Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Statistical Analysis
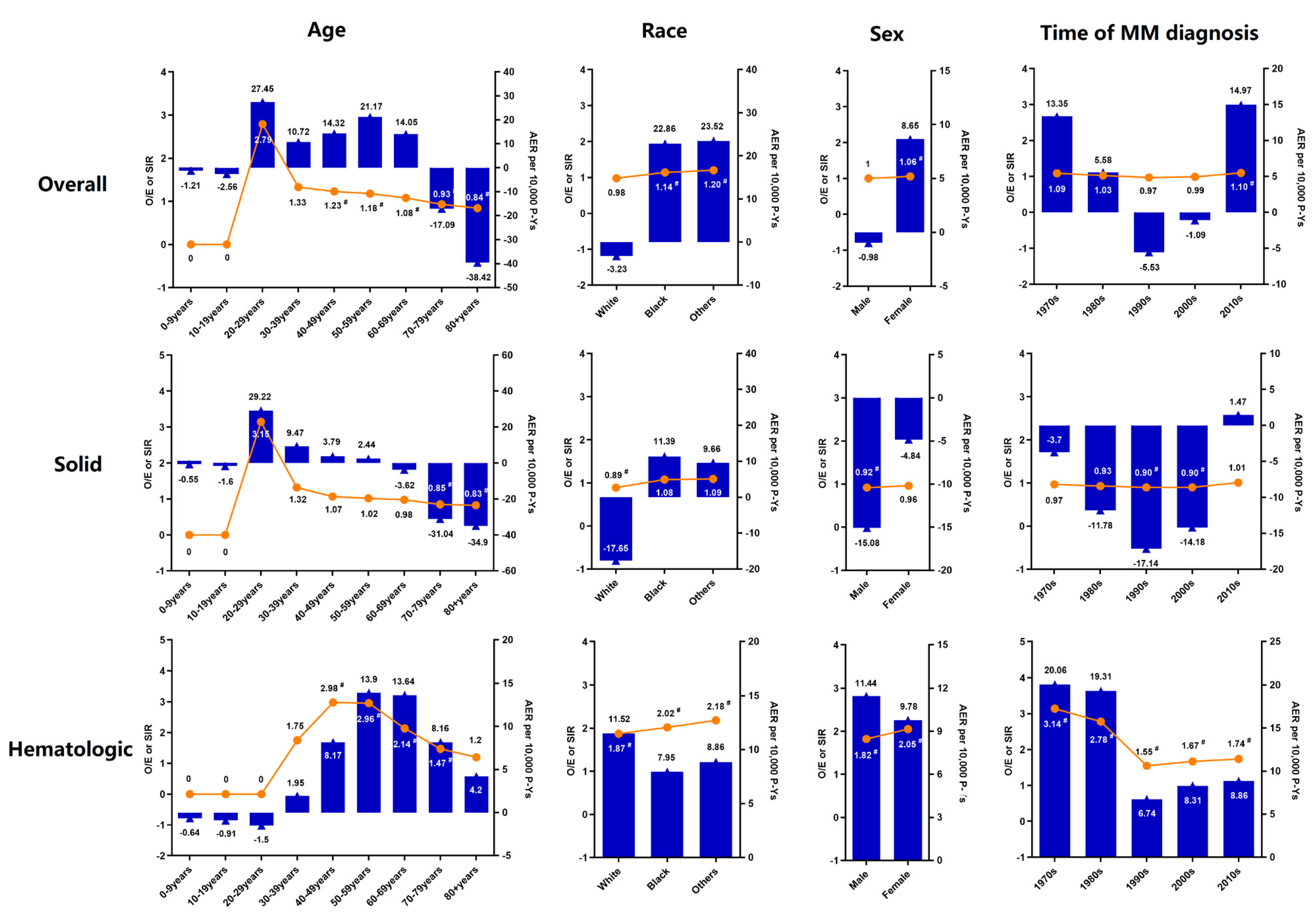
3. Results
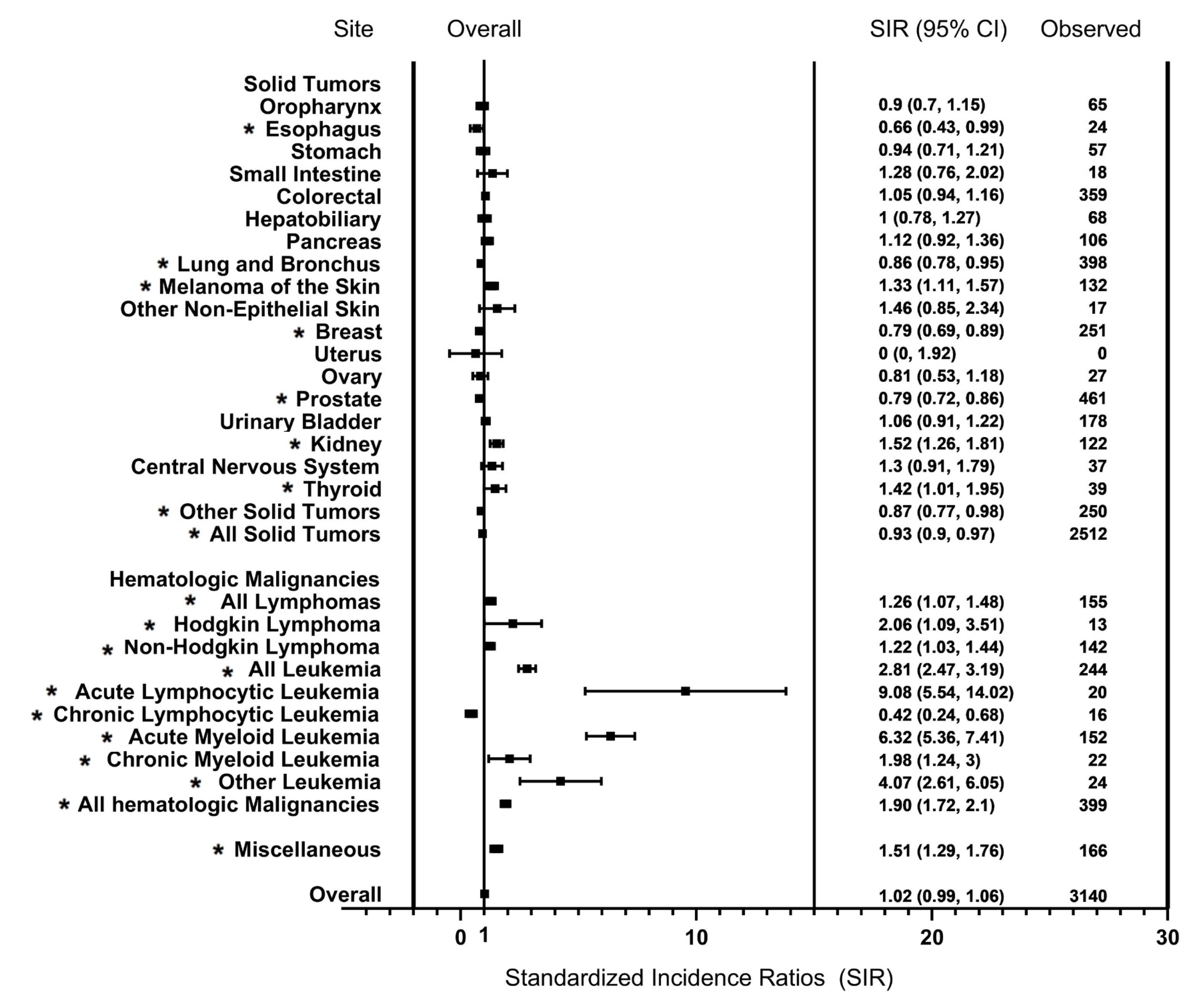
3.1. Distribution of SPM Sites
3.2. Risk of SPM
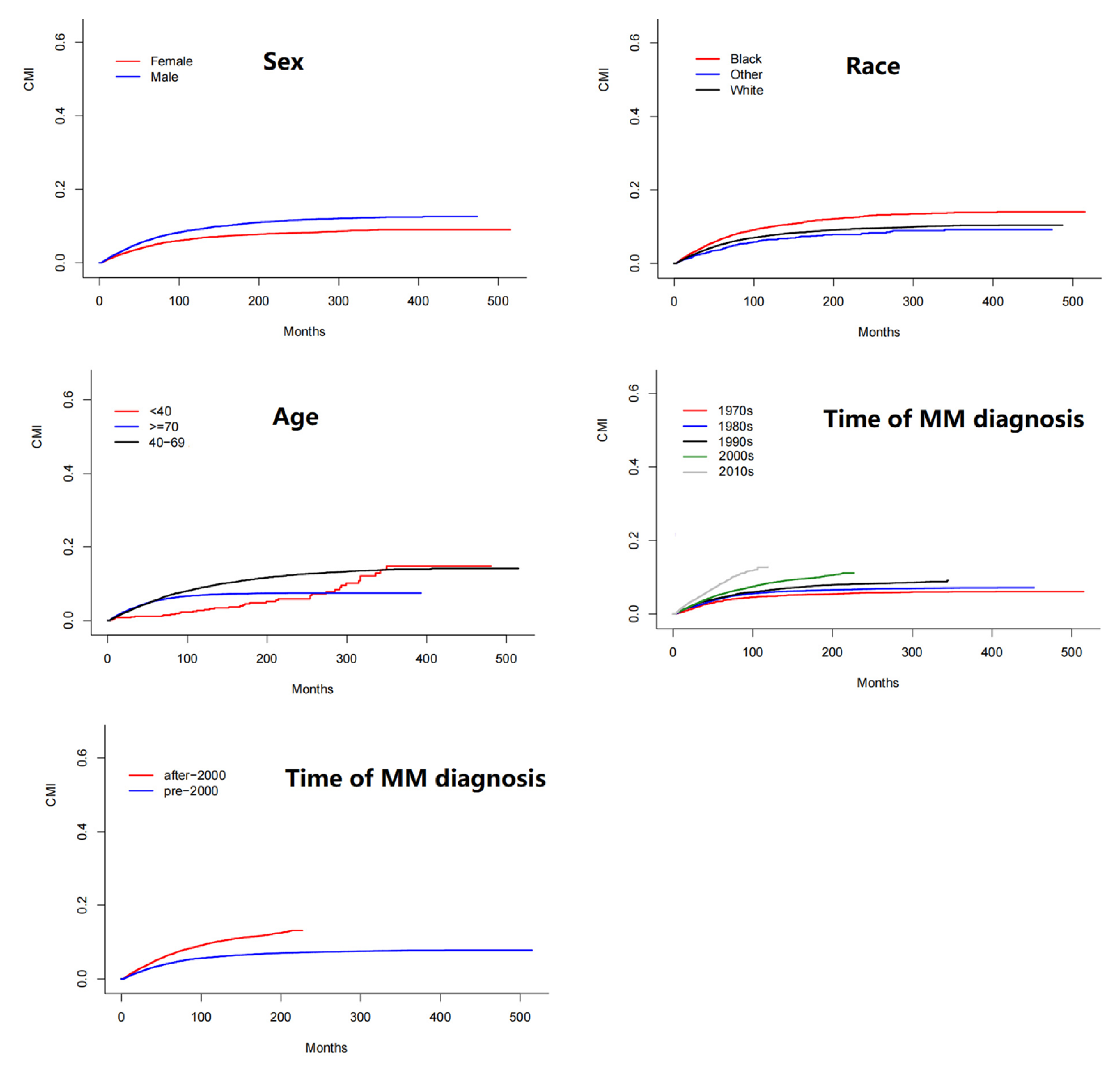
3.3. Cumulative Incidence and Risk Factors for SPM
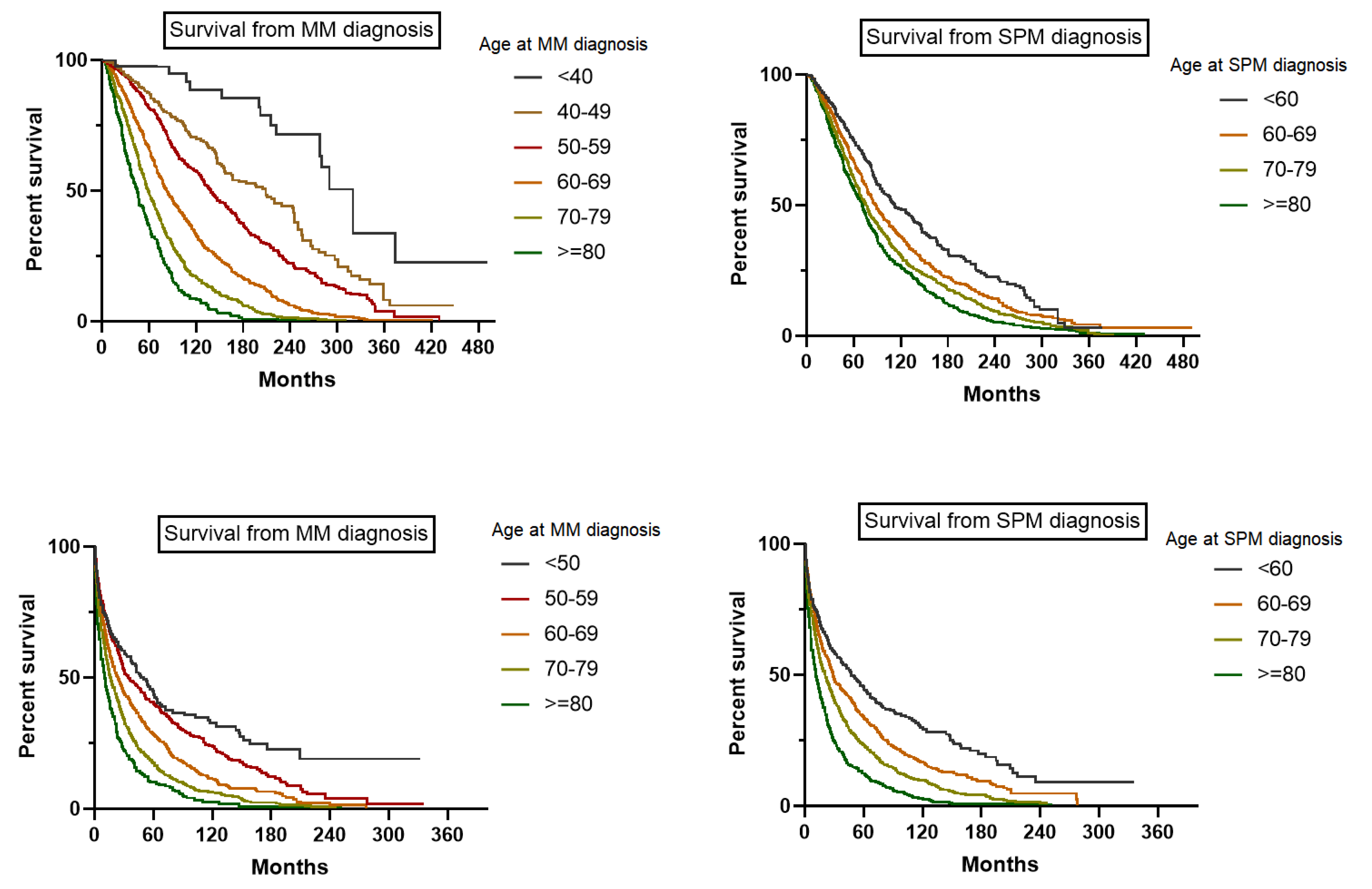
3.4. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Oortgiesen, B.E.; van Roon, E.N.; Joosten, P.; Kibbelaar, R.E.; Storm, H.; Hovenga, S.; Van Rees, J.B.; Woolthuis, G.; Veeger, N.; De Waal, E.G.; et al. The role of initial clinical presentation, comorbidity and treatment in multiple myeloma patients on survival: A detailed population-based cohort study. Eur. J. Clin. Pharmacol. 2017, 73, 771–778. [Google Scholar] [CrossRef]
- Poh, C.; Keegan, T.; Rosenberg, A.S. Second primary malignancies in multiple myeloma: A review. Blood Rev. 2021, 46, 100757. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, K.; Diamond, B.; Maura, F.; Hillengass, J.; Turesson, I.; Landgren, C.O.; Kazandjian, D. Second malignancies in multiple myeloma; emerging patterns and future directions. Best Pr. Res. Clin. Haematol. 2020, 33, 101144. [Google Scholar] [CrossRef]
- Rosenberg, A.S.; Brunson, A.; Tuscano, J.; Jonas, B.A.; Hoeg, R.; Wun, T.; Keegan, T.H.M. Effect of autologous hematopoietic stem cell transplant on the development of second primary malignancies in multiple myeloma patients. Blood Cancer J. 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef]
- Kyle, R.A.; Pierre, R.V.; Bayrd, E.D. Multiple myeloma and acute myelomonocytic leukemia. N. Engl. J. Med. 1970, 283, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, D.E.; Bailey, A.J.; Langley, G.R.; MacDonald, R.N.; White, D.F.; Miller, A.B. The chemotherapy on plasma-cell myeloma and the incidence of acute leukemia. N. Engl. J. Med. 1979, 301, 743–748. [Google Scholar] [CrossRef]
