Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier
Abstract
Simple Summary
Abstract
1. Introduction
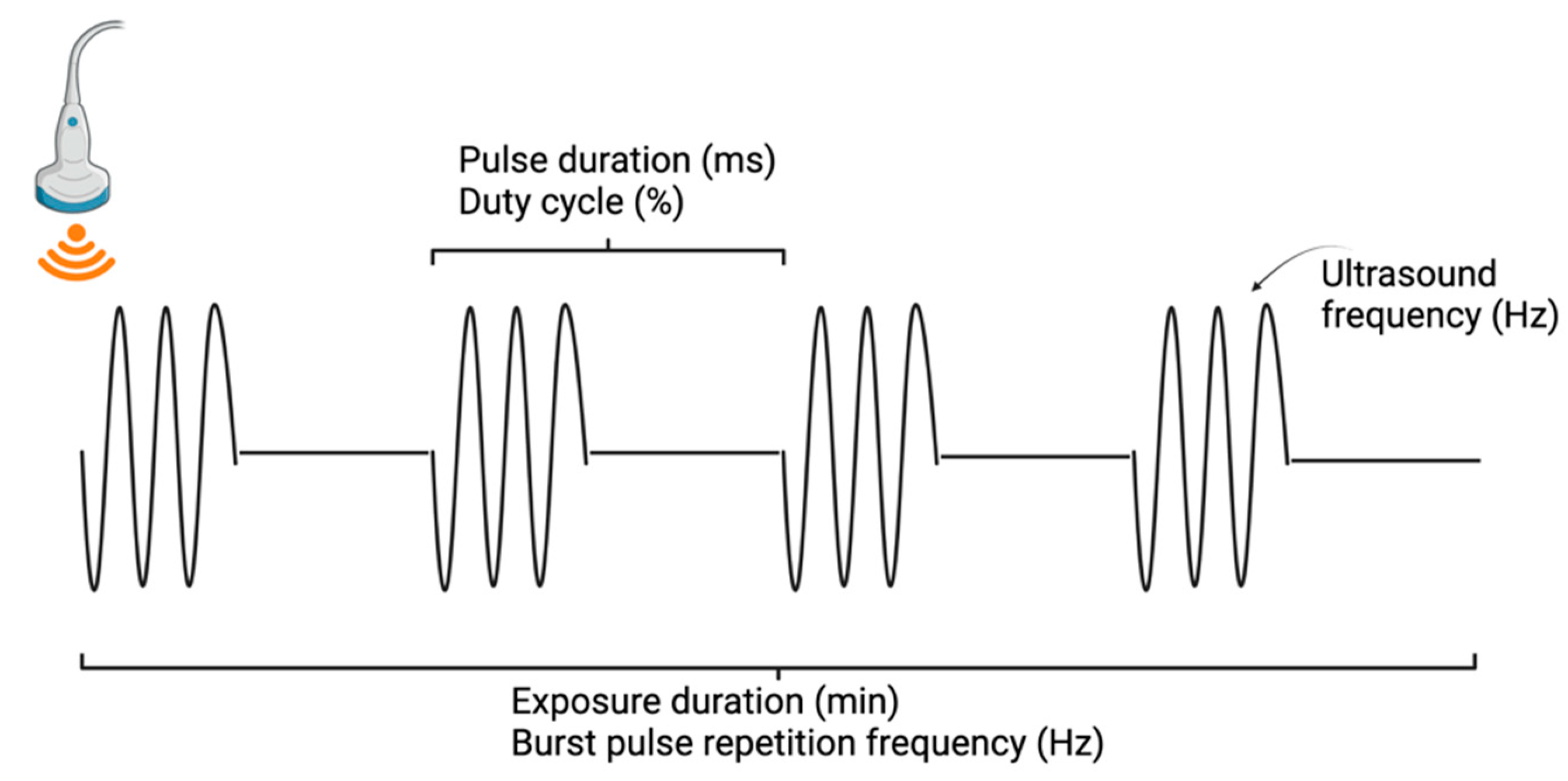
2. Overview of Focused Ultrasound
2.1. High and Low Intensity Focused Ultrasound
2.2. Mechanisms of Focused Ultrasound
2.3. Focused Ultrasound Technology
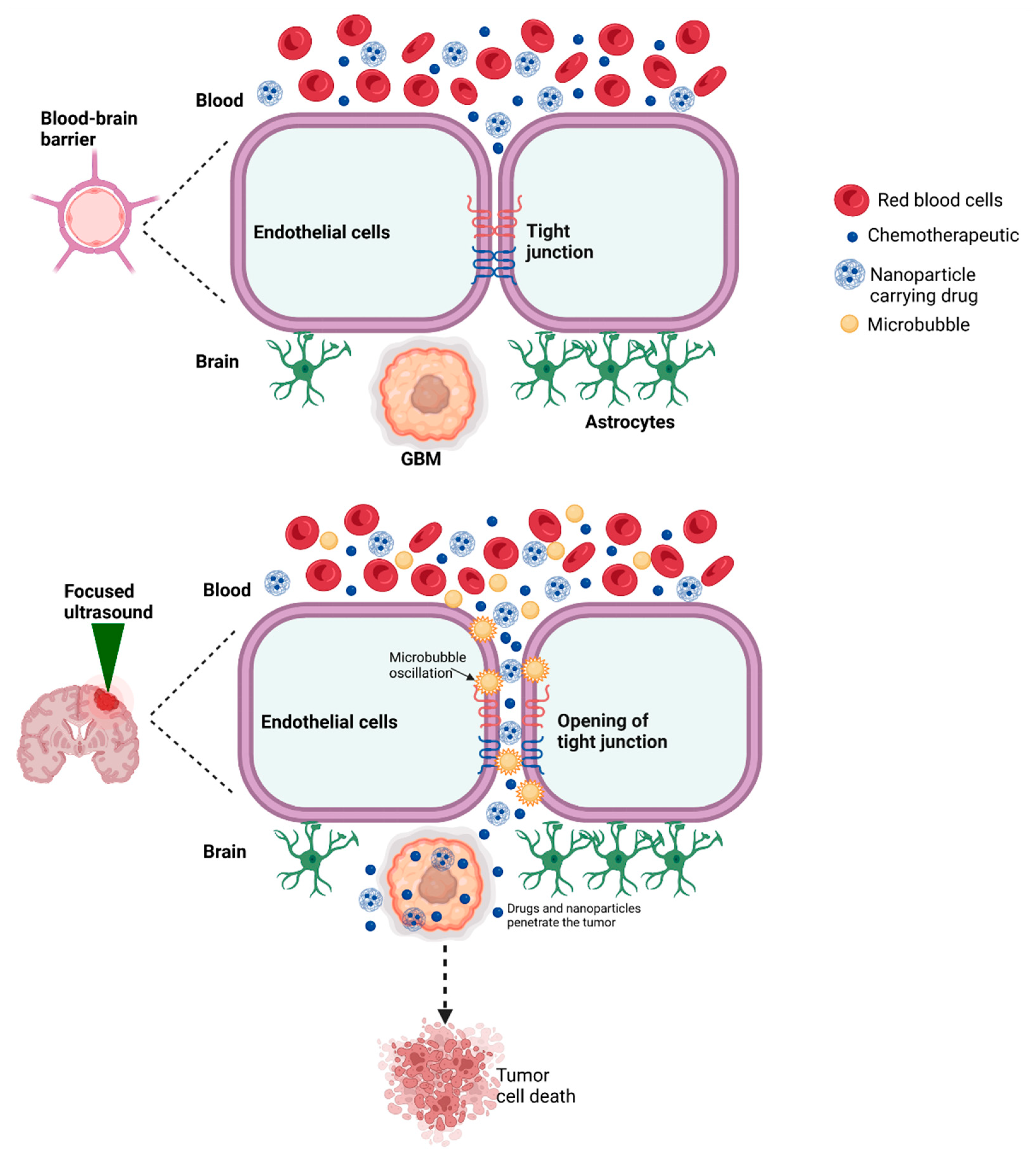
3. Enhanced Drug Delivery
3.1. FUS-Mediated Opening of the BBB
3.2. Applications of FUS for Drug Delivery
4. Tumor Ablation
5. Immunotherapy
6. Sensitization Strategies: Sonodynamic Therapy
6.1. Sonosensitizers
6.2. Sonodynamic Therapy: Preclinical and Clinical Trials
7. Sensitization Strategies: Radiosensitization
8. Histotripsy
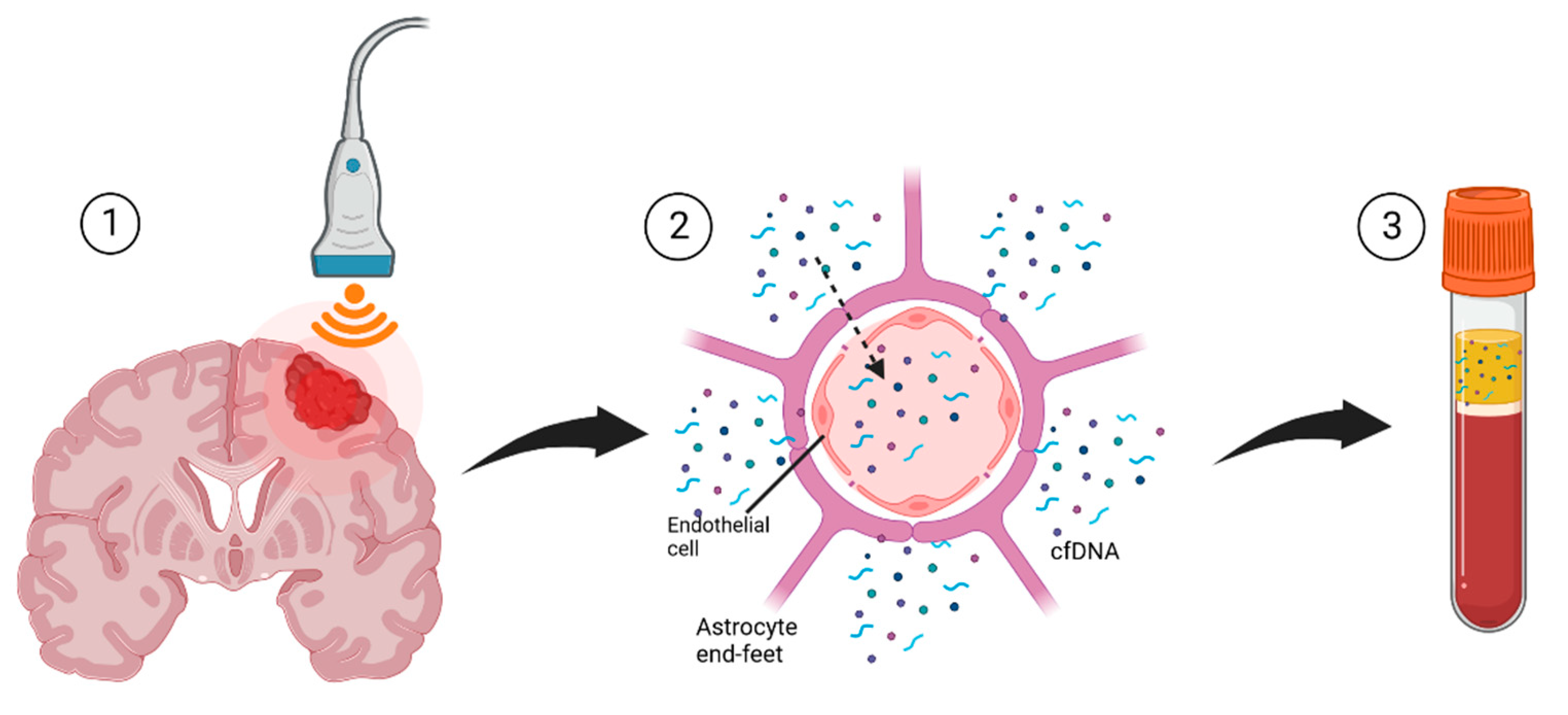
9. Liquid Biopsy
Focused Ultrasound for Liquid Biopsies
10. Challenges and Opportunities
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-ALA | 5-Aminolevulinic acid |
| BBB | Blood–brain barrier |
| cfDNA | Cell-free DNA |
| CNS | Central nervous system |
| FUS | Focused ultrasound |
| GBM | Glioblastoma multiforme |
| MRI | Magnetic resonance imaging |
| MRgFUS | Magnetic resonance guided focused ultrasound |
| RT | Radiation therapy |
| SDT | Sonodynamic therapy |
| TMZ | Temozolomide |
References
- Tamimi, A.F.; Juweid, M.; Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, QLD, Australia, 2017. [Google Scholar]
- DAS, K.K.; Kumar, R. Pediatric Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; pp. 297–312. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Haj, A.; Doenitz, C.; Schebesch, K.M.; Ehrensberger, D.; Hau, P.; Putnik, K.; Riemenschneider, M.J.; Wendl, C.; Gerken, M.; Pukrop, T.; et al. Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center. Brain Sci. 2018, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, M.; Hejmady, S.; Narayan Saha, R.; Damle, S.; Singhvi, G.; Alexander, A.; Kesharwani, P.; Kumar Dubey, S. Evolving New-Age Strategies to Transport Therapeutics across the Blood-Brain-Barrier. Int. J. Pharm. 2021, 599, 120351. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3. [Google Scholar] [CrossRef]
- Sampath, P.; Rhines, L.D.; DiMeco, F.; Tyler, B.M.; Park, M.C.; Brem, H. Interstitial Docetaxel (Taxotere), Carmustine and Combined Interstitial Therapy: A Novel Treatment for Experimental Malignant Glioma. J. Neuro-Oncol. 2006, 80, 9–17. [Google Scholar] [CrossRef]
- Mangraviti, A.; Tyler, B.; Brem, H. Interstitial Chemotherapy for Malignant Glioma: Future Prospects in the Era of Multimodal Therapy. Surg. Neurol. Int. 2015, 6, S78–S84. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358. [Google Scholar] [CrossRef]
- Stine, C.A.; Munson, J.M. Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow. Front. Oncol. 2019, 9, 966. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood-Brain Barrier 11 Medical and Health Sciences 1109 Neurosciences. Ital. J. Pediatr. 2018, 44, 127–133. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood-Brain Barrier with Nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Fortin, D. Blood-Brain Barrier Disruption in the Treatment of Brain Tumors. Methods Mol. Biol. 2011, 686, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Etame, A.B.; Diaz, R.J.; Smith, C.A.; Mainprize, T.G.; Hynynen, K.; Rutka, J.T. Focused Ultrasound Disruption of the Blood Brain Barrier: A New Frontier for Therapeutic Delivery in Molecular Neuro-Oncology. Neurosurg. Focus 2012, 32, E3. [Google Scholar] [CrossRef]
