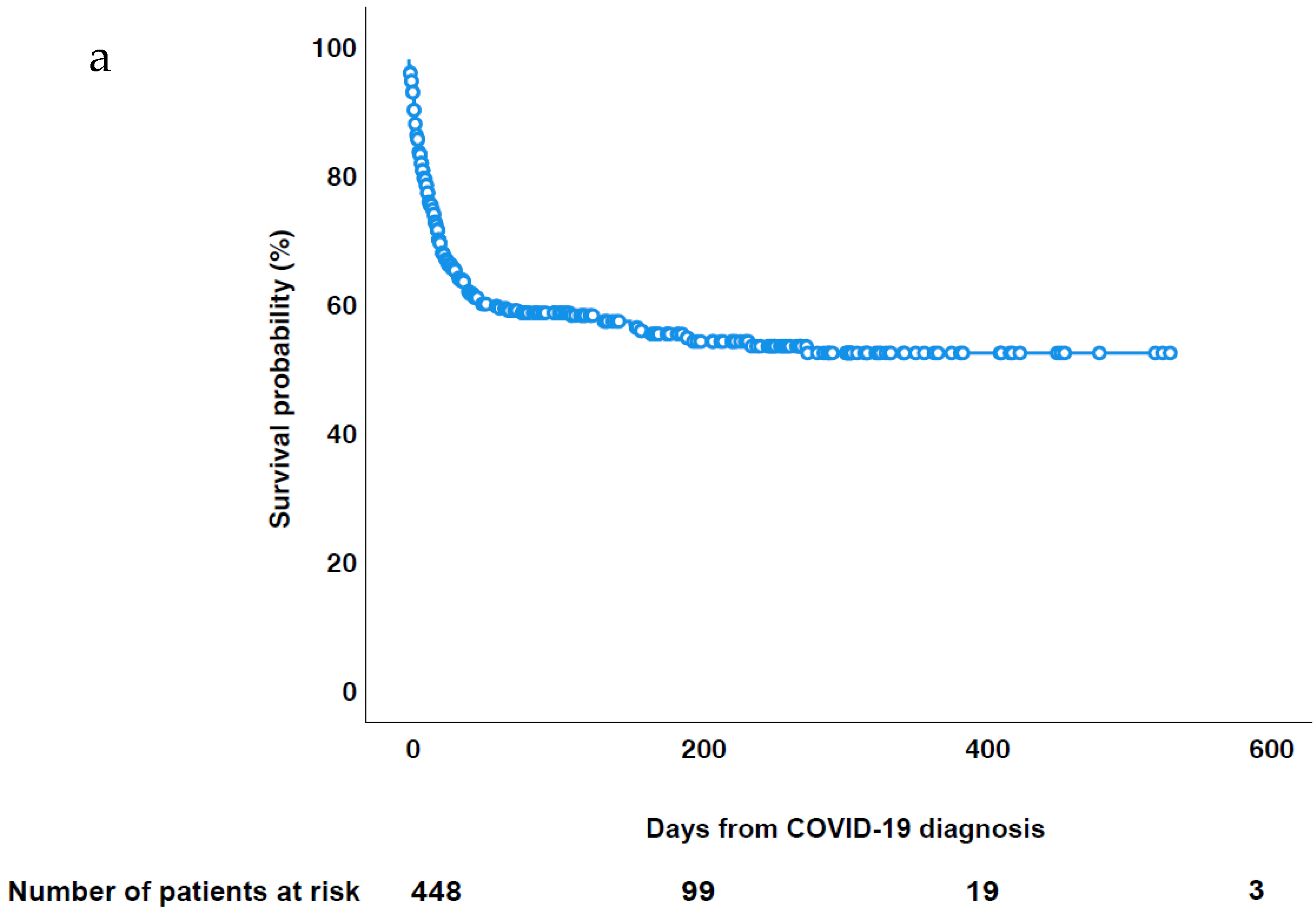
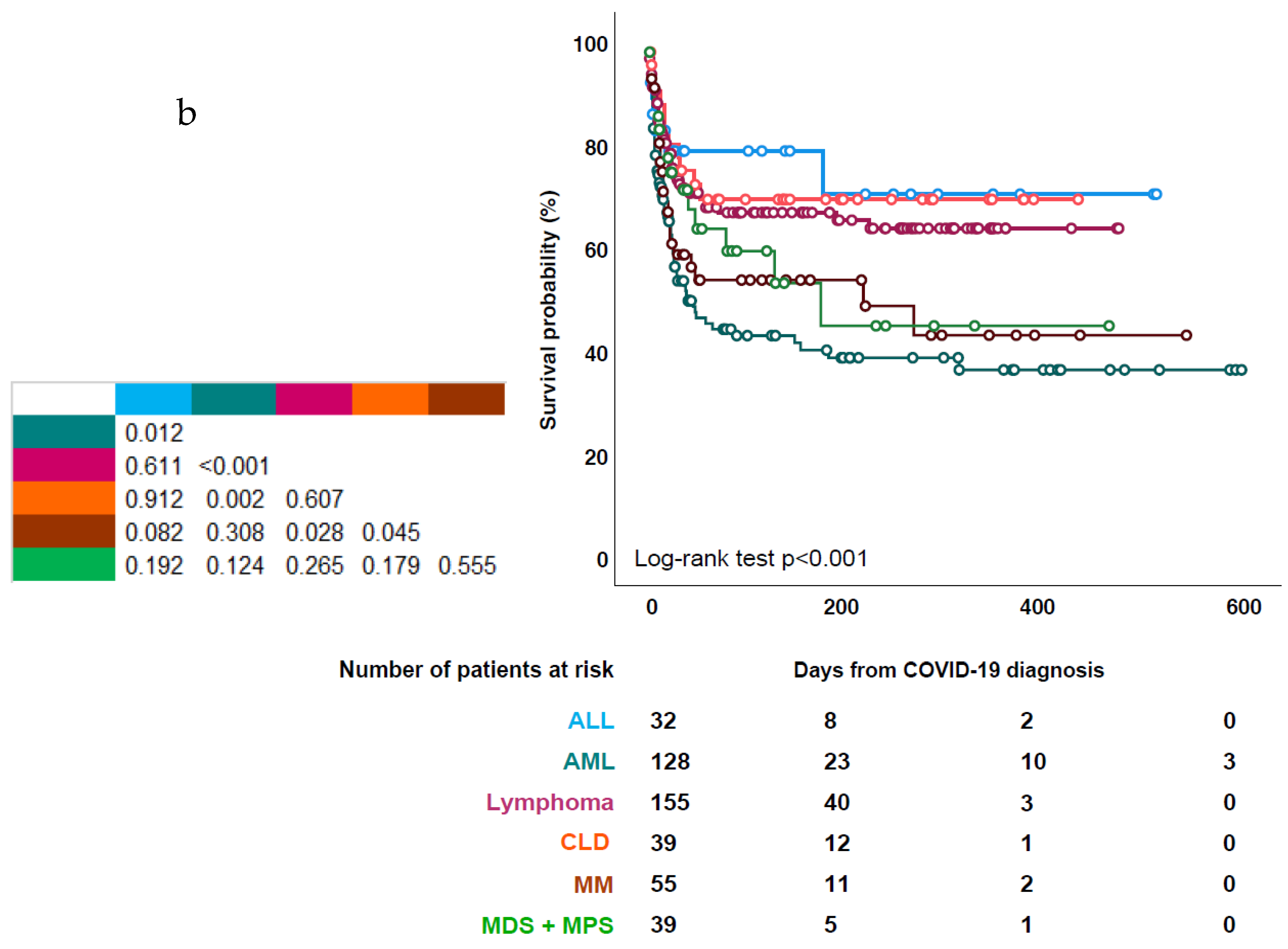
3.3. Patient Outcome
3.4. Risk Factors for Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, J.; de la Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.Á.; Gil-Manso, R.; Kwon, M.; Sánchez-Godoy, P.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Daffini, R.; Pagani, C.; Salvetti, M.; Mancini, V.; Borlenghi, E.; D’Adda, M.; Oberti, M.; Paini, A.; De Ciuceis, C.; et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer 2020, 126, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Boddu, P.C.; Patnaik, M.M.; Bewersdorf, J.P.; Stahl, M.; Rampal, R.K.; Shallis, R.; Steensma, D.P.; Savona, M.R.; Sekeres, M.A.; et al. Special considerations in the management of adult patients with acute leukemias and myeloid neoplasms in the COVID-19 era: Recommendations from a panel of international experts. Lancet Haematol. 2020, 7, e601–e612. [Google Scholar] [CrossRef]
- Marchesi, F.; Salmanton-García, J.; Emarah, Z.; Piukovics, K.; Nucci, M.; López-García, A.; Ráčil, Z.; Farina, F.; Popova, M.; Zompi, S.; et al. COVID-19 in adult acute myeloid leukemia patients: A long-term followup study from the