- Reddi, D.M.; Lu, C.M.; Fedoriw, G.; Liu, Y.C.; Wang, F.F.; Ely, S.; Boswell, E.L.; Louissaint, A., Jr.; Arcasoy, M.O.; Goodman, B.K.; et al. Myeloid neoplasms secondary to plasma cell myeloma: An intrinsic predisposition or therapy-related phenomenon? A clinicopathologic study of 41 cases and correlation of cytogenetic features with treatment regimens. Am. J. Clin. Pathol. 2012, 138, 855–866. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.S.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Hajek, R.; Delforge, M.; Kropff, M.; Petrucci, M.T.; Catalano, J.; Gisslinger, H.; Wiktor-Jędrzejczak, W.; Zodelava, M.; Weisel, K.; et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N. Engl. J. Med. 2012, 366, 1759–1769. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Kumar, S.K.; Lupparelli, G.; Usmani, S.; Waage, A.; Larocca, A.; van der Holt, B.; Musto, P.; Offidani, M.; et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: A meta-analysis of individual patient data. Lancet Oncol. 2014, 15, 333–342. [Google Scholar] [CrossRef]
- Attal, M.; Palumbo, A.; Holstein, S.A.; Lauwers-Cances, V.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide (LEN) maintenance (MNTC) after high-dose melphalan and autologous stem cell transplant (ASCT) in multiple myeloma (MM): A meta-analysis (MA) of overall survival (OS). J. Clin. Oncol. 2016, 34, 8001. [Google Scholar] [CrossRef]
- Jonsdottir, G.; Lund, S.H.; Björkholm, M.; Turesson, I.; Wahlin, A.; Mailankody, S.; Blimark, C.; Hultcrantz, M.; Porwit, A.; Landgren, O.; et al. Survival in multiple myeloma patients who develop second malignancies: A population-based cohort study. Haematologica 2016, 101, e145–e148. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Razavi, P.; Rand, K.A.; Cozen, W.; Chanan-Khan, A.; Usmani, S.; Ailawadhi, S. Patterns of second primary malignancy risk in multiple myeloma patients before and after the introduction of novel therapeutics. Blood Cancer J. 2013, 3, e121. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.S.; Huang, H.; Cai, L.; Zhao, L.; Peng, R.J.; Lin, Y.; Tang, J.; Zeng, J.; Zhang, L.; et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 2021, 325, 50–58. [Google Scholar] [PubMed]