- MacDonell, J.; Patel, N.; Rubino, S.; Ghoshal, G.; Fischer, G.; Clif Burdette, E.; Hwang, R.; Pilitsis, J.G. Magnetic Resonance-Guided Interstitial High-Intensity Focused Ultrasound for Brain Tumor Ablation. Neurosurg. Focus 2018, 44, E11. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, X.; Unger, M.; Patties, I.; Melzer, A.; Landgraf, L. Focused Ultrasound-Induced Cavitation Sensitizes Cancer Cells to Radiation Therapy and Hyperthermia. Cells 2020, 9, 2595. [Google Scholar] [CrossRef]
- Mauri, G.; Nicosia, L.; Xu, Z.; Di Pietro, S.; Monfardini, L.; Bonomo, G.; Varano, G.M.; Prada, F.; Della Vigna, P.; Orsi, F. Focused Ultrasound: Tumour Ablation and Its Potential to Enhance Immunological Therapy to Cancer. Br. J. Radiol. 2018, 91, 20170641. [Google Scholar] [CrossRef]
- Duc, N.M.; Keserci, B. Emerging Clinical Applications of High-Intensity Focused Ultrasound. Diagn. Interv. Radiol. 2019, 25, 398–409. [Google Scholar] [CrossRef]
- Brenin, D.R. Focused Ultrasound Ablation for the Treatment of Breast Cancer. Ann. Surg. Oncol. 2011, 18, 3088–3094. [Google Scholar] [CrossRef]
- Crouzet, S.; Rouviere, O.; Martin, X.; Gelet, A. High-Intensity Focused Ultrasound as Focal Therapy of Prostate Cancer. Curr. Opin. Urol. 2014, 24, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann. Biomed. Eng. 2021, 49, 1975. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Pahk, K.J.; Kim, H. A Review of Low-Intensity Focused Ultrasound for Neuromodulation. Biomed. Eng. Lett. 2017, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front. Oncol. 2022, 12, 903059. [Google Scholar] [CrossRef] [PubMed]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J.; Miller, A.E. A New method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef]
- Quadri, S.A.; Waqas, M.; Khan, I.; Khan, M.A.; Suriya, S.S.; Farooqui, M.; Fiani, B. High-Intensity Focused Ultrasound: Past, Present, and Future in Neurosurgery. Neurosurg. Focus 2018, 44, E16. [Google Scholar] [CrossRef]
- Elhelf, I.A.S.; Albahar, H.; Shah, U.; Oto, A.; Cressman, E.; Almekkawy, M. High Intensity Focused Ultrasound: The Fundamentals, Clinical Applications and Research Trends. Diagn. Interv. Imaging 2018, 99, 349–359. [Google Scholar] [CrossRef]
- Kim, Y.S.; Rhim, H.; Min, J.C.; Hyo, K.L.; Choi, D. High-Intensity Focused Ultrasound Therapy: An Overview for Radiologists. Korean J. Radiol. 2008, 9, 291. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Hum. Neurosci. 2021, 15, 568. [Google Scholar] [CrossRef]
- Rezayat, E.; Toostani, I.G. A Review on Brain Stimulation Using Low Intensity Focused Ultrasound. Basic Clin. Neurosci. 2016, 7, 187. [Google Scholar] [CrossRef]
- Fomenko, A.; Lozano, A.M. Neuromodulation and Ablation with Focused Ultrasound—Toward the Future of Noninvasive Brain Therapy. Neural Regen. Res. 2019, 14, 1509. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First MRI-Guided Focused Ultrasound Device to Treat Essential Tremor | FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-mri-guided-focused-ultrasound-device-treat-essential-tremor (accessed on 31 August 2022).
- Exablate Neuro for Essential Tremor Treatment | Insightec. Available online: https://insightec.com/exablate-neuro/ (accessed on 1 September 2022).