European Hematology Association survey (EPICOVIDEHA). Haematologica, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Hungria, V.; Garnica, M.; Crusoé, E.D.Q.; Filho, R.J.P.D.M.; Martinez, G.; Bittencourt, R.; de Farias, D.L.C.; Braga, W.M.; Neto, J.V.P.; Ribeiro, G.N.; et al. Managing patients with multiple myeloma during the COVID-19 pandemic: Recommendations from an expert panel–ABHH monoclonal gammopathies committe. Hematol. Transfus. Cell Ther. 2020, 42, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.; Andritsos, L.; Banerji, V.; Barrientos, J.C.; Bhat, S.; Blachly, J.S.; Call, T.; Cross, M.; Dearden, C.; Demeter, J.; et al. Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia 2021, 35, 1864–1872. [Google Scholar] [CrossRef]
- Lamure, S.; Salmanton-García, J.; Marieton, E.R.; Jaksic, O.; Kohn, M.; Marchesi, F.; Marchetti, M.; El-Ashwah, S.; Demirkan, F.; Valković, T.; et al. COVID-19 and Hairy-Cell Leukemia: An EPICOVIDEHA Survey. Blood Adv. 2022, 6, 3870–3874. [Google Scholar] [CrossRef]
- Taurino, D.; Frigeni, M.; Grassi, A.; Cavallaro, G.; Salmoiraghi, S.; Spinelli, O.; Rambaldi, A.; Lussana, F. Concurrent diagnosis of acute myeloid leukemia and symptomatic COVID-19 infection: A case report successfully treated with Azacitidine-Venetoclax combination. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021057. [Google Scholar] [CrossRef]
- Orf, K.; Rogosic, S.; Dexter, D.; Ancliff, P.; Badle, S.; Brierley, J.; Cheng, D.; Dalton, C.; Dixon, G.; Du Pré, P.; et al. Remdesivir during induction chemotherapy for newly diagnosed paediatric acute lymphoblastic leukaemia with concomitant SARS-CoV-2 infection. Br. J. Haematol. 2020, 190, e274–e276. [Google Scholar] [CrossRef]
- Ghandili, S.; Pfefferle, S.; Roedl, K.; Sonnemann, P.; Karagiannis, P.; Boenisch, O.; Kluge, S.; Schmiedel, S.; Ittrich, H.; Rohde, H.; et al. Challenges in treatment of patients with acute leukemia and COVID-19: A series of 12 patients. Blood Adv. 2020, 4, 5936–5941. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Busca, A.; Cornely, O.A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Marchesi, F.; Pagliuca, A.; Passamonti, F.; Koehler, P.; et al. EPICOVIDEHA: A Ready to Use Platform for Epidemiological Studies in Hematological Patients With COVID-19. HemaSphere 2021, 5, e612. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms (accessed on 1 May 2022).