- Minard-Colin, V.; Aupérin, A.; Pillon, M.; Burke, G.A.A.; Barkauskas, D.A.; Wheatley, K.; Delgado, R.F.; Alexander, S.; Uyttebroeck, A.; Bollard, C.M.; et al. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin's Lymphoma in Children. N. Engl. J. Med. 2020, 382, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Pfeiffer, R.M.; Kristinsson, S.Y.; Korde, N.; Bjorkholm, M.; Goldin, L.R.; Turesson, I.; Landgren, O. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 2011, 118, 4086–4092. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hemminki, K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: A search for common mechanisms. Br. J. Cancer 2001, 85, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hauke, R.J.; Bonthu, N.; Tarantolo, S.R. Increased incidence of a second lymphoproliferative malignancy in patients with multiple myeloma--a SEER based study. Anticancer. Res. 2012, 32, 4507–4515. [Google Scholar] [PubMed]
- Tzeng, H.E.; Lin, C.L.; Tsai, C.H.; Tang, C.H.; Hwang, W.L.; Cheng, Y.W.; Sung, F.-C.; Chung, C.-J. Time trend of multiple myeloma and associated secondary primary malignancies in Asian patients: A Taiwan population-based study. PLoS ONE. 2013, 8, e68041. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Swaika, A.; Razavi, P.; Yang, D.; Chanan-Khan, A. Variable risk of second primary malignancy in multiple myeloma patients of different ethnic subgroups. Blood Cancer J. 2014, 4, e243. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Cairns, D.A.; Gregory, W.M.; Collett, C.; Pawlyn, C.; Sigsworth, R.; Striha, A.; Henderson, R.; Kaiser, M.F.; Jenner, M.; et al. Second malignancies in the context of lenalidomide treatment: An analysis of 2732 myeloma patients enrolled to the Myeloma XI trial. Blood Cancer J. 2016, 6, e506. [Google Scholar] [CrossRef] [PubMed]
- Duray, A.; Demoulin, S.; Petermans, J.; Moutschen, M.; Saussez, S.; Jerusalem, G.; Delvenne, P. Aging and cancer: Coincidence or etiologic relationship? Rev. Med. Liege 2014, 69, 276–281. [Google Scholar] [PubMed]