- NaviFUS—Breakthrough Therapeutic Focused Ultrasound Technology. Available online: https://navifus.com/about/ (accessed on 1 September 2022).
- Our Solutions—Carthera. Available online: https://carthera.eu/our-solutions/ (accessed on 1 September 2022).
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood–Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, B.K.; Cohen-Gadol, A.A.; Miller, J.C. Novel Delivery Methods Bypassing the Blood-Brain and Blood-Tumor Barriers. Neurosurg. Focus 2015, 38, E10. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Bortot, B.; Ruozi, B.; Dolcetta, D.; Vandelli, M.A.; Forni, F.; Severini, G.M. Potential Use of Polymeric Nanoparticles for Drug Delivery across the Blood-Brain Barrier. Curr. Med. Chem. 2013, 20, 2212–2225. [Google Scholar] [CrossRef]
- Golden, P.L.; Pollack, G.M. Blood-Brain Barrier Efflux Transport. J. Pharm. Sci. 2003, 92, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. One-Year Outcome of Multiple Blood–Brain Barrier Disruptions with Temozolomide for the Treatment of Glioblastoma. Front. Oncol. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Kirkwood, J.M. Temozolomide, a Novel Alkylating Agent with Activity in the Central Nervous System, May Improve the Treatment of Advanced Metastatic Melanoma. Oncologist 2000, 5, 144–151. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177. [Google Scholar] [CrossRef]
- Hersh, A.M.; Gaitsch, H.; Alomari, S.; Lubelski, D.; Tyler, B.M. Molecular Pathways and Genomic Landscape of Glioblastoma Stem Cells: Opportunities for Targeted Therapy. Cancers 2022, 14, 3743. [Google Scholar] [CrossRef]
- Burgess, A.; Hynynen, K. Drug Delivery across the Blood-Brain Barrier Using Focused Ultrasound. Expert Opin. Drug Deliv. 2014, 11, 711. [Google Scholar] [CrossRef]
- Sorum, B.; Rietmeijer, R.A.; Gopakumar, K.; Adesnik, H.; Brohawn, S.G. Ultrasound Activates Mechanosensitive TRAAK K+ Channels through the Lipid Membrane. Proc. Natl. Acad. Sci. USA 2021, 118, e2006980118. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Huang, Y.; Dumont, D.J.; Hynynen, K. Focused Ultrasound-Mediated Bbb Disruption Is Associated with an Increase in Activation of AKT: Experimental Study in Rats. BMC Neurol. 2010, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper. Neurosurg. 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Anastasiadis, P.; Mohammadabadi, A.; Nguyen, B.A.; Guo, S.; Winkles, J.A.; Kim, A.J.; Gullapalli, R.; Keller, A.; Frenkel, V.; et al. MR-Guided Transcranial Focused Ultrasound Safely Enhances Interstitial Dispersion of Large Polymeric Nanoparticles in the Living Brain. PLoS ONE 2018, 13, e0192240. [Google Scholar] [CrossRef]
- Hersh, D.S.; Nguyen, B.A.; Dancy, J.G.; Adapa, A.R.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J.; Frenkel, V. Pulsed Ultrasound Expands the Extracellular and Perivascular Spaces of the Brain. Brain Res. 2016, 1646, 543–550. [Google Scholar] [CrossRef]
- Dauba, A.; Delalande, A.; Kamimura, H.A.S.; Conti, A.; Larrat, B.; Tsapis, N.; Novell, A. Recent Advances on Ultrasound Contrast Agents for Blood-Brain Barrier Opening with Focused Ultrasound. Pharmaceutics 2020, 12, 1125. [Google Scholar] [CrossRef]
- Wei, K.C.; Chu, P.C.; Wang, H.Y.J.; Huang, C.Y.; Chen, P.Y.; Tsai, H.C.; Lu, Y.J.; Lee, P.Y.; Tseng, I.C.; Feng, L.Y.; et al. Focused Ultrasound-Induced Blood-Brain Barrier Opening to Enhance Temozolomide Delivery for Glioblastoma Treatment: A Preclinical Study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef]
- Liu, H.L.; Huang, C.Y.; Chen, J.Y.; Wang, H.Y.J.; Chen, P.Y.; Wei, K.C. Pharmacodynamic and Therapeutic Investigation of Focused Ultrasound-Induced Blood-Brain Barrier Opening for Enhanced Temozolomide Delivery in Glioma Treatment. PLoS ONE 2014, 9, e114311. [Google Scholar] [CrossRef]
- Wei, H.J.; Upadhyayula, P.S.; Pouliopoulos, A.N.; Englander, Z.K.; Zhang, X.; Jan, C.I.; Guo, J.; Mela, A.; Zhang, Z.; Wang, T.J.C.; et al. Focused Ultrasound-Mediated Blood-Brain Barrier Opening Increases Delivery and Efficacy of Etoposide for Glioblastoma Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 539. [Google Scholar] [CrossRef]
- Nance, E.; Timbie, K.; Miller, G.W.; Song, J.; Louttit, C.; Klibanov, A.L.; Shih, T.Y.; Swaminathan, G.; Tamargo, R.J.; Woodworth, G.F.; et al. Non-Invasive Delivery of Stealth, Brain-Penetrating Nanoparticles across the Blood-Brain Barrier Using MRI-Guided Focused Ultrasound. J. Control. Release 2014, 189, 123–132. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Melander, F.; Thomsen, M.S.; Burkhart, A.; Kempen, P.J.; Andresen, T.L.; Moos, T. Modulating the Antibody Density Changes the Uptake and Transport at the Blood-Brain Barrier of Both Transferrin Receptor-Targeted Gold Nanoparticles and Liposomal Cargo. J. Control. Release 2019, 295, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent Progress of Drug Nanoformulations Targeting to Brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ding, Y.; Zhou, L.; Shi, D.; Sun, L.; Webster, T.J.; Shen, Y. Noninvasive Nanoparticle Strategies for Brain Tumor Targeting. Nanomedicine 2017, 13, 2605–2621. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.J.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing Glioblastoma Treatment Using Cisplatin-Gold-Nanoparticle Conjugates and Targeted Delivery with Magnetic Resonance-Guided Focused Ultrasound. Nanomedicine 2018, 14, 1137–1148. [Google Scholar] [CrossRef]
- Shen, Y.; Pi, Z.; Yan, F.; Yeh, C.K.; Zeng, X.; Diao, X.; Hu, Y.; Chen, S.; Chen, X.; Zheng, H. Enhanced Delivery of Paclitaxel Liposomes Using Focused Ultrasound with Microbubbles for Treating Nude Mice Bearing Intracranial Glioblastoma Xenografts. Int. J. Nanomed. 2017, 12, 5613. [Google Scholar] [CrossRef]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Wilson Miller, G.; Suk, J.S.; Hanes, J.; Price, R.J. MR Image-Guided Delivery of Cisplatin-Loaded Brain-Penetrating Nanoparticles to Invasive Glioma with Focused Ultrasound. J. Control. Release 2017, 263, 120. [Google Scholar] [CrossRef]
- Chan, M.H.; Chen, W.; Li, C.H.; Fang, C.Y.; Chang, Y.C.; Wei, D.H.; Liu, R.S.; Hsiao, M. An Advanced In Situ Magnetic Resonance Imaging and Ultrasonic Theranostics Nanocomposite Platform: Crossing the Blood-Brain Barrier and Improving the Suppression of Glioblastoma Using Iron-Platinum Nanoparticles in Nanobubbles. ACS Appl. Mater. Interfaces 2021, 13, 26759–26769. [Google Scholar] [CrossRef]
- Anastasiadis, P.; Gandhi, D.; Guo, Y.; Ahmed, A.K.; Bentzen, S.M.; Arvanitis, C.; Woodworth, G.F. Localized Blood-Brain Barrier Opening in Infiltrating Gliomas with MRI-Guided Acoustic Emissions-Controlled Focused Ultrasound. Proc. Natl. Acad. Sci. USA 2021, 118, e2103280118. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical Trial of Blood-Brain Barrier Disruption by Pulsed Ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Kim, A.J.; Winkles, J.A.; Eisenberg, H.M.; Woodworth, G.F.; Frenkel, V. Emerging Applications of Therapeutic Ultrasound in Neuro-Oncology: Moving Beyond Tumor Ablation. Neurosurgery 2016, 79, 643. [Google Scholar] [CrossRef] [PubMed]
- Ram, Z.; Cohen, Z.R.; Harnof, S.; Tal, S.; Faibel, M.; Nass, D.; Maier, S.E.; Hadani, M.; Mardor, Y. Magnetic Resonance Imaging-Guided, High-Intensity Focused Ultrasound for Brain Tumor Therapy. Neurosurgery 2006, 59, 949–955. [Google Scholar] [CrossRef]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial MRI-Guided Focused Ultrasound Surgery of Brain Tumors: Initial Findings in Three Patients. Neurosurgery 2010, 66, 323. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O’Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First Noninvasive Thermal Ablation of a Brain Tumor with MR-Guided Focused Ultrasound. J. Ther. Ultrasound 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025. [Google Scholar] [CrossRef]