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 1 May 2022).
- Palanques-Pastor, T.; Megías-Vericat, J.E.; Martínez, P.; López Lorenzo, J.L.; Cornago Navascués, J.; Rodriguez Macias, G.; Cano, I.; Arnan Sangerman, M.; Vidriales Vicente, M.B.; Algarra Algarra, J.L.; et al. Characteristics, clinical outcomes, and risk factors of SARS-COV-2 infection in adult acute myeloid leukemia patients: Experience of the PETHEMA group. Leuk. Lymphoma 2021, 62, 2928–2938. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.-M.; Morgades, M.; Coll, R.; Barba, P.; López-Lorenzo, J.-L.; Montesinos, P.; Foncillas, M.-A.; Cabrero, M.; Gómez-Centurión, I.; Morales, M.-D.; et al. Frequency, Clinical Characteristics and Outcome of Adults With Acute Lymphoblastic Leukemia and COVID 19 Infection in the First vs. Second Pandemic Wave in Spain. Clin. Lymphoma Myeloma Leuk. 2021, 21, e801–e809. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Bonifacio, M.; Agrippino, R.; Giglio, F.; Annunziata, M.; Curti, A.; Del Principe, M.I.; Salutari, P.; Sciumè, M.; Delia, M.; et al. COVID-19 infection in acute lymphoblastic leukemia over 15 months of the pandemic. A Campus ALL report. Haematologica 2022, 107, 1955–1959. [Google Scholar] [CrossRef]
- Yarza, R.; Bover, M.; Paredes, D.; López-López, F.; Jara-Casas, D.; Castelo-Loureiro, A.; Baena, J.; Mazarico, J.M.; Folgueira, M.D.; Meléndez-Carmona, M.Á.; et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: Analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer 2020, 135, 242–250. [Google Scholar] [CrossRef]
- Ljungman, P.; de la Camara, R.; Mikulska, M.; Tridello, G.; Aguado, B.; Zahrani, M.A.; Apperley, J.; Berceanu, A.; Bofarull, R.M.; Calbacho, M.; et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicentre prospective survey. Leukemia 2021, 35, 2885–2894. [Google Scholar] [CrossRef]
- Piñana, J.L.; Martino, R.; García-García, I.; Parody, R.; Morales, M.D.; Benzo, G.; Gómez-Catalan, I.; Coll, R.; De La Fuente, I.; Luna, A.; et al. Risk factors and outcome of COVID-19 in patients with haematological malignancies. Exp. Hematol. Oncol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Stahl, M.; Narendra, V.; Jee, J.; Derkach, A.; Maloy, M.; Geyer, M.B.; Mato, A.R.; Roeker, L.E.; Tallman, M.S.; Shah, G.L.; et al. Neutropenia in adult acute myeloid leukemia patients represents a powerful risk factor for COVID-19 related mortality. Leuk. Lymphoma 2021, 62, 1940–1948. [Google Scholar] [CrossRef]