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence Trends of Breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US From 2010 to 2016. JAMA Netw. Open 2020, 3, e2013226. [Google Scholar] [CrossRef] [PubMed]
- Ohri, A.; Robinson, A.; Liu, B.; Bhuket, T.; Wong, R. Updated Assessment of Colorectal Cancer Incidence in the U.S. by Age, Sex, and Race/Ethnicity. Dig. Dis. Sci. 2020, 65, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Hurwitz, L.M.; Geczik, A.M.; Butler, E.N. An Up-to-date Assessment of US Prostate Cancer Incidence Rates by Stage and Race: A Novel Approach Combining Multiple Imputation with Age and Delay Adjustment. Eur. Urol. 2021, 79, 33–41. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Singal, A.G.; Waljee, A.K.; Elmunzer, B.J.; Pruitt, S.L.; McKey, T.; Rubenstein, J.H.; Scheiman, J.M.; Murphy, C.C. Racial Disparities and Trends in Pancreatic Cancer Incidence and Mortality in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gopalani, S.V.; Janitz, A.E.; Campbell, J.E. Cervical Cancer Incidence and Mortality among Non-Hispanic African American and White Women, United States, 1999–2015. J. Natl. Med. Assoc. 2020, 112, 632–638. [Google Scholar] [CrossRef]
- Acute leukaemia and other secondary neoplasms in patients treated with conventional chemotherapy for multiple myeloma: A Finnish Leukaemia Group study. Eur. J. Haematol. 2000, 65, 123–127. [CrossRef]
- Yamasaki, S.; Yoshimoto, G.; Kohno, K.; Henzan, H.; Aoki, T.; Tanimoto, K.; Sugio, Y.; Muta, T.; Kamimura, T.; Ohno, Y.; et al. Risk of secondary primary malignancies in multiple myeloma patients with or without autologous stem cell transplantation. Int. J. Hematol. 2019, 109, 98–106. [Google Scholar] [CrossRef] [PubMed]
| Year | 1975–2018 (n = 3101) | 1975–1979 (n = 186) | 1980–1989 (n = 532) | 1990–1999 (n = 752) | 2000–2009 (n = 951) | 2010–2018 (n = 680) | |
|---|---|---|---|---|---|---|---|
| Characteristics | |||||||