- Man, J.; Shoemake, J.D.; Ma, T.; Rizzo, A.E.; Godley, A.R.; Wu, Q.; Mohammadi, A.M.; Bao, S.; Rich, J.N.; Yu, J.S. Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling. Cancer Res. 2015, 75, 1760–1769. [Google Scholar] [CrossRef]
- Zhou, Y.-F. High Intensity Focused Ultrasound in Clinical Tumor Ablation. World J. Clin. Oncol. 2011, 2, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hsieh, H.Y.; Huang, C.Y.; Lin, C.Y.; Wei, K.C.; Liu, H.L. Focused Ultrasound-Induced Blood-Brain Barrier Opening to Enhance Interleukin-12 Delivery for Brain Tumor Immunotherapy: A Preclinical Feasibility Study. J. Transl. Med. 2015, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Hsu, P.H.; Lin, C.Y.; Huang, C.W.; Chai, W.Y.; Chu, P.C.; Huang, C.Y.; Chen, P.Y.; Yang, L.Y.; Kuo, J.S.; et al. Focused Ultrasound Enhances Central Nervous System Delivery of Bevacizumab for Malignant Glioma Treatment. Radiology 2016, 281, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yuan, J.; Yue, Y.; Rubin, J.B.; Chen, H. Focused Ultrasound-Enhanced Delivery of Intranasally Administered Anti-Programmed Cell Death-Ligand 1 Antibody to an Intracranial Murine Glioma Model. Pharmaceutics 2021, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, N.D.; Breza, V.R.; Paul, S.; McCauley, K.S.; Berr, S.S.; Miller, G.W.; Neumann, K.D.; Price, R.J. ImmunoPET-Informed Sequence for Focused Ultrasound-Targeted MCD47 Blockade Controls Glioma. J. Control. Release 2021, 331, 19–29. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Witter, A.R.; Garrison, W.J.; Miller, G.W.; Price, R.J.; Bullock, T.N.J. Profiling of the Immune Landscape in Murine Glioblastoma Following Blood Brain/Tumor Barrier Disruption with MR Image-Guided Focused Ultrasound. J. Neuro-Oncol. 2022, 156, 109–122. [Google Scholar] [CrossRef]
- Chen, K.T.; Chai, W.Y.; Lin, Y.J.; Lin, C.J.; Chen, P.Y.; Tsai, H.C.; Huang, C.Y.; Kuo, J.S.; Liu, H.L.; Wei, K.C. Neuronavigation-Guided Focused Ultrasound for Transcranial Blood-Brain Barrier Opening and Immunostimulation in Brain Tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Batts, A.J.; Mathew, A.S.; Andrew Thim, E.; Price, R.J. Focused Ultrasound Hyperthermia Augments Release of Glioma-Derived Extracellular Vesicles with Differential Immunomodulatory Capacity. Theranostics 2020, 10, 7436. [Google Scholar] [CrossRef]
- Li, J.; Xi, A.; Qiao, H.; Liu, Z. Ultrasound-Mediated Diagnostic Imaging and Advanced Treatment with Multifunctional Micro/Nanobubbles. Cancer Lett. 2020, 475, 92–98. [Google Scholar] [CrossRef]
- Bunevicius, A.; Pikis, S.; Padilla, F.; Prada, F.; Sheehan, J. Sonodynamic Therapy for Gliomas. J. Neuro-Oncol. 2022, 156, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, J.; Yang, D.; Raymond, J.; Roy, R.; Zhang, D. Photo- and Sono-Dynamic Therapy: A Review of Mechanisms and Considerations for Pharmacological Agents Used in Therapy Incorporating Light and Sound. Curr. Pharm. Des. 2019, 25, 401–412. [Google Scholar] [CrossRef]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound Activated Sensitizers and Applications. Angew. Chem. Int. Ed. 2019, 59, 14212–14233. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Demidova, T.; Hamblin, M. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Kruger, C.; Evans, D.; Abrahamse, H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. The History of PDT in Norway. Photodiagn. Photodyn. Ther. 2007, 4, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Buytaert, E.; Breyssens, H.; Hendrickx, N. Regulatory Pathways in Photodynamic Therapy Induced Apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical Properties of Human Skin, Subcutaneous and Mucous Tissues in the Wavelength Range from 400 to 2000 Nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Um, W.; Kumar, E.; Lee, J.; Kim, C.; You, D.; Park, J. Recent Advances in Nanomaterial-Based Augmented Sonodynamic Therapy of Cancer. Chem. Commun. 2021, 57, 2854–2866. [Google Scholar] [CrossRef]
- Li, X.; Geng, X.; Chen, Z.; Yuan, Z. Recent Advances in Glioma Microenvironment-Response Nanoplatforms for Phototherapy and Sonotherapy. Pharmacol. Res. 2022, 179, 106218. [Google Scholar] [CrossRef]
- Guo, Q.-L.; Dai, X.-L.; Yin, M.-Y.; Cheng, H.-W.; Qian, H.; Wang, H.; Zhu, D.; Wang, X. Nanosensitizers for Sonodynamic Therapy for Glioblastoma Multiforme: Current Progress and Future Perspectives. Mil. Med. Res. 2022, 9, 26. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kitahara, S.; Kusuda, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Current Landscape of Sonodynamic Therapy for Treating Cancer. Cancers 2021, 13, 6184. [Google Scholar] [CrossRef]
- Pitt, W.; Husseini, G.; Staples, B. Ultrasonic Drug Delivery—A General Review. Expert Opin. Drug Deliv. 2004, 1, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Matsuura, K.; Takagi, R.; Yamamoto, M.; Umemura, S.-I. Detection of Tissue Coagulation by Decorrelation of Ultrasonic Echo Signals in Cavitation-Enhanced High-Intensity Focused Ultrasound Treatment. J. Ther. Ultrasound 2016, 4, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.; Alnashef, I. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Yamaguchi, F.; Zhan, G.; Higuchi, T.; Asakura, T.; Morita, A.; Orimo, H.; Hu, S. Hyperthermotherapy Enhances Antitumor Effect of 5-Aminolevulinic Acid-Mediated Sonodynamic Therapy with Activation of Caspase-Dependent Apoptotic Pathway in Human Glioma. Tumor Biol. 2016, 37, 10415–10426. [Google Scholar] [CrossRef]
- Sheehan, K.; Sheehan, D.; Sulaiman, M.; Padilla, F.; Moore, D.; Sheehan, J.; Xu, Z. Investigation of the Tumoricidal Effects of Sonodynamic Therapy in Malignant Glioblastoma Brain Tumors. J. Neuro-Oncol. 2020, 148, 9–16. [Google Scholar] [CrossRef]
- Li, J.-H.; Yue, W.; Huang, Z.; Chen, Z.-Q.; Zhan, Q.; Ren, F.-B.; Liu, J.-Y.; Fu, S.-B. Calcium Overload Induces C6 Rat Glioma Cell Apoptosis in Sonodynamic Therapy. Int. J. Radiat. Biol. 2011, 87, 1061–1066. [Google Scholar] [CrossRef]
- Chen, L.; Cong, D.; Li, Y.; Wang, D.; Li, Q.; Hu, S. Combination of Sonodynamic with Temozolomide Inhibits C6 Glioma Migration and Promotes Mitochondrial Pathway Apoptosis via Suppressing NHE-1 Expression. Ultrason. Sonochem. 2017, 39, 654–661. [Google Scholar] [CrossRef]
- Dammando, A.; Raspagliesi, L.; Gionso, M.; Franzini, A.; Porto, E.; Meco, F.; Durando, G.; Pellegatta, S.; Prada, F. Sonodynamic Therapy for the Treatment of Intracranial Gliomas. J. Clin. Med. 2021, 10, 1101. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Wang, X.; Gu, C.; Gao, Z.; Cao, W.; Zheng, J. 5-Aminolevulinic Acid–Mediated Sonodynamic Therapy Reverses Macrophage and Dendritic Cell Passivity in Murine Melanoma Xenografts. Ultrasound Med. Biol. 2014, 40, 2125–2133. [Google Scholar] [CrossRef]
- Wood, A.; Sehgal, C. A Review of Low-Intensity Ultrasound for Cancer Therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/Nanoparticle-Augmented Sonodynamic Therapy (SDT): Breaking the Depth Shallow of Photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Q.; Hu, Z.; Yang, B.; Li, Q.; Wang, J.; Zheng, J.; Cao, W. 5-Aminolevulinic Acid-Based Sonodynamic Therapy Induces the Apoptosis of Osteosarcoma in Mice. PLoS ONE 2015, 10, e0132074. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What Is the Surgical Benefit of Utilizing 5-ALA for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663. [Google Scholar] [CrossRef]