- Cesaro, S.; Ljungman, P.; Mikulska, M.; Hirsch, H.H.; von Lilienfeld-Toal, M.; Cordonnier, C.; Meylan, S.; Mehra, V.; Styczynski, J.; Marchesi, F.; et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 2022, 36, 1467–1480. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; García-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19–Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Salmanton-García, J.; Maertens, J.; Bourgeois, M.; Reynders, M.; Rutsaert, L.; Van Regenmortel, N.; Lormans, P.; et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—A multinational observational study by the European Confederation of Medical Mycology. Clin. Microbiol. Infect. 2022, 28, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Zappasodi, P.; Cattaneo, C.; Ferretti, V.; Mina, R.; Ferreri, A.J.M.; Merli, F.; Oberti, M.; Krampera, M.; Romano, A.; Zerbi, C.; et al. Secondary infections worsen the outcome of COVID-19 in patients with hematological malignancies a report from the ITA-HEMA-COV. Hematol. Oncol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cattaneo, C.; Pagani, C.; Cancelli, V.; Imberti, L.; Roccaro, A.M.; Notarangelo, L.D.; Rossi, G. Reduction in the rate and improvement in the prognosis of COVID-19 in haematological patients over time. Leukemia 2021, 35, 632–634. [Google Scholar] [CrossRef] [PubMed]
| n | % | |
|---|---|---|
| Sex | ||
| Female | 186 | 41.3% |
| Male | 264 | 58.7% |
| Age, median (IQR) [absolute range] | 65 (53 to 75) [18 to 95] | |
| 18–25 years old | 15 | 3.3% |
| 26–50 years old | 84 | 18.7% |
| 51–69 years old | 174 | 38.7% |
| ≥70 years old | 177 | 39.3% |
| Comorbidities before COVID-19 | ||
| No comorbidities | 168 | 37.3% |
| 1 comorbidity | 140 | 31.1% |
| 2 comorbidities | 81 | 18% |
| 3 or more comorbidities | 61 | 13.6% |
| Chronic cardiopathy | 156 | 34.7% |
| Chronic pulmonary disease | 61 | 13.6% |
| Diabetes mellitus | 77 | 17.1% |
| Liver disease | 18 | 4% |
| Obesity | 43 | 9.6% |
| Renal impairment | 43 | 9.6% |
| Smoking history | 52 | 11.6% |
| No risk factor identified | 168 | 37.3% |
| Baseline malignancy | ||
| Days between malignancy diagnosis and COVID-19 diagnosis, median (IQR) [absolute range] | −11 (−21 to −2) [−33 to 3] | |
| Acute lymphoid leukaemia | 32 | 7.1% |
| Chronic lymphoid leukaemia | 27 | 6% |
| Acute myeloid leukaemia | 129 | 28.7% |
| Chronic myeloid leukaemia | 9 | 2% |
| Hodgkin lymphoma | 16 | 3.6% |
| Non-Hodgkin lymphoma | 142 | 31.6% |
| Indolent | 42 | 9.3% |
| Aggressive | 91 | 20.2% |
| Unknown | 7 | 1.6% |
| Essential thrombocythemia | 2 | 0.4% |
| Multiple myeloma | 55 | 12.2% |
| Myelodysplastic syndrome | 25 | 5.6% |
| Myelofibrosis | 3 | 0.7% |
| Hairy cell leukaemia | 10 | 2.2% |
| Neutrophils at COVID-19 diagnosis | ||
| ≤500 | 69 | 15.3% |
| 501–999 | 28 | 6.2% |
| ≥1000 | 307 | 68.2% |
| Lymphocytes at COVID-19 diagnosis | ||
| ≤200 | 47 | 10.4% |
| 201–499 | 54 | 12% |
| ≥500 | 315 | 70% |
| COVID-19 infection | ||
| Asymptomatic | 106 | 23.6% |
| Mild infection | 58 | 12.9% |