| Median age at MM diagnosis, years (IQR) | 67.0 (59.0–74.0) | 65.0 (56.75–72.0) | 68.0 (61.0–74.0) | 67.5 (58.0–74.0) | 66.0 (58.0–73.0) | 68.0 (61.0–75.0) | |
| Median age at SPM diagnosis, years (IQR) | 71.0 (65.0–78.0) | 70.0 (62.75–77.25) | 73.0 (67.0–79.0) | 72.0 (65.0–78.0) | 71.0 (64.0–78.0) | 70.0 (64.0–77.0) | |
| Median latency, months (IQR) | 44.0 (19.0–83.0) | 45.5 (23.75–89.0) | 46.0 (19.25–82.75) | 50.5 (22.0–101.0) | 48.0 (22.0–89.0) | 26.0 (11.0–47.0) | |
| Age at MM diagnosis (%) | |||||||
| 20–29 years | 2 (0.06) | 1 (0.54) | 0 (0) | 1 (0.13) | 0 (0) | 0 (0) | |
| 30–39 years | 32 (1.03) | 3 (1.61) | 7 (1.32) | 10 (1.33) | 6 (0.63) | 6 (0.88) | |
| 40–49 years | 168 (5.42) | 13 (6.99) | 27 (5.07) | 62 (8.25) | 49 (5.15) | 17 (2.50) | |
| 50–59 years | 593 (19.12) | 45 (24.19) | 79 (14.85) | 142 (18.88) | 211 (22.19) | 116 (17.06) | |
| 60–69 years | 1086 (35.02) | 65 (34.95) | 197 (37.03) | 230 (30.59) | 340 (35.75) | 254 (37.35) | |
| 70–79 years | 897 (28.93) | 42 (22.58) | 167 (31.39) | 236 (31.38) | 262 (27.55) | 190 (27.94) | |
| 80+ years | 323 (10.42) | 17 (9.14) | 55 (10.34) | 71 (9.44) | 83 (8.73) | 97 (14.27) | |
| Age at SPM diagnosis (%) | |||||||
| 0–9 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 10–19 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 20–29 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 30–39 years | 5 (0.16) | 0 (0) | 1 (0.19) | 0 (0) | 1 (0.11) | 3 (0.44) | |
| 40–49 years | 55 (1.77) | 6 (3.23) | 6 (1.13) | 16 (2.13) | 10 (1.05) | 17 (2.50) | |
| 50–59 years | 319 (10.29) | 24(12.90) | 35 (6.58) | 72 (9.58) | 113 (11.88) | 75 (11.03) | |
| 60–69 years | 921 (29.70) | 59 (31.72) | 135 (25.37) | 203 (26.99) | 301 (31.65) | 223 (32.79) | |
| 70–79 years | 1177 (37.96) | 59 (31.72) | 242 (45.49) | 303 (40.29) | 348 (36.59) | 225 (33.09) | |
| 80+ years | 624 (20.12) | 38 (20.43) | 113 (21.24) | 158 (21.01) | 178 (18.72) | 137 (20.15) | |
| Sex (%) | |||||||
| Male | 1884 (60.75) | 111 (59.68) | 319 (59.96) | 463 (61.57) | 576 (60.57) | 408 (60.00) | |
| Female | 1209 (39.25) | 75 (40.32) | 213 (40.04) | 294 (38.43) | 375 (39.43) | 272 (40.00) | |