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of Antitumor Activity by Using 5-ALA–Mediated Sonodynamic Therapy to Induce Apoptosis in Malignant Gliomas: Significance of High-Intensity Focused Ultrasound on 5-ALA-SDT in a Mouse Glioma Model. J. Neurosurg. 2018, 129, 1416–1428. [Google Scholar] [CrossRef]
- Pi, Z.; Huang, Y.; Shen, Y.; Zeng, X.; Hu, Y.; Chen, T.; Li, C.; Yu, H.; Chen, S.; Chen, X. Sonodynamic Therapy on Intracranial Glioblastoma Xenografts Using Sinoporphyrin Sodium Delivered by Ultrasound with Microbubbles. Ann. Biomed. Eng. 2018, 47, 549–562. [Google Scholar] [CrossRef]
- An, Y.; Liu, H.-Q.; Zhou, Z.; Wang, J.; Jiang, G.; Li, Z.; Wang, F.; Jin, H. Sinoporphyrin Sodium Is a Promising Sensitizer for Photodynamic and Sonodynamic Therapy in Glioma. Oncol. Rep. 2020, 44, 1596–1604. [Google Scholar] [CrossRef]
- Yoshida, M.; Kobayashi, H.; Terasaka, S.; Endo, S.; Yamaguchi, S.; Motegi, H.; Itay, R.; Suzuki, S.; Brokman, O.; Shapira, Y.; et al. Sonodynamic Therapy for Malignant Glioma Using 220-KHz Transcranial Magnetic Resonance Imaging-Guided Focused Ultrasound and 5-Aminolevulinic Acid. Ultrasound Med. Biol. 2018, 45, 526–538. [Google Scholar] [CrossRef]
- Wu, S.-K.; Santos, M.; Marcus, S.; Hynynen, K. MR-Guided Focused Ultrasound Facilitates Sonodynamic Therapy with 5-Aminolevulinic Acid in a Rat Glioma Model. Sci. Rep. 2019, 9, 10465. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Wang, P.; Zhang, K.; Geng, X.; Liu, Q.; Wang, X. Tumor Targeting DVDMS-Nanoliposomes for Enhanced Sonodynamic Therapy of Glioma. Biomater. Sci. 2018, 7, 985–994. [Google Scholar] [CrossRef]
- Song, D.; Yue, W.; Li, Z.; Li, J.; Zhao, J.; Zhang, N. Study of the Mechanism of Sonodynamic Therapy in a Rat Glioma Model. OncoTargets Ther. 2014, 7, 1801. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Kudo, N.; Yamaguchi, S.; Sumiyoshi, K.; Motegi, H.; Kobayashi, H.; Terasaka, S.; Houkin, K. Porphyrin Derivatives-Mediated Sonodynamic Therapy for Malignant Gliomas In Vitro. Ultrasound Med. Biol. 2015, 41, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Bilmin, K.; Kujawska, T.; Secomski, W.; Nowicki, A.; Grieb, P. 5-Aminolevulinic Acid-Mediated Sonosensitization of Rat RG2 Glioma Cells in Vitro. Folia Neuropathol. 2016, 54, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, T.; Secomski, W.; Bilmin, K.; Nowicki, A.; Grieb, P. Impact of Thermal Effects Induced by Ultrasound on Viability of Rat C6 Glioma Cells. Ultrasonics 2014, 54, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, Y.; Huang, Y.; Zeng, X.; Huang, L.; Diao, X.; Chen, S.; Chen, X. An in Vitro Study on the Antitumor Effect of Sonodynamic Therapy Using Sinoporphyrin Sodium on Human Glioblastoma Cells. Ultrasonics 2021, 110, 106272. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Zhang, K.; Liu, J.; Wang, P.; Wang, X.; Liu, Q. Sonodynamic Therapy Induces Oxidative Stress, DNA Damage and Apoptosis in Glioma Cells. RSC Adv. 2018, 8, 36245–36256. [Google Scholar] [CrossRef]
- Prada, F.; Sheybani, N.; Franzini, A.; Moore, D.; Cordeiro, D.; Sheehan, J.; Timbie, K.; Xu, Z. Fluorescein-Mediated Sonodynamic Therapy in a Rat Glioma Model. J. Neuro-Oncol. 2020, 148, 445–454. [Google Scholar] [CrossRef]
- Dai, S.; Xu, C.; Tian, Y.; Cheng, W.; Li, B. In Vitro Stimulation of Calcium Overload and Apoptosis by Sonodynamic Therapy Combined with Hematoporphyrin Monomethyl Ether in C6 Glioma Cells. Oncol. Lett. 2014, 8, 1675–1681. [Google Scholar] [CrossRef]
- Hao, D.; Song, Y.; Che, Z.; Liu, Q. Calcium Overload and in Vitro Apoptosis of the C6 Glioma Cells Mediated by Sonodynamic Therapy (Hematoporphyrin Monomethyl Ether and Ultrasound). Cell Biochem. Biophys. 2014, 70, 1445–1452. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, P.; Li, Y.; Zhang, L.; Zong, Y.; Wan, M. Synergistic Therapy for Orthotopic Gliomas via Biomimetic Nanosonosensitizer-Mediated Sonodynamic Therapy and Ferroptosis. Biomater. Sci. 2022, 10, 3911–3923. [Google Scholar] [CrossRef]
- Qu, F.; Wang, P.; Zhang, K.; Shi, Y.; Li, Y.; Li, C.; Lu, J.; Liu, Q.; Wang, X. Manipulation of Mitophagy by “All-in-One” Nanosensitizer Augments Sonodynamic Glioma Therapy. Autophagy 2020, 16, 1413. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zou, C.; Hu, D.; Zhou, J.; Chen, M.; Tie, C.; Qiao, Y.; Yan, F.; Cheng, C.; Sheng, Z.; et al. Imaging-Guided Focused Ultrasound-Induced Thermal and Sonodynamic Effects of Nanosonosensitizers for Synergistic Enhancement of Glioblastoma Therapy. Biomater. Sci. 2019, 7, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, M.; Sheng, Z.; Chen, Y.; Yeh, C.K.; Chen, W.; Liu, J.; Liu, X.; Yan, F.; Zheng, H. Theranostic Nanosensitizers for Highly Efficient MR/Fluorescence Imaging-Guided Sonodynamic Therapy of Gliomas. J. Cell. Mol. Med. 2018, 22, 5394–5405. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, Y.; Cao, Y.; Liu, Z.Y. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv. Mater. 2022, 34, 2110364. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Chen, Q.; Hou, J.; Wang, J.; Zhong, Y.; Wang, X.; Jiang, W.; Ran, H.; Guo, D. Multifunctional Nanozyme for Multimodal Imaging-Guided Enhanced Sonodynamic Therapy by Regulating the Tumor Microenvironment. Nanoscale 2021, 13, 14049–14066. [Google Scholar] [CrossRef]
- Wu, P.; Dong, W.; Guo, X.; Qiao, X.; Guo, S.; Zhang, L.; Wan, M.; Zong, Y. ROS-Responsive Blended Nanoparticles: Cascade-Amplifying Synergistic Effects of Sonochemotherapy with On-demand Boosted Drug Release During SDT Process. Adv. Healthc. Mater. 2019, 8, 1900720. [Google Scholar] [CrossRef]
- Lv, Z.; Jin, L.; Cao, Y.; Zhang, H.; Xue, D.; Yin, N.; Zhang, T.; Wang, Y.; Liu, J.; Liu, X.; et al. A Nanotheranostic Agent Based on Nd3+-Doped YVO4 with Blood-Brain-Barrier Permeability for NIR-II Fluorescence Imaging/Magnetic Resonance Imaging and Boosted Sonodynamic Therapy of Orthotopic Glioma. Light Sci. Appl. 2022, 11, 116. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Zhang, W.; Lee, C.S.; Wang, P. A Novel Hypocrellin-Based Assembly for Sonodynamic Therapy against Glioblastoma. J. Mater. Chem. B 2021, 10, 57–63. [Google Scholar] [CrossRef]
- Liang, K.; Li, Z.; Luo, Y.; Qiuhong, Z.; Yin, F.; Xu, L.; Chen, H.; Wang, H.; Liang, K.; Zhang, H.; et al. 906985 (1 of 12) Intelligent Nanocomposites with Intrinsic Blood- Brain-Barrier Crossing Ability Designed for Highly Specific MR Imaging and Sonodynamic Therapy of Glioblastoma. Small 2020, 16, e1906985. [Google Scholar] [CrossRef]
- Shono, K.; Mizobuchi, Y.; Yamaguchi, I.; Nakajima, K.; Fujiwara, Y.; Fujihara, T.; Kitazato, K.; Matsuzaki, K.; Uto, Y.; Sampetrean, O.; et al. Elevated Cellular PpIX Potentiates Sonodynamic Therapy in a Mouse Glioma Stem Cell-Bearing Glioma Model by Downregulating the Akt/NF-ΚB/MDR1 Pathway. Sci. Rep. 2021, 11, 15105. [Google Scholar] [CrossRef]