| Severe infection | 170 | 37.8% |
| Critical infection | 116 | 25.8% |
| COVID-19 symptoms at infection onset | ||
| Pulmonary | 151 | 33.6% |
| Pulmonary + extrapulmonary | 106 | 23.6% |
| Extrapulmonary | 66 | 14.7% |
| Screening | 127 | 28.2% |
| COVID-19 vaccination | ||
| Not vaccinated | 360 | 80% |
| One dose | 15 | 3.3% |
| Two doses | 46 | 10.2% |
| Three doses | 29 | 6.4% |
| COVID-19 treatment | ||
| No specific treatment reported | 277 | 61.6% |
| Antivirals + monoclonal antibodies ± corticosteroids ± plasma | 8 | 1.8% |
| Antivirals ± corticosteroids ± plasma | 52 | 11.6% |
| Corticosteroids | 72 | 16% |
| Monoclonal antibodies ± plasma ± corticosteroids | 31 | 6.9% |
| Plasma ± corticosteroids | 10 | 2.2% |
| Stay during COVID-19 episode | ||
| Hospital | 380 | 84.4% |
| Days of the stay in ICU, median (IQR) [absolute range] | 15 (7 to 28) [1 to 150] | |
| ICU | 116 | 25.8% |
| Days of the stay in ICU, median (IQR) [absolute range] | 8 (4 to 20) [1 to 56] | |
| Invasive mechanical ventilation | 74 | 16.4% |
| Non-invasive mechanical ventilation | 42 | 9.3% |
| Home | 93 | 20.7% |
| No HM Tx at All | HM Tx at Least after COVID-19 dx | p Value | No HM Tx at All | HM Tx | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Sex | 0.031 | 0.021 | ||||||||
| Female | 34 | 31.8% | 96 | 44.2% | 34 | 31.8% | 152 | 44.3% | ||
| Male | 73 | 68.2% | 121 | 55.8% | 73 | 68.2% | 191 | 55.7% | ||
| Age, median (IQR) [absolute range] | 68 (56–79) [24–92] | 61 (47–72) [18–95] | <0.001 | 68 (56–79) [24–92] | 64 (50–74) [18–95] | 0.003 | ||||
| Comorbidities Before COVID-19 | 0.031 | 0.093 | ||||||||
| No comorbidities | 37 | 34.6% | 86 | 39.6% | 37 | 34.6% | 131 | 38.2% | ||
| 1 comorbidity | 29 | 27.1% | 71 | 32.7% | 29 | 27.1% | 111 | 32.4% | ||
| 2 comorbidities | 28 | 26.2% | 28 | 12.9% | 28 | 26.2% | 53 | 15.5% | ||
| 3 or more comorbidities | 13 | 12.1% | 32 | 14.7% | 13 | 12.1% | 48 | 14.0% | ||
| Baseline malignancy | <0.001 | <0.001 | ||||||||
| ALL | 4 | 3.7% | 19 | 8.8% | 4 | 3.7% | 28 | 8.2% | ||
| AML | 17 | 15.9% | 62 | 28.6% | 17 | 15.9% | 112 | 32.7% | ||
| Lymphoma | 32 | 29.9% | 82 | 37.8% | 32 | 29.9% | 124 | 36.2% | ||
| CLD | 26 | 24.3% | 9 | 4.1% | 26 | 24.3% | 13 | 3.8% | ||
| MM | 12 | 11.2% | 29 | 13.4% | 12 | 11.2% | 43 | 12.5% | ||
| MDS/MPS | 16 | 15.0% | 16 | 7.4% | 16 | 15.0% | 23 | 6.7% | ||
| Anti-SARS-CoV-2 vaccination | 26 | 24.3% | 38 | 17.5% | 0.149 | 26 | 24.3% | 64 | 18.7% | 0.203 |
| COVID-19 severity | 0.019 | 0.092 | ||||||||
| Asymptomatic | 20 | 18.7% | 64 | 29.5% | 20 | 18.7% | 86 | 25.1% | ||
| Mild infection | 11 | 10.3% | 27 | 12.4% | 11 | 10.3% | 47 | 13.7% | ||
| Severe infection | 39 | 36.4% | 83 | 38.2% | 39 | 36.4% | 131 | 38.2% | ||