| Race (%) | |||||||
| White | 2302 (74.23) | 150 (80.65) | 401 (75.38) | 570 (75.80) | 709 (74.55) | 472 (69.41) | |
| Black | 626 (20.18) | 31 (16.66) | 111 (20.86) | 141 (18.75) | 189 (19.87) | 154 (22.65) | |
| Others | 172 (5.55) | 5 (2.69) | 20 (3.76) | 41 (5.45) | 53 (5.58) | 53 (7.79) | |
| Type of SPM (%) | |||||||
| Prostate | 488 (14.32) | 26 (12.44) | 81 (14.04) | 117 (14.10) | 175 (16.51) | 89 (12.18) | |
| Lung and Bronchus | 420 (12.33) | 30 (14.35) | 80 (13.86) | 104 (12.53) | 117 (11.04) | 89 (12.18) | |
| Breast | 272 (7.98) | 11 (5.26) | 50 (8.67) | 64 (7.71) | 87 (8.21) | 60 (8.21) | |
| Urinary Bladder | 187 (5.49) | 19 (9.09) | 44 (7.63) | 40 (4.82) | 50 (4.72) | 34 (4.65) | |
| Melanoma | 160 (4.70) | 5 (2.39) | 12 (2.08) | 33 (3.98) | 65 (6.13) | 45 (6.16) | |
| Kidney and Renal Pelvis | 131 (3.85) | 4 (1.91) | 14 (2.43) | 25 (3.01) | 36 (3.40) | 52 (7.11) | |
| AML | 158 (4.64) | 19 (9.09) | 39 (6.76) | 24 (2.89) | 45 (4.25) | 31 (4.24) | |
| ALL | 22 (0.65) | 3 (1.44) | 0 (0) | 6 (0.72) | 3 (0.28) | 10 (1.37) | |
| CML | 23(0.74) | 2(1.08) | 2(0.04) | 10(1.33) | 5(0.53) | 4(0.59) | |
| MDS | 102 (2.99) | 0 (0) | 0 (0) | 26 (3.13) | 48 (4.53) | 28 (3.83) | |
| NHL—Nodal | 107 (3.14) | 8 (3.83) | 20 (3.47) | 25 (3.01) | 36 (3.40) | 18 (2.46) | |
| NHL—Extranodal | 69 (2.03) | 1 (0.48) | 9 (1.56) | 14 (1.69) | 28 (2.64) | 17 (2.33) | |
| Characteristics | 1975–2018 SIR (95% CI) | 1970s SIR (95% CI) | 1980s SIR (95% CI) | 1990s SIR (95% CI) | 2000s SIR (95% CI) | 2010s SIR (95% CI) |
| 1.90 (1.72–2.10) # | 3.14 (2.18–4.36) # | 2.78 (2.23–3.43) # | 1.55 (1.22–1.95) # | 1.67 (1.39–2.00) # | 1.74 (1.39–2.16) # | |
| Latency period (Months) | ||||||
| 2–5 | 1.78 (1.19–2.55) # | 0.00 (0.00–4.32) | 2.83 (1.14–5.84) # | 2.06 (0.83–4.25) | 2.48 (1.24–4.44) # | 0.77 (0.21–1.98) |
| 6–11 | 0.97 (0.60–1.48) | 0.88 (0.02–4.93) | 1.83 (0.67–3.99) | 0.22 (0.01–1.23) | 0.50 (0.10–1.46) | 1.47 (0.70–2.70) |