- Chen, L.; Yan, Y.; Kong, F.; Wang, J.; Zeng, J.; Fang, Z.; Wang, Z.; Liu, Z.; Liu, F. Contribution of Oxidative Stress Induced by Sonodynamic Therapy to the Calcium Homeostasis Imbalance Enhances Macrophage Infiltration in Glioma Cells. Cancers 2022, 14, 2036. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Chen, Z.-Q.; Huang, Z.; Zhan, Q.; Ren, F.-B.; Liu, J.-Y.; Yue, W.; Wang, Z. In Vitro Study of Low Intensity Ultrasound Combined with Different Doses of PDT: Effects on C6 Glioma Cells. Oncol. Lett. 2013, 5, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.P.; Willis, A.; Pernal, S.; Phakatkar, A.; Shokuhfar, T.; Blot, V.; Engelhard, H.H. Targeted Sonodynamic Destruction of Glioblastoma Cells Using Antibody-Titanium Dioxide Nanoparticle Conjugates. Nanomedicine 2021, 16, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Wang, Z.; Zhao, L.; Mao, H.; Wang, G.; Zhang, K.; Liu, X.; Wu, D.; Zheng, Y.; Lu, J.; et al. Hypoxia-Responsive Lipid-Poly-(Hypoxic Radiosensitized Polyprodrug) Nanoparticles for Glioma Chemo- and Radiotherapy. Theranostics 2018, 8, 5088–5105. [Google Scholar] [CrossRef]
- Kanazawa, T.; Taki, H.; Okada, H. Nose-to-Brain Drug Delivery System with Ligand/Cell-Penetrating Peptide-Modified Polymeric Nano-Micelles for Intracerebral Gliomas. Eur. J. Pharm. Biopharm. 2020, 152, 85–94. [Google Scholar] [CrossRef]
- Geng, J.; Li, J.; Huang, T.; Zhao, K.; Chen, Q.; Guo, W.; Gao, J. A Novel Manganese Complex Selectively Induces Malignant Glioma Cell Death by Targeting Mitochondria. Mol. Med. Rep. 2016, 14, 1970–1978. [Google Scholar] [CrossRef][Green Version]
- Nonaka, M.; Yamamoto, M.; Yoshino, S.; Umemura, S.-I.; Sasaki, K.; Fukushima, T. Sonodynamic Therapy Consisting of Focused Ultrasound and a Photosensitizer Causes a Selective Antitumor Effect in a Rat Intracranial Glioma Model. Anticancer Res. 2009, 29, 943–950. [Google Scholar]
- Raspagliesi, L.; Dammando, A.; Gionso, M.; Sheybani, N.; Lopes, M.-B.; Moore, D.; Allen, S.; Gatesman, J.; Porto, E.; Timbie, K.; et al. Intracranial Sonodynamic Therapy With 5-Aminolevulinic Acid and Sodium Fluorescein: Safety Study in a Porcine Model. Front. Oncol. 2021, 11, 2229. [Google Scholar] [CrossRef]
- Jeong, E.-J.; Seo, S.-J.; Ahn, Y.-J.; Choi, K.H.; Kim, K.-H.; Kim, J.-K. Sonodynamically Induced Antitumor Effects of 5-Aminolevulinic Acid and Fractionated Ultrasound Irradiation in an Orthotopic Rat Glioma Model. Ultrasound Med. Biol. 2012, 38, 2143–2150. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Song, X.; Wang, Z.; Yue, W. Use of a Novel Sonosensitizer in Sonodynamic Therapy of U251 Glioma Cells in Vitro. Exp. Ther. Med. 2012, 3, 273–278. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Wang, K.; Li, X.-Q.; Chen, S.; Deng, J.-M.; Cheng, Y.; Wang, Z.-G. The ABCG2 Transporter Is a Key Molecular Determinant of the Efficacy of Sonodynamic Therapy with Photofrin in Glioma Stem-like Cells. Ultrasonics 2012, 53, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.; Cacaccio, J.; Durrani, F.; Bshara, W.; Turowski, S.; Spernyak, J.; Pandey, R. Sonodynamic Therapy in Combination with Photodynamic Therapy Shows Enhanced Long-Term Cure of Brain Tumor. Sci. Rep. 2020, 10, 21791. [Google Scholar] [CrossRef] [PubMed]
- Tzerkovsky, D.; Alexandrova, E.; Chalau, V.; Istomin, Y. Effects of Combined Sonodynamic and Photodynamic Therapies with Photolon on a Glioma C6 Tumor Model. Exp. Oncol. 2012, 34, 332–335. [Google Scholar]
- Madsen, S.; Gonzalez, J.; Zamora, G.; Berg, K.; Nair, R.; Hirschberg, H. Comparing the Effects of Light or Sonic Activated Drug Delivery: Photo/Sono Chemical Internalization. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Makita, K.; Miura, H.; Matsuda, A.; Kuchiike, D.; Kubo, K.; Mette, M.; Uto, Y.; Nishikata, T.; Hori, H.; et al. Case Report: A Breast Cancer Patient Treated with GcMAF, Sonodynamic Therapy and Hormone Therapy. Anticancer Res. 2014, 34, 4589–4593. [Google Scholar]
- Inui, T.; Amitani, H.; Kubo, K.; Kuchiike, D.; Uto, Y.; Nishikata, T.; Mette, M. Case Report: A Non-Small Cell Lung Cancer Patient Treated with GcMAF, Sonodynamic Therapy and Tumor Treating Fields. Anticancer Res. 2016, 36, 3767–3770. [Google Scholar] [PubMed]
- Sørensen, B.; Horsman, M. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- de Ridder, M.; Verellen, D.; Verovski, V.; Storme, G. Hypoxic Tumor Cell Radiosensitization through Nitric Oxide. Nitric Oxide 2008, 19, 164–169. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A Key Regulatory Factor in Tumour Growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Sharma, D.; Leong, K.; Czarnota, G. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4393. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wan, J.; Yu, A. Membrane Perforation and Recovery Dynamics in Microbubble-Mediated Sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Tharkar, P.; Varanasi, R.; Wong, W.S.; Jin, C.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Tarapacki, C.; Tran, W.; el Kaffas, A.; Lee, J.; Hupple, C.; Iradji, S.; Giles, A.; Al-Mahrouki, A.; Czarnota, G. Breast Tumor Response to Ultrasound Mediated Excitation of Microbubbles and Radiation Therapy in Vivo. Oncoscience 2016, 3, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, V.; Prakash, P.; Rieke, V.; Ozhinsky, E.; Plata, J.; Kurhanewicz, J.; Hsu, I.-C.; Diederich, C. Model-Based Feasibility Assessment and Evaluation of Prostate Hyperthermia with a Commercial MR-Guided Endorectal HIFU Ablation Array. Med. Phys. 2014, 41, 033301. [Google Scholar] [CrossRef] [PubMed]
- Tran, W.; Iradji, S.; Sofroni, E.; Giles, A.; Eddy, D.; Czarnota, G. Microbubble and Ultrasound Radioenhancement of Bladder Cancer. Br. J. Cancer 2012, 107, 469–476. [Google Scholar] [CrossRef]
- El Kaffas, A.; Al-Mahrouki, A.; Hashim, A.; Law, N.; Giles, A.; Czarnota, G. Role of Acid Sphingomyelinase and Ceramide in Mechano-Acoustic Enhancement of Tumor Radiation Responses. J. Natl. Cancer Inst. 2018, 110, 1009–1018. [Google Scholar] [CrossRef]
- Jang, K.; Seol, D.; Ding, L.; Lim, T.-H.; Frank, J.; Martin, J. Ultrasound-Mediated Microbubble Destruction Suppresses Melanoma Tumor Growth. Ultrasound Med. Biol. 2018, 44, 831–839. [Google Scholar] [CrossRef]
- Shi, J.; Fu, C.; Su, X.; Feng, S.; Wang, S. Ultrasound-Stimulated Microbubbles Inhibit Aggressive Phenotypes and Promotes Radiosensitivity of Esophageal Squamous Cell Carcinoma. Bioengineered 2021, 12, 3000–3013. [Google Scholar] [CrossRef]
- Peng, C.; Wu, Y.; Yang, Y.; Li, N.; Chen, X.; Gu, L.; Xu, D.; Yang, C. Using Ultrasound-Targeted Microbubble Destruction to Enhance Radiotherapy of Glioblastoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1355–1363. [Google Scholar] [CrossRef]
- He, Y.; Dong, X.-H.; Zhu, Q.; Xu, Y.-L.; Chen, M.-L.; Liu, Z. Ultrasound-Triggered Microbubble Destruction Enhances the Radiosensitivity of Glioblastoma by Inhibiting PGRMC1-Mediated Autophagy in Vitro and in Vivo. Mil. Med. Res. 2022, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-S.; Shih, I.-J.; Shueng, P.-W.; Kao, M.; Zhang, L.-W.; Chen, S.-F.; Chen, M.-H.; Liu, T.Y. Tumor Cell-Targeting Radiotherapy in the Treatment of Glioblastoma Multiforme Using Linear Accelerators. Acta Biomater 2021, 125, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bobeica, M.; Unger, M.; Bednarz, A.; Gerold, B.; Patties, I.; Melzer, A.; Landgraf, L. Focused Ultrasound Radiosensitizes Human Cancer Cells by Enhancement of DNA Damage. Strahlenther. Onkol. 2021, 197, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, N.; Heijman, E.; Köhler, M.; Ylihautala, M.; Ehnholm, G.; Simonetti, A.; Grüll, H. Tumour Hyperthermia and Ablation in Rats Using a Clinical MR-HIFU System Equipped with a Dedicated Small Animal Set-Up. Int. J. Hyperth. 2012, 28, 141–155. [Google Scholar] [CrossRef]
- Sukovich, J.; Cain, C.; Pandey, A.; Chaudhary, N.; Camelo-Piragua, S.; Allen, S.; Hall, T.; Snell, J.; Xu, Z.; Cannata, J.; et al. In Vivo Histotripsy Brain Treatment. J. Neurosurg. 2018, 131, 1331–1338. [Google Scholar] [CrossRef]