| Critical infection | 37 | 34.6% | 43 | 19.8% | 37 | 34.6% | 79 | 23.0% | ||
| COVID-19 diagnostic period | <0.001 | 0.001 | ||||||||
| March 2020–September 2020 | 27 | 25.2% | 56 | 25.8% | 27 | 25.2% | 91 | 26.5% | ||
| October 2020–February 2021 | 25 | 23.4% | 99 | 45.6% | 25 | 23.4% | 142 | 41.4% | ||
| March 2021–November 2021 | 24 | 22.4% | 29 | 13.4% | 24 | 22.4% | 47 | 13.7% | ||
| December 2021–February 2022 | 31 | 29.0% | 33 | 15.2% | 31 | 29.0% | 63 | 18.4% | ||
| Any COVID-19 treatment | 46 | 43.0% | 66 | 30.4% | 0.025 | 46 | 43.0% | 127 | 37.0% | 0.268 |
| Day-30 mortality | 45 | 42.1% | 33 | 15.2% | <0.001 | 45 | 42.1% | 94 | 27.4% | 0.004 |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||
| Sex | ||||||||
| Female | - | - | - | - | ||||
| Male | 0.965 | 0.993 | 0.735 | 1.342 | ||||
| Age | <0.001 | 1.035 | 1.024 | 1.046 | <0.001 | 1.033 | 1.019 | 1.047 |
| Baseline malignancy | ||||||||
| ALL | - | - | - | - | - | - | - | - |
| AML | 0.015 | 2.632 | 1.210 | 5.726 | 0.080 | 2.307 | 0.906 | 5.873 |
| Lymphoma | 0.656 | 1.198 | 0.541 | 2.650 | 0.957 | 0.974 | 0.369 | 2.567 |
| CLD | 0.832 | 1.108 | 0.430 | 2.860 | 0.082 | 0.365 | 0.117 | 1.137 |
| MM | 0.085 | 2.090 | 0.904 | 4.833 | 0.941 | 1.040 | 0.374 | 2.889 |
| MDS/MPS | 0.245 | 1.704 | 0.694 | 4.180 | 0.752 | 0.838 | 0.282 | 2.495 |
| COVID-19 infection severity | ||||||||
| Asymptomatic | - | - | - | - | - | - | - | - |
| Mild infection | 0.919 | 1.036 | 0.525 | 2.045 | 0.862 | 1.068 | 0.509 | 2.239 |
| Severe infection | 0.080 | 1.545 | 0.949 | 2.515 | 0.026 | 1.804 | 1.074 | 3.032 |
| Critical infection | <0.001 | 4.598 | 2.896 | 7.299 | <0.001 | 5.523 | 3.328 | 9.166 |
| Comorbidities at COVID-19 onset | ||||||||
| No comorbidities | - | - | - | - | - | - | - | - |
| 1 comorbidity | 0.033 | 1.527 | 1.034 | 2.256 | 0.618 | 1.113 | 0.732 | 1.692 |
| 2 comorbidities | <0.001 | 2.630 | 1.746 | 3.960 | 0.015 | 1.767 | 1.119 | 2.793 |
| 3 or more comorbidities | 0.014 | 1.817 | 1.129 | 2.924 | 0.066 | 1.680 | 0.967 | 2.918 |
| Season COVID-19 diagnosis | ||||||||
| March 2020–September 2020 | - | - | - | - | - | - | - | - |
| October 2020–February 2021 | 0.003 | 0.603 | 0.430 | 0.845 | 0.699 | 1.084 | 0.720 | 1.634 |
| March 2021–November 2021 | 0.060 | 0.639 | 0.400 | 1.019 | 0.402 | 1.280 | 0.719 | 2.279 |
| December 2021–February 2022 | <0.001 | 0.384 | 0.222 | 0.666 | 0.728 | 0.880 | 0.428 | 1.810 |
| Malignancy treatment? | ||||||||
| No | - | - | - | - | ||||
| Before COVID-19 diagnosis | 0.053 | 0.717 | 0.512 | 1.004 | ||||
| After COVID-19 diagnosis | <0.001 | 0.251 | 0.154 | 0.408 | ||||
| Neutrophils at COVID-19 onset | ||||||||
| ≤500 | - | - | - | - | ||||
| 501–999 | 0.650 | 0.858 | 0.442 | 1.665 | ||||
| ≥1000 | 0.082 | 0.706 | 0.475 | 1.045 | ||||