| 12–59 | 1.63 (1.39–1.89) # | 2.54 (1.35–4.34) # | 2.19 (1.51–3.07) # | 1.06 (0.67–1.59) | 1.59 (1.19–2.09) # | 1.64 (1.22–2.17) # |
| 60–119 | 2.87 (2.40–3.42) # | 7.26 (4.15–11.80) # | 4.38 (2.86–6.42) # | 2.59 (1.69–3.79) # | 1.95 (1.40–2.66) # | 3.39 (2.07–5.23) # |
| ≥120 | 2.25 (1.68–2.96) | 2.70 (0.88–6.30) | 3.30 (1.85–5.44) | 2.08 (1.23–3.28) | 1.71 (0.91–2.93) | NA |
| Age (Years) | ||||||
| 0–9 | 0.00 (0.00–16402.87) | 0.00 (0.00–0.00) | 0.00 (0.00–16402.87) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| 10–19 | 0.00 (0.00–11395.49) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–47317.55) | 0.00 (0.00–15010.46) |
| 20–29 | 0.00 (0.00–52.58) | 0.00 (0.00–270.34) | 0.00 (0.00–232.66) | 0.00(0.00–200.09) | 0.00 (0.00–236.48) | 0.00 (0.00–556.75) |
| 30–39 | 1.75 (0.21–6.32) | 10.28 (0.26–57.27) | 3.45 (0.09–19.22) | 0.00 (0.00–11.05) | 0.00 (0.00–11.32) | 0.00 (0.00–37.93) |
| 40–49 | 2.98 (1.89–4.46) # | 5.70 (1.18–16.65) # | 3.90 (1.27–9.10) # | 3.39 (1.46–6.68) # | 1.96 (0.64–4.57) | 1.98 (0.24–7.17) |
| 50–59 | 2.96 (2.38–3.64) # | 4.58 (2.10–8.70) # | 3.10 (1.69–5.19) # | 2.19 (1.25–3.56) # | 2.98 (2.06–4.16) # | 3.24 (1.89–5.19) # |
| 60–69 | 2.14 (1.80–2.53) # | 3.20 (1.60–5.73) # | 3.52 (2.45–4.90) # | 1.32 (0.79–2.06) | 1.94 (1.39–2.62) # | 2.07 (1.39–2.95) # |
| 70–79 | 1.47 (1.20–1.77) # | 2.74 (1.31–5.03) # | 2.29 (1.49–3.39) # | 1.22 (0.76–1.87) | 1.35 (0.92–1.90) | 1.09 (0.65–1.70) |
| 80+ | 1.20 (0.86–1.64) | 0.68 (0.02–3.79) | 1.60 (0.64–3.30) | 1.65 (0.82–2.95) | 0.53 (0.19–1.15) | 1.60 (0.89–2.64) |
| Race | ||||||
| White | 1.87 (1.67–2.09) # | 3.31 (2.27–4.68) # | 2.65 (2.06–3.35) # | 1.35 (1.02–1.76) # | 1.73 (1.41–2.09) # | 1.75 (1.35–2.22) # |
| Black | 2.02 (1.52–2.64) # | 2.36 (0.49–6.90) | 3.5 (1.91–5.87) # | 2.22 (1.18–3.80) # | 1.24 (0.62–2.22) | 1.95 (1.04–3.34) # |
| Other | 2.18 (1.31–3.41) # | 0.00 (0.00–16.05) | 3.41 (0.70–9.96) | 3.99 (1.60–8.21) # | 1.94 (0.71–4.21) | 1.09 (0.23–3.20) |
| Sex | ||||||
| Female | 2.05 (1.75–2.40) # | 2.98 (1.63–5.00) # | 2.82 (1.97–3.90) # | 1.87 (1.30–2.62) # | 1.74 (1.27–2.33) # | 1.88 (1.28–2.67) # |