- Khokhlova, V.; Fowlkes, J.; Roberts, W.; Schade, G.; Xu, Z.; Khokhlova, T.; Hall, T.; Maxwell, A.; Wang, Y.-N.; Cain, C. Histotripsy Methods in Mechanical Disintegration of Tissue: Toward Clinical Applications. Int. J. Hyperth. 2015, 31, 145–162. [Google Scholar] [CrossRef]
- Xu, Z.; Ludomirsky, A.; Eun, L.Y.; Hall, T.L.; Tran, B.C.; Fowlkes, J.; Cain, C.A. Controlled Ultrasound Tissue Erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 726–736. [Google Scholar] [CrossRef]
- Xu, Z.; Owens, G.; Gordon, D.; Cain, C.; Ludomirsky, A. Noninvasive Creation of an Atrial Septal Defect by Histotripsy in a Canine Model. Circulation 2010, 121, 742–749. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.; Vlaisavljevich, E.; Lee, F. Histotripsy: The First Noninvasive, Non-Ionizing, Non-Thermal Ablation Technique Based on Ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Senapati, N.; Lele, P.; Caulfield, J. On Mechanisms of Cavitational Damage to Biological Tissues. J. Acoust. Soc. Am. 1974, 55, S6. [Google Scholar] [CrossRef]
- Gambihler, S.; Delius, M.; Brendel, W. Biological Effects of Shock Waves: Cell Disruption, Viability, and Proliferation of L1210 Cells Exposed to Shock Waves in Vitro. Ultrasound Med. Biol. 1990, 16, 587–594. [Google Scholar] [CrossRef]
- Giorgio, A.; Tarantino, L.; de Stefano, G.; Carmine, C.; Ferraioli, G. Complications After Percutaneous Saline-Enhanced Radiofrequency Ablation of Liver Tumors: 3-Year Experience with 336 Patients at a Single Center. AJR Am. J. Roentgenol. 2005, 184, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Maxwell, A.; Warnez, M.; Johnsen, E.; Cain, C.; Xu, Z. Histotripsy-Induced Cavitation Cloud Initiation Thresholds in Tissues of Different Mechanical Properties. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 341–352. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Kim, Y.; Owens, G.; Roberts, W.; Cain, C.; Xu, Z. Effects of Tissue Mechanical Properties on Susceptibility to Histotripsy-Induced Tissue Damage. Phys. Med. Biol. 2013, 59, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Edsall, C.; Khan, Z.M.; Mancia, L.; Hall, S.; Mustafa, W.; Johnsen, E.; Klibanov, A.L.; Durmaz, Y.Y.; Vlaisavljevich, E. Bubble Cloud Behavior and Ablation Capacity for Histotripsy Generated from Intrinsic or Artificial Cavitation Nuclei. Ultrasound Med. Biol. 2021, 47, 620–639. [Google Scholar] [CrossRef]
- Bader, K.; Vlaisavljevich, E.; Maxwell, A. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med. Biol. 2019, 45, 1056–1080. [Google Scholar] [CrossRef]
- Parsons, J.; Cain, C.; Abrams, G.; Fowlkes, J. Pulsed Cavitational Ultrasound Therapy for Controlled Tissue Homogenization. Ultrasound Med. Biol. 2006, 32, 115–129. [Google Scholar] [CrossRef]
- Xu, J.; Bigelow, T. Experimental Investigation of the Effect of Stiffness, Exposure Time and Scan Direction on the Dimension of Ultrasound Histotripsy Lesions. Ultrasound Med. Biol. 2011, 37, 1865–1873. [Google Scholar] [CrossRef]
- Ivey, J.; Bonakdar, M.; Kanitkar, A.; Davalos, R.; Verbridge, S. Improving Cancer Therapies by Targeting the Physical and Chemical Hallmarks of the Tumor Microenvironment. Cancer Lett. 2015, 380, 330–339. [Google Scholar] [CrossRef]
- Roberts, W. Development and Translation of Histotripsy: Current Status and Future Directions. Curr. Opin. Urol. 2013, 24, 104–110. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.; Ganguly, A.; Beems, M.; Hubbard, R.; Pepple, A.; Kevelin, A.; Garavaglia, H.; Dib, J.; et al. Non-Thermal Histotripsy Tumor Ablation Promotes Abscopal Immune Responses That Enhance Cancer Immunotherapy. J Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Kim, Y.; Allen, S.; Owens, G.; Pelletier, S.; Cain, C.; Ives, K.; Xu, Z. Image-Guided Non-Invasive Ultrasound Liver Ablation Using Histotripsy: Feasibility Study in an In Vivo Porcine Model. Ultrasound Med. Biol. 2013, 39, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Smolock, A.; Cristescu, M.; Vlaisavljevich, E.; Gendron-Fitzpatrick, A.; Green, C.; Cannata, J.; Ziemlewicz, T.; Lee, F. Robotically Assisted Sonic Therapy as a Noninvasive Nonthermal Ablation Modality: Proof of Concept in a Porcine Liver Model. Radiology 2018, 287, 171544. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Kieran, K.; Ives, K.; Fowlkes, J.; Cain, C.; Roberts, W. Histotripsy of Rabbit Renal Tissue in Vivo: Temporal Histologic Trends. J. Endourol. 2007, 21, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, T.; Schade, G.; Wang, Y.-N.; Buravkov, S.; Chernikov, V.; Simon, J.; Starr, F.; Maxwell, A.; Bailey, M.; Kreider, W.; et al. Pilot in Vivo Studies on Transcutaneous Boiling Histotripsy in Porcine Liver and Kidney. Sci. Rep. 2019, 9, 20176. [Google Scholar] [CrossRef]
- Styn, N.; Hall, T.; Fowlkes, J.; Cain, C.; Roberts, W. Histotripsy of Renal Implanted VX-2 Tumor in a Rabbit Model: Investigation of Metastases. Urology 2012, 80, 724–729. [Google Scholar] [CrossRef]
- Schade, G.; Wang, Y.-N.; D’Andrea, S.; Hwang, J.; Liles, W.; Khokhlova, T. Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response. Ultrasound Med. Biol. 2018, 45, 137–147. [Google Scholar] [CrossRef]
- Schade, G.; Keller, J.; Ives, K.; Cheng, X.; Rosol, T.; Keller, E.; Roberts, W. Histotripsy Focal Ablation of Implanted Prostate Tumor in an ACE-1 Canine Cancer Model. J. Urol. 2012, 188, 1957–1964. [Google Scholar] [CrossRef]
- Sukovich, J.; Xu, Z.; Kim, Y.; Cao, H.; Nguyen, T.-S.; Pandey, A.; Hall, T.; Cain, C. Targeted Lesion Generation Through the Skull Without Aberration Correction Using Histotripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 671–682. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, G.; Ye, D.; Nazeri, A.; Yue, Y.; Liu, W.; Wang, X.; Dunn, G.P.; Petti, A.A.; Leuthardt, E.C.; et al. Focused Ultrasound-Enabled Brain Tumor Liquid Biopsy. Sci. Rep. 2018, 8, 6553. [Google Scholar] [CrossRef]
- Rincon-Torroella, J.; Khela, H.; Bettegowda, A.; Bettegowda, C. Biomarkers and Focused Ultrasound: The Future of Liquid Biopsy for Brain Tumor Patients. J. Neuro-Oncol. 2022, 156, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Pacia, C.P.; Zhu, L.; Yang, Y.; Yue, Y.; Nazeri, A.; Michael Gach, H.; Talcott, M.R.; Leuthardt, E.C.; Chen, H. Feasibility and Safety of Focused Ultrasound-Enabled Liquid Biopsy in the Brain of a Porcine Model. Sci. Rep. 2020, 10, 7449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bettegowda, C. Applications of DNA-Based Liquid Biopsy for Central Nervous System Neoplasms. J. Mol. Diagn. 2017, 19, 24–34. [Google Scholar] [CrossRef]
- Azad, T.D.; Jin, M.C.; Bernhardt, L.J.; Bettegowda, C. Liquid Biopsy for Pediatric Diffuse Midline Glioma: A Review of Circulating Tumor DNA and Cerebrospinal Fluid Tumor DNA. Neurosurg. Focus 2020, 48, E9. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Suppiah, S.; Llinas, M.; Huang, Y.; Sahgal, A.; Perry, J.; Keith, J.; Davidson, B.; Hamani, C.; et al. MR-Guided Focused Ultrasound Liquid Biopsy Enriches Circulating Biomarkers in Patients with Brain Tumors. Neuro-Oncology 2021, 23, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Nazeri, A.; Pacia, C.P.; Yue, Y.; Chen, H. Focused Ultrasound for Safe and Effective Release of Brain Tumor Biomarkers into the Peripheral Circulation. PLoS ONE 2020, 15, e0234182. [Google Scholar] [CrossRef]
- Lozano, A.; Zadeh, G. BRAINFUL (BRAIN Tumor Focused Ultrasound-Enabled Liquid Biopsy) Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT04940507 (accessed on 27 August 2022).