| Lymphocytes at COVID-19 onset | ||||||||
| ≤200 | - | - | - | - | - | - | - | - |
| 201–499 | 0.058 | 0.582 | 0.333 | 1.018 | 0.210 | 0.683 | 0.377 | 1.239 |
| ≥500 | 0.005 | 0.553 | 0.366 | 0.836 | 0.024 | 0.573 | 0.352 | 0.931 |
| Chemotherapy induced neutropenia | ||||||||
| No | - | - | - | - | - | - | - | - |
| Yes | 0.071 | 0.722 | 0.506 | 1.029 | 0.239 | 1.285 | 0.846 | 1.950 |
| No treatment administered | 0.011 | 1.602 | 1.112 | 2.308 | <0.001 | 3.449 | 2.224 | 5.349 |
| Secondary infection after COVID-19 | 0.125 | 0.740 | 0.503 | 1.087 | ||||
| COVID-19 treatment | ||||||||
| No specific treatment reported | - | - | - | - | - | - | - | - |
| Antivirals + monoclonal antibodies ± corticosteroids ± plasma | 0.213 | 0.286 | 0.040 | 2.049 | 0.105 | 0.190 | 0.025 | 1.414 |
| Antivirals ± corticosteroids ± plasma | 0.793 | 1.064 | 0.668 | 1.695 | 0.395 | 0.798 | 0.476 | 1.341 |
| Corticosteroids | 0.791 | 0.944 | 0.617 | 1.444 | 0.033 | 0.605 | 0.381 | 0.961 |
| Monoclonal antibodies ± plasma ± corticosteroids | 0.026 | 0.360 | 0.147 | 0.882 | 0.081 | 0.442 | 0.177 | 1.107 |
| Plasma ± corticosteroids | 0.905 | 1.063 | 0.392 | 2.880 | 0.269 | 0.557 | 0.198 | 1.571 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattaneo, C.; Salmanton-García, J.; Marchesi, F.; El-Ashwah, S.; Itri, F.; Weinbergerová, B.; Gomes Da Silva, M.; Dargenio, M.; Dávila-Valls, J.; Martín-Pérez, S.; et al. Simultaneous Onset of Haematological Malignancy and COVID: An Epicovideha Survey. Cancers 2022, 14, 5530. https://doi.org/10.3390/cancers14225530
Cattaneo C, Salmanton-García J, Marchesi F, El-Ashwah S, Itri F, Weinbergerová B, Gomes Da Silva M, Dargenio M, Dávila-Valls J, Martín-Pérez S, et al. Simultaneous Onset of Haematological Malignancy and COVID: An Epicovideha Survey. Cancers. 2022; 14(22):5530. https://doi.org/10.3390/cancers14225530
Chicago/Turabian StyleCattaneo, Chiara, Jon Salmanton-García, Francesco Marchesi, Shaimaa El-Ashwah, Federico Itri, Barbora Weinbergerová, Maria Gomes Da Silva, Michelina Dargenio, Julio Dávila-Valls, Sonia Martín-Pérez, and et al. 2022. "Simultaneous Onset of Haematological Malignancy and COVID: An Epicovideha Survey" Cancers 14, no. 22: 5530. https://doi.org/10.3390/cancers14225530
APA StyleCattaneo, C., Salmanton-García, J., Marchesi, F., El-Ashwah, S., Itri, F., Weinbergerová, B., Gomes Da Silva, M., Dargenio, M., Dávila-Valls, J., Martín-Pérez, S., Farina, F., Van Doesum, J., Valković, T., Besson, C., Poulsen, C. B., López-García, A., Žák, P., Schönlein, M., Piukovics, K., ... Pagano, L. (2022). Simultaneous Onset of Haematological Malignancy and COVID: An Epicovideha Survey. Cancers, 14(22), 5530. https://doi.org/10.3390/cancers14225530