| Male | 1.82 (1.59–2.06) # | 3.25 (2.01–4.96) # | 2.75 (2.05–3.62) # | 1.36 (0.98–1.84) # | 1.63 (1.28–2.05) # | 1.67 (1.24–2.18) # |
| Factors | HR and 95% CI | p |
|---|---|---|
| Age at MM diagnosis | ||
| ≥70 vs. <40–69 | 0.67 (0.63–0.72) | 4.3 × 10−10 |
| ≥70 vs. <39 | 1.11 (0.82–1.51) | 0.510 |
| 40–69 vs. <39 | 1.65 (1.21–2.23) | 0.0014 |
| Sex | ||
| Female vs. Male | 0.72 (0.67–0.77) | 0 |
| Race | ||
| Black vs. White | 1.33 (1.22–1.45) | 3.9 × 10−11 |
| Other vs. White | 0.80 (0.69–0.93) | 3.3 × 10−3 |
| Other vs. Black | 0.60 (0.51–0.71) | 1.1 × 10−9 |
| Time of MM diagnosis | ||
| post-2000 vs. pre-2000 | 1.31 (1.23–1.40) | 1.8 × 10−15 |
| 2010s vs. 2000s | 1.10 (1.00–1.21) | 5.3 × 10−2 |
| 2010s vs. 1990s | 1.24 (1.13–1.37) | 1.6 × 10−5 |
| 2010s vs. 1980s | 1.47 (1.32–1.64) | 3.6 × 10−12 |
| 2010s vs. 1970s | 1.73 (1.48–2.01) | 2.7 × 10−12 |
| 2000s vs. 1990s | 1.13 (1.04–1.24) | 6.5 × 10−3 |
| 2000s vs. 1980s | 1.34 (1.21–1.48) | 1.2 × 10−8 |
| 2000s vs. 1970s | 1.57 (1.36–1.82) | 1.7 × 10−9 |
| 1990s vs. 1980s | 1.18 (1.07–1.32) | 1.8 × 10−3 |
| 1990s vs. 1970s | 1.39 (1.19–1.61) | 2.0 × 10−5 |
| 1980s vs. 1970s | 1.17 (1.00–1.37) | 4.7 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lv, C.; Zhou, M.; Xu, J.-Y.; Chen, B.; Wan, Y. Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018. Cancers 2022, 14, 4919. https://doi.org/10.3390/cancers14194919
Wang J, Lv C, Zhou M, Xu J-Y, Chen B, Wan Y. Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018. Cancers. 2022; 14(19):4919. https://doi.org/10.3390/cancers14194919
Chicago/Turabian StyleWang, Jing, Chenglan Lv, Min Zhou, Jing-Yan Xu, Bing Chen, and Yuan Wan. 2022. "Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018" Cancers 14, no. 19: 4919. https://doi.org/10.3390/cancers14194919
APA StyleWang, J., Lv, C., Zhou, M., Xu, J.-Y., Chen, B., & Wan, Y. (2022). Second Primary Malignancy Risk in Multiple Myeloma from 1975 to 2018. Cancers, 14(19), 4919. https://doi.org/10.3390/cancers14194919