- Huang, Y.; Alkins, R.; Schwartz, M.L.; Hynynen, K. Opening the Blood-Brain Barrier with MR Imaging-Guided Focused Ultrasound: Preclinical Testing on a Trans-Human Skull Porcine Model. Radiology 2017, 282, 123–130. [Google Scholar] [CrossRef]
- Conti, A.; Kamimura, H.A.S.; Novell, A.; Duggento, A.; Toschi, N. Magnetic Resonance Methods for Focused Ultrasound-Induced Blood-Brain Barrier Opening. Front. Phys. 2020, 8, 393. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-Invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Weeks, E.M.; Platt, M.W.; Gedroyc, W. MRI-Guided Focused Ultrasound (MRgFUS) to Treat Facet Joint Osteoarthritis Low Back Pain—Case Series of an Innovative New Technique. Eur. Radiol. 2012, 22, 2822–2835. [Google Scholar] [CrossRef] [PubMed]
- Schoen, S.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards Controlled Drug Delivery in Brain Tumors with Microbubble-Enhanced Focused Ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Antar, A.; Pennington, Z.; Aygun, N.; Patel, J.; Goldsborough, E.; Porras, J.L.; Elsamadicy, A.A.; Lubelski, D.; Wolinsky, J.-P.; et al. Predictors of Survival and Time to Progression Following Operative Management of Intramedullary Spinal Cord Astrocytomas. J. Neuro-Oncol. 2022, 1, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Patel, J.; Pennington, Z.; Porras, J.L.; Goldsborough, E.; Antar, A.; Elsamadicy, A.A.; Lubelski, D.; Wolinsky, J.-P.; Jallo, G.; et al. Perioperative Outcomes and Survival after Surgery for Intramedullary Spinal Cord Tumors: A Single-Institutional Series of 302 Patients. J. Neurosurg. Spine 2022, 37, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.S.; Iannessi, A.; Natale, R.; Beaumont, H.; Patriti, S.; Xiong-Ying, J.; Baudin, G.; Thyss, A. Focused Ultrasound for the Treatment of Bone Metastases: Effectiveness and Feasibility. J. Ther. Ultrasound 2018, 6, 8. [Google Scholar] [CrossRef]
| Sonosensitizer | Application/Additional Treatment | Cell Line | Intensity (W/cm2) | Frequency (MHz) | Exposure Time (min) | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| 5-ALA | Standard SDT | C6/U87 | 10 | 1.1 | 3 | Reduction in tumor cell size and viability | [101] |
| 5-ALA | Standard SDT | C6 | 5.5 | 1.06 | 20 | Inhibition of tumor growth, significantly improved survival. | [115] |
| 5-ALA | Standard SDT | F98 | 20 | 0.22 | 4 | Reduced tumor cell viability, induction of apoptosis, suppression of tumor proliferation and invasion, minimal damage to normal brain tissue. | [114] |
| 5-ALA | Standard SDT | U87/U251 | 2 | 3 | 3 | Inhibition of tumor cell growth, increased apoptotic death, prolonged survival. | [111] |
| 5-ALA | Standard SDT | RG2 | 2–6 | 1 | 3 | Decreased cell viability, increased chromatin condensation and apoptosis. | [119] |
| 5-ALA | Standard SDT | C6 | 0.33–8 | 1.06 | — | Average threshold intensity causing tumor cell death determined as 5.7 W/cm2. | [120] |
| 5-ALA/PPIX | Standard SDT | C6/U87 | 0.16 | 1 | 1 | Enhanced tumor cell cytotoxicity and increased induction of apoptosis. | [118] |
| DVDMS | Standard SDT | U87 | 0.32 | 0.97 | 3 | Significant cytotoxicity | [121] |
| DVDMS | Standard SDT | U373 | 0.45 | 1 | 1 | Significant loss of tumor cell viability and increased apoptosis, caspase-3, and DNA fragmentation. | [122] |
| Fluorescein | Standard SDT | C6 | 2–6 | 0.35 | 20 | Significant inhibition of ectopic glioma outgrowth. | [123] |
| HMME | Standard SDT | C6 | 0.5 | 1 | 2 | Inhibition of tumor growth and angiogenesis, induction of apoptosis. | [117] |
| HMME | Standard SDT | C6 | 1 | 0.5 | 1 | Increased induction of apoptosis, ROS production, and cyt-c along with decreased MMP. | [124] |
| HMME | Standard SDT | C6 | 1 | 0.5 | 1 | Apoptosis, ROS production, decreased MMP, and release of cytochrome c. | [125] |
| Nanoparticles | |||||||
| Ce6 | Fe3O4 + Ce6 NPs | C6 | 1 | 1 | 1 | Significant inhibition of tumor growth, prolonged median survival, no adverse effects on healthy tissues | [126] |
| Ce6 | Ce6 + HCQ liposomal NPs | GL261 | 1 | 1 | 1 | Significant inhibition of tumor growth, prolonged survival time | [127] |
| Ce6 | Mn2+-chelated Ce6 NPs | U87 | 1 | 0.8 | — | Complete suppression of subcutaneous tumor growth and delayed progression of orthotopic tumor growth. | [128] |
| DVDMS | DVDMS Liposomal NPs | C6 | 1 | 1 | 1 | Suppression of tumor growth, increased median survival time and good biocompatibility | [116] |
| DVDMS | Mn2-chelated DVDMS NPs | U87 | 0.5 | 0.5 | 5 | Inhibition of tumor growth. | [129] |
| Indocyanine green | Silica NPs loaded with indocyanine green | U87 | 1.5 | 1 | 5 | Significant inhibition of tumor growth, increased median survival | [130] |
| IR780 | Angiopep-2 + PLGA + IR780 + MnO2 NPs | U87 | 1 | 1 | 1 | Improved targeting and deeper penetration into tumors, significant inhibition of tumor growth and distal metastasis, lack of systemic toxicity. | [131] |
| IR780 | IR780 NPs | U87 | 0.2–0.4 | 1 | 3 | Significant inhibition of tumor growth, induction of apoptosis in tumors, no obvious toxicity. | [132] |
| HMME | YVO4:Nd3+-HMME NPs with MnO2 shell | C6 | 0.7 | 3 | 4 | Inhibition of tumor growth | [133] |
| Hypocrellin | PEG-PGLA NPs with hypocrellin | U87 | 0.8 | 1 | 5 | Slower tumor growth rates | [134] |
| PPIX | MnO2—transferrin NPs loaded with PPIX | C6 | 1.5 | 1 | 3 | Suppression of tumor growth, favorable biocompatibility, and safety. | [135] |
| Additional therapies | |||||||
| 5-ALA | Combined with hyperthermotherapy | SNB19/U87 | 1–2 | 1 | 2 | Significant reduction in tumor cell viability, increased apoptosis induction | [100] |
| 5-ALA | Combined with celecoxib | Mouse glioma cells | 2 | 1 | 2 | Decreased tumor volume, improved survival | [136] |
| DVDMS | Combined with PDT | U118/U87 | 0.5 | 1 | 1–3 | Inhibition of glioma cell proliferation, induction of tumor cell apoptosis | [113] |
| HMME | Combined with Ca2+ channel antagonist | U87 | 0.5 | 0.04 | 1 | Tumor volume significantly suppressed. | [137] |
| HMME | Combined with PDT | C6 | 0.5 | 1 | 1.5 | Significantly higher tumor growth inhibition rate, apoptosis rate ROS generation. | [138] |
| TiO2 | Combined with anti-EGFR antibody | U87/U87de2–7 | 1 | 1 | 1 | Reduced tumor cell viability | [139] |
| Transducer | Transducer Focus | Acoustic Pressure | Duty Cycle | Pulse Repetition Frequency | Exposure Duration | Refs. |
|---|---|---|---|---|---|---|
| Animal Studies | ||||||
| VIFU 2000; Alpinion US Inc., Bothell, WA, USA | 1.5 MHz | 3.82 MPa | 1% | 1 Hz | 2 min | [196] |
| Sonalleve V2, Profound Medical Inc., Mississauga, ON, Canada | 1.44 MHz | 1.48 MPa 2.74 MPa 3.53 MPa | 1% | 1 Hz | 2 min | |
| Sonalleve V2, Profound Medical Inc., Mississauga, ON, Canada | 1.44 MHz | 0.59 MPa, 1.29 MPa, 1.58 MPa | 1% | 1 Hz | 4 min | [203] |
| Imasonics, Voray sur l’Ognon, France | 650 kHz | 1.5 MPa | 1% | 1 Hz | 3 min | [198] |
| Human Studies | ||||||
| ExAblate Neuro hemispheric device (InSightec, Tirat Carmel, Israel) | 220 kHz | 500 kPa | 0.74% | 33 Hz | 50 s | [202,204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hersh, A.M.; Bhimreddy, M.; Weber-Levine, C.; Jiang, K.; Alomari, S.; Theodore, N.; Manbachi, A.; Tyler, B.M. Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier. Cancers 2022, 14, 4920. https://doi.org/10.3390/cancers14194920
Hersh AM, Bhimreddy M, Weber-Levine C, Jiang K, Alomari S, Theodore N, Manbachi A, Tyler BM. Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier. Cancers. 2022; 14(19):4920. https://doi.org/10.3390/cancers14194920
Chicago/Turabian StyleHersh, Andrew M., Meghana Bhimreddy, Carly Weber-Levine, Kelly Jiang, Safwan Alomari, Nicholas Theodore, Amir Manbachi, and Betty M. Tyler. 2022. "Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier" Cancers 14, no. 19: 4920. https://doi.org/10.3390/cancers14194920
APA StyleHersh, A. M., Bhimreddy, M., Weber-Levine, C., Jiang, K., Alomari, S., Theodore, N., Manbachi, A., & Tyler, B. M. (2022). Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier. Cancers, 14(19), 4920. https://doi.org/10.3390/cancers14194920







